Introduction
The incidence rate of diabetes, particularly type 1,
is rising rapidly worldwide and increases by >7 million every
year (1). In China, the diabetes
incidence rate has become increasingly urgent and severe; the New
England Journal of Medicine reported that China topped the list in
the number of diabetic patients in the world in 2010 (2). Currently, 1 patient succumbs to
diabetes every 10 sec on average around the world, and the average
annual cost for the treatment of diabetes is >$200 billion,
accounting for ~18.2% of total medical expenses (3,4).
Diabetes severely affects human health, and patients with diabetes
often experience complications in heart, brain and kidney, among
which cardiovascular diseases are the main cause of mortality in
patients with diabetes (5).
With rapid economic development, prolonging
lifespans and changes in lifestyle, cardiovascular diseases have
become the major cause of mortality in China (6). As a major risk factor of
cardiovascular disease, diabetes increases the incidence of
cardiovascular diseases (2).
Previous studies have reported that mortality from cardiovascular
complications in patients with diabetes is 3–5 times higher
compared with those without diabetes, and the incidence rate of
ischemic heart disease in patients with diabetes is twice that of
those without diabetes (6,7).
The phosphoinositide 3-kinase (PI3K)/Akt signaling
pathway participates in cell differentiation, proliferation,
apoptosis and migration, and overactivation of this pathway may
lead to cellular dysfunction (8).
PI3K/Akt signaling pathway serves biological functions through
activation of a number of processes (9). It has been >10 years since the
PI3K/Akt pathway was first identified; it was initially examined in
studies on the metabolism, differentiation, proliferation,
apoptosis and angiogenesis of tumor cells (9). The biological functions of PI3K/Akt
signaling occur through the activation and phosphorylation of Akt
(10). The PI3K/Akt/endothelial
nitric oxide synthase (eNOS)/nitric oxide (NO) pathway is a
cellular ‘survival signal’ pathway that serves an important role in
protecting the cardiovascular system (11).
As a nuclear transcription factor with wide
biological activities, nuclear factor (NF)-κB serves an important
role in signal transduction and gene expression (11). High blood glucose levels may
improve the activity of NF-κB, and activated NF-κB is involved in
the production of a number of inflammatory cytokines, proliferation
and differentiation of fibroblasts, as well as extracellular matrix
cross-linking and cellular apoptosis (12). NF-κB also regulates the
transcriptional activity of NOS and cyclooxygenase-2, along with
other DNA binding proteins (13).
Icariside II is a flavonoid extracted from
Epimedium that has been reported to reduce the expression of
rat hippocampal brain-derived neurotrophic factor, induced by
D-galactose and its receptor TrkB tyrosine kinase, to facilitate
axon regeneration, thus promoting nerve regeneration in rats with
dementia and improving their cognitive function (14). Previous studies have also
demonstrated that Epimedium, icariside II and
Epimedium extract aid in recovering the functions of injured
peripheral nerves (14,15). The present study aimed to
investigate whether icariside II treatment was able to protect
against diabetic cardiomyopathy in streptozotocin-induced type I
diabetic rats and to explore the underlying molecular mechanism
in vivo.
Materials and methods
Animal model and treatment
Male Sprague-Dawley rats (weight, 180–200 g; age 7–8
weeks) were purchased from the Experimental Animal Center of
Beijing University (Beijing, China), housed at 22–23°C, 55–60%
humidity, SPF atmosphere (0.03% CO2), 12 h light/dark
cycle and freely access to food and water. All experiments in this
study were approved by the Institutional Animal Care and Use
Committee of Navy General Hospital of PLA (Beijing, China). A total
of 36 rats were randomly divided into three groups (n=12/group): i)
Normal control group (Control group) group, which received an
intraperitoneal (i.p.) injection of citrate-phosphate buffer (0.1
mol/l); ii) streptozotocin (STZ)-induced diabetic rat group (STZ
group), which received an i.p. injection of STZ (60 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to induce type 1
diabetes; and iii) STZ-induced diabetic rats + icariside II therapy
group (STZ + icariside II group), which received an i.p. injection
of STZ (60 mg/kg), followed by icariside II (5 mg/kg/day;
Sigma-Aldrich; Merck KGaA) i.p. injection for 8 weeks. Following 8
weeks of treatment, body weight and heart weight were measured and
calculated as (heart weight)/(body weight).
Measurement of fasting blood
glucose
Following treatments, blood was collected by tail
vein puncture and the fasting blood glucose levels (following 12 h
of fasting) under anesthesia (35 mg/kg pentobarbital sodium) were
measured using a glucometer (Sinocare Inc., Changsha, China). The
serum was separated by centrifuging at 1,500 × g for 10 min at 4°C
and stored at −70°C until use.
Inflammatory cytokines detection and
oxidative stress measurement
Serum creatine kinase (CK, cat. no. A032) and
lactate dehydrogenase (LDH, cat. no. A020-2) levels were measured
using ELISA kits (Nanjing Jiancheng Biology Engineering Institute).
Protein homogenates were prepared from cardiac tissues in Lysis
Buffer (Nanjing Keygen Biotech Co., Ltd., Nanjing, China) on ice
for 15 min, and protein contents were determined using the
bicinchoninic acid protein assay kit (Nanjing Keygen Biotech Co.,
Ltd.). Tumor necrosis factor-α (TNF-α, cat. no. H052), interleukin
(IL)-1β (cat no. H002) and interleukin-6 (IL-6, cat. no. H007),
malondialdehyde (MDA, cat. no. A003-1), superoxide dismutase (SOD,
cat. no. A001-1), glutathione (GSH, cat. no. A006-2) and GSH
peroxidase (GPx, cat. no. A005) expression levels were measured
using ELISA kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China). The absorbance was determined using an automatic
microplate reader (PerkinElmer, Inc., Waltham, MA, USA) at 450
nm.
Western blotting
Protein homogenates were prepared from cardiac
tissues in Lysis Buffer (Nanjing Keygen Biotech Co., Ltd., Nanjing,
China) on ice for 15 min, and protein contents were determined
using the BCA Protein Assay kit (Nanjing Keygen Biotech Co., Ltd.).
