Introduction
The Repair of tissue defects following trauma or
tumor ablation is one of the major challenges surgeons face today.
Especially in anatomical regions where a lesion results in
significant impairment of organ function or socially stigmatizing
disfiguration, plastic reconstruction with autologous material is
appropriate and necessary. The unsatisfactory outcomes of previous
attempts to repair muscle defects, e.g., with flaps, triggered the
development of alternative treatment approaches, such as tissue
engineering (1).
The aim of skeletal muscle tissue engineering is to
obtain autologous tissue by isolating and growing stem cells
capable of myogenic differentiation. This engineered tissue is then
used for tissue reconstruction. However, the induction of complete
differentiation in these stem cells is still challenging and
virtually only achieved in immortalized cell lines, such as C2C12
(mouse) or L6 (rat), but not in primary human stem cells (2). Since other studies have shown that
human myoblast/mesenchymal stem cell (MSC) co-cultures resulted in
an increased degree of differentiation and stimulation with static
magnetic fields resulted in enhanced maturation (3,4), the
aim of this study was to investigate the effect of statistic
magnetic fields (SMFs) on the growth of human myoblast/mesenchymal
stem cell (MSC) co-cultures. Satellite cells, also called
myoblasts, and human mesenchymal stem cells (MSCs) are the
preferred stem cells for growing skeletal muscle tissue since their
extraction is easily realized by tissue biopsies and are capable of
stable myogenic differentiation (5). Their ability to replicate without
losing the ability of differentiation is an advantage of MSCs
(3), enabling the generation of
larger numbers of cells from a smaller population. In addition,
MSCs are suitable for autologous grafting and can improve tissue
regeneration by means of immunomodulation (6,7). For
this reason, combining the two types of stem cells to increase the
degree of myogenic differentiation appears to be a promising
approach. Studies on MSC/myoblast co-cultures showed a significant
increase in myoblast proliferation and up-regulation of the
expression of Notch-1, both as mRNA and as protein, indicating
myoblast activation (8). Beier
et al demonstrated that rats' MSCs/myoblasts form hybrid
myotubes as well as an upregulation of the myogenic marker MEF2
(myogenic enhancer factor 2) and α-sarcomeric actin, representing
indicators of myogenic differentiation in MSCs (3). Since the effects of static magnetic
stimulation on myoblasts and MSCs are not yet fully understood and
heterogeneous in terms of proliferation and differentiation,
depending on cell type and strength of the magnetic field, it is
necessary to undertake further studies in this field. Eldashev
et al showed that shielding of the earth's magnetic field
and thus reduction to 0.3 mT resulted in the inhibition of
proliferation and maturation of newborn rat satellite cells, while
60–160 mT magnetic fields had a stimulatory effect (9). Sakurai et al demonstrated that
strong SMFs of 10T induced the formation of orientated myotubes in
immortalized C2C12 mouse myoblast cell cultures (10). Coletti et al found that in
immortalized rat myoblasts (L6) 80 mT SMFs increased the degree of
differentiation, resulting in elevated actin and myosin levels and
the formation of myotubes (4).
However, it was not possible to apply this finding to human
myoblasts. Our working group demonstrated that the effect of
magnetic stimulation on human myoblasts correlates to the serum
concentration in the cell culture medium (11). Myoblasts cultivated in growth
medium (GM) under stimulation of SMF showed a higher fusion index,
indicative of a higher degree of differentiation, compared to
myoblast cell cultures exposed to additional stimulation with a
differentiation medium (DM). Likewise, the additional stimulation
of human myoblasts with SMFs und hepatocyte growth factor (HGF) did
not result in the assumed increase in myogenic differentiation
(12). While an increase in marker
gene expression in human myoblast cultures under SMF and by adding
insulin-like growth factor (IGF) was detected, no contractile
skeletal muscle was found (13).
To determine the degree of differentiation in
co-cultures under stimulation with SMFs, semi-quantitative gene
expression measurements of the following marker genes were
performed: myogenic factor 5 (MYF5), myogenic differentiation
antigen 1 (MYOD1), myogenin (MYOG), adult myosin heavy chain 1
(MYH1), and skeletal muscle α1 actin (ACTA1). Transcription factor
MYF5 is along with MYOD1 and MYOG part of the myogenic regulatory
factors. As promoters of numerous muscle-specific genes, they
control the fusion of mononucleatd muscle fibers (14). MYF5 promotes myoblast proliferation
and is activated together with MYOD1 in the early stage of
myogenesis and thus regarded as an early differentiation marker.
MYOD1 promotes the exit from the cell cycle and induces myogenesis
via positive regulation of cell-cycle inhibitors, such as p21 and
Rb. In addition, it inhibits cell cycle activators, such as cyclins
and cyclin-dependent kinases (15). MYOG acts at a later stage than
MYOD1 and more specificly on the formation of myofibrils (16). The myosin heavy chain (MYH) is a
component of the contractile protein myosin, a hexamer consisting
of four light chains and two heavy chains. Myosin produces a muscle
contraction by transforming chemical energy, derived from the
hydrolysis of ATP, into mechanical force. MYH accounts for almost
50% of the total protein content in skeletal muscle fibers and
occurs in at least 10 different isoforms, which are used for the
characterization of skeletal muscle fibers in fast-twitch and
slow-twitch fibers. During myogenesis, MYH occurs in embryonic,
perinatal and adult isoforms. The expression patterns of the MYH
isoforms are controlled in a development-specific manner and can
thus act as differentiation markers (17). In this study, the adult isoform was
used as a differentiation marker. As a further late differentiation
marker, ACTA1, a key component of the contractile apparatus, was
analyzed. In their studies, Coletti et al showed that in rat
myoblasts the stimulation with SMFs resulted in an accumulation of
ACTA1 in myotubes (4). For this
reason, we conducted proliferation, gene and protein expression
studies in human myoblast/MSC co-cultures with and without
stimulation by an 80 mT SMF and cultivated in GM and DM to
potentially identify a new adequate myogenic stimulus.
