Introduction
Depressive disorder (DD) imposes a severe burden on
the afflicted individuals and is becoming increasingly prevalent in
modern society (1). The most
generally accepted hypothesized pathogenesis of DD entails a
depletion in levels of monoamines including 5-hydroxytryptamine
(5-HT), norepinephrine and dopamine in the central nervous system
(2). Therefore, selective
serotonin reuptake inhibitors (SSRIs) including fluoxetine,
paroxetine, fluvoxamine, sertraline and citalopram are currently
the first line antidepressants (3,4).
Monoaminergic antidepressants serve crucial roles in depression
therapy, however their efficacy is only 60–70% and the side effects
include sexual problems, drowsiness, fatigue, sleep difficulties,
nausea, weight gain, nervousness, dry mouth and blurred vision
(3,4) indicating that alternative treatments
are necessary (5).
Chinese herbal formulas have advantages in
antidepressant treatment with broad prospects for development
(6). The classical Chinese herbal
formula Xiao Yao San (XYS) has been verified to have an
antidepressive effect with few side effects in clinical studies
(7,8). However, its composition of six
Chinese herbs leads to difficulties in identifying its
pharmacological mechanism.
The Shuyu capsule (SYC), a novel Chinese herbal
formula preparation developed by the authors' research team based
on a modification of XYS, was used in a clinical therapy for
depression approved by the China Food and Drug Administration
(Approval Number: 2008L11169). The SYC is composed of active
ingredients extracted from four Chinese herbs, namely Bupleurum
chinensis, Paeonia sufruticosa, Cyperus rotundus
and Glycyrrhiza uralensis Fisch. Although previous
pharmacological studies have revealed that components of the SYC
have antidepressant effects, the antidepressant mechanism of the
SYC remains unclear (9–12). The authors' previous study
demonstrated that levels of the 5-HT1A receptor (5-HT1AR) decrease
in the hippocampus of rats with chronic mild stress (CMS)-induced
depression and increase with use of the SYC (13).
5-HT1AR is of primary research interest, due to its
involvement in depression and anxiety states (14). The therapeutic effect of the most
commonly prescribed antidepressants, including SSRIs and the
partial 5-HT1AR agonist buspirone, may in part be associated with
the enhancement of 5-HT neurotransmission in the hippocampus,
involving the 5-HT1AR (15–17).
In line with these results, a reduced binding potential and mRNA
level of the 5-HT1AR have been observed in the hippocampus of major
DD patients (18,19).
The 5-HT1AR has been tied to a variety of
physiological and pathological processes as it is involved in a
number of signal transduction pathways. A previous study observed
that 5-HT1AR is an inhibitory G-protein coupled receptor (20). Agonist binding to 5-HT1ARs
exchanges GDP for GTP on the subunit of Gi/o and then inhibits
adenylyl cyclase (AC), resulting in decreased intracellular cyclic
adenosine monophosphate (cAMP) production (21). In turn, cAMP, as an important
second messenger, mediates a number of intracellular signaling
cascades, including the protein kinase A (PKA)-cAMP response
element-binding (CREB) signaling pathway (22). A number of animal tests have
indicated that the cAMP-PKA-CREB signaling pathway in hippocampus
is closely related to depression and the pathogenesis of cognitive
function impairments (23).
Chronic unpredictable stress reduced the expression of cAMP, PKA,
CREB in the hippocampus of model rats (24). This signaling pathway contributes
to impaired neurogenesis, similar to depressive-like behaviors
(25).
To determine whether the SYC serves antidepressive
roles partly by acting on 5-HT1ARs in the brain, the effects of the
SYC on the expression level of the 5-HT1AR and activation of the
5-HT1AR-mediated AC-cAMP-PKA-CREB signal transduction pathway were
investigated in hippocampal neurons in vitro, using a serum
pharmacological method.
Materials and methods
Animals and primary reagents
A total of 40 male adult Wistar rats [Charles River
Laboratories, Beijing, China, License No. SCXK (Beijing, China)
2012–0001], weighing 110–130 g and 6–8 weeks old, were used in the
experiment. The rats were given 1 week upon arrival to adjust to
the novel environment (21±1°C, 50–60% humidity, white noise (40±10
dB) and a 12-h light/dark cycle with light from 8:00 p.m. to 8:00
a.m.). Food and water were available freely prior to the
experimental procedures. The rats were housed separately according
to the chronic mild stress (CMS) procedure. Wistar rats born within
24 h were purchased from the Laboratory Animal Center, Shandong
University of Traditional Chinese Medicine, Shandong China, [SCXK
(Lu) 20120003] and sacrificed upon arrival. All animal care
procedures were carried out in accordance with the National
Institutes of Health Guide for Care and were approved by the
Institutional Committee for Animal Care and Use of Shandong
University of Traditional Chinese Medicine (Approval ID:
DWSY201206102).
The SYC was purchased from the Haichuan Research
Center for Innovative Biological Natural Drug Discovery (Qingdao,
China). The quality and stability of the SYC were the same as those
in a previous study (26).
Neurobasal A media, L-glutamine, a B27 supplement and fetal bovine
serum were obtained from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The rat cAMP ELISA kit (cat. no. F15181) was
purchased from Xi Tang Biotechnology Co., Ltd., (Shanghai, China).
The 5-HT1AR primary antibody (cat. no. ab44635) and 5-HT1AR
antagonist WAY-100635 (cat. no. ab120550) were purchased from Abcam
(Cambridge, UK). Primary antibodies against PKA (cat. no. 5842) and
CREB (cat. no. 9197) in addition to the activated (phosphorylated)
versions of these proteins (cat. nos. 4781 and 9191) were purchased
from Cell Signaling Technology, Inc., (Danvers, MA, USA). Mouse
monoclonal antibodies against β-actin (cat. no. A1978) and the
5-HT1AR agonist (R)-(+)-8-hydroxy-DPAT hydrobromide (cat. no. H140)
were obtained from Sigma-Aldrich; Merck KGaA, (Darmstadt,
Germany).
Animal treatments
Experimental procedure and
grouping
The depression rat model was established using CMS
as described below. Prior to stress, all adult rats were randomly
divided into the following four groups (n=10 per group): i) Control
group [received intragastric (ig) administration daily with normal
saline at 5 ml/kg·d]; ii) model group (received stress plus normal
saline at 5 ml/kg·d, ig, daily); iii) SYC group (received stress
and SYC at 0.408 g/kg·d ig, daily); and iv) fluoxetine group
(received stress and fluoxetine 2.67 mg/kg·d, ig, daily). The
treatments lasted for 4 weeks. Prior to treatments and at the end
of every week during treatments, a sucrose preference test and body
weight measurement were performed. Finally, 1.5 h following the
last dose, sodium pentobarbital anesthesia at 45 mg/kg,
intraperitoneally, was administered to the rats. Abdominal aorta
blood (as much as possible) was collected, left for 1 h and then
centrifuged at 800 × g for 15 min at 4°C; subsequently, the serum
was obtained from all rats in all groups and inactivated in a 56°C
water bath for 30 min, filter sterilized through a 0.45-µm
membrane, sealed, and preserved at −70°C for cell treatment.