Equal amounts of protein (50 µg) were separated by 8–12% SDS/PAGE
and transferred to nitrocellulose membranes (EMD Millipore,
Billerica, USA). Following blocking with 5% nonfat milk for 1 h,
the membranes were individually incubated with primary antibodies
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) against apoptosis
regulator Bax (cat. no. sc-526, 1:500), caspase-3 (cat. no.
sc-98785, 1:500), PI3K (cat. no. sc-7174, 1:500), phosphorylated
(p)-Akt (cat. no. sc-135650, 1:500), inducible (i) NOS (cat. no.
sc-649, 1:500), NF-κB (cat. no. sc-109, 1:500) and GAPDH (cat. no.
sc-25778, 1:500, to normalize protein expressions) overnight at 4°C
with agitation. Membranes were washed with TBS with 0.1% Tween-20,
incubated with horseradish peroxidase-conjugated secondary antibody
(cat. no. sc-2030, 1:2,000; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h and were visualized using an Enhanced
Chemiluminescence Detection kit (GE Healthcare, Chicago, IL, USA
and analyzed Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical significance was analyzed by one-way
analysis of variance and Tukey's post hoc test using SPSS version
18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
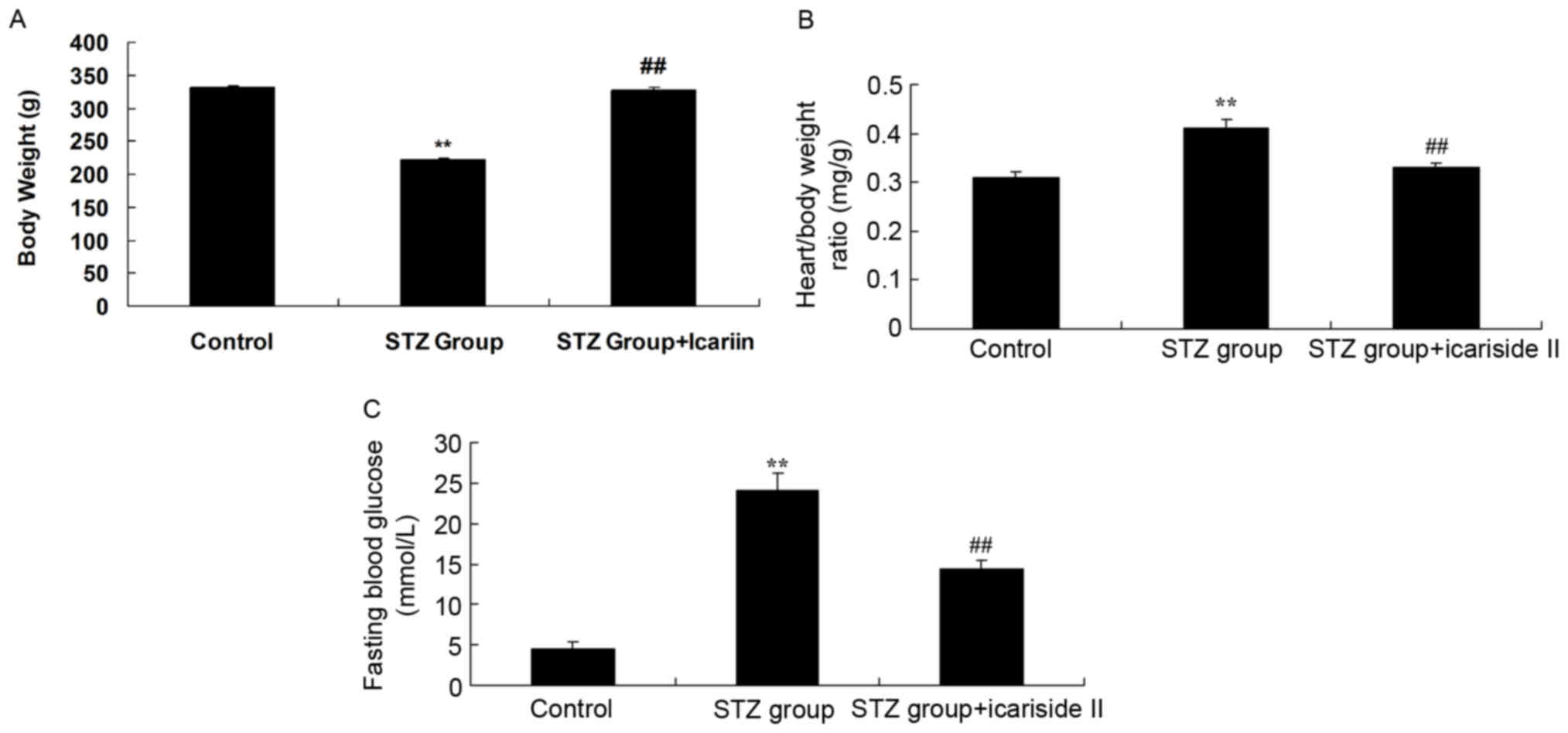
Icariside II treatment improves body
weight, heart/body weight Ratio and fasting blood glucose in
diabetic rat
Body weight was significantly reduced in rats in the
STZ group compared with Control rats (Fig. 1A). Treatment with STZ resulted in
significant increases in the heart/body weight ratio and fasting
blood glucose compared with the untreated Control group (Fig. 1B and C, respectively). Notably,
rats co-treated with STZ and icariside II exhibited a significant
increase in body weight and significant reductions in heart/body
weight ratios and fasting blood glucose levels, compared with
diabetic rats in the STZ group (Fig.
1).
