Introduction
Keloids are a pathological wound healing response to
cutaneous injury in genetically susceptible individuals. Keloid
formation is characterized by hyperproliferation of secretory and
responsive keloid fibroblasts (KFs), overproduction of
extracellular matrix (ECM) (1).
Although there are various methods widely applied for the treatment
of keloids, including intralesional steroid injection,
dermabrasion, pressure therapy, surgical excision, radiotherapy,
cryotherapy, pulse dye, and carbon dioxide laser ablation (2,3), the
pathogenesis of keloids is not fully understood and there is no
standard treatment method. Accumulating data has demonstrated that
the proliferation and migration of KFs are involved in keloid
formation by synthesizing ECM (4–6).
Transforming growth factor (TGF)-β1 is one of the
most important cytokines that promote keloid formation (7,8).
Keloid-derived fibroblasts demonstrated a unique sensitivity to
TGF-β, coupled with an increased expression of TGF-β1 and 2 and
TGF-β type I and II receptors, and an increased proliferation and
collagen production (9,10). Therefore, inhibiting the TGF-β1
pathway may have therapeutic potential for keloid treatment.
The eukaryotic translation initiation factor 3
subunit A (eIF3a) is one of the core subunits of the translation
initiation complex eIF3, responsible for ribosomal subunit joining
and mRNA recruitment to the ribosome (11). Multiple lines of evidence support
that eIF3a is involved in regulating cell cycle and cell
differentiation (12,13). For example, Liu et al
(13) reported that the expression
of eIF3a was significantly reduced in colon cancer cell lines prior
to differentiation. The overexpression of eIF3a inhibited the
progression of colon cancer; however, eIF3a knockdown greatly
induced colon cancer cell differentiation (13). In addition, it was reported that
knockdown of elF3a inhibits collagen synthesis in renal fibroblasts
via inhibition of TGF-β1/Smad signaling pathway (14). However, the role of eIF3a in keloid
formation has not yet been investigated. Therefore, in the current
study, the effect of eIF3a on TGF-β1-mediated ECM production in KFs
was examined. The present study demonstrated that eIF3a was highly
expressed in human keloid tissues, and knockdown of eIF3a
efficiently suppressed ECM expression in KFs, at least in part, via
the TGF-β1/Smad signaling pathway. Thus, eIF3a may be a potential
target for treatment of keloids.
Materials and methods
Tissue sample collection
Fresh keloid and normal skin samples were obtained
from 8 healthy subjects with keloid and another 8 healthy subjects.
All the subjects were patients of the Department of Burns and
Plastic Surgery, Plastic and Cosmetic Center, Nanyang Nanshi
Hospital, Affiliated Hospital of Henan University (Nanyang, China).
The present study was approved by Ethics Committee of Nanyang
Nanshi Hospital. Informed consent was obtained from all individual
subjects for all procedures.
KF culture
Primary KF cultures were established as previously
described (15). The specimens
were digested in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
0.5% dispase overnight at 4°C, then cultured in DMEM with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
0.1 g/ml streptomycin at 37°C in a humidified incubator with 5%
CO2.
Transfection of small interfering
(si)RNA
siRNA-eIF3a (5′-GCAGATGGTCTTAGATATA-3′) and the
non-silencing control siRNA (siRNA-mock, 5′-GCAAAAAATGGGTTTTCGT-3′)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). For in vitro transfection, KFs were plated and
cultures to 70–90% confluency without antibiotics, and then
incubated with a mixture of siRNA and Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in 100 µl serum-free
DMEM, according to the manufacturer's instructions. The cells were
harvested with extraction buffer after transfection for 48 h. The
relative expression of eIF3a was determined using western blot
analysis.
Cell proliferation assay
Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen,
China) according to manufacturer's instructions. In brief, KFs at a
density of 1×104 cells/well were transfected with
siRNA-eIF3a or siRNA-mock, and treated with TGF-β1 (10 ng/ml).