CMS procedure
The CMS protocol consisted of water deprivation for
24 h, food deprivation for 24 h, cage rotation for 30 min, a cage
tilt of 45°C for 16 h, white noise (100 dB) for 3 h, wet bedding
(100 ml water/individual cage) for 17 h, empty water bottles for 1
h, restricted access to food for 2 h, gauze bundling for 1 h,
continuous lighting for 24 h, mothball odor for 24 h, a foreign
object in the cage for 17 h and stroboscopic lighting (100
frequency/min) for 1 h. These stressors were sequential and mild,
as described by Willner et al (27). The stressors were applied in a
random sequence. The CMS procedure was administered to the stressed
rats once per day for 4 weeks.
Sucrose preference test (SPT)
The sucrose preference test was performed on days 0,
7, 14, 21 and 28. Prior to the start of the test, the rats were
trained to consume 1% sucrose solution. They were habituated for 48
h to two bottles, one with 1% sucrose and the other with tap water,
followed by a period of 24 h with no food or water available, and a
1 h exposure to the two identical bottles again for testing fluid
consumption. To have a concordance measure for all groups, each rat
in a control group was randomly selected and housed individually at
the beginning of this test. Two-bottle tests for each cage were
adopted throughout the procedure. Sucrose solution consumption was
recorded by calculating the volume of the test solution. Sucrose
preference=sucrose consumption (g)/[sucrose consumption (g) + tap
water consumption (g)]x100%.
Hippocampal neuron culture and
treatments
Cell culture and appraisal
Primary cultures of dissociated hippocampal neurons
were performed as previously described (28). Hippocampi were dissected from
neonatal rats within 24 h and collected in Hank's balanced salt
solution (HBSS) containing 1% penicillin/streptomycin and 14
isolated hippocampi were then dissociated at 37°C for 15 min in
0.25% trypsin-EDTA. The digestion medium was then replaced with a
dissection buffer (HBSS solution containing 1%
penicillin/streptomycin) and they were centrifuged at 200 × g for 1
min at room temperature twice. Pelleted cells were centrifuged at
300 × g for 5 min at room temperature and washed with inoculated
culture medium (Neurobasal A media plus 0.5 mM L-glutamine, the B27
supplement and 10% fetal bovine serum). Hippocampus cells
(5×106 cells/cm2) were plated into six-well
plates precoated with poly-L-lysine in inoculated culture medium
followed by incubation at 37°C in a humidified atmosphere
containing 5% CO2. The entire medium was replaced with
fresh inoculated culture medium following 12 h. Half of the medium
was then replaced with fresh serum-free neuronal maintenance medium
(Neurobasal A media plus 0.5 mM L-glutamine and the B27 supplement)
twice weekly. The cell viability was measured using 5 mg/ml MTT
with the formazan dissolved in dimethyl sulfoxide and absorbance
read at 490 nm.
Cell treatment
To detect the effect of the SYC on 5-HT1AR protein
levels in primary cultured hippocampal neurons, cells were randomly
divided into the following groups: i) Control group (with no serum
treatment), ii) normal group (treatment with serum from normal
rats), iii) model group (treatment with serum from CMS rats), iv)
SYC group (treatment with serum from SYC rats) and v) fluoxetine
group (treatment with serum from fluoxetine rats). All serum
treatments were administered for 48 h at 10% concentration.
To investigate the role of 5-HT1AR and the
AC-cAMP-PKA-CREB signaling cascade activity in the antidepressive
effect of SYC treatment on hippocampal neurons, primary cultured
hippocampal neurons were divided randomly into seven groups: i)
Control group (with no serum treatment); ii) normal group
(treatment with serum from normal rats); iii) model group
(treatment with serum from CMS rats); iv) SYC group (treatment with
serum from SYC rats); v) fluoxetine group (treatment with serum
from fluoxetine rats); vi) model plus 5-HT1AR agonist group
(treatment with serum from CMS rats plus 1 µM
(R)-(+)-8-hydroxy-DPAT hydrobromide stimulation for 45 min); and
vii) model plus 5-HT1AR antagonist group (treatment with serum from
CMS rats plus 0.5 µM WAY-100635 stimulation for 60 min). All serum
treatments lasted for 24 h at 10% concentration.
To further verify the role of the 5-HT1AR-mediated
AC-cAMP-PKA-CREB postreceptor signal transduction pathway in the
antidepressive effect of the SYC, hippocampal neurons that
underwent primary culture in vitro were divided into four
groups: i) Normal group (with no serum treatment); ii) model plus
5-HT1AR agonist group (treatment with serum from CMS rats plus 1 µM
(R)-(+)-8-hydroxy-DPAT hydrobromide stimulation for 45 min); iii)
SYC plus the agonist group (treatment with serum from SYC rats plus
1 µM (R)-(+)-8-hydroxy-DPAT hydrobromide stimulation for 45 min);
and iv) fluoxetine plus the agonist group (treatment with serum
from fluoxetine rats plus 1 µM (R)-(+)-8-hydroxy-DPAT hydrobromide
stimulation for 45 min). All serum treatments lasted for 24 h at
10% concentration.
Cell protein extraction
Following treatments, all cell groups
(5×106 cells/group) were washed twice with HBSS and then
lysed in 100 µl radioimmunoprecipitation lysis buffer (containing 1
µl phenylmethylsulfonyl fluoride and 10 µl phosphatase inhibitors).
Following incubation on ice for 30 min, cell lysates were
centrifuged at 13,000 × g for 15 min at 4°C. The supernatants were
collected and protein concentrations were determined using a
commercial bicinchoninic Protein Assay kit (cat. no. P0010S;
Beyotime Institute of Biotechnology, Haimen, China).
ELISA assay
The concentration of cAMP in the cell lysate of
hippocampal neurons in each group was measured using a rat cAMP
Elisa kit (cat. no. F15181; Westang Biotech Co., Ltd., Shanghai,
China) according to the manufacturer's protocol.