Materials and methods
Cell culture
Following the approval of the Ethics Committee II of
the Medical Faculty Mannheim, University of Heidelberg (Mannheim,
Germany)-valid for the collection of all cell lines-stem cells were
obtained by skeletal muscle biopsies during head and neck
surgeries. The biopsy-derived primary human myoblasts were pooled
and expanded for three passages. The degree of purity of the
satellite culture was determined to be more than 80% by testing the
muscle-specific intermediate filament desmin, using
immunohistochemical staining. For myoblast cultivation, cell
culture flasks with 0.2% gelatin coating (culture medium: Ham's F10
Medium + 10% fetal bovine serum + 2 mM
L-glutamine+penicillin/streptomycin/fungizone [PSF]) were used.
Cells were cultivated in an incubator at 37°C, 95% relative
humidity and 5% CO2 in growth medium.
Isolation and cultivation of
mesenchymal stem cells from bone marrow
Isolation and cultivation of human mesenchymal stem
cells from adult bone marrow of the femoral shaft was carried out
as already described by Stern-Straeter (18) by diluting the aspirate with PBS/2
mM EDTA on a Ficoll-Hypaque solution. Cell counting was performed
after density gradient centrifugation (30 min, 435 g, seeded at a
concentration of 1×106 cells/cm2) of the
mononuclear cell (MNC) fraction (bone marrow monocytic cells).
After specification as ‘bone marrow-derived fibroblastoid adherent
cells’, these cells were cultivated in MSCGM or DMEM-lg plus 10%
MSC growth supplements. Once confluence between 70 and 90% was
reached, the FACs were cultivated and passaged.
Co-culture of satellite cell cultures
and MSCs
The satellite cell cultures (myoblasts) and MSC
cultures were mixed in a ratio of 1:1 and cultivated in three
cultures in a growth medium (GM) [Ham's F-10, 1%
penicillin/streptomycin/fungizone (PSF), 2 mM L-glutamine
(PromoCell GmbH, Heidelberg, Germany) and 10% fetal bovine serum
(PAA Laboratories, Linz, Austria)] or a differentiation medium (DM)
[minimal essential medium (PromoCell GmbH), 2% horse serum (PAA
Laboratories), 2 mM L-glutamine, and PSF]. Supernatants were pooled
together later. GM and DM were changed every 72 h and cells were
cultivated in an incubator at 37°C, 95% relative humidity and 5%
CO2 in growth medium.
Use of static magnetic fields for cell
stimulation
As described by Coletti et al, a magnetic
field of 80±5 mT was set underneath the cell culture containers
(distance cell layer-magnet: 1 mm; magnetic field axial to the
magnet's north pole). A control group of cell cultures treated in
the same way (see above) was not exposed to this magnetic
field.
Immunohistochemistry
The cells cultured on the chamber slides were
immunohistochemically characterized to determine the degree of
differentiation of the cells with greater accuracy. For this
purpose, staining with primary antibodies [peroxidase-producing AB,
concentration 1:50 (except for desmin with 1:100, MYH1 1:20)
against MYF5, MYOG, desmin, MYH1, and ACTA1] was carried out.
Corresponding biotinylated antibodies were used as
secondary antibodies based on a peroxidase reaction and
immunologically bound via IgG. For the actual peroxidase reaction,
amino-ethylcarbazole (Dako, Glostrup, Denmark) was used as the
chromogen.
The slides were washed with PBS and incubated in
sheep serum dissolved in PBS for 30 min at room temperature to
prevent unspecific antibody reactions. Harris' hematoxylin was used
for counterstaining the cell nuclei. For the final assessment of
the immunohistochemical stainings, a Zeiss Axiophot light
microscope was used.
Proliferation analysis
The proliferation analysis was carried out using the
alamarBlue® assay (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). In the first batch, cells were fed with growth
medium and differentiation medium and proliferation was measured on
the days 0, 2, 6, 8, and 12 followed by incubation with
alamarBlue® for 24 h and measured via florescence at a
wave length of 540 nm. In the second batch, the effect of the SMF
was determined. Here, myoblast proliferation was measured under
magnetic field stimulation on the days 0, 2, 6, 8, and 12.
RNA isolation
In accordance with the manufacturer's instructions,
total RNA was isolated, using the RNeasy mini kit (Qiagen GmbH,
Hilden, Germany).
RNA concentration, integrity and degree of purity
was measured at A260 and A280 nm (A250/A280=1.7–2.0) using the
NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, Inc.)
and the Agilent 2100 bioanalyzer (Agilent Technologies, Inc., Santa
Clara, CA, USA).
cDNA synthesis and semi-quantitative
PCR
For cDNA synthesis, total RNA was used and treated
with 1 U DNAse for 30 min at 37°C. Reverse transcription of the RNA
(0.5 µg) was carried out using the oligo(dT)-primed first-strand
cDNA synthesis kit (Roche Diagnostics GmbH,, Mannheim, Germany) for
1 h at 42°C. Using Taq DNA polymerase (Amersham Pharmacia Biotech,
Buckinghamshire, UK) and using 2–5 µl from each
reverse-transcription products, all cDNA samples were tested for
the following genes: MYOG, ACTA1, MYF5, MYOD1, desmin, MYH1, and
GAPDH. Therefor a Primus 96 Plus thermal cycler (MWG Biotech,
Freiburg, Germany) was used.
Electrophoresis and analysis
Electrophoresis was run in 2% agarose gel with added
ethidium bromide. Subsequently, images of the PCR products were
displayed under UV light. Using GAPDH as a standard, relative gene
expression was calculated with the software ImageJ (National
Institutes of Health, Bethesda, MD, USA).