Following cultivation for 72 h, CCK-8 solution (10 µl) was added to
each well and the plates were incubated for 2 h. The absorbance was
determined at 490 nm (optical density value) using a Synergy HT
microplate reader (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from keloid tissues and KFs
using the TRIzol® Plus RNA Purification kit (Invitrogen;
Thermo Fisher Scientific, Inc.). Complementary DNA was synthesized
from 2 µg of total RNA with an AMV reverse transcriptase kit
(Promega Corporation, Madison, WI, USA) according to the
manufacturer's instructions. RT-qPCR amplification was carried out
on an IQ5 real-time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using a SYBR Premix Ex Taq kit (Takara
Biotechnology Co., Ltd., Dalian, China) following the
manufacturer's instructions. The specific primers used were as
follows: eIF3a sense, 5′-TCAAGTCGCCGGACGATA-3′ and anti-sense,
5′-CCTGTCATCAGCACGTCTCCA-3′; and for β-actin were sense,
5′-AAATCGTGCGTGACATCAAAGA-3′ and antisense,
5′-GGCCATCTCCTGCTCGAA-3′. The PCR cycling program was 95°C for 5
min, then 35 cycles of 94°C for 20 sec, 59°C for 20 sec and 72°C
for 25 sec, and a final extension at 72°C for 4 min. The
specificity of the PCR product was examined by dissociation curve
analysis, and the relative quantification of gene expression was
analyzed by the 2−ΔΔCq method (16) and normalized to β-actin that served
as internal standard.
Western blot analysis
The proteins were extracted from keloid tissues and
KFs using radioimmunoprecipiation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's instructions. Protein concentration was determined
using the Bradford method. Protein samples (20 µg) were separated
by 10% SDS-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes (GE Healthcare Life Sciences, Little
Chalfont, UK). After blocking with 10% fat-free milk in TBS-Tween
[20 mmol/l Tris, 0.15 mol/l NaCl (pH 7.0), 0.1% Tween-20], the
membranes were incubated with primary antibodies [anti-eIF3a
(1:3,000, sc-376651), anti-α-smooth muscle actin (SMA, 1:2,500,
sc-53142) (both from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-collagen I (1:3,000, SAB4200678), anti-TGF-β RI
(1:3,000, SAB4502958), and anti-TGF-β RII (1:2,500; SAB4504269)
(all from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) anti-Smad2