Western blot analysis
Equal denatured cell lysates (45 µg) were separated
using 10% SDS-PAGE and then electrotransferred onto nitrocellulose
membranes. Following being blocked with 5% nonfat milk in
Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at
room temperature, the membranes were incubated overnight at 4°C
with a respective primary antibody in TBST containing 5% nonfat
milk. The following primary antibodies were used: 5-HT1AR
(1:1,200), PKA (1:700), phosphorylated (p)-PKA (1:800), CREB
(1:1,500), p-CREB (1:1,500) and β-actin (1:3,000). All antibodies
were dissolved in 0.5% blocking reagent. Following being washed
three times with TBST, the membranes were incubated for 70 min at
room temperature with a secondary antibody, either goat antirabbit
immunoglobulin (Ig)G-horseradish peroxidase (HRP; Jingmei, cat. no.
SB200; 1:4,000) or goat antimouse IgG-HRP (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-2005; 1:4,000).
The protein bands were detected by enhanced chemiluminescence (EMD
Millipore, Billerica, MA). ImageJ software version 2.1.4.7
(National Institutes of Health, Bethesda, MD, USA) was used for
quantification.
Statistical analysis
All experiments were performed at least three times
and the values represent the means + standard deviations. The
significance of differences was determined using one-way analysis
of variance (ANOVA) followed by Bonferroni post hoc tests. When two
factors were assessed, the significance of the differences was
determined using two-way ANOVA followed by Bonferroni post hoc
tests. Prism software 5.0 (GraphPad software, Inc., La Jolla, CA,
USA) for statistical analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of SYC on depression-like
behavior and body weight of CMS-induced model rats
Rat body weight in all groups increased throughout
the experiment (Fig. 1A). The
model group had a significantly decreased body weight compared with
the control group following the implementation of 2 weeks of stress
(P<0.05). The SYC group and fluoxetine group had similar body
weight gain to that of the control group and the two groups had
significant weight gain in the third week (P<0.05) and in the
fourth week (P<0.05) compared with the model group (Fig. 1A).
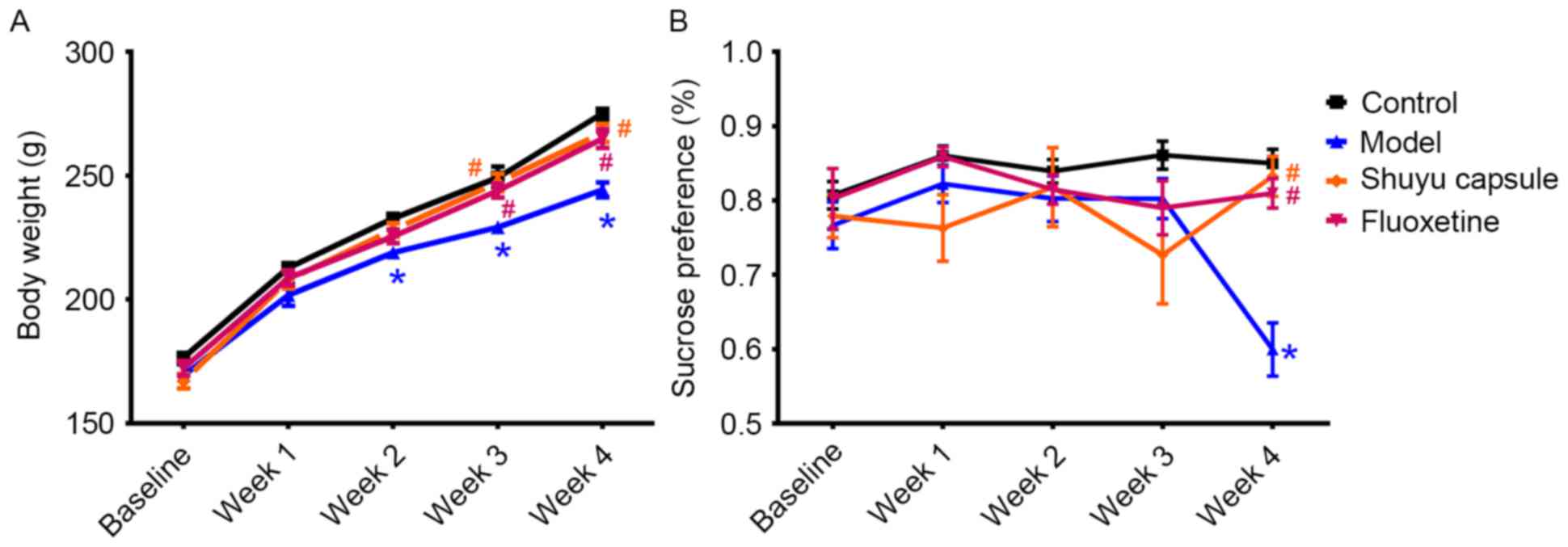 | Figure 1.Sucrose preference test and body
weight measurement of rats in all groups. (A) Body weight of rats
and (B) sucrose preference in groups prior to treatments (baseline)
and at the end of every week during treatments for 4 weeks. The
rats were exposed to different treatments as follows: Control group
(received ig administration daily with normal saline at 5 ml/kg·d),
model group (received stress plus normal saline at 5 ml/kg·d, ig,
daily), SYC group (received stress and SYC at 0.408 g/kg·d, ig,
daily) and fluoxetine group (received stress and fluoxetine 2.67
mg/kg·d, ig, daily). *P<0.01 vs. the control.
#P<0.01 vs. the model group. SYC, Shuyu capsule; ig,
intragastric; daily, four times daily. |
Anhedonia, defined as the inability to feel pleasure
from usually enjoyable activities, is a core syndrome of depression
(29). The SPT is a common
depression-like behavior test for anhedonia in rats (30). At the 28th day, sucrose solution
intake was significantly lower in the CMS-induced model group
compared with the control group (P<0.05; Fig. 1B). SYC and fluoxetine treatment led
to an increase in sucrose intake in the fourth week, indicating a
significant difference compared with the model group (P<0.05).
However, there were no significant differences among the SYC group,
fluoxetine group and the control group, suggesting that the SYC and
fluoxetine groups did not increase the sucrose intake compared with
control. They simply prevented it from decreasing (keeping it at
control levels) as happened in the model group. There was no
substantial difference between the SYC group and fluoxetine group
(Fig. 1B).
