Introduction
The cytochrome P450 enzymes have an important role
in the metabolism of various therapeutic agents, in which different
enzymatic activities lead to variability in drug responses among
individuals (1). Cytochrome P450
2C19 (CYP2C19) is a highly polymorphic isoenzyme of the cytochrome
P450 superfamily, affecting the metabolism of an extensive range of
therapeutic drugs (2). The CYP2C19
gene, which includes nine exons and eight introns, is located at
the 10q24.1–10q24.3 locus of chromosome 10, where coding sequences
is 1,473 bp and resulting in a protein of 490 amino acid residues
(3,4). Approximately 25 genetic variants in
the exonic region of the CYP2C19 have been identified (4). CYP2C19 is involved in metabolizing
several important therapeutic drugs, including omeprazole,
lansoprazole, proguanil, propranolol, imipramine, mephenytoin,
chloroguanide, hexabarbitone, diazepam and certain antidepressants
(1,5). Common variants of the CYP2C19 gene
are associated with impaired drug metabolism. CYP2C19*2 and
CYP2C19*3 were identified in individuals who exhibited a reduced
capability for metabolizing the probe drugs, and variant CYP2C19*17
is associated with ultra-rapid metabolism of CYP2C19 substrates
(3).
The principal detrimental allele, CYP2C19*2, results
from a guanine (G) to adenine (A) transition at position 681 in
exon 5 (rs4244285), producing an aberrant splice site and it
represents the most frequent CYP2C19 defect in all populations
(6). CYP2C19*2 and CYP2C19*3 are
the most common alleles, encoding enzymes with decreased activity
(7). CYP2C19*3 (636G>A) is
considered the most important allele, in which a point mutation in
exon 4 results in a premature stop codon, and therefore
nonfunctional protein (1,4).
The prevalence of the CYP2C19 poor metabolizer (PM)
phenotype is 2-5% among Caucasians and Africans, and ~15% in Asians
(8), while CYP2C19*3 is considered
to be an Asian mutation (1).
CYP2C19*2 and *3 alleles have been proposed to explain <50%, to
>90%, of the PM phenotype (2).
The CYP2C19*17 allele was previously reported to be
associated with high CYP2C19 activity, and identified at 18-28% in
European populations, in 17-18% of Africans and in 0.3–4% of Asian
populations (9). CYP2C19*17 is a
−806 C>T single nucleotide polymorphism that causes specific
nuclear protein binding to the 5′-flanking region. This binding
results in increased gene transcription and high enzyme activities
(10).
In this study, the frequency of the CYP2C19*2, *3,
and *17 was examined among an Iranian cohort of different
ethnicities.
Materials and methods
Specimen collection and ethical
approval
A total of 1,229 blood specimen of unrelated healthy
donors were obtained from Iranians through the Special Medical
Centre (SMC; Tehran, Iran) including: 180 Fars, 110 Turk (Azari),
73 Caspian (Mazani, Gilaki), 80 Lure and 95 Kurd individuals to
examine the CYP2C19*2 allele; 120 Fars, 82 Turk (Azari), 75 Caspian
(Mazani, Gilaki), 73 Lure and 70 Kurd individuals to examine the
CYP2C19*3 allele; and 156 Fars, 56 Turk (Azari), 32 Caspian
(Mazani, Gilaki), 13 Lure and 14 Kurd individuals to examine the
CYP2C19*17 allele. Blood samples (2 ml) with
ethylenediaminetetraacetic acid were collected from participants.
Informed consent to participate in genetic and molecular analyses,
and consent to publish results were obtained from individuals. The
Medical Ethics Committee of the SMC specifically approved this
study (approval no. AA/27/2008). The exclusion criteria to select
individuals in this study were any background of familial and
sporadic cancer, metabolic, nuclear and mitochondrial
DNA-associated disorders.