(1:2,500, sc-101153; Santa Cruz Biotechnology, Inc.), anti-phospho
(p)-Smad2 (1:3,000, SAB4301395; Sigma-Aldrich; Merck KGaA),
anti-Smad3 (1:1,500, sc-101154; Santa Cruz Biotechnology, Inc.),
anti-p-Smad3 (1:3,000, SAB4301395; Sigma-Aldrich; Merck KGaA) and
anti-GAPDH (1:3,000, sc-59540; Santa Cruz Biotechnology, Inc.) at
4°C overnight. Membranes were then washed and incubated with
horseradish peroxidase-conjugated secondary antibodies (1:2,500,
sc-2005; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Proteins were visualized using the enhanced
chemiluminescence western blotting detection system according to
the manufacturer's protocol (GE Healthcare Life Sciences).
Quantification analysis was performed using Gel-Pro Analyzer
software (version 4.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation based on at least three independent experiments. The
significance of differences was analyzed using Student's t-test or
by one-way analysis of variance followed by a Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
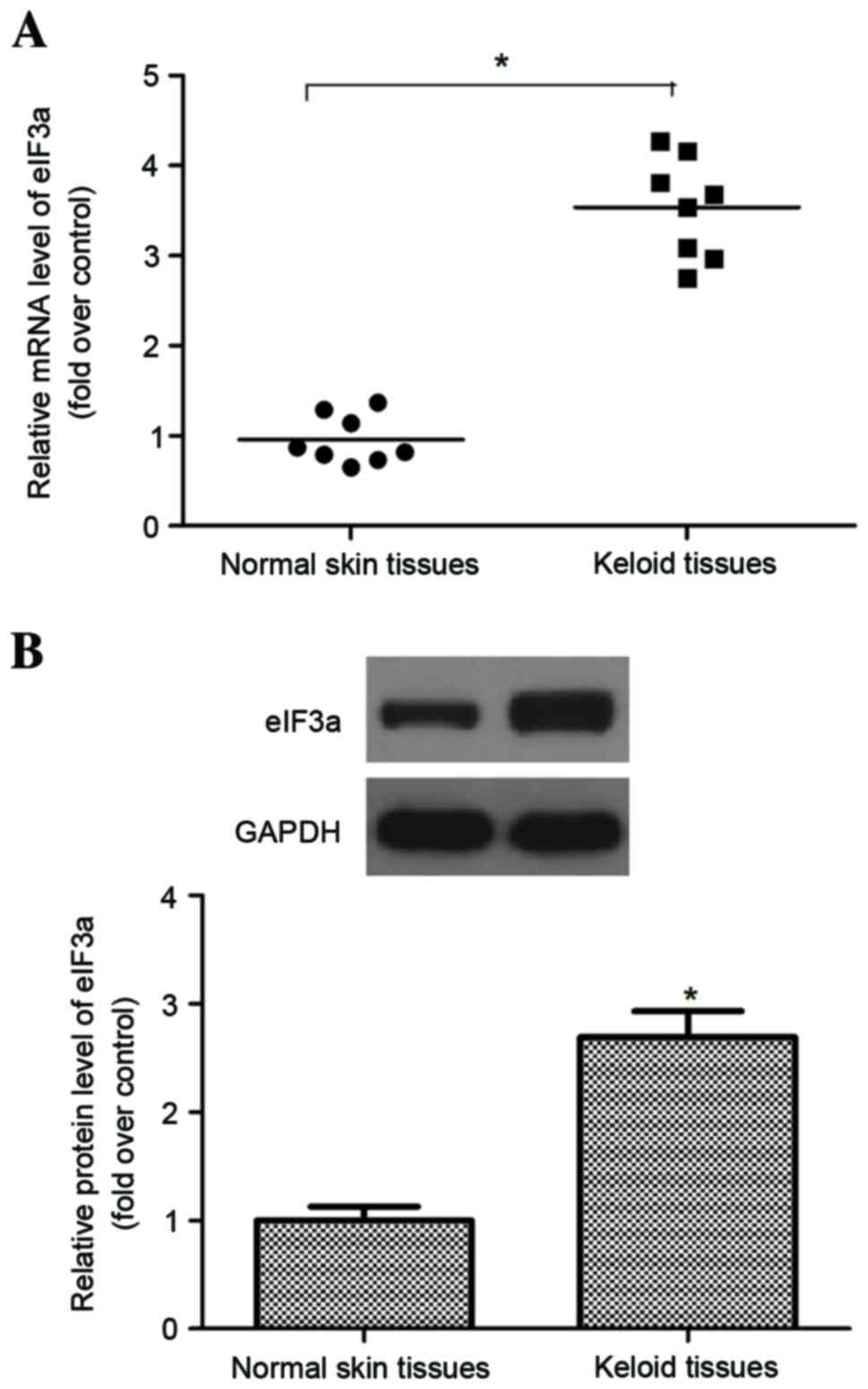
eIF3a is highly expressed in human
keloid tissues
To investigate the role of eIF3a in keloid
pathogenesis, the expression of eIF3a in keloid tissues was
measured using RT-qPCR and western blot analyses. The data
demonstrated that the mRNA expression of eIF3a in keloids was
significantly higher than those in normal skin (P<0.05; Fig. 1A). Similarly, western blot analysis
demonstrated that the protein of eIF3a in keloid was also
significantly higher than those in normal skin (P<0.05; Fig. 1B).
Effects of eIF3a on KF
proliferation
In order to investigate the effect of eIF3a on cell
proliferation and migration in TGF-β1-induced KFs, an eIF3a siRNA
was used to knockdown the expression of eIF3a. As presented in
Fig. 2A, siRNA-eIF3a significantly
decreased the expression levels of eIF3a in KFs compared with the
control group and mock siRNA (P<0.05). The effect of eIF3a
silencing on KF proliferation induced by TGF-β1 was then examined.
The results indicated that TGF-β1 treatment promoted KF
proliferation compared with the control group. Whereas the cell
proliferation induced by TGF-β1 was inhibited in eIF3a-silenced KFs
compared with cells transfected with the mock control siRNA
(P<0.05; Fig. 2B).