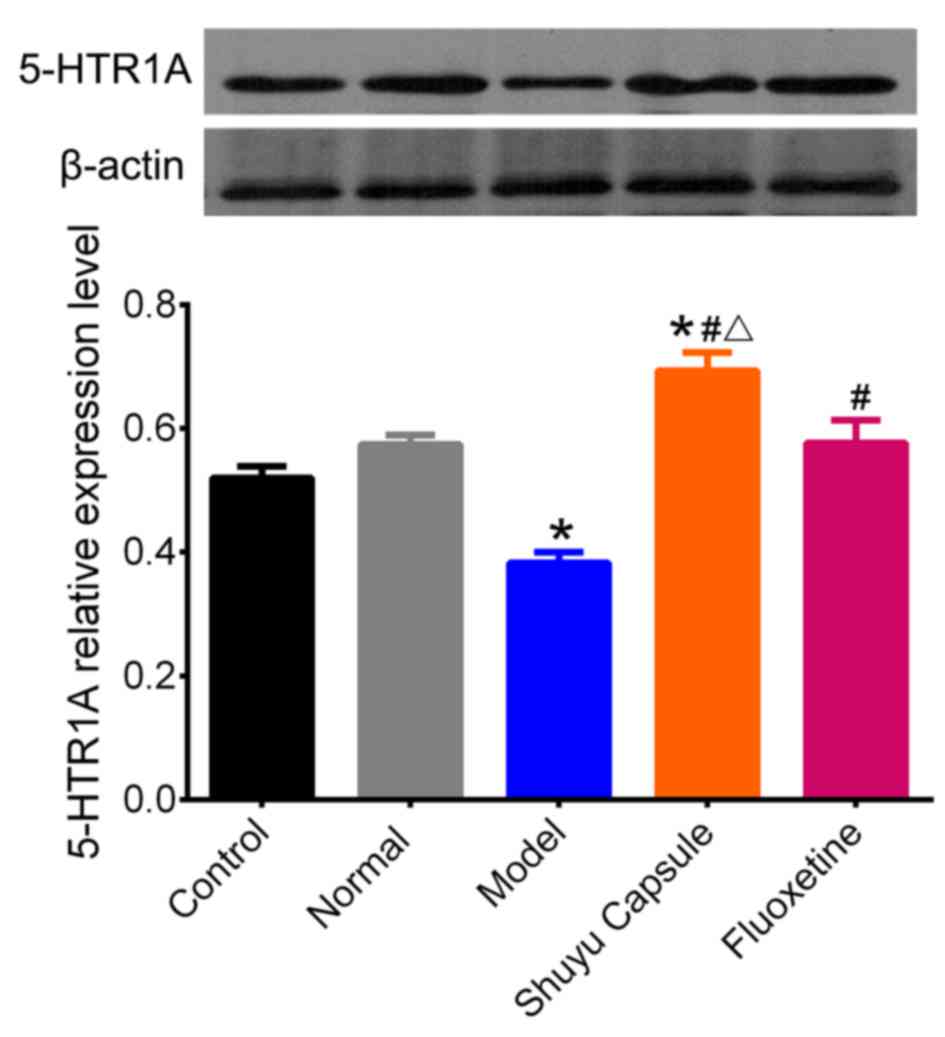
Effect of SYC on 5-HT1AR expression in
cultured hippocampal neurons treated with serum from depressive
rats
To investigate the effect of the SYC on 5-HT1AR
protein levels in hippocampal neurons in vitro, western
blotting was performed following exposure of cultured hippocampal
neurons to serum from rats in the different groups for 48 h. There
was no significant difference in 5-HT1AR protein levels between
cultured neurons with no serum treatment and those with serum from
normal rats (Fig. 2). A one-way
ANOVA revealed that the serum of the depression model rats induced
a significant reduction of 5-HT1AR expression in hippocampal
neurons compared with the control (P<0.05). Compared with the
serum from the model rats, the sera of the rats in the SYC and
fluoxetine groups elicited a significant increase in 5-HT1AR
expression (P<0.05). Additionally, there was a significant
difference between the SYC group and the normal group (P<0.05).
Compared with the fluoxetine group, the expression of the 5-HT1AR
in the SYC group increased significantly (P<0.05). These results
indicated that treatment with the depression model rat serum for 48
h resulted in a decrease of the 5-HT1AR in hippocampal neurons,
whereas the SYC and fluoxetine effectively prevented 5-HT1AR
reduction, demonstrating that the 5-HT1AR was the target of SYC and
fluoxetine. The effect of the SYC on 5-HT1AR protein expression was
increased compared with fluoxetine, with the expression being
greater than normal levels, suggesting that SYC serves a stronger
regulation effect on the expression of 5-HT1AR.
Effect of SYC on cAMP-PKA-CREB pathway
in cultured hippocampal neurons
The cAMP-PKA-CREB signaling pathway is the primary
second messenger cascade mediated by the 5-HT1AR, in addition to
other receptors. To investigate the effect of the SYC on 5-HT1AR
activity in hippocampal neurons, the activation of this cascade was
measured. The concentration of cAMP was first measured in cultured
hippocampal neurons (Fig. 3A).
There was no significant difference between the control group and
the normal group. The model group exhibited a significant decrease
in the concentration of cAMP compared with the normal group
(P<0.05), however SYC and fluoxetine treatment significantly
increased the cAMP compared with the model group (P<0.05).
Treatment with model rat serum plus 8-hydroxy-DPAT resulted in a
significantly decreased cAMP concentration compared with the normal
group (P<0.05) however there was no significant difference with
the model group, whereas treatment with WAY-100635 was able to
return the concentration to normal levels compared with the model
group. Alterations in phosphorylation levels of PKA and CREB were
similar to those in cAMP concentrations (Fig. 3B and C). These results demonstrated
that treatment with the serum of depressive rats led to reduced
cAMP-PKA-CREB signaling pathway activity mediated by the 5-HT1AR,
whereas the SYC and fluoxetine reversed the reduction, having
similar effects to those of the 5HT1AR antagonist.