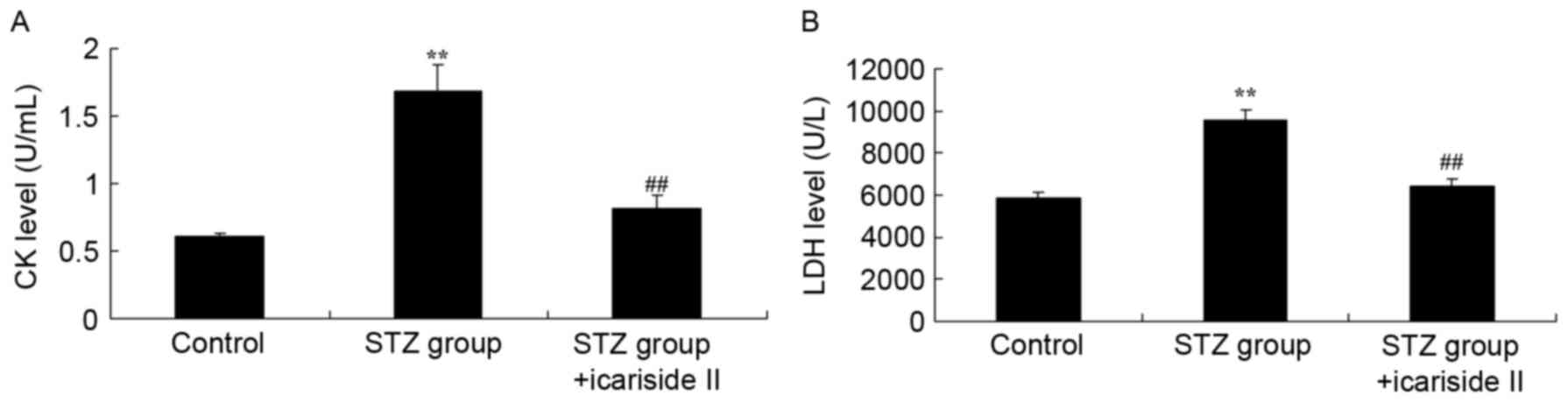
Icariside II lowers CK and LDH levels
in the serum of diabetic rats
To investigate whether icariside II protected
against diabetic cardiomyopathy, serum CK and LDH levels were
measured. CK and LDH levels in the serum of diabetic rats were
higher compared with the Control group (Fig. 2A and B, respectively). Co-treatment
with icariside II significantly reduced CK and LDH levels in serum
of diabetic rat (Fig. 2).
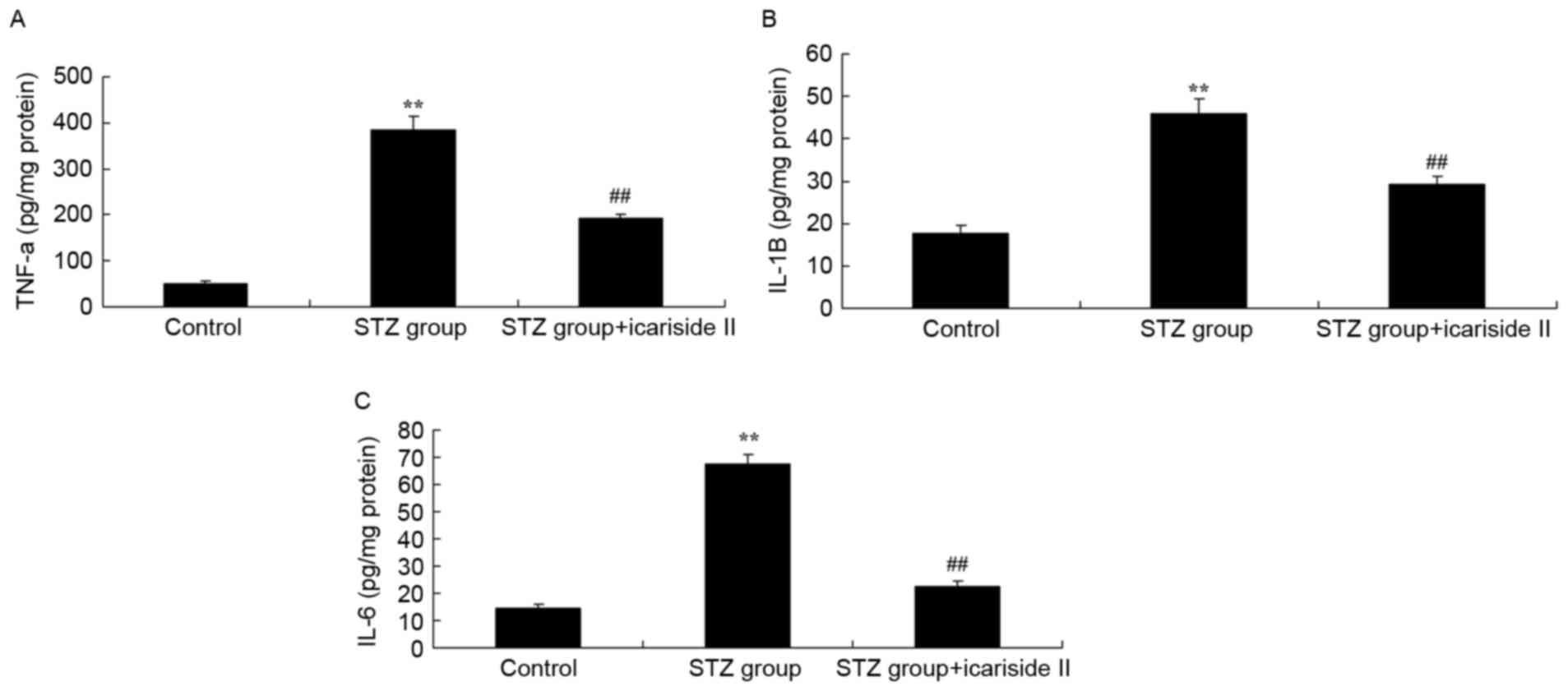
Icariside II reduces cardiac
inflammation in diabetic rats
To detect whether icariside II treatment in diabetic
rats exhibited anti-inflammatory effects, the expression levels of
TNF-α, IL-1β and IL-6 in myocardial tissues were measured by ELISA.
TNF-α, IL-1β and IL-6 levels in heart tissue were significantly
increased in STZ-treated diabetic rats (Fig. 3A-C, respectively). Rats co-treated
with icariside II exhibited reduced levels of TNF-α, IL-1β and IL-6
compared with the STZ-only group (Fig.
3).