Results
Proliferation analysis from satellite
cell/MSC co-cultures with and without static magnetic field (SMF)
stimulation
The proliferation behavior was determined using the
alamarBlue® proliferation assay from day 0 to day 12 in
human satellite cell/MSC co-cultures, cultivated in growth medium
(GM) or differentiation medium (DM). In addition, these co-cultures
were stimulated with SMFs. Cell cultures without SMF stimulation
served as controls. The proliferation behavior of the co-culture
showed in both GM and DM without SMF stimulation continuously
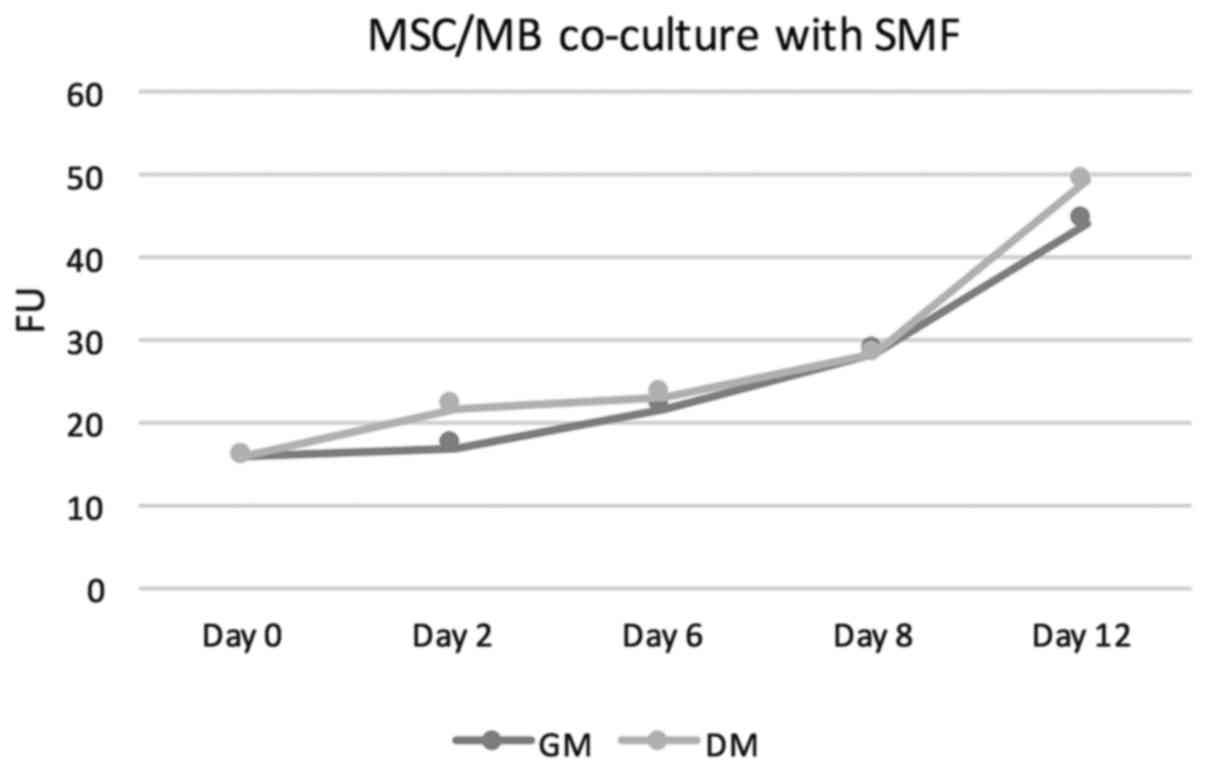
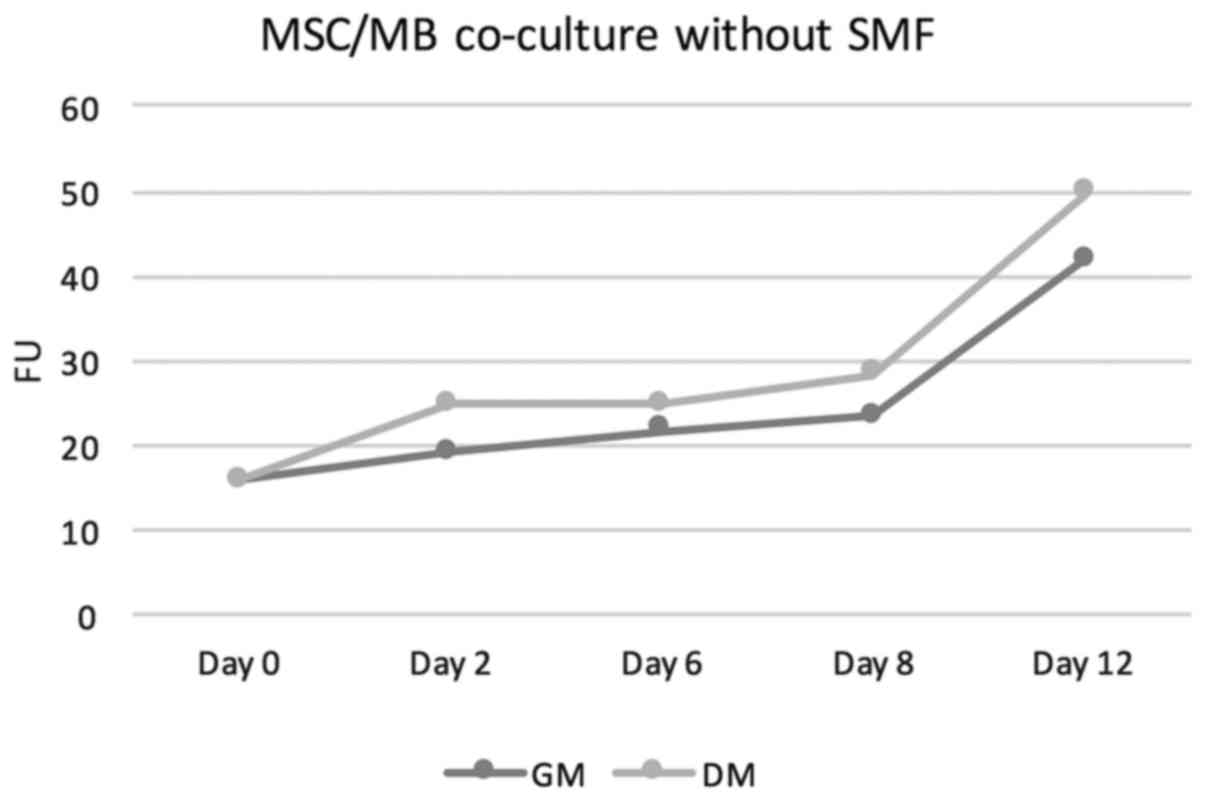
increasing proliferation rates. For details see Table I. The measured fluorescence units
(FUs) increased from a baseline value of 15,81 at the start of the
cell culture to values of 21,66 in GM and 24.92 in DM on day 6 to
values of 41.77 on day 12 in GM and 49.75 in DM. Thus, FUs in
DM-treated co-culture were at all points slightly above those
measured in GM-cultivated co-cultures. Under additional stimulation
with SMFs, no significant change in proliferation rates neither in
DM-cultivated co-cultures nor in GM-treated co-cultures was
overserved. In this group as well, the proliferation rate showed a
steady increase. On day 6, FUs of 21.86 were measured in GM+SMF and
FUs of 23.17 in DM+SMF. On day 12, the FUs were 44.29 in GM+SMF and
49.27 in DM+SMF. Figs. 1 and
2 provides graphical
interpretation.