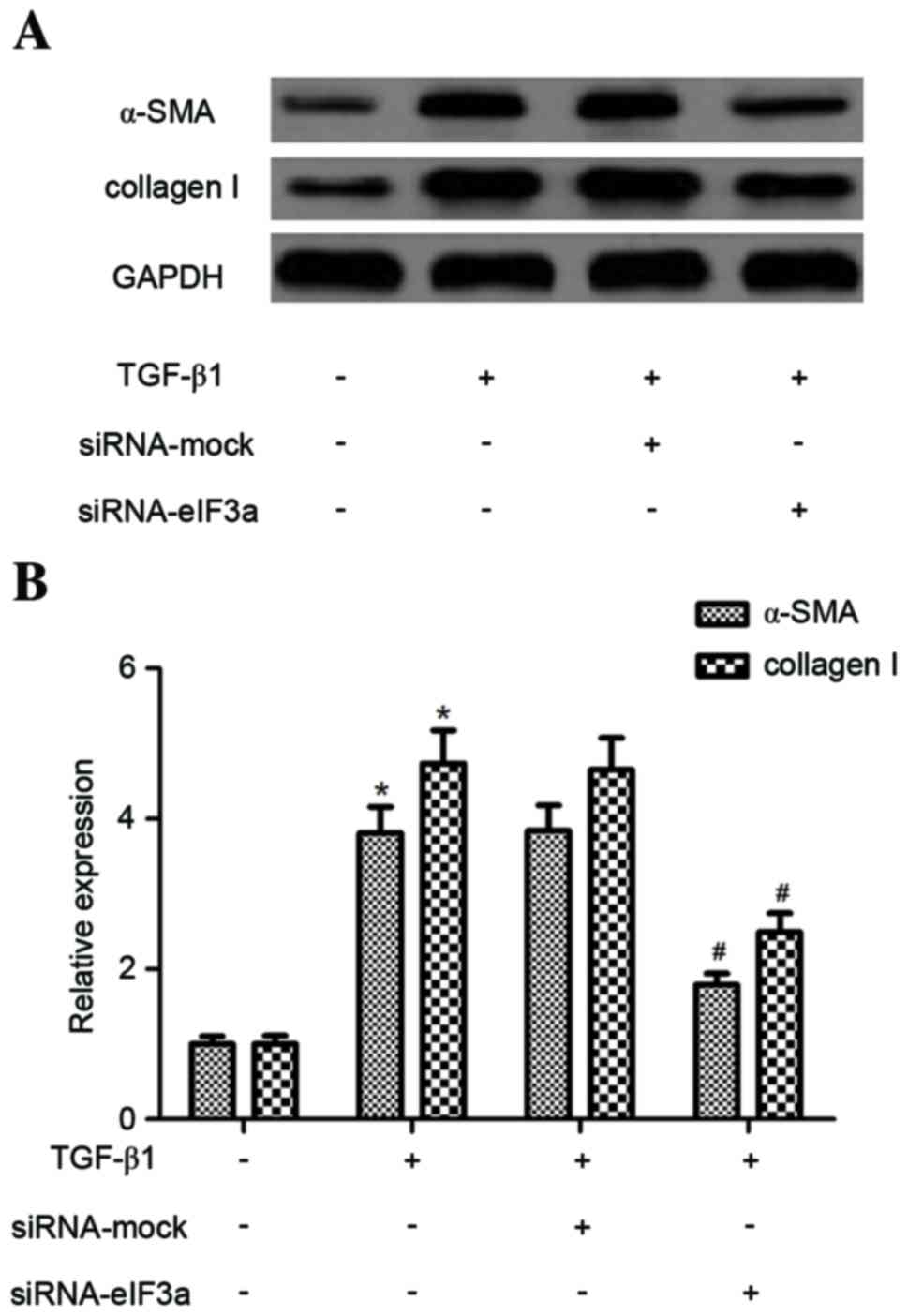
Effect of eIF3a on the expression of
α-SMA and collagen in human KFs
Subsequently, the effect of eIF3a on α-SMA and
collagen I protein levels in TGF-β1-induced KFs was examined. As
demonstrated in Fig. 3, TGF-β1
produced a significant increase in the expression of α-SMA and
collagen I compared with untreated cells (P<0.05). However,
eIF3a silencing significantly suppressed the TGF-β1-induced
expression levels of α-SMA and collagen I when compared with the
TGF-β1 + siRNA mock group (P<0.05).
