Introduction
Osteosarcoma is the most common primary malignancy
of bone in teenagers and accounts for 20–35% of all malignant
primary bone tumors (1,2). It originates from primitive
transformed cells of mesenchymal origin in bone, which exhibit
osteoblastic differentiation and produce malignant osteoid
(3). Currently, the most common
treatments for patients with OS are neoadjuvant chemotherapy,
surgery and adjuvant chemotherapy (4). With development in the therapeutic
strategies, the 5-year overall and disease-free survival rates for
patients with OS have improved in the last 30 years (5). However, the curative effects in the
treatment of those patients with metastasis or at an advanced
clinical stage have been less effective in this period (6). The molecular mechanisms underlying
the occurrence and development of OS remain to be fully elucidated
(7). Therefore, it is important to
fully understand the mechanism underlying the metastasis and
progression in OS, and to investigate therapeutic targets to
improve the prognosis of this disease.
MicroRNAs (miRNAs) are an abundant class of
non-coding, conserved, single-strand RNA molecules containing 18–25
nucleotides (8,9). It is well established that miRNAs are
important in several diverse biological processes, including cell
proliferation, cell cycle, apoptosis, metastasis, invasion,
angiogenesis, stress responses and differentiation (10). miRNAs exert their functions through
interaction with the 3′ untranslated regions (3′UTRs) of target
genes in a base pairing manner, and mainly result in translational
repression or target mRNA degradation (11,12).
At present, >1,000 miRNAs have been identified in the human
genome, and these can modulate the expression of thousands of human
protein-encoding genes (9,13). Accumulated evidence shows that
numerous miRNAs are abnormally expressed in several types of human
cancer (14–16). These miRNAs are necessary to
maintain the malignant phenotype of cancer cells, and can function
as either tumor suppressors or oncogenes depending on the tumor
type and effects of their target genes (17). This suggests there is merit in
investigating miRNAs as novel and efficient therapeutic targets for
antitumor therapy.
Previous studies have reported that miR-129-5p is
involved in the carcinogenesis and progression of several types of
human cancer, including breast cancer (18), gastric cancer (19), lung cancer (20), thyroid carcinoma (21), ovarian cancer (22) and colorectal cancer (23). However, the expression, effects and
mechanisms underlying the effects of miR-129-5p in OS remain to be
elucidated. Therefore, in the present study, the expression levels
of miR-129-5p in OS tissues and cell lines were examined, and the
biological roles of the direct target genes of miR-129-5p in OS
were investigated.
Materials and methods
Cell lines and tumor specimens
The human OS cell lines, MG-63, SAOS-2, HOS, 143B
and U2OS, were purchased from American Type Culture Collection
(Manassas, VA, USA). A human normal osteoblastic cell line (hFOB
1.19) was also obtained from American Type Culture Collection. The
cells were grown in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). The cultures were
maintained at 37°C in a 5% CO2 atmosphere. In addition,
19 pairs of OS tissues and corresponding adjacent normal bone
tissues (13 male, 6 female; age range 23–67 years; mean age, 42
years; I+IIA stage, 11; IIB/III stage, 8) were collected from the
Department of Orthopedic Surgery, First Affiliated Hospital of
Harbin Medical University (Harbin, China) between August 2013 to
December 2015. The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Harbin Medical
University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The NanoDrop ND-1000 spectrophotometer
(Thermo Fisher Scientific, Inc.) was used to measure the
concentration. Reverse transcription was performed using M-MLV
(Promega Corporation, Madison, WI, USA). The temperature protocol
for reverse transcription was as follows: 95°C for 2 min; 20 cycles
of 94°C for 1 min, 55°C for 1 min and 72°C for 2 min; then 72°C for
5 min. The expression levels of miR-129-5p and the mRNA expression
levels of Rho-associated protein kinase 1 (ROCK1) were determined
using SYBR Premix Ex Taq™ kits (Takara Bio, Inc., Tokyo, Japan).