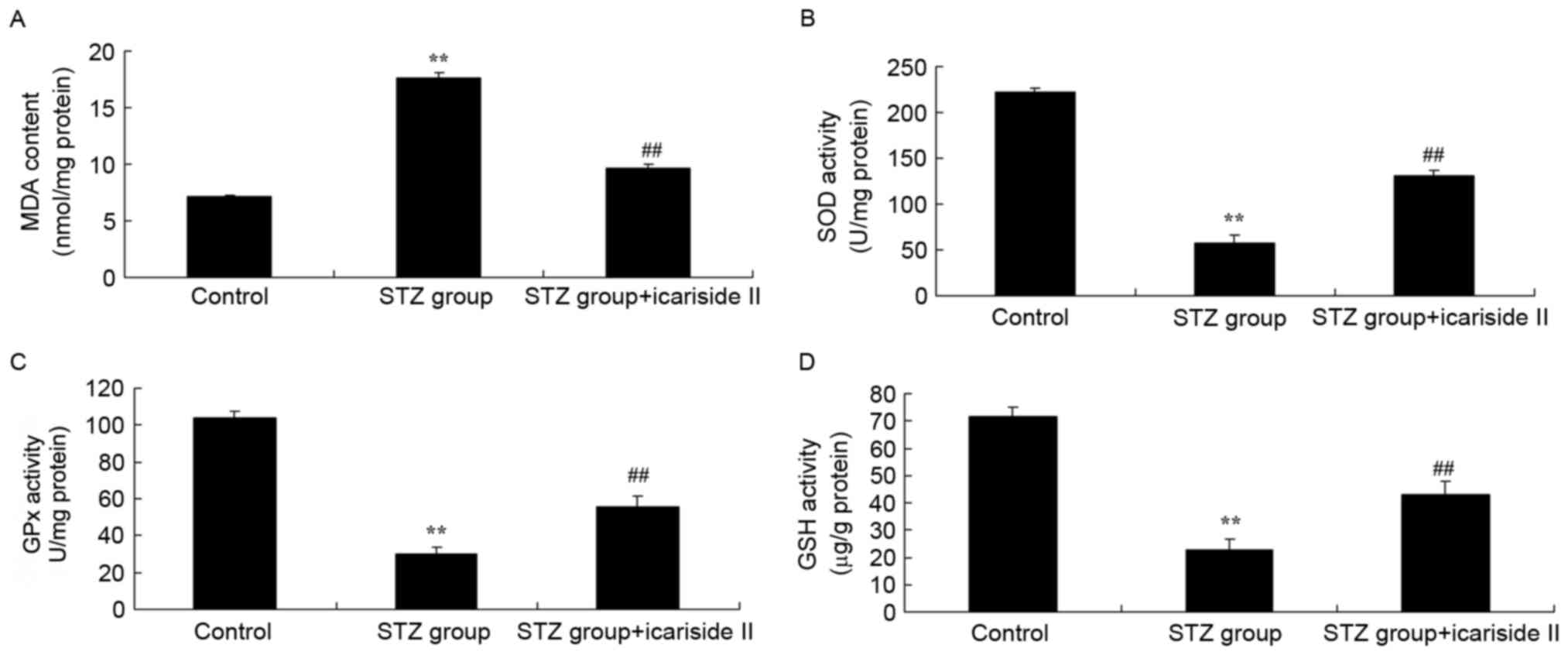
Icariside II reduces cardiac oxidative
stress in diabetic rats
To evaluate the anti-oxidative effects of icariside
II treatment in diabetic rats, the expression levels of MDA, SOD,
GPx and GSH in myocardial tissues were assessed by ELISA. Rats
treated with STZ exhibited a significant increase in MDA content
(Fig. 4A), and a reduction in SOD,
GPx and GSH levels compared with the Control rats (Fig. 4B-D, respectively). STZ-induced
diabetic rats co-treated with icariside II exhibited a decrease in
MDA and an increase in SOD, GPx and GSH levels (Fig. 4). These results indicated that
icariside II treatment may attenuate diabetes-induced oxidative
stress in heart tissues.
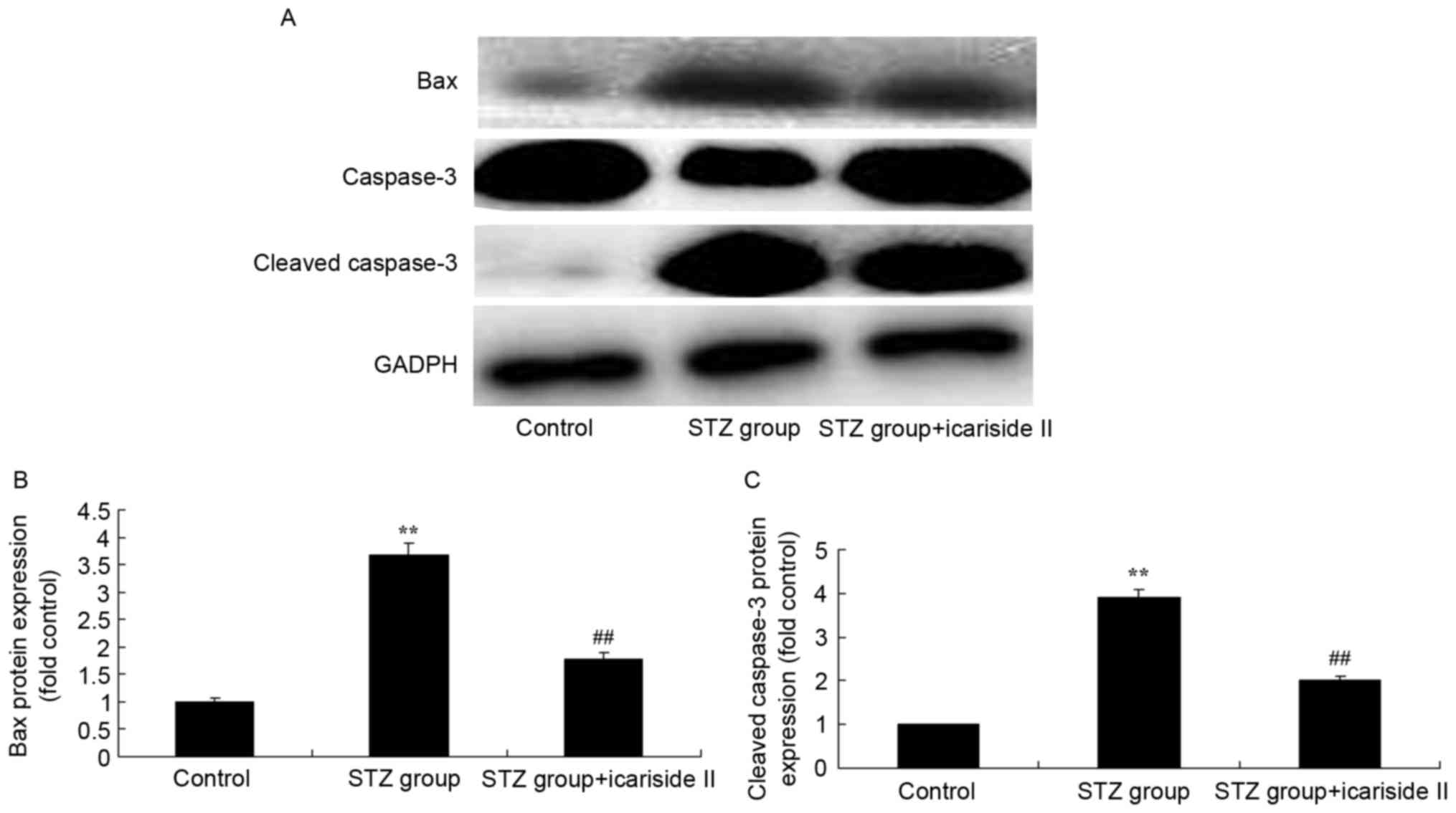
Icariside II treatment decreases
cardiac apoptosis levels in diabetic rats
To further investigate the potential anti-apoptotic
role of icariside II in diabetic rats, the levels of Bax and
caspase-3 protein expression were measured by western blotting
(Fig. 5A). Bax and cleaved
caspase-3 protein expression in diabetic rats in STZ group were
significantly higher compared with expression levels in the Control
group (Fig. 5B and C,
respectively). Icariside II co-treatment significantly reduced Bax
and cleaved caspase-3 protein expression in the heart tissues of
diabetic rats (Fig. 5).