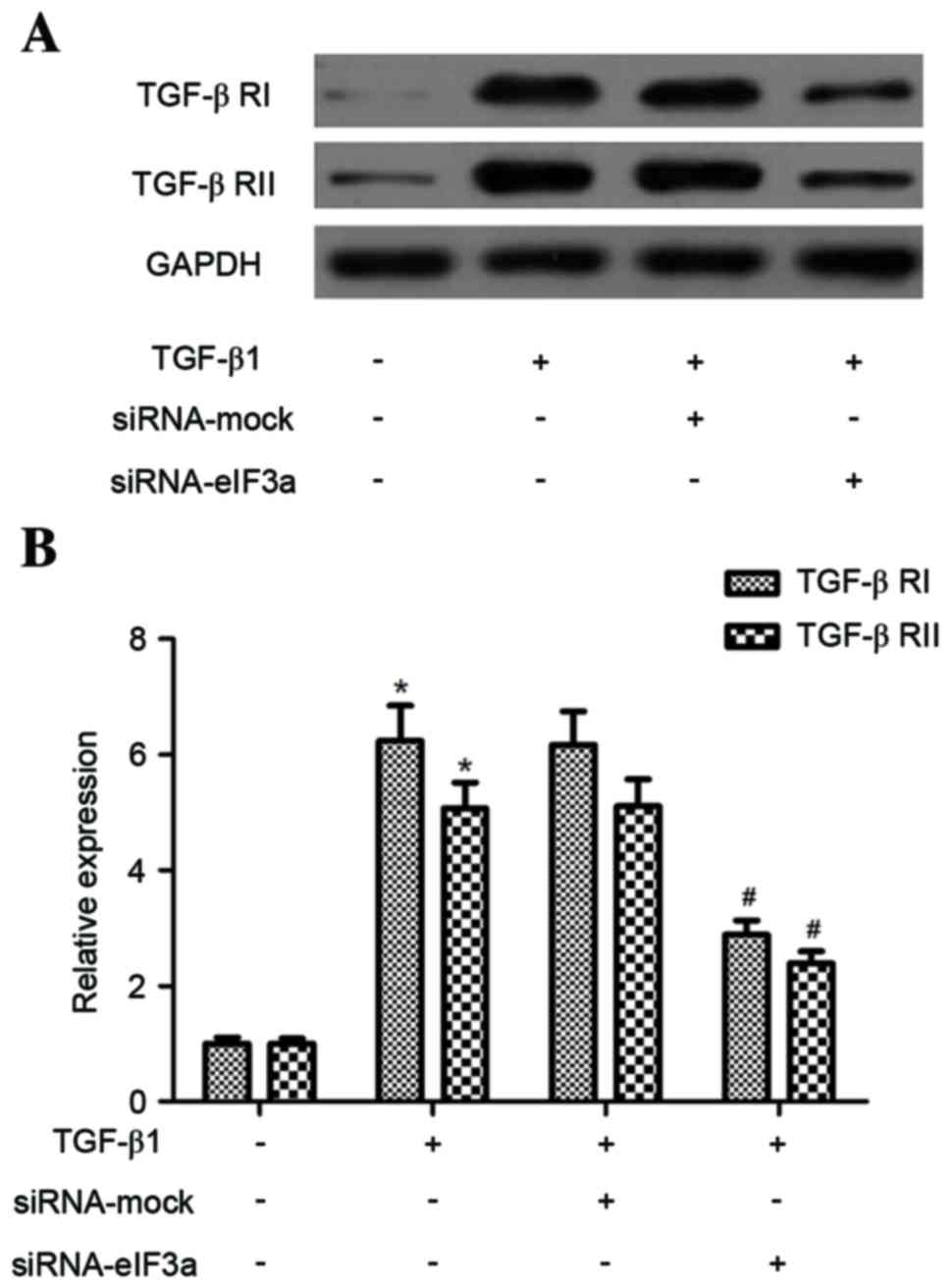
Effect of eIF3a on the expression of
TGF-β1 receptor (TGF-β R) I and II in human keloid fibroblasts
Next, the effect of eIF3a on TGF-β receptor I (TGF-β
RI) and II expression levels in TGF-β1-stimulated KFs was examined.
As demonstrated in Fig. 4, TGF-β1
significantly increased TGF-β RI and TGF-β RII expression in KFs
when compared with the control group (P<0.05). However, eIF3a
silencing dramatically suppressed the TGF-β1-enhanced TGF-β RI and
TGF-β RII expression in KFs, compared to the TGF-β1 + siRNA-mock
group (P<0.05).