 | Table I.alamarBlue® proliferation
assay results in FU of the of human MSC/MB co-cultures on GM and DM
without SMF. |
Table I.
alamarBlue® proliferation
assay results in FU of the of human MSC/MB co-cultures on GM and DM
without SMF.
|
| Day 0 | Day 2 | Day 6 | Day 8 | Day 12 |
|---|
| GM | 15.87 | 17.09 | 21.86 | 28.36 | 44.29 |
| DM | 15.87 | 21.79 | 23.17 | 28.19 | 49.27 |
Gene expression analysis
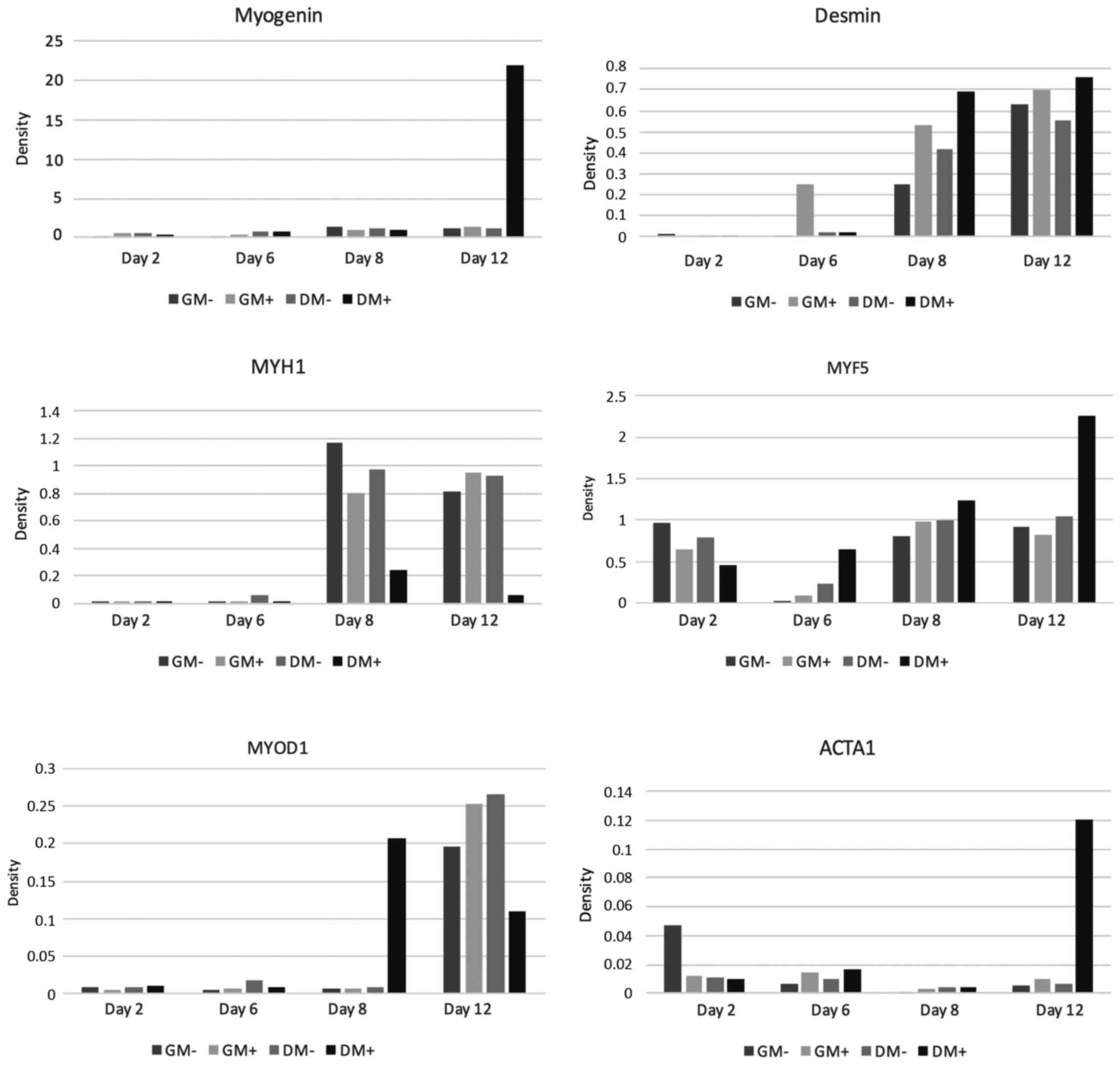
MYF 5
Gene expression analysis of the early myogenic
differentiation marker MYF5 showed positive findings in all tested
co-cultures. On day 2, the relative expression values, both in GM
and in DM, were lower in the co-cultures stimulated with SMF
compared to the non-stimulated cultures. On day 6 and on day 8,
however, the relative expression rates in co-cultures treated with
SMF, both in GM and in DM, were above those in non-stimulated
co-cultures. On day 12, MYF5 expression of non-stimulated
co-cultures was slightly higher in GM, whereas in DM a
significantly higher expression rate was found in stimulated cells.