The reaction system contained 10 µl SYBR Premix Ex Taq, 2 µl cDNA
(200 ng), 0.8 µl forward primer, 0.8 µl reverse primer, 0.4 µl ROX
reference dye and 6 µl ddH2O. The amplification was
performed with cycling conditions as follows: 5 min at 95°C,
followed by 40 cycles of 95°C for 30 sec and 65°C for 45 sec. GAPDH
and U6 were used as the internal reference for ROCK1 mRNA and
miR-129-5p, respectively. RT-qPCR analysis was performed on an
Applied Biosystems® 7900 HT Real-Time PCR system (Thermo
Fisher Scientific, Inc.). The primers were designed as follows:
miR-129-5p, 5′-GATACTCACTTTTTGCGGTCT-3′ (forward) and
5′-GTGCAGGGTCCGAGGT-3′ (reverse); U6, 5′-CTCGCTTCGGCAGCACA-3′
(forward) and 5′-AACGCTTCACGAATTTGCGT-3′ (reverse); ROCK1,
5′-ATGAGTTTATTCCTACACTCTACCACTTTC-3′ (forward) and
5′-TAACATGGCATCTTCGACACTCTAG-3′ (reverse); and GAPDH,
5′-ACACCCACTCCTCCACCTTT-3′ (forward) and
5′-TAGCCAAATTCGTTGTCATACC-3′ (reverse). Data were calculated and
normalized using the 2−ΔΔCq method (24).
Transfection of cells
The miR-129-5p mimics and negative control miRNA
mimics (miR-NC) were synthesized by GenePharma (Shanghai, China).
Small interfering (si)RNA targeting ROCK1 (si-ROCK1) and siRNA
negative control (si-NC) were purchased from Biomics
Biotechnologies Co., Ltd. (Nantong, China). The si-ROCK1 sequence
was 5′-GGGUAACUCAUCUGGUAAATT-3′ and the si-NC sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. The cells were seeded into 6-well
plates at a density of 50–60% 1 day prior to transfection. Cell
transfection was performed using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell proliferation was evaluated using an MTT assay
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) according to
the manufacturer's protocol. Briefly, cells grown in the
logarithmic phase were seeded in 96-well plates at a density of
2,500 cells/well. Following adherence, the cells were transfected
with the miRNA mimics or siRNA, followed by incubation at 37°C in
5% CO2 atmosphere for 24, 48, 72 and 96 h. The MTT assay
was then performed at each time point; 20 µl MTT solution was added
to the culture medium and incubated at 37°C for an additional 4 h.
Subsequently, the MTT formazan was dissolved by 150 µl DMSO
(Sigma-Aldrich; Merck Millipore). The absorbance was determined at
490 nm using an automatic multi-well spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Transwell migration and invasion
assays
The cell migration and invasion capacities were
assessed using Transwell chambers (8-µm pores; BD Biosciences, San
Jose, CA, USA). For the invasion assay, the chambers were coated
with 60 µl of Matrigel (5 mg/ml; BD Biosciences). The cells were
collected 48 h following transfection and, at a density of
4×104 in 200 µl FBS-free medium, were seeded in the
upper chamber. Culture medium (600 µl) supplemented with 20% FBS
was added to the lower chamber. Following incubation at 37°C in a
5% CO2 atmosphere for 48 h, cotton swabs were used to
wipe the cells remaining in the upper chamber. The invaded cells
were stained with 0.5% crystal violet for 30 min and then counted
in five randomly selected fields under a light microscope
(magnification, ×200; Olympus Corporation, Tokyo, Japan). For the
migration assay, the procedures were similar to those in the
invasion assay, with the exception that the upper chambers were not
coated with Matrigel.
miRNA target prediction and luciferase
reporter assay
To determine the potential target genes of
miR-129-5p, bioinformatics analysis was performed with TargetScan
7.0 (http://www.targetscan.org/) and miRanda
(August 2010 release; http://www.microrna.org). psiCHECK™2-ROCK1-wild-type
(WT)-3′UTR and psiCHECK™2-ROCK1-mutant (MUT)-3′UTR were synthesized
by GenePharma. At 1 day prior to transfection, HEK293T cells
(American Type Culture Collection) were seeded in 24-well plates at
a density of 1×105 cells/well. psiCHECK™2-ROCK1-WT-3′UTR
or psiCHECK™2-ROCK1-MUT-3′UTR, together with the miR-129-5p mimics
or miR-NC were transfected into the HEK293T cells using
Lipofectamine 2000. The culture medium was replaced with DMEM
containing 10% FBS at 8 h post-transfection. The cells were
incubated at 37°C in 5% CO2 atmosphere for 48 h and
luciferase activities were determined using a Dual-Luciferase
Reporter Assay system (Promega Corporation). Renilla
luciferase activities were normalized to firefly luciferase
activities.