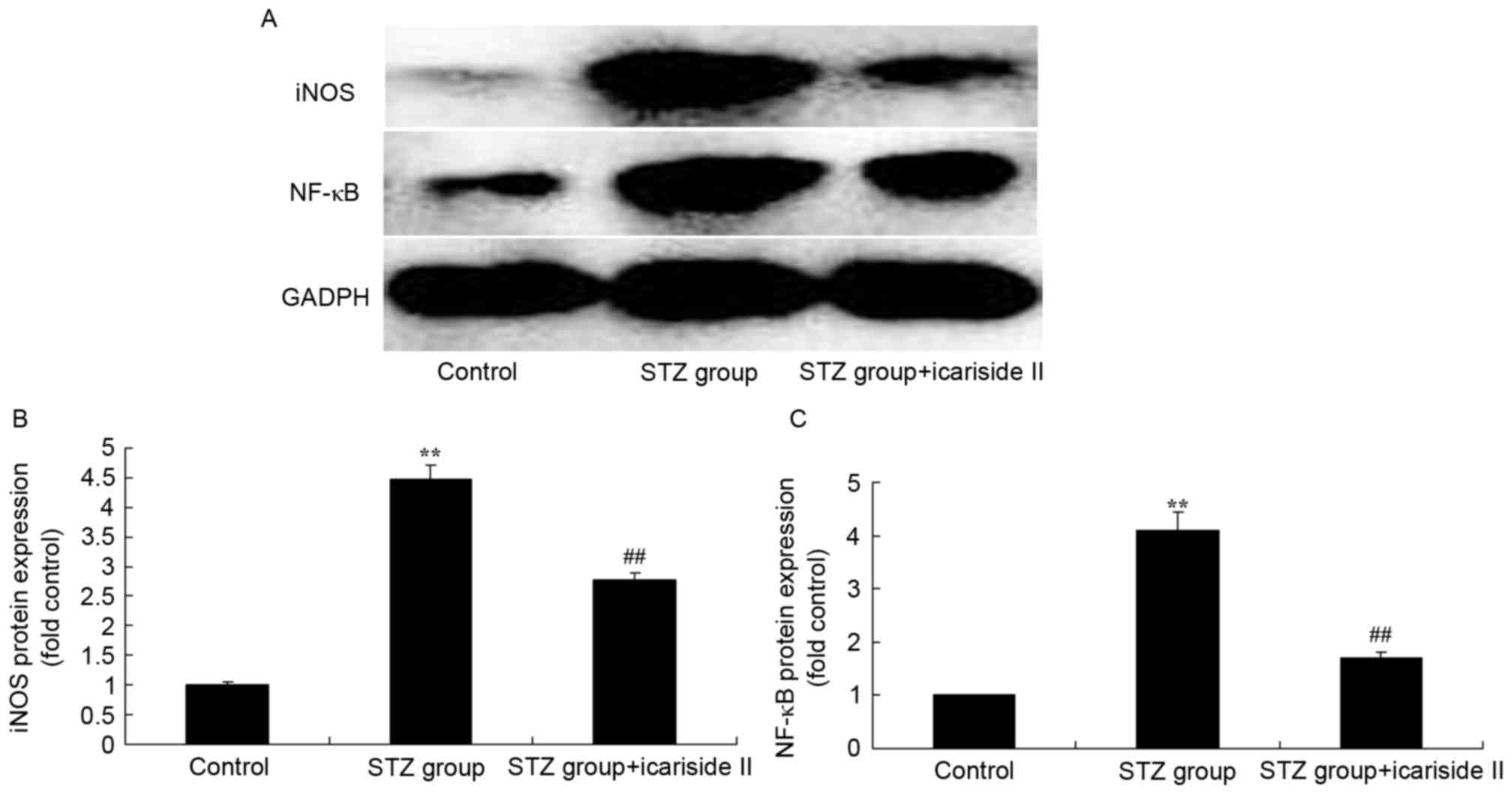
Icariside II reduces iNOS and NF-κB
protein expression in diabetic rats
To further elucidate the potential regulatory
mechanism of icariside II on iNOS and NF-κB protein expression in
STZ-induced diabetic rats, iNOS and NF-κB protein expression levels
were measured using in cardiac tissue by western blotting (Fig. 6A). Results from western blotting
demonstrated that iNOS and NF-κB protein expression levels of
diabetic rats were significantly higher compared with rats in the
Control group (Fig. 6B and C,
respectively). Icariside II co-treatment significantly reduced iNOS
and NF-κB protein expression in STZ-induced diabetic rats (Fig. 6).