For graphical interpretation see Fig.
3.
MYOD1
As shown in Fig. 3,
during the first days of the cell culture, MYOD1 expression
analysis showed initially low expression rates, both in
SMF-stimulated and non-SMF-stimulated co-cultures. On day 2 and day
6, the expression rates found in both SMF-stimulated and
non-SMF-stimulated co-cultures were almost identical, regardless of
the culture medium. On day 8, relative expression of MYOD1
significantly increased in SMF-stimulated co-cultures cultivated in
DM. The highest expression rates were detected on day 12. Here,
relative expression in co-cultures growing in GM were higher in
SMF-stimulated cells compared to non-SMF-stimulated cells. In
DM-cultivated co-cultures, by contrast, higher expression rates
were detected in non-SMF-stimulated cells. In the gene expression
analysis of MYOD 1, the initially similarly low values are
noteworthy, then, strikingly, there was a high value for cell
proliferation in the differentiation medium under the influence of
the magnetic field on day 8, while on day 12 these values were
below those in the growth medium with and without SMF and in the DM
without SMF.
MYOG
Gene expression measurement of myogenin showed a
mild time-dependent increase in all groups examined; the highest
expression was detected on day 12 in SMF-stimulated co-cultures
cultivated in DM. For details see Fig.
3.
ACTA1
On day 2, ACTA1 expression initially showed slightly
lower values for SMF-stimulated co-cultures compared to the
non-SMF-stimulated groups. On days 6, 8 and 12, however, the
expression values of SMF-treated cultures, both in GM and DM
cultivated cells, were above those in non-SMF-stimulated cells. The
highest expression was detected on day 12 in SMF-stimulated
co-cultures cultivated in DM (Fig.
3).
MYH1
Gene expression measurement of MYH1 as a terminal
differentiation marker showed significantly higher expression
values on day 8 compared to days 2 and 6. Non-SMF-stimulated
co-cultures demonstrated higher expression rates compared to
SMF-stimulated ones. On day 12, however, MYH1 expression in
stimulated co-cultures was higher compared to non-stimulated
co-cultures cultivated in GM. In SMF-stimulated co-cultures
cultivated in DM, MYH1 expression was markedly depressed (Fig. 3).
Desmin
Expression analysis of desmin showed a continuous
rise in expression with increasing cultivation length. Starting
from day 6, the expression values of SMF-stimulated co-cultures
were higher compared to those of non-stimulated cells, regardless
of the cell culture medium used (Fig.
3).
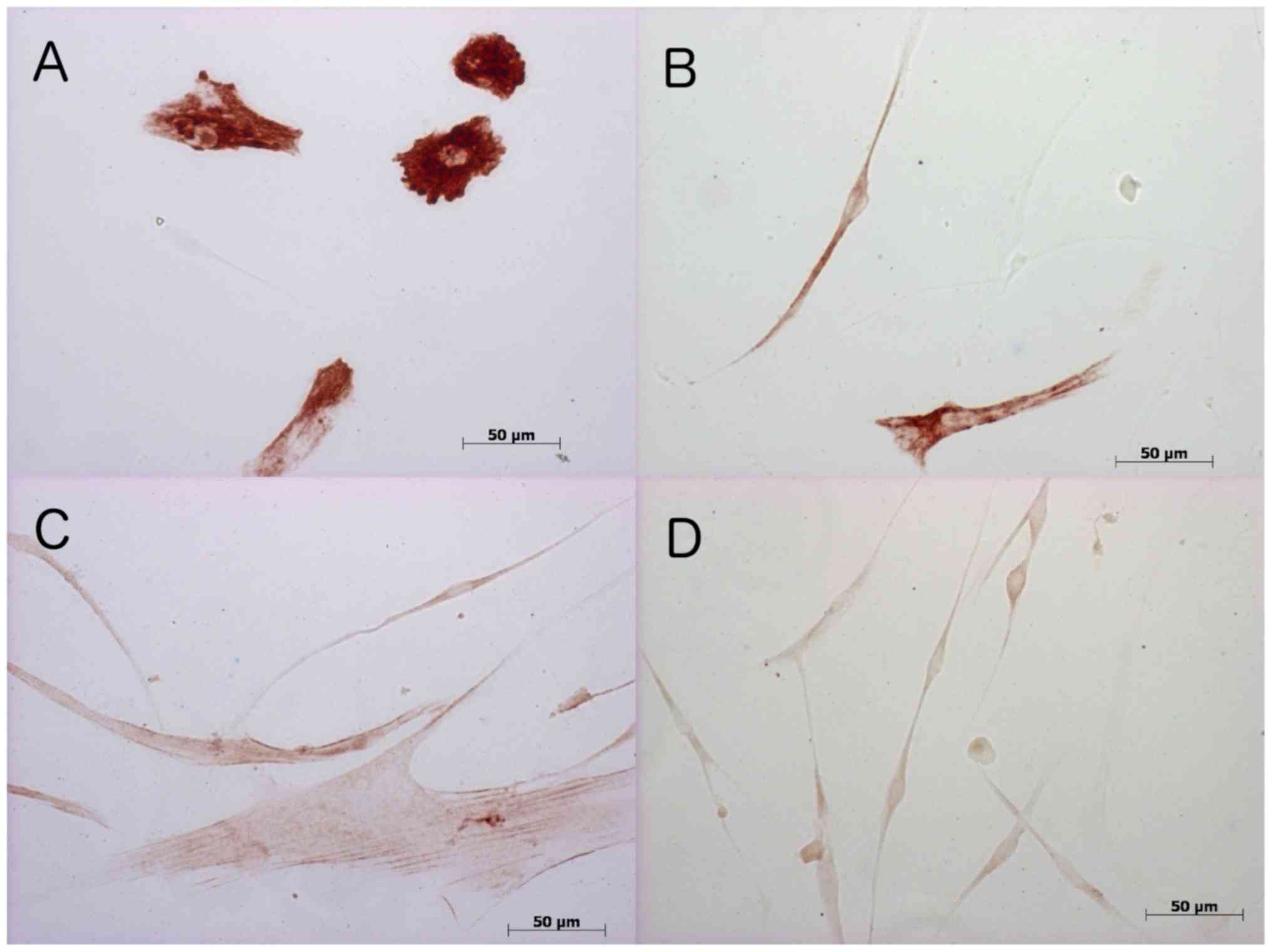
Immunohistochemistry
To validate mRNA measurements and to identify
conceivable differences to the protein form, immunohistochemical
stainings using monoclonal antibodies against desmin, MYOD1, MYOG,
and ACTA1 were performed. Stain distribution is shown in GM at
Table II and in DM in Table III. Fig. 4 provides examples of
immunohistochemical stainings.