Western blot analysis
Total protein was isolated from the tissues and cell
lines using RIPA buffer (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) supplemented with protease inhibitors and phosphate
inhibitors (Roche Diagnostics, Indianapolis, IN, USA). A
Bicinchoninic Acid Protein assay kit (Thermo Fisher Scientific,
Inc.) was used to detect protein concentrations. Equal quantities
of protein (30 µg) were separated by 10% SDS-PAGE gel and
transferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Bedford, MA, USA) followed by blocking with 5% non-fat
milk in Tris-buffered saline-Tween 20 (TBST) for 1 h at room
temperature. The membranes were then immunoblotted with primary
antibodies at 4°C overnight. Following washing three times with
TBST, the membranes were incubated with goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (cat no. sc-2005; 1:2,000;
Santa Cruz Biotechnology, Inc.) for 2 h at room temperature.
Finally, immunoreactive protein bands were visualized using the
enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.). The primary antibodies used in the present study
were as follows: Mouse anti-human monoclonal ROCK1 antibody (cat
no. sc-365628; 1:1,000) and mouse anti-human monoclonal GAPDH
antibody (cat no. sc-47724; 1:1,000) (both from Santa Cruz
Biotechnology, Inc.). The densitometry of protein bands was
quantified using ImageJ version 1.49 (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences between groups were compared using two-tailed
Student's t-test or one-way analysis of variance using SPSS 19.0
statistical software (IBM SPSS Inc., Armonk, NY, USA). SNK analysis
was performed as a post hoc test following analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-129-5p is downregulated in OS
tissues and OS cell lines
The expression of miR-129-5p was determined in OS
tissues and corresponding adjacent normal bone tissues using
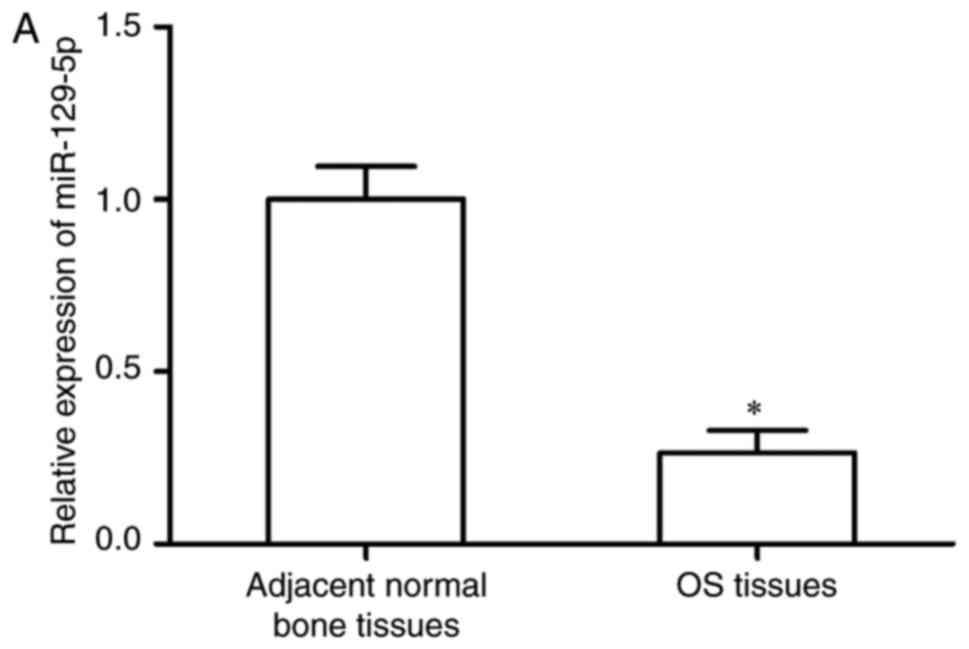
RT-qPCR analysis. As shown in Fig.
1A, the expression of miR-129-5p was significantly lower in the
OS tissues, compared with that in the corresponding adjacent normal
bone tissues (P<0.05). The expression levels of miR-129-5p in
the MG-63, SAOS-2, HOS, 143B and U2OS cells, and in the human
normal osteoblast cell line (hFOB 1.19) were also detected.