Genomic DNA extraction and primer
sequences
Genomic DNA from blood samples was extracted using
the MBST salting-out kit (CinnaGen, Tehran, Iran). The
oligonucleotide forward and reverse primers used for the
amplification of CYP2C19*2, *3, and *17 alleles were from previous
published studies as follows: CYP2C19*2, forward
5′-AATTACAACCAGAGCTTGGC-3′ and reverse 5′-TATCACTTTCCATAAAAGCAAG-3′
(11); CYP2C19*3, forward
5′-AACATCAGGATTGTAAGCAC-3′ and reverse 5′-TCAGGGCTTGGTCAATATAG-3′
(11); and CYP2C19*17, forward
5′-GCCCTTAGCACCAAATTCTC-3′ and reverse
5′-ATTTAACCCCCTAAAAAAACACG-3′ primers (10).
Restriction fragment length
polymorphism (RFLP)
Genotyping analysis of the CYP2C19*2, *3 and *17
alleles was performed using polymerase chain reaction-RFLP
(PCR-RFLP). The PCR amplification was performed using 60 ng genomic
DNA, 0.3 U Taq DNA polymerase (CinnaGen), 5 pmol each primers, 10X
PCR buffer, 1.5 mM MgCl2 and 0.5 mM dNTP. The reaction
mixture was initially denatured at 95°C for 3 min, followed by 35
cycles of 95°C for 1 min, 54°C for 1 min, and 72°C for 2 min for
CYP2C19*2; 35 cycles of 94°C for 50 sec, 54.2°C for 50 sec, and
72°C for 50 sec for CYP2C19*3; 35 cycles of 94°C for 1 min, 56.3°C
for 1 min, and 72°C for 1 min for CYP2C19*17; and all had and a
final extension at 72°C for 10 min.
The 168-bp, 119-bp and 473-bp amplified fragments
for *2, *3 and *17 alleles, respectively, were run on a 1.5%
agarose gel and then stained using silver nitrate (CinnaGen) prior
to restriction digestion. Restriction endonucleases, including
SmaI, BamHI or LweI, were then added to PCR
products in a 31 µl reaction volume consisting of 10 µl PCR
products, 2 µl 10X SmaI or BamHI or LweI
buffer (Thermo Fisher Scientific, Inc.), 1 µl SmaI or
BamHI or LweI enzyme (Thermo Fisher Scientific, Inc.)
and 18 µl double distilled water, and were then incubated at 37°C
for 16 hr. The digested PCR products were separated by 8%
polyacrylamide gel electrophoresis (PAGE) at 180 V for 110 min. The
DNA bands were then visualized using silver nitrate staining. The
PCR products with length of 168-bp, 119-bp, and 473-bp were
subsequently digested with restriction endonuclease SmaI,
BamHI, and LweI, respectively.
Single-strand conformation
polymorphism (SSCP)
The PCR-amplified DNA fragments were denatured prior
to loading on a polyacrylamide gel. Briefly, 10 µl PCR mixture was
mixed with 7 µl denaturation buffer (990 µl of 100% formamide, 10
µl of 1 M sodium hydroxide, and a few granules of bromophenol
blue). Samples were then incubated at 45°C for 30 min, and 12 µl
SSCP color (CinnaGen) was added to sample after 23 min. The samples
were analyzed using 6% PAGE at 80 V for 16 h for *2 and *3, and 150
V for 16 h for *17 and then stained by silver nitrate. The
homozygous (as mutated or wild type) and heterozygous variants of
the CYP2C19*2, *3, and *17 were identified as several bands.