 | Table II.Stain distribution of Desmin, Myogenin
and ACTA1 in human MSC/(MB) co-cultures on GM with and without SMF
exposure.a |
Table II.
Stain distribution of Desmin, Myogenin
and ACTA1 in human MSC/(MB) co-cultures on GM with and without SMF
exposure.a
|
| GM without SMF | GM with SMF |
|---|
|
|
|
|
|---|
| Antibodies | Day 0 | Day 2 | Day 6 | Day 8 | Day12 | Day 0 | Day 2 | Day 6 | Day 8 | Day 12 |
|---|
| Desmin | ++ | + | ++ | ++ | ++ | ++ | + | ++ | ++ | + |
| Myogenin | ++ | ++ | ++ | +-++ | +-++ | ++ | neg | ++ | + | + |
| ACTA1 | neg | + | + | + | +-++ | neg | + | neg | + |
|
 | Table III.Stain distribution of Desmin,
Myogenin and ACTA1 in human MSC/MB co-cultures on DM with and
without SMF exposure.a |
Table III.
Stain distribution of Desmin,
Myogenin and ACTA1 in human MSC/MB co-cultures on DM with and
without SMF exposure.a
|
| DM without SMF | DM with SMF |
|---|
|
|
|
|
|---|
| Antibodies | Day 0 | Day 2 | Day 6 | Day 8 | Day 12 | Day 0 | Day 2 | Day 6 | Day 8 | Day 12 |
|---|
| Desmin | ++ | + | + | + | + | ++ | + | + | + | + |
| Myogenin | ++ | ++ | neg | neg | neg | ++ | ++ | + | neg | neg |
| ACTA1 | neg | + | + | neg | neg | neg | neg | neg | + |
|
Desmin
Immunohistochemical staining of MSC/satellite cell
co-cultures to the muscle-specific intermediate filament desmin was
detected at all points in time in both GM and DM cultivated cells.
In co-cultures cultivated with GM, SMF stimulation did not result
in any difference in expression rate until day 8. On day 12, the
proportion of desmin measured in non-SMF-stimulated co-cultures was
higher compared to the stimulated co-cultures. By contrast, in
cultures cultivated with DM, no difference between stimulated and
non-stimulated co-cultures was detected.
MYOG
Immunohistochemical staining to the transcription
factor MYOG in co-cultures cultivated in GM revealed a trend
towards higher detection rates in non-SMF-stimulated cultures
compared to stimulated cultures. Without SMF stimulation, the
detection rates on days 2 and 6 were higher than on days 8 and 12.
In DM, myogenin as an early differentiation marker was only
detected on the first days of cell culture, regardless of with or
without SMF stimulation.
ACTA1
As a component of the contractile apparatus, the
late differentiation marker ACTA 1 was detected in
non-SMF-stimulated cell cultures cultivated in GM, starting from
day 2 of the cell culture. The highest detection rates in this
group were achieved on day 12. Under the influence of SMFs, ACTA1
detection in the GM group declined and only on day 6 expression
rates were the same. At all other times, the detection rates were
below those found for non-SMF-stimulated MSC/satellite cell
co-cultures. ACTA1-positive cells were reduced by using DM and only
detectable without SMF stimulation on days 2 and 6. With additional
SMF stimulation, ACTA1 was solely detected on day 12.
Discussion
Induction of stable myogenic differentiation in
human stem cells is a basic requirement for skeletal muscle tissue
engineering intended to generate adequate amounts of tissue for the
repair of skeletal muscle defects, resulting from injuries or tumor
ablation procedures. Given their muscle origin and stable myogenic
differentiation potential, satellite cells are the most promising
and most often used primary cells for the cultivation of skeletal
muscle (19). However, satellite
cells loose their differentiation ability. Therefore, the
production of large volumes of muscle tissue sufficient to meet
today's clinical demand is still a very challenging task (20). One reason for the loss of
differentiation ability is the heterogeneity in the satellite cell
population (21).
Mesenchymal stem cells (MSCs) are regarded as an
alternative, promising cell type, because they do not loose their
differentiation potential following expansion (3) and can be extracted from a variety of
tissue types, including bone marrow, adipose tissue, umbilical cord
blood, and placental tissue. However, whether all types of MSCs or
only subpopulations can be differentiated into skeletal muscle,
remains unclear (18,22,23).
The phenotype and myogenic differentiation potential of the
different MSCs vary with the respective tissue from which the cells
originate (24). It was shown that
MSCs from bone marrow were capable of supporting muscle
regeneration in vivo (5,25)
and thus appeared to be suitable for tissue engineering. However,
attempts to achieve myogenic differentiation of human MSCs of bone
marrow origin solely by stimulation with cell culture media failed
(18). Thus, it was assumed that
paracrine factors, such as cytokines, and the extracellular matrix
play an important role in the process of myogenic differentiation.