Compared with the expression in hFOB 1.19 cells, the expression of
miR-129-5p was downregulated in all five OS cell lines (Fig. 1B; P<0.05).
miR-129-5p inhibits cell
proliferation, migration and invasion in OS
To examine the potential roles of miR-129-5p in OS,
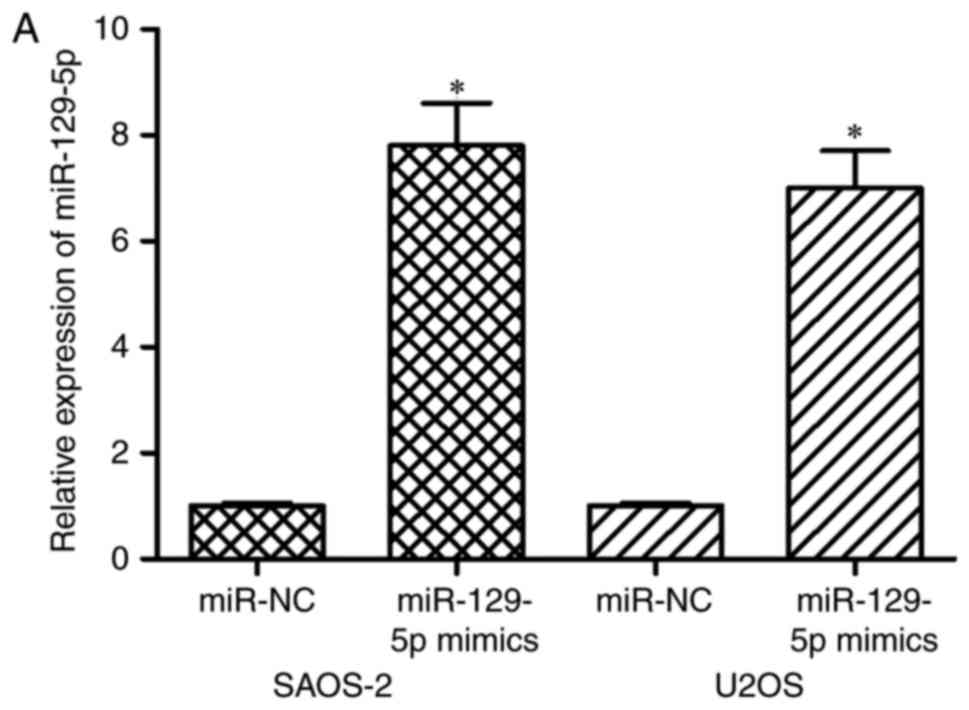
the SAOS-2 and U2OS cells were transfected with miR-129-5-mimics or
miR-NC. Following transfection, the expression of miR-129-5p was
significantly increased in the SAOS-2 and U2OS cells transfected
with the miR-129-5p mimics (Fig.
2A; P<0.05). The MTT assay showed that cell proliferation
was inhibited in the miR-129-5p mimic-transfected SAOS-2 and U2OS
cells, compared with that in the miR-NC-transfected cells (Fig. 2B; P<0.05). Similarly, the
Transwell migration and invasion assays indicated that the ectopic
expression of miR-129-5p decreased the migration and invasion
capacities of the SAOS-2 and U2OS cells (Fig. 2C; P<0.05). Taken together, these
data suggested that miR-129-5p acted as a tumor suppressor in OS
via inhibiting cell proliferation, migration and invasion.
ROCK1 is a direct target of
miR-129-5p
To examine the molecular mechanism underlying the
tumor suppressive roles of miR-129-5p in OS, bioinformatics
analysis was performed using TargetScan 7.0 and miRanda. A number
of genes were predicated as potential direct target genes of
miR-129-5p, however, ROCK1 was selected for further analysis as it
has been reported to be involved in the carcinogenesis and
progression of OS (25) (Fig. 3A; P<0.05).
A luciferase reporter assay was then performed to
investigate whether ROCK1 is a direct target of miR-129-5p. HEK293T
cells were co-transfected with psiCHECK™2-ROCK1-WT-3′UTR or
psiCHECK™2-ROCK1-MUT-3′UTR and miR-129-5p mimics or miR-NC. The
results showed that the upregulation of miR-129-5p suppressed the
luciferase activities of psiCHECK™2-ROCK1-WT-3′UTR (Fig. 3B; P<0.05) but had no inhibitory
effects on the luciferase activities of psiCHECK™2-ROCK1-MUT-3′UTR,
suggesting that ROCK1 may be a direct target of miR-129-5p.