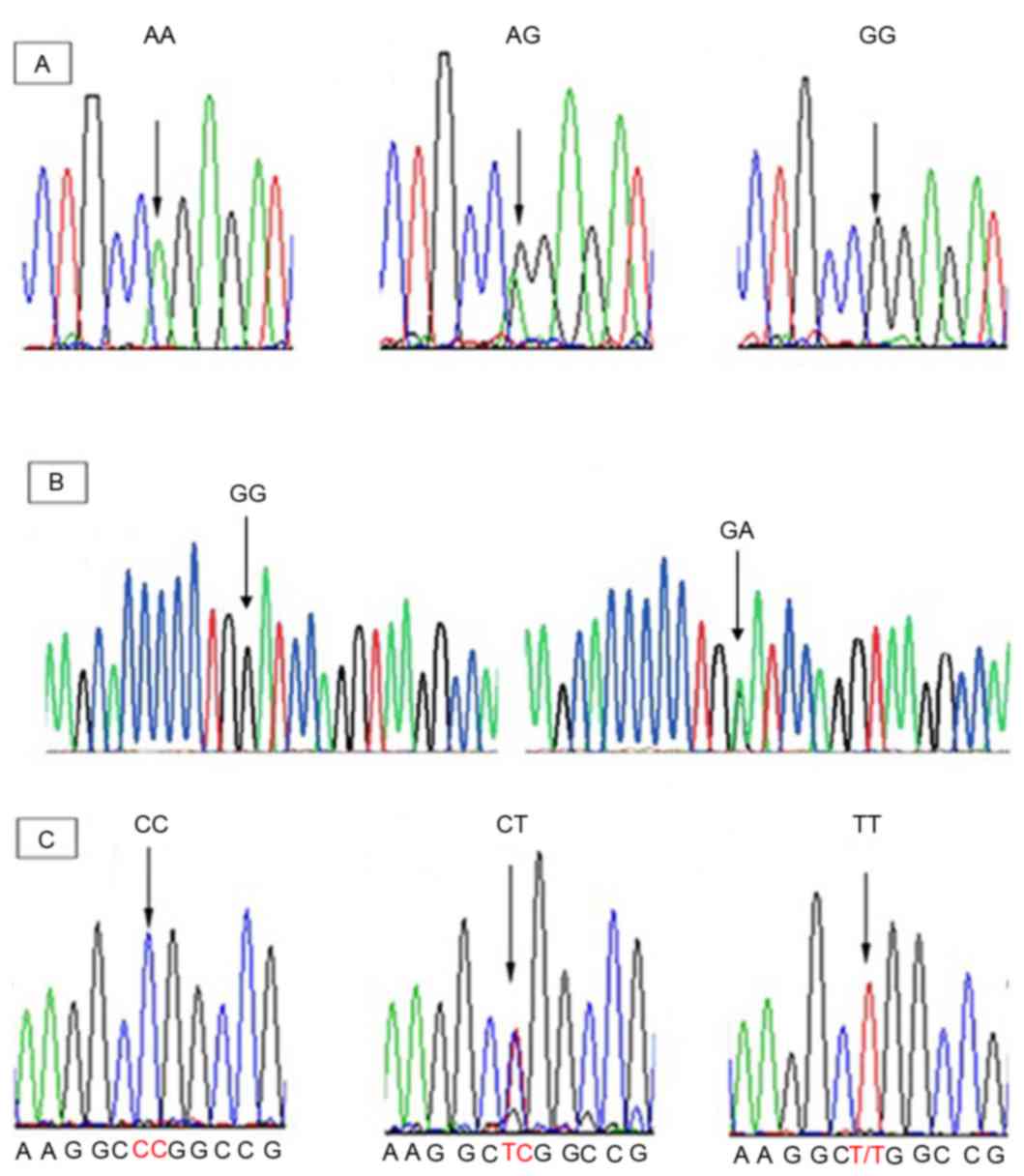
Sequencing analysis
The PCR products were sequenced with forward and
reversed primers on an automated ABI 3100 sequencing machine
(Applied Biosystems, Kavosh Fanavaran Kawsar Company, Iran). All
DNA fragments were then sequenced and analyzed using the Finch TV
program (Geospiza, Inc., Seattle, WA, USA) in order to confirm any
nucleotide variations (Fig.
1).
Statistical analysis
The statistical analyses were conducted using SPSS
(version 22; IBM Corp., Armonk, NY, USA) software to perform
χ2 analysis of the association between the frequency of
alleles among the different ethnicities within the Iranian
population and confidence interval test (95%) was used to calculate
the frequency of alleles. P<0.05 was considered to indicate a
statistically significant difference.