Another way to accomplish myogenic differentiation of MSCs is to
grow them in co-culture with satellite cells. Beier et al
showed that rat MSCs in co-culture with myoblasts formed myotubes
(3). Likewise, Di Rocco et
al demonstrated that a co-culture combining adipose mouse MSCs
and myoblasts boosted the myogenic phenotype (26). However, there is a lack of studies
investigating human satellite cell/MSC co-cultures, even though
such data are crucial for tissue engineering. To gain a better
understanding, we conducted this study. Static magnetic fields are
another myogenic differentiation stimulus that has the potential to
be clinically useful. For example, Coletti et al showed that
in the L6 rat cell line SMFs promoted actin and MYH1 formation,
indicative of increased differentiation (4). However, studies with human satellite
cells, the preferred stem cell for skeletal muscle tissue
engineering, found that the effect of SMF stimulation depends on
the growth factor concentration in the cell culture medium and that
a combination of differentiation medium (low growth factor
concentration) and SMF did not result in the desired increase in
the degree of differentiation (11). For this reason, it is of interest
to investigate the effect of SMF stimulation on human MSC/satellite
cell co-cultures and to assess its impact on myogenic
differentiation potential.
To determine the effect of SMFs on proliferation
behavior in co-cultures, alamarBlue® cell proliferation
assays were performed. These showed that 80 mT SMF stimulation had
no effect on proliferation behavior in these co-cultures,
regardless of the growth factor concentration in the cell culture
medium. This result is in line with our data from human satellite
cell cultures. It is also confirmed by data obtained from myoblast
cultures derived from other species, showing that SMFs of this
strength do not influence the proliferation behavior of the cells
studied (4,11). That the proliferation rate in DM
were slightly higher compared to those of the co-cultures in GM, is
an unexpected finding, since in cultures with only satellite cells
the high growth-factor concentration resulted in increased
proliferation (11). Apparently,
this effect does not occur in human MSC/satellite cell
co-cultures-a new insight. Given the continued proliferation of
MSCs cultured under low growth factor conditions, MSC proliferation
capacity is apparently to some extent independent of growth factor
concentrations in the cell culture medium used. This phenomenon
appears to offset the inhibited proliferation capacity of human
satellite cells, as the proliferation measurements in MSC/satellite
cell co-cultures yielded comparable proliferation rates for growth
medium and differentiation medium (high and low growth factor
concentrations). This confirms the results of our previous studies
where we showed that the percentage of growth factor in cell
culture medium had no significant effect on the proliferation
capacity of human MSCs derived from adipose tissue or bone marrow
(18). Analysis of quantitative
gene expression measurements of the early myogenic marker genes
MYF5, MYOD1 and myogenin revealed a rise in expression rates in the
co-culture with advancing cell culture duration. The highest
expression rates of MYF5, MYOD1 and myogenin were detected on day
12. However, neither in co-cultures cultivated in GM nor in those
cultivated in DM, a repeated effect of SMF stimulation was
detectable. At all points of measurement, the muscle, specific
intermediate filament desmin, which, due to its early expression
during myogenesis, is an early myogenic marker (27), was detected. As with MYF5, MYOD1
and myogenin, the highest expression rates were detected on day 12.
This shows that myogenic differentiated cells were present in the
co-cultures at all points in time and that the degree of
differentiation increased with time. However, no evidence of a
significant, continuous effect of SMF stimulation, independent of
growth factor concentrations in the cell culture medium, was
found.
For myogenic markers indicative of late myogenesis,
such as ACTA1 and MYH1, increased expression values were measured
during the later days of cell culture monitoring. However, for
these markers too, no significant effect of SMFs on myogenic
differentiation behavior, in terms of an increase in marker gene
expression, was detected. Therefore, the cells of the co-culture do
undergo myogenic differentiation with advancing cell culture
duration, but this differentiation process is not enhanced by SMF
exposure, regardless of the growth factor concentration in the cell
culture medium. Consequently, the results obtained for SMF
stimulation of human satellite cell monocultures are not consistent
with those obtained for human MSC/satellite cell co-cultures. For
monocultures we demonstrated that the SMF-induced pro-myogenic
stimulation effect was dependent on growth factor concentration
(11). We found that only cultures
grown in GM showed increased fusion as an indicator of myogenic
maturation, but not satellite cells cultured in DM. Since we could
not demonstrate this effect in the co-culture, it represents a new
research finding. While the exact mechanism underlying the effect
of SMFs remains unclear, we know that it is influenced by cell type
and cell origin. Contrary to our expectations, this study did not
find a pro-myogenic effect of SMFs in human MSC/satellite cell
co-cultures. Another factor which may explain the difference
between our results and those of Beier et al is their use of
rat myoblasts and rat MSCs as well as other stimulating agents
(basic fibroblast growth factor; dexamethasone) (3). This shows that the results obtained
in studies using cells from other species cannot always be applied
to human stem cells-an insight which is of fundamental importance
for tissue engineering.
Overall, the analysis revealed marked heterogeneity
in the expression rates of the analyzed markers. This can be
explained by the fact that MSCs represent a heterogeneous group of
cells with diverse myogenic differentiation potential. Similarly,
the studies by Di Rocco et al found significant variability
in the analyzed myogenic markers (26). The results of the quantitative gene
expression measurements are partly confirmed by the results of the
immunohistochemical examinations. Immunohistochemical staining
succeeded in detecting myogenic markers in the co-culture,
regardless of the growth factor concentration in the cell culture
medium. However, here again, SMF stimulation did not result in any
significant increase in myogenic markers, such as desmin and ACTA1.
Overall, immunohistochemical staining showed high variability in
the measurements of the myogenic markers which is explained by the
heterogeneity of the MSCs.
In conclusion, 80 mT SMF stimulation had no
pro-myogenic effect on human satellite cell/MSC co-culture,
regardless of the growth factor concentrations in the cell culture
medium.
Acknowledgements
This study contains parts of the doctoral thesis of
Cornelia Emika Müller.
References
|
1
|
Stern-Straeter J and Hörmann K: New
perspectives in skeletal muscle tissue engineering. HNO.