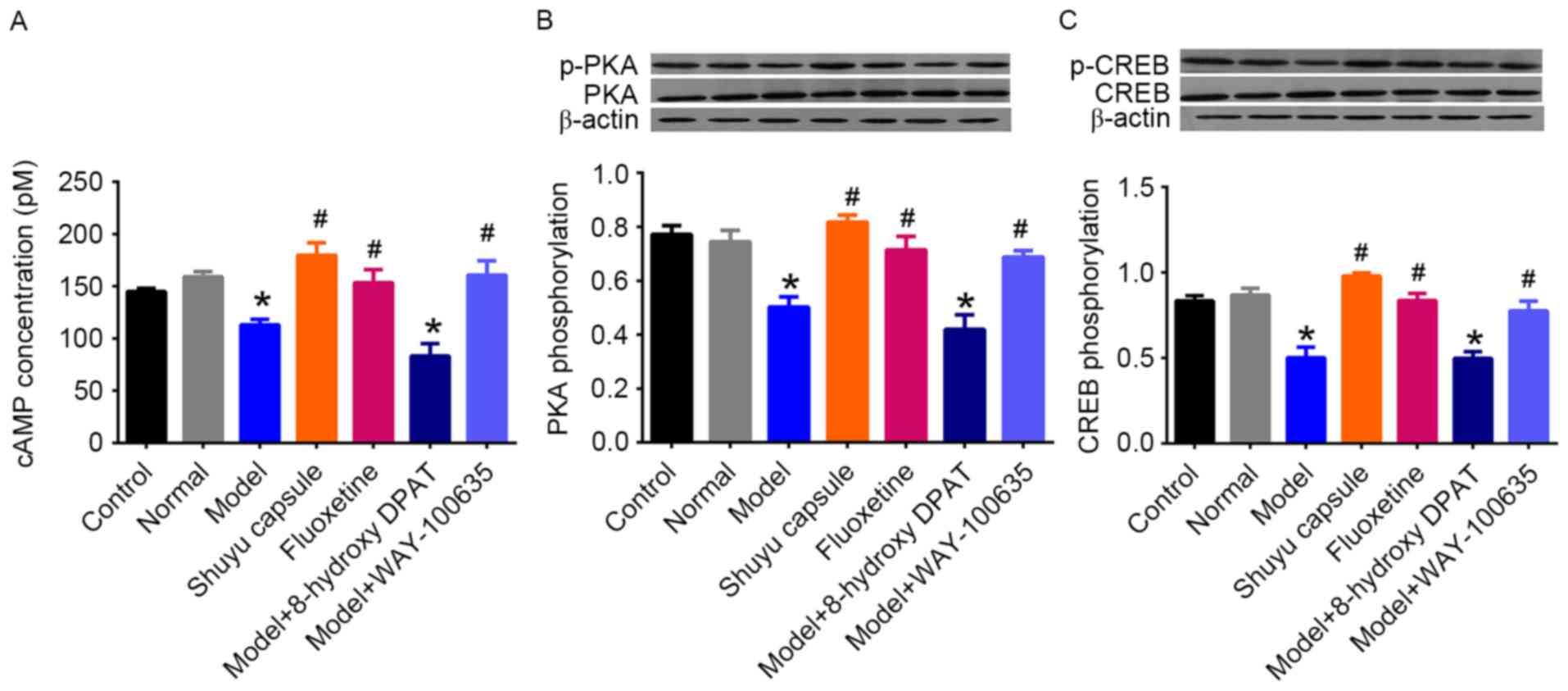 | Figure 3.Activation of cAMP-PKA-CREB pathway
in primary cultured hippocampal neurons treated with serum of rats
in different groups. ELISA and western blotting analyses were
performed to detect the intracellular (A) cAMP concentration and
phosphorylation of (B) PKA and (C) CREB following exposure of cells
to different serum treatments at the concentration of 10% for 24 h.
The cells were divided into the following groups: Control (no
serum), normal (treatment with serum of normal rats), model
(treatment with serum of rats in CMS group), SYC (treatment with
serum of rats in SYC group), fluoxetine (treatment with serum of
rats in fluoxetine group), model plus 5-hydroxytryptamine 1A
receptor agonist (treatment with serum of rats in CMS group plus 1
µM (R)-(+)-8-hydroxy-DPAT hydrobromide stimulation for 45 min) and
model plus 5-HT1AR antagonist (treatment with serum of CMS rats
plus 0.5 µM WAY-100635 stimulation for 60 min). *P<0.05 vs. the
normal group. #P<0.05 vs. the model group. CMS,
chronic mild stress; SYC, Shuyu capsule; cAMP, cyclic adenosine
monophosphate; PKA, protein kinase A; CREB, cAMP response
element-binding; p, phosphorylated. |
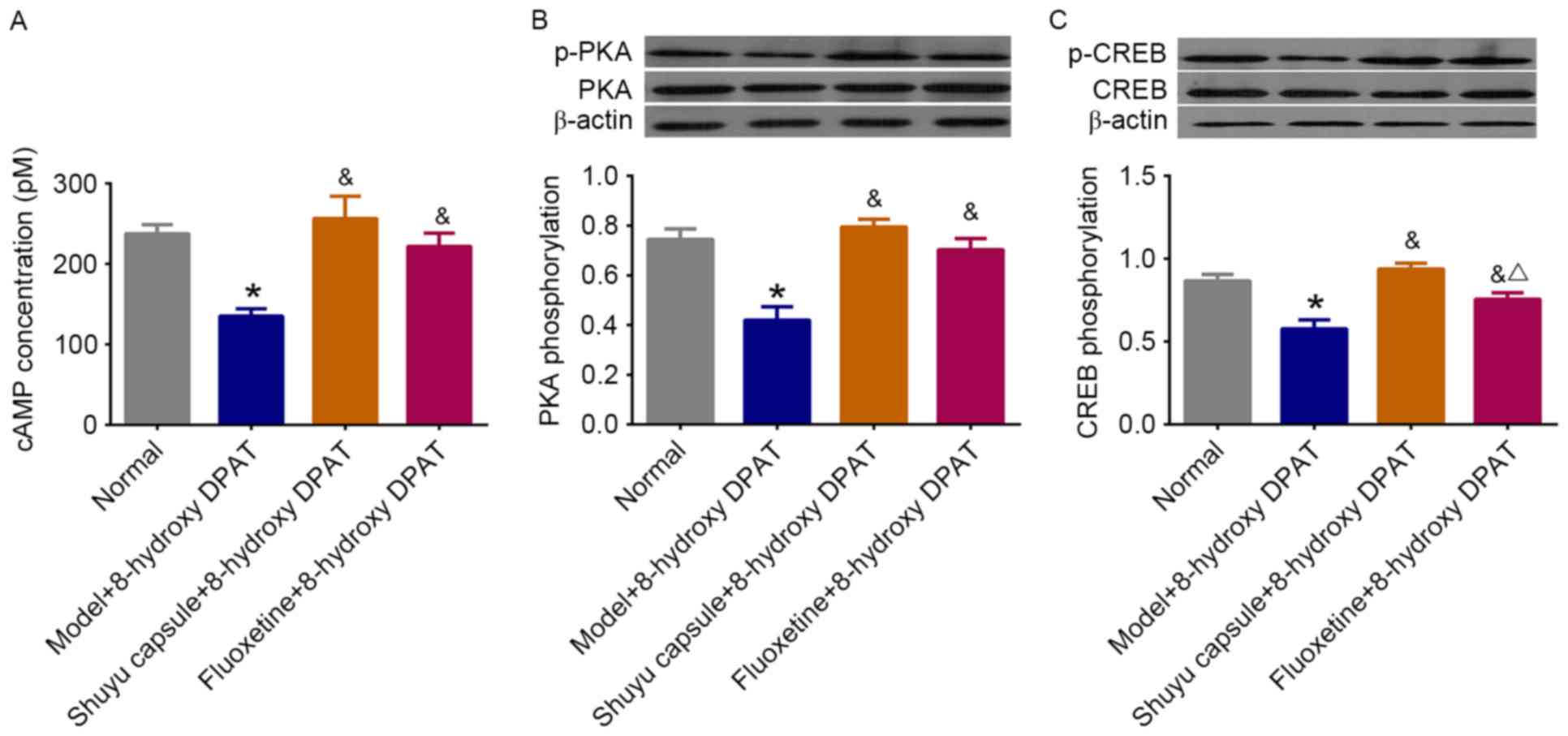
Effect of SYC on 5-HT1AR-mediated
cAMP-PKA-CREB signaling pathway in cultured hippocampal
neurons
To further investigate the effect of the SYC on the
5-HT1AR-mediated cAMP-PKA-CREB cascade, the activities of the
components of the cascade were measured again in hippocampal
neurons treated with the serum of SYC or fluoxetine rats plus the
5-HT1AR agonist 8-hydroxy-DPAT. As exhibited in Fig. 4, the treatment resulted in a
significant increase in the concentration of cAMP to normal levels
(P<0.05) as well as the phosphorylation levels of PKA and CREB.