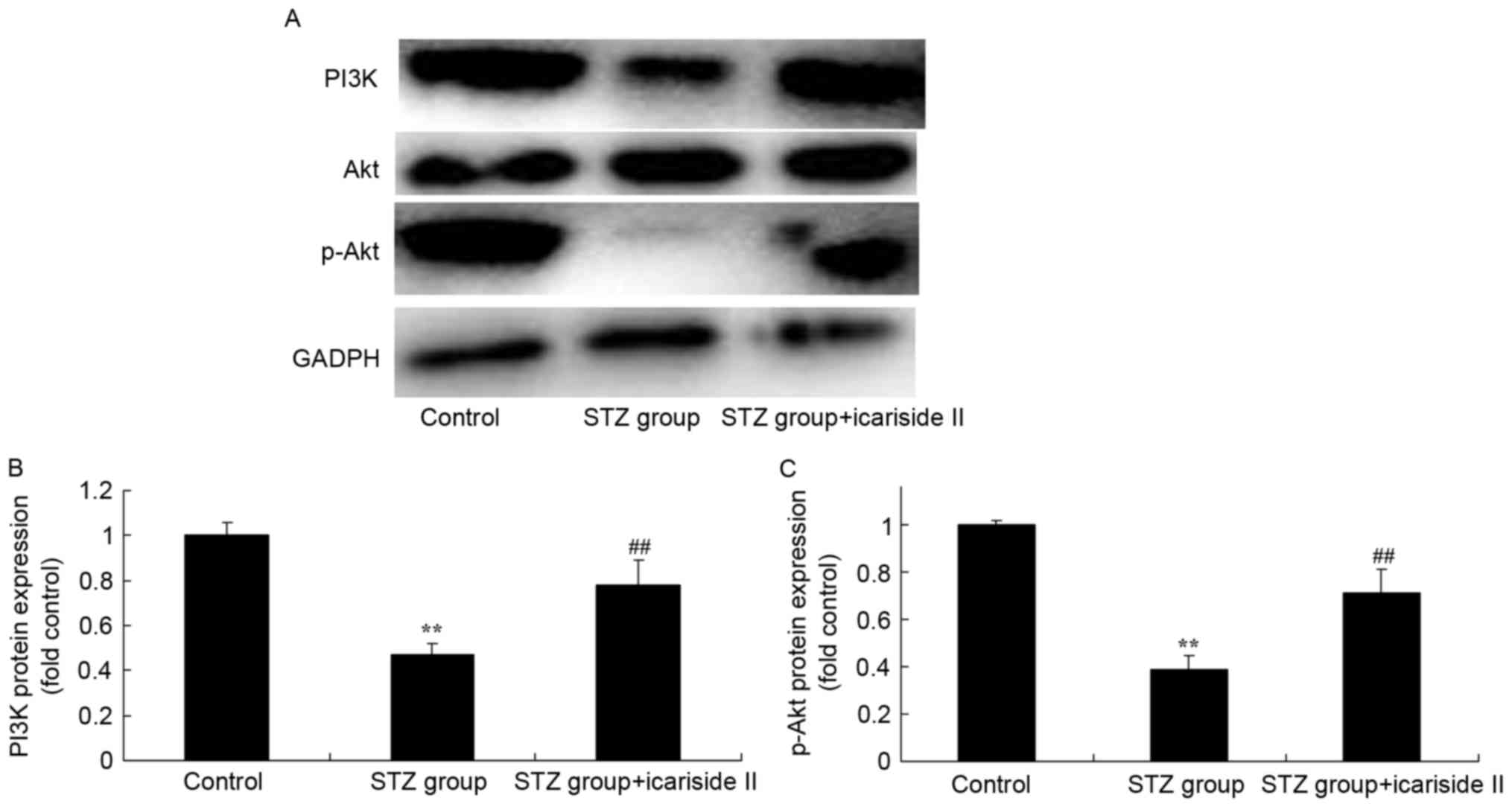
Icariside II induces PI3K and p-Akt
protein expression in diabetic rats
To further assess whether the PI3K/Akt signaling
pathway is a functional target of icariside II, the PI3K/Akt
signaling pathway was examined in cardiac tissue using western
blotting (Fig. 7A). Significant
reductions in PI3K and p-Akt protein expression in heart tissues of
diabetic rats were observed, compared with the Control group
(Fig. 7B and C, respectively).
Icariside II co-treatment significantly induced PI3K/Akt signaling
in heart tissues of diabetic rats (Fig. 7).
Discussion
Diabetic cardiomyopathy is a severe diabetic
complication that is characterized by ventricular diastolic or
systolic dysfunction (16). As the
number of people diagnosed as obese increases, the number of
patients with diabetes continue to rise, and diabetic
cardiomyopathy has become an urgent clinical problem that needs to
be solved (17). A previous study
reported that diabetes increases the risk of heart failure by 2–3
times, and even accelerates the deterioration of heart function in
patients with hypertension, ischemic heart disease and atrial
fibrillation (18). Inflammatory
reactions and apoptosis in myocardial tissues are key mechanisms in
the incidence and development of diabetic cardiomyopathy. The
present study demonstrated that icariside II treatment improved
body weight, heart/body weight ratio and fasting blood glucose, and
reduced CK and LDH levels in the serum of STZ-induced type I
diabetic rats. A previous study demonstrated that icariside II
reduced the STZ-induced cognitive deficits in rats through
anti-inflammatory effects (14).
Oxidative stress has also been demonstrated to be an
important factor that may lead to the occurrence and development of
type 2 diabetes (19). Oxidative
stress is an imbalance between the production and removal of
reactive oxygen species (ROS), leading to excessive production of
ROS and reactive nitrogen species, thereby injuring tissue cells
and biological macromolecules, including proteins and nucleic acids
(20). However, obesity or high
glucose levels may significantly increase the production of
superoxide, and oxidative stress is induced when the production
rate of superoxide exceeds its removal rate (21). A previous study has demonstrated
that oxidative stress may lead to functional injury of islet β
cells and peripheral insulin resistance, thereby inducing diabetes
and even diabetic neuropathy, diabetic retinopathy and diabetic
cardiovascular disease and other complications under the severe
cases (22). In the present study,
icariside II treatment was demonstrated to reduce the expression
levels of TNF-α, IL-1β and IL-6 levels in the heart tissue of
STZ-induced type I diabetic rats. A previous study revealed that
icariside II reduces diabetic nephropathy in STZ-induced diabetic
rats through antioxidative effects (15).