To confirm the regulatory roles of miR-129-5p in the
expression of ROCK1, the expression levels of ROCK1 were measured
in SAOS-2 and U2OS cells following transfection with miR-129-5p
mimic or miR-NC. The results of RT-qPCR and western blot analyses
indicated that the mRNA (Fig. 3C;
P<0.05) and protein (Fig. 3D;
P<0.05) levels of ROCK1 were downregulated in the miR-129-5p
mimic-transfected SAOS-2 and U2OS cells, compared with those in the
miR-NC group. Taken together, these results suggested that ROCK1
may be a direct target of miR-129-5p.
ROCK1 is upregulated in OS tissues and
inversely correlated with the expression levels of miR-129-5p
As the results demonstrated that ROCK1 was a direct
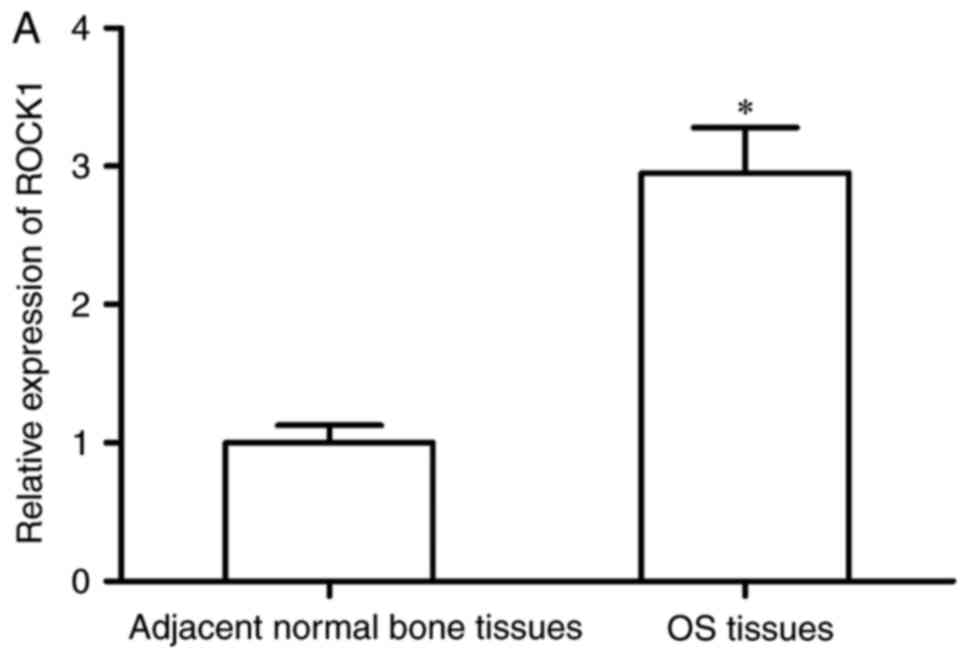
target of miR-129-5p in OS, the present study detected the
expression of ROCK1 in OS tissues and corresponding adjacent normal
bone tissues using RT-qPCR analysis. The results showed that the
mRNA expression of ROCK1 was significantly higher in the OS
tissues, compared with that in corresponding adjacent normal bone
tissues (Fig. 4A; P<0.05). The
associations between the expression of miR-129-5p and the mRNA
expression of ROCK1 in OS tissues were analyzed using Spearman's
correlation analysis, and it was found that the mRNA expression of
ROCK1 was inversely correlated with the expression of miR-129-5p in
OS tissues (R=−0.6203; P=0.0046; Fig.
4B).