The SYC had a stronger effect on CREB phosphorylation compared with
fluoxetine. These results suggest that the SYC effectively restores
the 5-HT1AR-mediated cAMP-PKA-CREB signaling pathway in primary
cultured hippocampal neurons treated with the serum of DD rats.
Discussion
The 5-HT1AR and its linked signaling pathways are
known to serve crucial roles in depression. In the present study,
it was demonstrated that the 5-HT1AR protein level and activation
of the 5-HT1AR-AC-cAMP-PKA-CREB signaling pathway in hippocampal
neurons are antidepressant targets of the SYC, a traditional
Chinese herbal compound composed of four herbs (Bupleurum
chinensis, Paeonia sufruticosa, Cyperus rotundus and
Glycyrrhiza uralensis Fisch). Although previous reports have
demonstrated the antidepressant effects of these herbs and formulas
with these herbs have been employed as primary constituents in
animal experiments, the 5-HT1AR and the signaling pathway that it
mediates have not been implicated in the antidepressant mechanism
of these natural products (9–12).
The present study, to the best of the author's knowledge, was the
first to provide evidence of the aforementioned herbal
antidepressant effects, with the 5-HT1AR and its linked
AC-cAMP-PKA-CREB signaling pathway as targets.
It is generally believed that chronic exposure to
stress serves a primary role in the onset and relapse of DD.
Therefore, chronic stress paradigms in laboratory animals
constitute important tools in this field. The CMS model, originally
developed by Willner, has been extensively used to study the
pathophysiology of and therapy for DD due to its high predictive,
face and construct validity (27,31).
Animals have been reported to exhibit a persistent reduction in
responsiveness to pleasurable stimuli, measured by a decrease in
their consumption of 1% sucrose solution and decreases in sucrose
consumption have been validated as a reliable behavioral measure
that may be associated with the anhedonia involved in depression
(32–34). Consistent with other reports, the
present study demonstrated that CMS induced a decrease in SPT,
which was reversed by the SYC and fluoxetine respectively,
suggesting that the SYC may be as effective as SSRIs in treating
depression.
In addition, alterations were observed in the body
weight of rats. The results of the present study demonstrated that
the model group had lower body weight compared with control group
following implementation of 2 weeks' stress, which was not
demonstrated by the SYC or fluoxetine groups. The SYC treatment and
fluoxetine treatment groups demonstrated similar body weight gain
to that of the control group, suggesting that the SYC or fluoxetine
had an effect on body weight in CMS-induced DD model rats. In a
previous DD study, a number of behavioral and physiological
alterations were reported, including an ~0–10% loss of body weight
(27). However, certain studies
have demonstrated that antidepressants, including fluoxetine,
elicit even lower body weight compared with CMS group and that CMS
rats gain body weight (35,36).
In the present study, the serum pharmacological method was used, to
the best of the author's knowledge, for the first time, to
investigate effects of the SYC on the 5-HT1AR-mediated
AC-cAMP-PKA-CREB postreceptor signal transduction pathway in
vitro. The serum pharmacological method was developed in the
1980s and is now frequently used for herbal mixture pharmacological
studies in vitro (37). To
ensure that certain active components of the SYC in serum pass
through the blood-brain barrier, the authors previously measured
the serum and cerebrospinal fluid from rats treated orally with the
SYC by liquid chromatography electrospray ionization tandem mass
spectrometry (38). According to
the results, 13 prototype compounds were absorbed into the rat
serum in the prototype and identified as quinic acid, dibenzoyl
paeoniflorin, 6′-O-galloyldibenzoyl paeoniflorin, paeoniflorin
sulfonate, albiflorin, paeoniflorin, liquiritin apioside,
liquiritin, hydrated-liquiritin, galloylpaeoniflorin,
galloylalbiflorin, apigenin-7-O-β-D-glucuronide and wogonoside.
Downregulation of the 5-HT1AR has been proposed as a
general hypothesis to explain alterations in the 5-HTR function in
subjects with depressive symptoms (39). The results of the present study
demonstrated that the levels of 5-HT1AR protein expression in
primary cultured hippocampal neurons were decreased in the CMS
model group compared with the control group, which is in line with
previous studies (40). It has
been reported that fluoxetine has an antidepressant function
partially mediated by the 5-HT1AR and is involved in brain
region-dependent 5-HT1AR gene transcription alterations (41,42).
The serum from fluoxetine-treated rats was observed to reverse the
decrease of 5-HT1AR protein levels in the cultured hippocampal
neurons. Furthermore, the SYC demonstrated a similar role to that
of fluoxetine, which implied that the beneficial action of the SYC
or fluoxetine on the CMS-induced depressive state may in part be
based on 5-HT1AR dysfunction in the hippocampal neurons. Notably,
the increasing effect of the SYC on 5-HT1AR protein levels was
statistically greater compared with fluoxetine, indicating that the
receptor is involved more in the antidepressant-like effect of the
SYC than in that of fluoxetine.
The AC-cAMP-PKA-CREB cascade is regulated by
serotonin and norepinephrine. Dysfunction of the AC-cAMP-PKA
cascade, including decreased G protein and cAMP levels, reduced AC
and PKA activity and altered PKA-mediated phosphorylation, have
been observed in DD patients (43,44).