Apoptosis serves an important role in diabetic
myocardial injury and is one of the important manifestations of
ischemic myocardial injury (23).
Experimental results have shown that ischemia/reperfusion-induced
apoptotic injuries in rats with diabetes are more serious than in
rats without diabetes. Previous studies also have demonstrated that
myocardial cell injury due to apoptosis may be an important cause
of various heart diseases (23,24).
As myocardial cell apoptosis is the main pathological mechanism of
ischemia/reperfusion injury, the development of antiapoptotic drugs
is required to prevent further injury to cardiomyocytes and to
reduce ischemia/reperfusion-induced myocardial injury, thus slowing
down or preventing the occurrence of various heart diseases, which
may provide a new strategy for the treatment of cardiovascular
complications in patients with diabetes (25). The present study demonstrated that
icariside II treatment significantly suppressed the protein
expression levels of Bax and cleaved caspase-3 in heart tissue of
type I diabetic rats.
Activation of PI3K-Akt signaling may reduce
apoptosis and regulate glucose transport and glycogen synthesis
(26). Akt may have a
cytoprotective function by activating its downstream effector
molecules, such as eNOS and anti-apoptotic Bcl-2, to prevent cell
death through the mitochondrial pathway (27). The present data revealed that
icariside II significantly suppressed iNOS and NF-κB protein
expression in the heart tissue of type I diabetic rats. A previous
study reported that icariside II protects dexamethasone treated
osteoblasts and activates epidermal growth factor
receptor/Akt/nuclear factor erythroid 2-related factor 2 signaling
(28).
As a type of information transfer molecule, NO
easily diffuses with only several sec of half-life, which makes it
difficult to directly determine NO levels in tissues (29). iNOS expression is regulated by a
number of transcription factors, including NF-κB (29). NF-κB is activated at the initial
stage of inflammation to upregulate the expression of iNOS and to
increase the production of NO (20). In addition, iNOS and
proinflammatory factors improve the activity of NF-κB, leading to
the expression of NF-κB inflammatory protein target genes in large
amount, thus promoting the development of inflammation (13). Results from the present study
suggested that icariside II significantly induced PI3K/Akt
signaling in heart tissue of type I diabetic rats. A previous study
demonstrated that icariside II inhibited the epithelial-mesenchymal
transition of A549 and H1299 lung carcinoma cells in an
inflammatory microenvironment, through the Akt/NF-κB signaling
pathway (30). Another study
reported that icariside II induced apoptosis in human PC-3 prostate
cancer cells through iNOS expression (31).
In conclusion, the present study demonstrated that
treatment with icariside II reduced diabetic cardiomyopathy in
STZ-induced diabetic rats through anti-inflammatory, anti-oxidative
stress and antiapoptotic effects. Future studies will investigate
the morphological alterations in cardiac tissues and assess cardiac
function in response to icariside II treatment in a mouse model of
diabetes. In addition, future studies will aim to explore the
effects of icariside II on glucose metabolism and cardiomyopathy in
a mouse model of type II diabetes. It is thus suggested that
icariside II may be a promising candidate drug for the clinical
treatment of diabetic cardiomyopathy through the Akt/NOS/NF-κB
pathway.
Acknowledgements
The present study was supported by grants from the
the National Natural Science Foundation of China (grant no.
81671279), the Natural Science Foundation of Hainan Province (grant
no. 20168361), the Science and Technology Special Foundation for
Social Development in Hainan Province (grant no. 2015SF05) and
Sanya Municipal Scientific and Technological Innovation Project in
Health Care (grant no. 2014YW41).
References
|
1
|
Agrawal V, Agrawal A, Dwivedi AN and
Tripathi K: Correlation between 2D echocardiography and
multidetector row CT for early detection of diastolic dysfunction
in normotensive diabetic patients. J Clin Diagn Res. 10:OC27–30.
2016.PubMed/NCBI
|
|
2
|
Ding Y, Wang Y, Chen J, Hu Y, Cao Z, Ren P
and Zhang Y: p21 overexpression sensitizes osteosarcoma U2OS cells
to cisplatin via evoking caspase-3 and Bax/Bcl-2 cascade. Tumour
Biol. 35:3119–3123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pham I, Cosson E, Nguyen MT, Banu I,
Genevois I, Poignard P and Valensi P: Evidence for a specific
diabetic cardiomyopathy: An observational retrospective
echocardiographic study in 656 asymptomatic type 2 diabetic
patients. Int J Endocrinol. 7435032015.PubMed/NCBI
|
|
4
|
Zhuo C, Jiang R, Lin X and Shao M: LncRNA
H19 inhibits autophagy by epigenetically silencing of DIRAS3 in
diabetic cardiomyopathy. Oncotarget. 8:1429–1437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holscher ME, Bode C and Bugger H: Diabetic
Cardiomyopathy: Does the type of diabetes matter? Int J Mol Sci.
17:21362016. View Article : Google Scholar :
|
|
6
|
Xu G, Kuang G, Jiang W, Jiang R and Jiang
D: Polydatin promotes apoptosis through upregulation the ratio of
Bax/Bcl-2 and inhibits proliferation by attenuating the
beta-catenin signaling in human osteosarcoma cells. Am J Transl
Res. 8:922–931. 2016.PubMed/NCBI
|
|
7
|
Li Z, Zhang J, Mulholland M and Zhang W:
mTOR activation protects liver from ischemia/reperfusion-induced
injury through NF-kappaB pathway. FASEB J. 31:3016–3026. 2017.