miR-129-5p triggers the inhibition of
OS cell proliferation, migration and invasion, in part, via
targeting ROCK1
To investigate whether the downregulation of ROCK1
contributed to the tumor suppressive roles of miR-129-5p in cell
proliferation, migration and invasion, the biological functions of
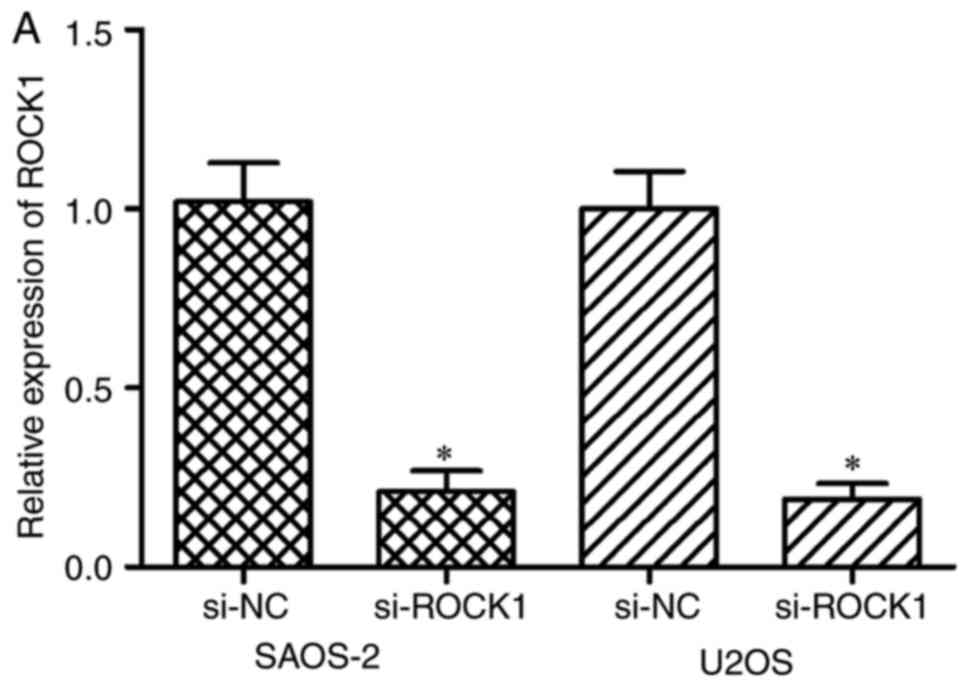
ROCK1 were determined using an RNA interference method. SAOS-2 and
U2OS cells were injected with si-ROCK1 or si-NC. The results of the
RT-qPCR and western blot analyses indicated that the mRNA (Fig. 5A; P<0.05) and protein (Fig. 5B; P<0.05) expression levels of
ROCK1 were reduced in the SAOS-2 and U2OS cells transfected with
si-ROCK1. Subsequently, an MTT assay, and Transwell migration and
invasion assays were performed to evaluate the roles of ROCK1 in
OS. As shown in Fig. 5C and D, the
downregulation of ROCK1 suppressed SAOS-2 and U2OS cell
proliferation (P<0.05), migration and invasion (P<0.05).
These findings demonstrated that the biological functions of the
underexpression of ROCK1 were similar to those induced by the
overexpression of miR-129-5p in OS cells, suggesting that the
antitumor effect of miR-129-5p was, in part, dependent on the
downregulation of ROCK1.
Discussion
miR-129 is transcribed from two genes, mir-129-1 and
mir-129-2. The mature miRNAs, miR-129-5p and miR-129-3p, are
processed from 5′ and 3′ precursors, respectively (26). In the present study, it was shown
that miR-129-5p was downregulated in OS tissues and cell lines,
compared with corresponding adjacent normal bone tissues and a
human normal osteoblast cell line, respectively. In addition, the
re-expression of miR-129-5p inhibited cell proliferation, migration
and invasion in OS. ROCK1 was identified as a novel direct target
gene of miR-129-5p, and miR-129-5p negatively regulated the
expression of ROCK1 via binding to its 3′UTR. Taken together, these
findings suggested that miR-129-5p may be a novel potential
therapeutic target for the treatment of patients with OS.
miR-129-5p has been found to be abnormally expressed
in various types of cancer. For example, Yu et al (18) reported that the expression of
miR-129-5p was lower in breast cancer tissues than paired adjacent
normal breast tissues; the downregulation of miR-129-5p was
significantly associated with advanced clinical stage and poor
prognosis in patients with breast cancer. Jiang et al
(19) found that the levels of
miR-129-5p were reduced in gastric cancer tissues and blood
samples, compared with those in matched non-tumor adjacent tissues
and healthy volunteers, respectively. Ma et al (27) demonstrated that miR-129-5p was
downregulated in hepatocellular carcinoma, and was correlated with
vascular invasion, intrahepatic metastasis and recurrence rate. In
addition, patients with hepatocellular carcinoma with a low
expression of miR-129-5p had a poorer outcome, compared with
patients with a high level of miR-129-5p. Similarly, Zhai et
al (28) revealed that a low
expression of miR-129-5p was correlated with advanced stage and
vascular invasion. miR-129-5p has also been found to be expressed
at low levels in lung cancer (20), thyroid carcinoma (21), ovarian cancer (22) and colorectal cancer (23). These findings suggest that
miR-129-5p is involved in carcinogenesis and cancer
progression.