Results
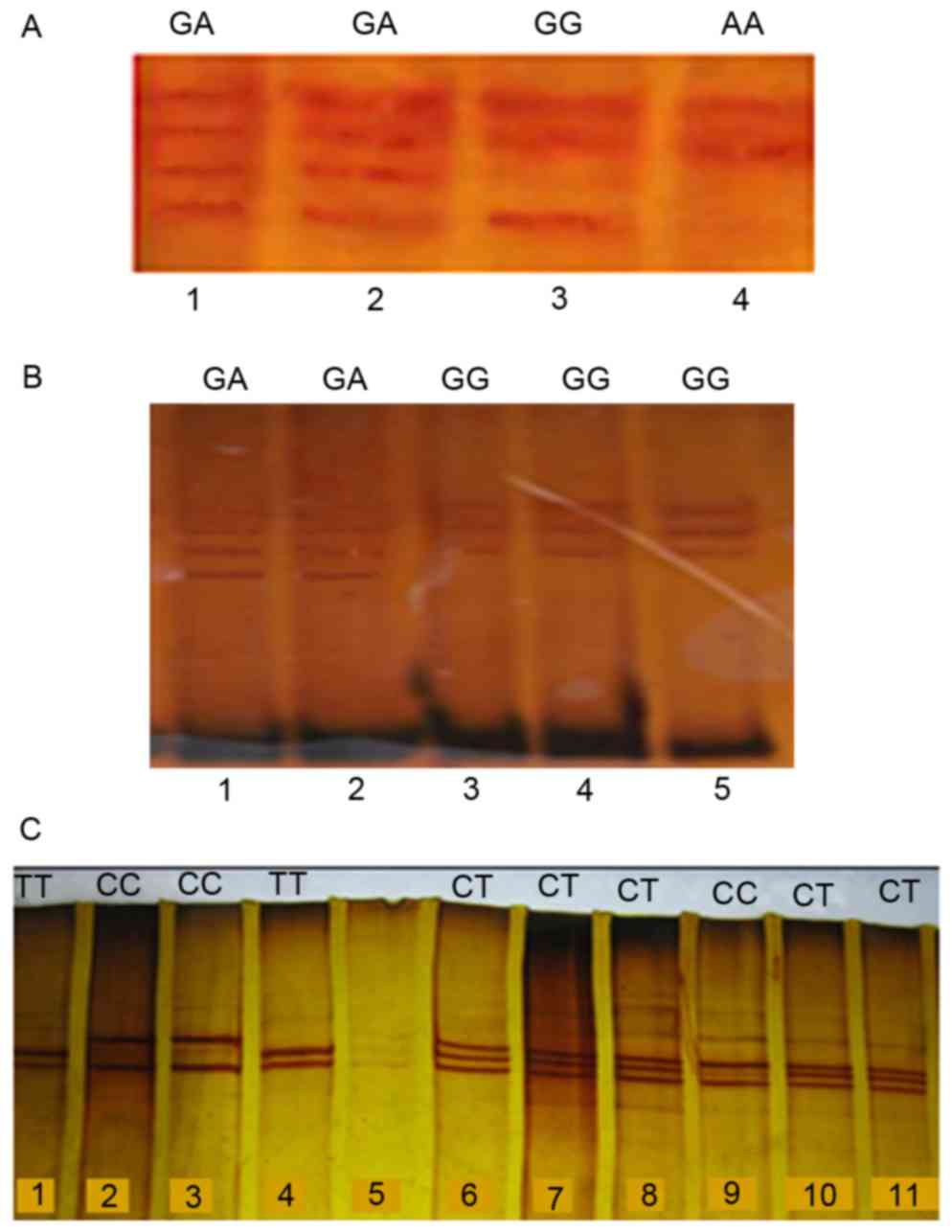
Allelic and genotypic frequency distributions of
CYP2C19*2, *3 and *17 were analyzed using blood samples of 1,229
unrelated healthy individuals from the Iranian population with
different ethnicities. The homozygous (mutated and wild type) and
heterozygous variants of the CYP2C19*2, *3 and *17 were analyzed
using the PCR-RFLP and PCR-SSCP (Figs.
2 and 3).