View Article : Google Scholar
|
|
8
|
Korkmaz-Icöz S, Al Said S, Radovits T, Li
S, Brune M, Hegedűs P, Atmanli A, Ruppert M, Brlecic P, Lehmann LH,
et al: Oral treatment with a zinc complex of acetylsalicylic acid
prevents diabetic cardiomyopathy in a rat model of type-2 diabetes:
activation of the Akt pathway. Cardiovasc Diabetol. 15:752016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Xiong Z, Wang J, Zhang S, Lei L,
Yang L and Zhang Z: Glucagon-like peptide-1 protects cardiomyocytes
from advanced oxidation protein product-induced apoptosis via the
PI3K/Akt/Bad signaling pathway. Mol Med Rep. 13:1593–1601. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He H, Qiao X and Wu S: Carbamylated
erythropoietin attenuates cardiomyopathy via PI3K/Akt activation in
rats with diabetic cardiomyopathy. Exp Ther Med. 6:567–573. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas CM, Yong QC, Rosa RM, Seqqat R,
Gopal S, Casarini DE, Jones WK, Gupta S, Baker KM and Kumar R:
Cardiac-specific suppression of NF-kappaB signaling prevents
diabetic cardiomyopathy via inhibition of the renin-angiotensin
system. Am J Physiol Heart Circ Physiol. 307:H1036–1045. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZQ, Chen MT, Zhang R, Zhang Y, Li W
and Li YG: Docosahexaenoic acid attenuates doxorubicin-induced
cytotoxicity and inflammation by suppressing NF-kappaB/iNOS/NO
signaling pathway activation in H9C2 cardiac cells. J Cardiovasc
Pharmacol. 67:283–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren XM, Zuo GF, Wu W, Luo J, Ye P, Chen SL
and Hu ZY: Atorvastatin alleviates experimental diabetic
cardiomyopathy by regulating the GSK-3beta-PP2Ac-NF-kappaB
signaling axis. PLoS One. 11:e01667402016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin C, Deng Y, Gao J, Li X, Liu Y and Gong
Q: Icariside II, a novel phosphodiesterase-5 inhibitor, attenuates
streptozotocin-induced cognitive deficits in rats. Neuroscience.
328:69–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian W, Lei H, Guan R, Xu Y, Li H, Wang L,
Yang B, Gao Z and Xin Z: Icariside II ameliorates diabetic
nephropathy in streptozotocin-induced diabetic rats. Drug Des Devel
Ther. 9:5147–5157. 2015.PubMed/NCBI
|
|
16
|
Frustaci A, Ciccosanti F, Chimenti C,
Nardacci R, Corazzari M, Verardo R, Ippolito G, Petrosillo N, Fimia
GM and Piacentini M: Histological and proteomic profile of diabetic
versus non-diabetic dilated cardiomyopathy. Int J Cardiol.
203:282–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Z and Hu X: HMGB1 induced endothelial
permeability promotes myocardial fibrosis in diabetic
cardiomyopathy. Int J Cardiol. 227:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lebeche D: Diabetic cardiomyopathy: is
resistin a culprit? Cardiovasc Diagn Ther. 5:387–393.
2015.PubMed/NCBI
|
|
19
|
Wu H, Sheng ZQ, Xie J, Li R, Chen L, Li
GN, Wang L and Xu B: Reduced HMGB 1-mediated pathway and oxidative
stress in resveratrol-treated diabetic mice: A possible mechanism
of cardioprotection of resveratrol in diabetes mellitus. Oxid Med
Cell Longev. 2016:98368602016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo WW, Wang WJ, Tsai CY, Way CL, Hsu HH
and Chen LM: Diallyl trisufide (DATS) suppresses high
glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFkappaB
signaling via attenuating ROS generation. Int J Cardiol.
168:270–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Varga ZV, Giricz Z, Liaudet L, Hasko G,
Ferdinandy P and Pacher P: Interplay of oxidative,
nitrosative/nitrative stress, inflammation, cell death and
autophagy in diabetic cardiomyopathy. Biochim Biophys Acta.
1852:232–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki H, Kayama Y, Sakamoto M, Iuchi H,
Shimizu I, Yoshino T, Katoh D, Nagoshi T, Tojo K, Minamino T, et
al: Arachidonate 12/15-lipoxygenase-induced inflammation and
oxidative stress are involved in the development of diabetic
cardiomyopathy. Diabetes. 64:618–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao YZ, Zhang M, Wong HL, Tian XQ, Zheng
L, Yu XC, Tian FR, Mao KL, Fan ZL, Chen PP, et al: Prevent diabetic
cardiomyopathy in diabetic rats by combined therapy of aFGF-loaded
nanoparticles and ultrasound-targeted microbubble destruction
technique. J Control Release. 223:11–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu W, Zha W, Guo S, Cheng H, Wu J and Liu
C: Flos Puerariae extract prevents myocardial apoptosis via
attenuation oxidative stress in streptozotocin-induced diabetic
mice. PLoS One. 9:e980442014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Ding WY, Wang ZH, Tang MX, Wang
F, Li Y, Zhong M, Zhang Y and Wei Zhang: Erratum to: Early
administration of trimetazidine attenuates diabetic cardiomyopathy
in rats by alleviating fibrosis, reducing apoptosis and enhancing
autophagy. J Transl Med. 14:3092016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR
and Yan C: Higenamine protects ischemia/reperfusion induced cardiac
injury and myocyte apoptosis through activation of
beta2-AR/PI3K/AKT signaling pathway. Pharmacol Res. 104:115–123.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Ji SY, Liu SZ, Jing R and Lou WJ:
Cardioprotective effect of breviscapine: Inhibition of apoptosis in
H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following
simulated ischemia/reperfusion injury. Pharmazie. 70:593–597.
2015.PubMed/NCBI
|
|
28
|
Liu W, Mao L, Ji F, Chen F, Wang S and Xie
Y: Icariside II activates EGFR-Akt-Nrf2 signaling and protects
osteoblasts from dexamethasone. Oncotarget. 8:2594–2603.
2017.PubMed/NCBI
|
|
29
|
Jiang C, Tong YL, Zhang D, Liu LZ and Wang
JF: Sinomenine prevents the development of cardiomyopathy in
diabetic rats by inhibiting inflammatory responses and blocking
activation of NF-kappaB. Gen Physiol Biophys. 36:65–74. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Feng L, Zhong R, Xia Z, Zhang L,
Cui L, Yan H, Jia X and Zhang Z: Icariside II inhibits the EMT of
NSCLC cells in inflammatory microenvironment via down-regulation of
Akt/NF-kappaB signaling pathway. Mol Carcinog. 56:36–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee KS, Lee HJ, Ahn KS and Kim SH, Nam D,
Kim DK, Choi DY, Ahn KS, Lu J and Kim SH:
Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II
induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett.
280:93–100. 2009. View Article : Google Scholar : PubMed/NCBI
|