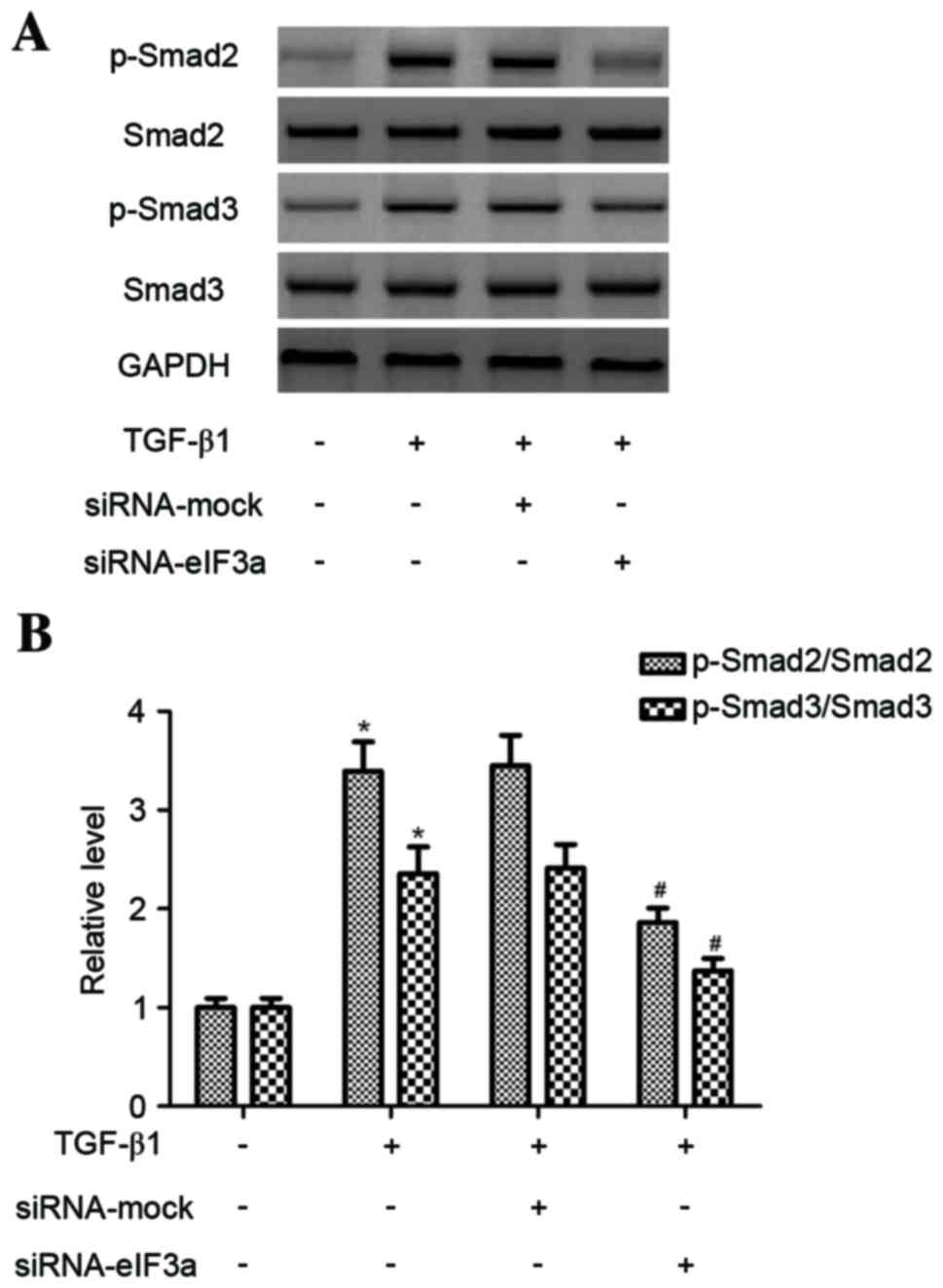
Effect of eIF3a on phosphorylation of
Smad3 in human KFs
To further explore the intracellular signaling
pathway, the effect of eIF3a on TGF-β1-induced Smad2 and Smad3
phosphorylation in KFs was investigated. As presented in Fig. 5, the phosphorylation of Smad2 and
Smad3 increased in TGF-β1-treated KFs compared to the control group
(P<0.05). However, eIF3a silencing inhibited the phosphorylation
levels of Smad2 and Smad3 in TGF-β1-induced KFs compared with the
TGF-β1 + siRNA-mock group (P<0.05).
Discussion
In the present study, a significant upregulation of
eIF3a mRNA and protein in human keloid tissues was observed when
compared with normal tissues. Knockdown of eIF3a inhibited KF
proliferation induced by TGF-β1. In addition, eIF3a silencing
significantly suppressed TGF-β1-induced expression of α-SMA and
collagen I, as well as expression of TGF-β RI and TGF-β RII in KFs.
In addition, eIF3a silencing inhibited the phosphorylation levels
of Smad2 and Smad3 in TGF-β1-induced KFs.
Previous studies identified that eIF3a has a
critical role in fibrotic disease. Li et al (17) reported that the expression of eIF3a
was obviously increased in the lungs of rats with pulmonary
fibrosis, and detected upregulation of α-SMA and collagens. An
additional study demonstrated that the expression of eIF3a was
significantly increased in the right ventricle (RV) of RV
remodeling rats (18). In line
with these results, the present study identified that there was
upregulation of eIF3a mRNA and protein and in human keloid tissues,
which suggested that eIF3a may be useful as a potential biomarker
for keloid formation.