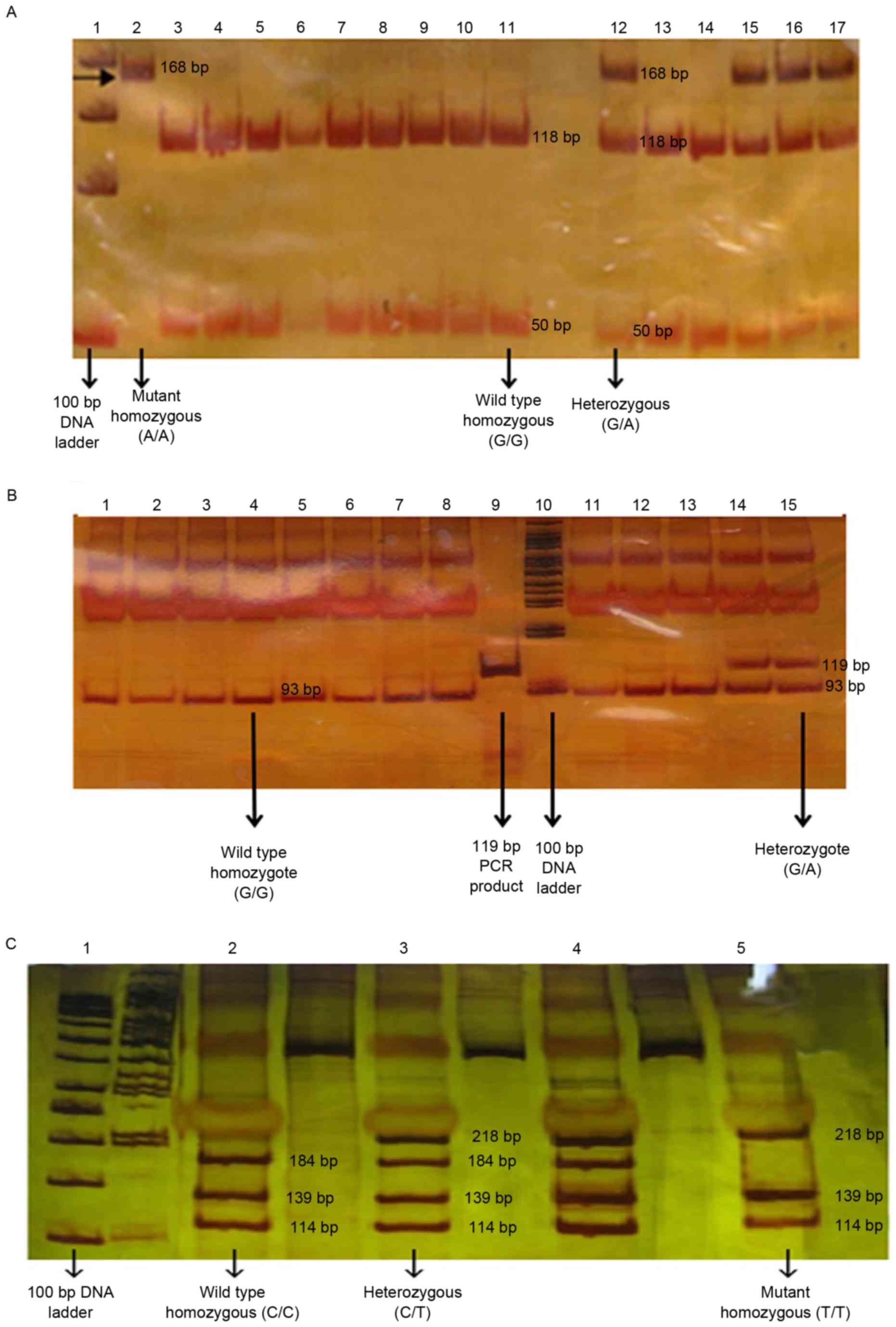 | Figure 2.PCR-RFLP analysis. (A) The PCR
products were disgusted with SmaI, producing 50-bp and 118-bp
fragments for wild type allele, and one fragment of 168-bp for the
allele carrying the CYP2C19*2681 G>A substitution. (B) The PCR
product is disgusted with BamHI, producing 26 and 93-bp fragments
for the wild type allele, and one fragment of 119-bp for the allele
carrying the CYP2C19*3636 G>A substitution. (C) The PCR product
was disgusted with LweI, producing 114, 184, 34 and 139-bp
fragments for the wild type allele, and three fragments in size
218, 139 and 114-bp for the allele carrying the CYP2C19*17-806
C>T substitution. DNA fragments with size of >50 bp were not
visible. PCR-RFLP, polymerase chain reaction-restriction fragment
length polymorphism; CYP2C19, cytochrome P450 2C19. |
RFLP and SSCP for CYP2C19*2
The frequency of the extensive metabolizer (EM;
G/G), intermediate metabolizer (IM; G/A) and PM (A/A) of CYP2C19*2
were 63.6, 30.1 and 6.3%, respectively (Table I), in which the frequency of the
homozygous A/A variants of the CYP2C19*2 allele was significantly
highest in Lure individuals (P<0.001), while Caspian individuals
had the lowest frequency (P<0.001) in comparison with rest of
Iranian ethnicities. The frequency of the heterozygous G/A variant
of CYP2C19*2 was significantly highest in Lure individuals
(P<0.001) and Caspians had the lowest frequency (P<0.001) in
comparison with rest of Iranian ethnicities. Furthermore, the
frequency of the wild-type G/G variant of CYP2C19*2 was
significantly high in Caspians (P<0.001) and low in Lures
(P<0.001) in comparison with other ethnicities.