Previous studies have reported that miR-129-5p
functions as a tumor suppressor in several types of human cancer.
For example, in breast cancer, the overexpression of miR-129-5p
inhibits cell mobility and migration, reverses
epithelial-mesenchymal transition, attenuates irradiation-induced
autophagy and decreases radioresistance (18,29,30).
In gastric cancer, the upregulation of miR-129-5p causes marked
suppression of cell viability, colony-forming ability, migration
and invasion (19). Ma et
al (27) showed that the
ectopic expression of miR-129-5p suppressed cell growth, invasion
and metastasis in hepatocellular carcinoma (28). Duan et al (21) reported that the re-expression of
miR-129-5p reduced cell migration and induces apoptosis of thyroid
carcinoma. Tan et al (22)
demonstrated that the enforced expression of miR-129-5p repressed
ovarian cancer cell proliferation, survival and tumorigenicity. In
colorectal cancer, miR-129-5p has been shown to inhibit cell
growth, induce apoptosis and enhance cell-cycle arrest in
colorectal cancer (23). These
findings indicate that miR-129-5p is important in these types of
human cancer, and may serve as a potential therapeutic target for
their treatment.
To investigate the potential molecular mechanism by
which miR-129-5p regulates the proliferation and metastasis in OS,
the present study performed bioinformatics analysis using two
prediction programs. ROCK1 was predicted as a potential target gene
of miR-129-5p. The experimental data further revealed that
miR-129-5p suppressed the luciferase activities of the
psiCHECK™2-ROCK1-WT-3′UTR reporter but did not affect that of a
‘seed region’ ROCK1-MUT-3′UTR reporter. In addition, the
overexpression of miR-129-5p decreased the mRNA and protein
expression of ROCK1 in OS cells. The mRNA expression of ROCK1 was
expressed at high levels in OS tissues, compared with corresponding
adjacent normal bone tissues, and its expression was inversely
correlated with the expression level of miR-129-5p. The biological
effects of the underexpression of ROCK1 were similar to those
induced by the overexpression of miR-129-5p in OS cells, suggesting
that the antitumor effect of miR-129-5p was, at least in part,
dependent on the downregulation of ROCK1. These data suggested that
miR-129-5p directly negatively regulated the expression of ROCK1
via binding to its 3′UTR.
ROCK is an essential downstream effector of the Rho
small GTPase and functions predominantly as a molecular switch,
which binds GTP and GDP to modulate cell proliferation, survival
and cytoskeleton organization, inducing changes in cell
shape/morphology, invasion and movement (31–33).
There is considerable and increasing evidence supporting the
importance of the ROCK1 in the development and progression of OS.
Liu et al (25) found that
ROCK1 was significantly upregulated in OS tissues and cell lines.
The high expression level of ROCK1 was correlated with poor
prognosis in patients with OS, whereas the downregulation of ROCK1
suppressed cell proliferation and viability, and increased
apoptosis in OS. Wang et al (34) indicated that the underexpression of
ROCK1 repressed OS cell migration and invasion. These findings
suggest that ROCK1 can be investigated as a promising therapeutic
target for the treatment of patients with OS.
In conclusion, the present study observed the
downregulation of miR-129-5p in OS tissues and OS cell lines,
whereas the ectopic expression of miR-129-5p suppressed tumor cell
proliferation, migration and invasion. ROCK1 was identified as a
direct target gene of miR-129-5p. The miR-129-5p/ROCK1 axis may be
a promising therapeutic target for the treatment of OS.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho Y, Jung GH, Chung SH, Kim JY, Choi Y
and Kim JD: Long-term survivals of stage IIb osteosarcoma: A
20-year experience in a single institution. Clin Orthop Surg.