It was previously reported that the proliferation
ability of KFs was higher than that of normal skin fibroblasts
(19). In addition, application of
exogenous TGF-β1 induces cell proliferation in cultured cardiac
fibroblasts and pulmonary fibroblasts, and the effect of
TGF-β1-induced proliferation is abolished by eIF3a siRNA (17,18).
Consistent with these previous studies, the present study observed
that TGF-β1 treatment promoted KF proliferation, whereas, knockdown
of eIF3a significantly inhibited KF proliferation induced by
TGF-β1.
Previous studies demonstrated that the excessive ECM
deposition derived from fibroblasts in the skin is the major
histopathological characteristic of keloids (20–22).
In addition, TGF-β1 has been demonstrated to greatly increase the
expression of ECM proteins in KFs (21). Similarly, in the present study, it
was identified that TGF-β1 increased the expression levels of α-SMA
and collagen I; however, eIF3a silencing significantly suppressed
TGF-β1-induced the expression levels of α-SMA and collagen I in
KFs. These results suggested that siRNA-eIF3a exhibits a
suppressive effect on α-SMA and collagen I expression in KFs in the
presence of TGF-β1. Therefore, α-SMA may serve a specific role in
keloid formation.
Multiple lines of evidence support that the
TGF-β1/Smad signaling pathway has an important role in keloid
formation (23–25). Upon phosphorylation by TGF-β
receptors, Smad2 and Smad3, known as receptor R-Smads, form
heteromeric complexes with Co-Smad or Smad4, and the Smad complex
translocates into the nucleus where it regulates the expression of
target genes (26). KFs have been
reported to express higher levels of TGF-β RI and TGF-β RII than
normal dermal fibroblasts (10).
Previous studies have indicated that Smad2 and Smad3 are
overexpressed and highly phosphorylated in KFs compared with normal
fibroblasts, and inhibition of Smad2 and Smad3 may decrease the
expression of type I and III procollagen in keloids (10,27,28).
The current novel data reveal that TGF-β1 treatment markedly
increased the expression of TGF-β RI and TGF-β RII, and this
enhancing effect was inhibited by siRNA-eIF3a in KFs. In addition,
an increase of p-Smad2 and p-Smad3 induced by TGF-β1 was reversed
by siRNA-eIF3a. These results suggest that siRNA-eIF3a inhibits ECM
expression via the TGF-β1/Smad signaling pathway in KFs.
In conclusion, the current results demonstrate that
siRNA-eIF3a inhibits ECM expression in KFs and the effect may be
mediated via the TGF-β1/Smad signaling pathway. Thus, eIF3a may be
a potential target for treatment of keloids.
References
|
1
|
Seifert O and Mrowietz U: Keloid scarring:
Bench and bedside. Arch Dermatol Res. 301:259–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
English RS and Shenefelt PD: Keloids and
hypertrophic scars. Dermatol Surg. 25:631–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic scarring and keloids:
Pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang F, Huang RL, Zheng Y, Liu M and Huo
R: Bone marrow derived mesenchymal stem cells inhibit the
proliferative and profibrotic phenotype of hypertrophic scar
fibroblasts and keloid fibroblasts through paracrine signaling. J
Dermatol Sci. 83:95–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu CS, Wu PH, Fang AH and Lan CC: FK506
inhibits the enhancing effects of transforming growth factor
(TGF)-β1 on collagen expression and TGF-β/Smad signaling in keloid
fibroblasts: Implication for new therapeutic approach. Br J
Dermatol. 167:532–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Makino S, Mitsutake N, Nakashima M, Saenko
VA, Ohtsuru A, Umezawa K, Tanaka K, Hirano A and Yamashita S:
DHMEQ, a novel NF-kappaB inhibitor, suppresses growth and type I
collagen accumulation in keloid fibroblasts. J Dermatol Sci.
51:171–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bran GM, Sommer UJ, Goessler UR, Hörmann
K, Riedel F and Sadick H: TGF-β1 antisense impacts the SMAD
signalling system in fibroblasts from keloid scars. Anticancer Res.