62:415–422. 2014.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stern-Straeter J, Riedel F, Bran G,
Hörmann K and Goessler UR: Advances in skeletal muscle tissue
engineering. In Vivo. 21:435–444. 2007.PubMed/NCBI
|
|
3
|
Beier JP, Bitto FF, Lange C, Klumpp D,
Arkudas A, Bleiziffer O, Boos AM, Horch RE and Kneser U: Myogenic
differentiation of mesenchymal stem cells co-cultured with primary
myoblasts. Cell Biol Int. 35:397–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coletti D, Teodori L, Albertini MC, Rocchi
M, Pristerà A, Fini M, Molinaro M and Adamo S: Static magnetic
fields enhance skeletal muscle differentiation in vitro by
improving myoblast alignment. Cytometry A. 71:846–856. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrari G, Cusella-De Angelis G, Coletta
M, Paolucci E, Stornaiuolo A, Cossu G and Mavilio F: Muscle
regeneration by bone marrow-derived myogenic progenitors. Science.
279:1528–1530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Castro J, Trigueros C, Madrenas J,
Pérez-Simón JA, Rodriguez R and Menendez P: Mesenchymal stem cells
and their use as cell replacement therapy and disease modelling
tool. J Cell Mol Med. 12:2552–2565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sassoli C, Pini A, Chellini F, Mazzanti B,
Nistri S, Nosi D, Saccardi R, Quercioli F, Zecchi-Orlandini S and
Formigli L: Bone marrow mesenchymal stromal cells stimulate
skeletal myoblast proliferation through the paracrine release of
VEGF. PLoS One. 7:e375122012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eldashev IS, Shchegolev BF, Surma SV and
Belostotskaia GB: Effect of low-intensity magnetic fields on the
development of satellite muscle cells of a newborn rat in the
primary culture. Biofizika. 55:868–874. 2010.(In Russian).
PubMed/NCBI
|
|
10
|
Sakurai T, Hashimoto A, Kiyokawa T,
Kikuchi K and Miyakoshi J: Myotube orientation using strong static
magnetic fields. Bioelectromagnetics. 33:421–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stern-Straeter J, Bonaterra GA, Kassner
SS, Faber A, Sauter A, Schulz JD, Hörmann K, Kinscherf R and
Goessler UR: Impact of static magnetic fields on human myoblast
cell cultures. Int J Mol Med. 28:907–917. 2011.PubMed/NCBI
|
|
12
|
Birk R, Sommer U, Faber A, Aderhold C,
Schulz JD, Hörmann K, Goessler UR and Stern-Straeter J: Evaluation
of the effect of static magnetic fields combined with human
hepatocyte growth factor on human satellite cell cultures. Mol Med
Rep. 9:2328–2334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Birk R, Sommer JU, Haas D, Faber A,
Aderhold C, Schultz JD, Hoermann K and Stern-Straeter J: Influence
of static magnetic fields combined with human insulin-like growth
factor 1 on human satellite cell cultures. In Vivo. 28:795–802.
2014.PubMed/NCBI
|
|
14
|
Christ B and Brand-Saberi B: Limb muscle
development. Int J Dev Biol. 46:905–914. 2002.PubMed/NCBI
|
|
15
|
Brand-Saberi B: Genetic and epigenetic
control of skeletal muscle development. Ann Anat. 187:199–207.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ridgeway AG, Petropoulos H, Wilton S and
Skerjanc IS: Wnt signaling regulates the function of MyoD and
myogenin. J Biol Chem. 275:32398–32405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pette D and Staron RS: Myosin isoforms,
muscle fiber types, and transitions. Microsc Res Tech. 50:500–509.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stern-Straeter J, Bonaterra GA, Juritz S,
Birk R, Goessler UR, Bieback K, Bugert P, Schultz J, Hörmann K,
Kinscherf R and Faber A: Evaluation of the effects of different
culture media on the myogenic differentiation potential of adipose
tissue- or bone marrow-derived human mesenchymal stem cells. Int J
Mol Med. 33:160–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stern-Straeter J, Bran G, Riedel F, Sauter
A, Hörmann K and Goessler UR: Characterization of human myoblast
cultures for tissue engineering. Int J Mol Med. 21:49–56.
2008.PubMed/NCBI
|
|
20
|
Carlson ME and Conboy IM: Loss of stem
cell regenerative capacity within aged niches. Aging Cell.
6:371–382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pietrangelo T, Puglielli C, Mancinelli R,
Beccafico S, Fanò G and Fulle S: Molecular basis of the myogenic
profile of aged human skeletal muscle satellite cells during
differentiation. Exp Gerontol. 44:523–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara
T, Hoshino M, Takeda S, Ide C and Nabeshima Y: Bone marrow stromal
cells generate muscle cells and repair muscle degeneration.
Science. 309:314–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corti S, Strazzer S, Del Bo R, Salani S,
Bossolasco P, Fortunato F, Locatelli F, Soligo D, Moggio M, Ciscato
P, et al: A subpopulation of murine bone marrow cells fully
differentiates along the myogenic pathway and participates in
muscle repair in the mdx dystrophic mouse. Exp Cell Res. 277:74–85.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de la Garza-Rodea AS, van der Velde-van
Dijke I, Boersma H, Gonçalves MA, van Bekkum DW, de Vries AA and
Knaän-Shanzer S: Myogenic properties of human mesenchymal stem
cells derived from three different sources. Cell Transplant.
21:153–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
LaBarge MA and Blau HM: Biological
progression from adult bone marrow to mononucleate muscle stem cell
to multinucleate muscle fiber in response to injury. Cell.
111:589–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Rocco G, Iachininoto MG, Tritarelli A,
Straino S, Zacheo A, Germani A, Crea F and Capogrossi MC: Myogenic
potential of adipose-tissue-derived cells. J Cell Sci.
119:2945–2952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Allen RE, Rankin LL, Greene EA, Boxhorn
LK, Johnson SE, Taylor RG and Pierce PR: Desmin is present in
proliferating rat muscle satellite cells but not in bovine muscle
satellite cells. J Cell Physiol. 149:525–535. 1991. View Article : Google Scholar : PubMed/NCBI
|