3:48–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bramer JA, van Linge JH, Grimer RJ and
Scholten RJ: Prognostic factors in localized extremity
osteosarcoma: A systematic review. Eur J Surg Oncol. 35:1030–1036.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pierz KA, Womer RB and Dormans JP:
Pediatric bone tumors: Osteosarcoma ewing's sarcoma and
chondrosarcoma associated with multiple hereditary
osteochondromatosis. J Pediatr Orthop. 21:412–418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poletajew S, Fus L and Wasiutynski A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumors. Ortop Traumatol Rehabil. 13:537–545. 2011.(In
English, Polish). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J: Control of protein synthesis and
mRNA degradation by microRNAs. Curr Opin Cell Biol. 20:214–221.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carrington JC and Ambros V: Role of
microRNAs in plant and animal development. Science. 301:336–338.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan X, Fan S, Wu W and Zhang Y:
MicroRNA-26a inhibits osteosarcoma cell proliferation by targeting
IGF-1. Bone Res. 3:150332015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao G, Dong L, Shi H, Li H, Lu X, Guo X
and Wang J: MicroRNA-1207-5p inhibits hepatocellular carcinoma cell
growth and invasion through the fatty acid synthase-mediated
Akt/mTOR signalling pathway. Oncol Rep. 36:1709–1716. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu D, Niu X, Pan H, Zhou Y, Qu P and Zhou
J: MicroRNA-335 is downregulated in bladder cancer and inhibits
cell growth, migration and invasion via targeting ROCK1. Mol Med
Rep. 13:4379–4385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Zhao Y, Sun XH, Ge J, Zhang B, Wang
X and Cao XC: Down-regulation of miR-129-5p via the Twist1-Snail
feedback loop stimulates the epithelial-mesenchymal transition and
is associated with poor prognosis in breast cancer. Oncotarget.
6:34423–34436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Wang H, Li Y, Hou Z, Ma N, Chen
W, Zong Z and Chen S: miR-129-5p is down-regulated and involved in
migration and invasion of gastric cancer cells by targeting
interleukin-8. Neoplasma. 63:673–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Wang H, Ke H and Ni S: miR-129
regulates MMP9 to control metastasis of non-small cell lung cancer.
Tumour Biol. 36:5785–5790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duan L, Hao X, Liu Z, Zhang Y and Zhang G:
miR-129-5p is down-regulated and involved in the growth, apoptosis
and migration of medullary thyroid carcinoma cells through
targeting RET. FEBS Lett. 588:1644–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan G, Cao X, Dai Q, Zhang B, Huang J,
Xiong S, Zhang Yy, Chen W, Yang J and Li H: A novel role for
microRNA-129-5p in inhibiting ovarian cancer cell proliferation and
survival via direct suppression of transcriptional co-activators
YAP and TAZ. Oncotarget. 6:8676–8686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karaayvaz M, Zhai H and Ju J: miR-129
promotes apoptosis and enhances chemosensitivity to 5-fluorouracil
in colorectal cancer. Cell Death Dis. 4:e6592013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma N, Chen F, Shen SL, Chen W, Chen LZ, Su
Q, Zhang LJ, Bi J, Zeng WT, Li W, et al: MicroRNA-129-5p inhibits
hepatocellular carcinoma cell metastasis and invasion via targeting
ETS1. Biochem Biophys Res Commun. 461:618–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhai J, Qu S, Li X, Zhong J, Chen X, Qu Z
and Wu D: miR-129 suppresses tumor cell growth and invasion by
targeting PAK5 in hepatocellular carcinoma. Biochem Biophys Res
Commun. 464:161–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang QY, Tang J, Zhou CX and Zhao Q: The
down-regulation of miR-129 in breast cancer and its effect on
breast cancer migration and motility. Sheng Li Xue Bao. 64:403–411.
2012.(In Chinese). PubMed/NCBI
|
|
30
|
Luo J, Chen J and He L: mir-129-5p
Attenuates Irradiation-Induced Autophagy and Decreases
Radioresistance of Breast Cancer Cells by Targeting HMGB1. Med Sci
Monit. 21:4122–4129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang C, Zhang S, Zhang Z, He J, Xu Y and
Liu S: ROCK has a crucial role in regulating prostate tumor growth
through interaction with c-Myc. Oncogene. 33:5582–5591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossman KL, Der CJ and Sondek J: GEF means
go: Turning on RHO GTPases with guanine nucleotide-exchange
factors. Nat Rev Mol Cell Biol. 6:167–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patel RA, Forinash KD, Pireddu R, Sun Y,
Sun N, Martin MP, Schönbrunn E, Lawrence NJ and Sebti SM: RKI-1447
is a potent inhibitor of the Rho-associated ROCK kinases with
anti-invasive and antitumor activities in breast cancer. Cancer
Res. 72:5025–5034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Zhao W and Fu Q: miR-335
suppresses migration and invasion by targeting ROCK1 in
osteosarcoma cells. Mol Cell Biochem. 384:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|