Chronic antidepressant treatment upregulates cAMP signal
transduction and PKA activity in the brain (45). Previous studies have demonstrated
that the pathophysiology of major depression may involve
abnormalities within critical effector systems responsible for
neurotransmitter effects on cells, including PKA and CREB, and
conventional antidepressants may ameliorate depression in part
thorough effects on the cAMP-PKA pathway (46–48).
In addition, chronic fluoxetine treatment exerts a marked effect on
phosphorylated-CREB in the hippocampus. Furthermore, various
studies have demonstrated that the cAMP-PKA cascade is involved in
SSRI-induced antidepressant actions (49,50).
The present study evaluated the association between
the 5-HT1AR and the AC-cAMP-PKA-CREB cascade in the context of SYC
treatment. Studies have demonstrated that CMS induced
downregulation of the AC-cAMP-PKA cascade, which was reversed by
SYC and fluoxetine treatment. The treatments plus 8-hydroxy-DPAT
and WAY-100635, the specific agonist and antagonist of the 5-HT1AR
(51,52), verified that the alterations in the
AC-cAMP-PKA cascade in primary cultured hippocampal neurons were
mediated by the 5-HT1AR. Furthermore, the results of the present
study indicated that the SYC or fluoxetine were able to restore
downregulation of the AC-cAMP-PKA cascade induced by the 5-HT1AR
agonist. Additionally, the present study revealed that the effect
of the SYC is superior to that of fluoxetine on the upregulation of
the 5-HT1AR and improvement of the CREB phosphorylation level.
In conclusion, the SYC is an effective
antidepressant treatment in CMS-induced depression model rats, as
effective as fluoxetine, and the AC-cAMP-PKA-CREB signal
transduction pathway mediated by the 5-HT1AR is crucial for its
antidepressant mechanism.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81072721). The
authors would like to thank Shandong University of Traditional
Chinese Medicine and the Key Laboratory of Traditional Chinese
Medical Classical Theory for technical assistance.
References
|
1
|
Isometsä E: Suicidal behaviour in mood
disorders-who, when and why? Can J Psychiatry. 59:120–130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song L, Che W, Min-Wei W, Murakami Y and
Matsumoto K: Impairment of the spatial learning and memory induced
by learned helplessness and chronic mild stress. Pharmacol Biochem
Behav. 83:186–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delgado PL: Depression: The case for a
monoamine deficiency. J Clin Psychiatry. 61 Suppl 6:S7–S11.
2000.
|
|
4
|
Dording CM, Mischoulon D, Petersen TJ,
Kornbluh R, Gordon J, Nierenberg AA, Rosenbaum JE and Fava M: The
pharmacologic management of SSRI-induced side effects: A survey of
psychiatrists. Ann Clin Psychiatry. 14:143–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fitzgerald KT and Bronstein AC: Selective
serotonin reuptake inhibitor exposure. Top Companion Anim Med.
28:13–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Butler L and Pilkington K: Chinese herbal
medicine and depression: The research evidence. Evid Based
Complement Alternat Med. 2013:7397162013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin F, Wu XA, Tang Y, Huang Q, Zhang ZJ
and Yuan JH: Meta-analysis of randomized controlled trials to
assess the effectiveness and safety of free and easy wanderer plus,
a polyherbal preparation for depressive disorders. J Psychiatr Res.
45:1518–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Han M, Liu Z, Wang J, He Q and
Liu J: Chinese herbal formula xiao yao san for treatment of
depression: A systematic review of randomized controlled trials.
Evid Based Complement Alternat Med. 2012:9316362012.PubMed/NCBI
|
|
9
|
Lee B, Yun HY, Shim I, Lee H and Hahm DH:
Bupleurum falcatum prevents depression and anxiety-like behaviors
in rats exposed to repeated restraint stress. J Microbiol
Biotechnol. 22:422–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu F, Zhong X, Mao Q and Huang Z: The
antidepressant-like effects of paeoniflorin in mouse models. Exp
Ther Med. 5:1113–1116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dhingra D and Sharma A:
Antidepressant-like activity of Glycyrrhiza glabra L. in mouse
models of immobility tests. Prog Neuropsychopharmacol Biol
Psychiatry. 30:449–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarris J, Panossian A, Schweitzer I,
Stough C and Scholey A: Herbal medicine for depression, anxiety and
insomnia: A review of psychopharmacology and clinical evidence. Eur
Neuropsychopharmacol. 21:841–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu K, Li Z and Gao J: Effect of Shuyu
capsule on liver-qi stagnation rat model of depressive disorder
based on the analysis of gene expression microarray technique. Chin
Pharmocol Bulletin. 30:437–438. 2014.(In Chinese).
|
|
14
|
Gordon JA and Hen R: The serotonergic
system and anxiety. Neuromolecular Med. 5:27–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robinson DS, Rickels K, Feighner J, Fabre
LF Jr, Gammans RE, Shrotriya RC, Alms DR, Andary JJ and Messina ME:
Clinical effects of the 5-HT1A partial agonists in depression: A
composite analysis of buspirone in the treatment of depression. J
Clin Psychopharmacol. 10:67S–76S. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong DT, Bymaster FP and Engleman EA:
Prozac (fluoxetine, Lilly 110140), the first selective serotonin
uptake inhibitor and an antidepressant drug: Twenty years since its
first publication. Life Sci. 57:411–441. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riad M, Zimmer L, Rbah L, Watkins KC,
Hamon M and Descarries L: Acute treatment with the antidepressant
fluoxetine internalizes 5-HT1A autoreceptors and reduces the in
vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe
dorsalis of rat. J Neurosci. 24:5420–4526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drevets WC, Thase ME, Moses-Kolko EL,
Price J, Frank E, Kupfer DJ and Mathis C: Serotonin-1A receptor
imaging in recurrent depression: Replication and literature review.
Nucl Med Biol. 34:865–877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savitz J, Lucki I and Drevets WC: 5-HT(1A)
receptor function in major depressive disorder. Prog Neurobiol.
88:17–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Birnbaumer L: Expansion of signal
transduction by G proteins. The second 15 years or so: From 3 to 16
alpha subunits plus betagamma dimers. Biochim Biophys Acta.