30:3459–3463. 2010.PubMed/NCBI
|
|
8
|
Chalmers RL: The evidence for the role of
transforming growth factor-beta in the formation of abnormal
scarring. Int Wound J. 8:218–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peltonen J, Hsiao LL, Jaakkola S, Sollberg
S, Aumailley M, Timpl R, Chu ML and Uitto J: Activation of collagen
gene expression in keloids: Co-localization of type I and VI
collagen and transforming growth factor-beta 1 mRNA. J Invest
Dermatol. 97:240–248. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chin GS, Liu W, Peled Z, Lee TY,
Steinbrech DS, Hsu M and Longaker MT: Differential expression of
transforming growth factor-beta receptors I and II and activation
of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 108:423–429.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saletta F, Suryo Rahmanto Y and Richardson
DR: The translational regulator eIF3a: The tricky eIF3 subunit!
Biochim Biophys Acta 1806. 1–286. 2010.
|
|
12
|
Dong Z, Liu Z, Cui P, Pincheira R, Yang Y,
Liu J and Zhang JT: Role of eIF3a in regulating cell cycle
progression. Exp Cell Res. 315:1889–1894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Dong Z, Yang Z, Chen Q, Pan Y, Yang
Y, Cui P, Zhang X and Zhang JT: Role of eIF3a (eIF3 p170) in
intestinal cell differentiation and its association with early
development. Differentiation. 75:652–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YF, Wang Q, Luo J, Yang S, Wang JL
and Li HY: Knockdown of elF3a inhibits collagen synthesis in renal
fibroblasts via Inhibition of transforming growth factor-β1/Smad
signaling pathway. Int Clin Exp Pathol. 8:8983–8989. 2015.
|
|
15
|
Ishihara H, Yoshimoto H, Fujioka M,
Murakami R, Hirano A, Fujii T, Ohtsuru A, Namba H and Yamashita S:
Keloid fibroblasts resist ceramide-induced apoptosis by
overexpression of insulin-like growth factor I receptor. J Invest
Dermatol. 115:1065–1071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XW, Wu YH, Li XH, Li D, Du J, Hu CP and
Li YJ: Role of eukaryotic translation initiation factor 3a in
bleomycin-induced pulmonary fibrosis. Eur J PharSmacol. 749:89–97.
2015. View Article : Google Scholar
|
|
18
|
Li WQ, Li XH, Wu YH, Du J, Wang AP, Li D
and Li YJ: Role of eukaryotic translation initiation factors 3a in
hypoxia-induced right ventricular remodeling of rats. Life Sci.
144:61–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Younai S, Nichter LS, Wellisz T, Reinisch
J, Nimni ME and Tuan TL: Modulation of collagen synthesis by
transforming growth factor-[beta] in keloid and hypertrophic scar
fibroblasts. Ann Plast Surg. 33:148–154. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sidgwick G and Bayat A: Extracellular
matrix molecules implicated in hypertrophic and keloid scarring. J
Eur Acad Dermatol Venereol. 26:141–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babu M, Diegelmann R and Oliver N: Keloid
fibroblasts exhibit an altered response to TGF-beta. J Invest
Dermatol. 99:650–655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara M, Muragaki Y and Ooshima A:
Keloid-derived fibroblasts show increased secretion of factors
involved in collagen turnover and depend on matrix
metalloproteinase for migration. Br J Dermatol. 153:295–300. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu YC, Chen MJ, Yu YM, Ko SY and Chang
CC: Suppression of TGF-β1/SMAD pathway and extracellular matrix
production in primary keloid fibroblasts by curcuminoids: Its
potential therapeutic use in the chemoprevention of keloid. Arch
Dermatol Res. 302:717–724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mun JH, Kim YM, Kim BS, Kim JH, Kim MB and
Ko HC: Simvastatin inhibits transforming growth factor-β1-induced
expression of type I collagen, CTGF, and α-SMA in keloid
fibroblasts. Wound Repair Regen. 22:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou Q, He WJ, Hao HJ, Han QW, Chen L, Dong
L, Liu JJ, Li X, Zhang YJ, Ma YZ, et al: The four-herb Chinese
medicine ANBP enhances wound healing and inhibits scar formation
via bidirectional regulation of transformation growth factor
pathway. PLoS One. 9:e1122742014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phan TT, Lim IJ, Aalami O, Lorget F, Khoo
A, Tan EK, Mukhopadhyay A and Longaker MT: Smad3 signalling plays
an important role in keloid pathogenesis via epithelial-mesenchymal
interactions. J Pathol. 207:232–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang
H, Hou T, Wang Q, Yuan X, Zhu X, et al: Inhibition of Smad3
expression decreases collagen synthesis in keloid disease
fibroblasts. J Plast Reconstr Aesthet Surg. 60:1193–1199. 2007.
View Article : Google Scholar : PubMed/NCBI
|