1768:772–793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pucadyil TJ, Kalipatnapu S and
Chattopadhyay A: The serotonin1A receptor: A representative member
of the serotonin receptor family. Cell Mol Neurobiol. 25:553–580.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohno Y: New insight into the therapeutic
role of 5-HT1A receptors in central nervous system disorders. Cent
Nerv Syst Agents Med Chem. 10:148–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MH and Leem YH: Chronic exercise
improves repeated restraint stress-induced anxiety and depression
through 5HT1A receptor and cAMP signaling in hippocampus. J Exerc
Nutrition Biochem. 18:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Guo J and Guo R: Effect of
XingPiJieYu decoction on spatial learning and memory and
cAMP-PKA-CREB-BDNF pathway in rat model of depression through
chronic unpredictable stress. BMC Complement Altern Med. 17:732017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thome J, Henn FA and Duman RS: Cyclic AMP
response element-binding protein and depression. Expert Rev
Neurother. 2:347–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang FR, Qiao MQ, Xue L and Wei S:
Possible involvement of µ opioid receptor in the
antidepressant-like effect of shuyu formula in restraint
stress-induced depression-like rats. Evid Based Complement Alternat
Med. 2015:4524122015.PubMed/NCBI
|
|
27
|
Willner P, Towell A, Sampson D,
Sophokleous S and Muscat R: Reduction of sucrose preference by
chronic unpredictable mild stress, and its restoration by a
tricyclic antidepressant. Psychopharmacology (Berl). 93:358–364.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nunez J: Primary culture of hippocampal
neurons from P0 newborn rats. J Vis Exp. 19:pii: 8952008.
|
|
29
|
Gaillard R, Gourion D and Llorca PM:
Anhedonia in depression. Encephale. 39:296–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Overstreet DH: Modeling depression in
animal models. Methods Mol Biol. 829:125–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Willner P: Validity, reliability and
utility of the chronic mild stress model of depression: A 10-year
review and evaluation. Psychopharmacology (Berl). 134:319–329.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muscat R and Willner P: Suppression of
sucrose drinking by chronic mild unpredictable stress: A
methodological analysis. Neurosci Biobehav Rev. 16:507–517. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bessa JM, Ferreira D, Melo I, Marques F,
Cerqueira JJ, Palha JA, Almeida OF and Sousa N: The mood-improving
actions of antidepressants do not depend on neurogenesis but are
associated with neuronal remodeling. Mol Psychiatry. 14:764–773.
2009.39. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leach G, Adidharma W and Yan L:
Depression-like responses induced by daytime light deficiency in
the diurnal grass rat (Arvicanthis niloticus). PLoS One.
8:e571152013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu J, Liu Q, Wang YQ, Wang J, Li XY, Cao
XD and Wu GC: Electroacupuncture combined with clomipramine
enhances antidepressant effect in rodents. Neurosci Lett. 421:5–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu X, Alberico SL, Moges H, De Taboada L,
Tedford CE and Anders JJ: Pulsed light irradiation improves
behavioral outcome in a rat model of chronic mild stress. Lasers
Surg Med. 44:227–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iwama H, Amagaya S and Ogihara Y: Effect
of shosaikoto, a Japanese and Chinese traditional herbal medicinal
mixture, on the mitogenic activity of lipopolysaccharide: A new
pharmacological testing method. J Ethnopharmacol. 21:45–53. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li F, Zhang YB, Wei X, Song CH, Qiao MQ
and Zhang HY: Metabolic profiling of Shu-Yu capsule in rat serum
based on metabolic fingerprinting analysis using HPLC-ESI-MSn. Mol
Med Rep. 13:4191–4204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stahl S: 5HT1A receptors and
pharmacotherapy. Is serotonin receptor down-regulation linked to
the mechanism of action of antidepressant drugs? Psychopharmacol
Bull. 30:39–43. 1994.PubMed/NCBI
|
|
40
|
Larsson LG, Stenfors C and Ross SB:
Differential regional antagonism of 8-OH-DPAT-induced decrease in
serotonin synthesis by two 5-HT1A receptor antagonists. Eur J
Pharmacol. 346:209–215. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De Vry J, Schreiber R, Melon C, Dalmus M
and Jentzsch KR: 5-HT1A receptors are differentially involved in
the anxiolytic- and antidepressant-like effects of 8-OH-DPAT and
fluoxetine in the rat. Eur Neuropsychopharmacol. 14:487–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shishkina GT, Kalinina TS and Dygalo NN:
Effects of swim stress and fluoxetine on 5-HT1A receptor gene
expression and monoamine metabolism in the rat brain regions. Cell
Mol Neurobiol. 32:787–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dowlatshahi D, MacQueen GM, Wang JF,
Reiach JS and Young LT: G Protein-coupled cyclic AMP signaling in
postmortem brain of subjects with mood disorders: Effects of
diagnosis, suicide, and treatment at the time of death. J
Neurochem. 73:1121–1126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shelton RC, Mainer DH and Sulser F:
cAMP-dependent protein kinase activity in major depression. Am J
Psychiatry. 153:1037–10342. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li YC, Wang FM, Pan Y, Qiang LQ, Cheng G,
Zhang WY and Kong LD: Antidepressant-like effects of curcumin on
serotonergic receptor-coupled AC-cAMP pathway in chronic
unpredictable mild stress of rats. Prog Neuropsychopharmacol Biol
Psychiatry. 33:435–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hsiung SC, Tin A, Tamir H, Franke TF and
Liu KP: Inhibition of 5-HT1A receptor-dependent cell survival by
cAMP/protein kinase A: Role of protein phosphatase 2A and Bax. J
Neurosci Res. 86:2326–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duman RS, Malberg J, Nakagawa S and D'Sa
C: Neuronal plasticity and survival in mood disorders. Biol
Psychiatry. 48:732–739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shaywitz AJ and Greenberg ME: CREB: A
stimulus-induced transcription factor activated by a diverse array
of extracellular signals. Annu Rev Biochem. 68:821–861. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tiraboschi E, Tardito D, Kasahara J,
Moraschi S, Pruneri P, Gennarelli M, Racagni G and Popoli M:
Selective phosphorylation of nuclear CREB by fluoxetine is linked
to activation of CaM kinase IV and MAP kinase cascades.
Neuropsychopharmacology. 29:1831–1840. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duman CH, Schlesinger L, Kodama M, Russell
DS and Duman RS: A role for MAP kinase signaling in behavioral
models of depression and antidepressant treatment. Biol Psychiatry.
61:661–670. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Seth P, Gajendiran M and Ganguly DK:
Desensitization of spinal 5-HT1A receptors to 8-OH-DPAT: An in vivo
spinal reflex study. Neuroreport. 8:2489–2493. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fletcher A, Forster EA, Bill DJ, Brown G,
Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ,
Critchley DJ, et al: Electrophysiological, biochemical,
neurohormonal and behavioural studies with WAY-100635, a potent,
selective and silent 5-HT1A receptor antagonist. Behav Brain Res.
73:337–353. 1996. View Article : Google Scholar : PubMed/NCBI
|