 | Table I.Statistical analysis of the CYP2C19
(*2, *3 and *17) allelic and genotypic frequencies (%) among
different ethnic groups in Iran. |
Table I.
Statistical analysis of the CYP2C19
(*2, *3 and *17) allelic and genotypic frequencies (%) among
different ethnic groups in Iran.
| A, CYP2C19*2 |
|---|
|
|---|
|
|
| Genotype frequency
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Ethnicity | CYP2C19*2 allele
frequency (%) | G/G | G/A | A/A | χ2 | P-value |
|---|
| Fars | 15.3 | 72.8
(66.1–79.4) | 23.9
(17.2–30.6) | 3.3 (1.1–6.1) | 137.4 |
<0.001a |
| Turk | 25.0 | 58.2
(49.1–67.3) | 33.6
(24.5–42.7) | 8.2 (3.6–13.6) | 41.2 |
<0.001a |
| Caspian | 9.6 | 83.6 (74–91.8) | 13.7
(5.5–21.9) | 2.7 (0.0 −6.8) | 84.1 |
<0.001a |
| Lure | 35.0 | 41.3
(31.3–52.5) | 47.5
(37.5–58.8) | 11.3 (5.0
−18.8) | 18.0 |
<0.001a |
| Kurd | 26.3 | 55.8
(46.3–66.3) | 35.8
(25.3–45.3) | 8.4 (3.2–14.7) | 32.2 |
<0.001a |
| Total
population | 21.4 | 63.6
(59.9–67.7) | 30.1 (26–33.8) | 6.3 (4.5–8.6) | 269.0 |
<0.001a |
|
| B,
CYP2C19*3 |
|
|
|
| Genotype
frequency (%) |
|
|
|
|
|
|
|
|
|
Ethnicity | CYP2C19*3 allele
frequency (%) | G/G | G/A | A/A |
χ2 | P-value |
|
| Fars | 2.9 | 94.2
(89.2–98.3) | 5.8 (1.7–10.8) | 0.0 | 93.6 |
<0.001a |
| Turk | 1.8 | 96.3
(91.5–100.0) | 3.6 (0.0–8.5) | 0.0 | 70.4 |
<0.001a |
| Caspian | 1.3 | 97.3
(93.3–100.0) | 2.6 (0.0–6.7) | 0.0 | 67.2 |
<0.001a |
| Lure | 0.6 | 98.6
(95.9–100.0) | 1.3 (0.0–4.1) | 0.0 | 69.0 |
<0.001a |
| Kurd | 1.4 | 97.1
(92.9–100.0) | 2.8 (0.0–7.1) | 0.0 | 62.2 |
<0.001a |
| Total
population | 1.7 | 96.4
(94.5–98.1) | 3.5 (1.9–5.5) | 0.0 | 362.1 |
<0.001a |
|
| C,
CYP2C19*17 |
|
|
|
| Genotype
frequency |
|
|
|
|
|
|
|
|
|
Ethnicity | CYP2C19*17
allele frequency (%) | C/C | C/T | T/T |
χ2 | P-value |
|
| Fars | 28.2 | 51.9
(44.2–60.3) | 39.7
(32.1–47.4) | 8.3 (3.8–12.8) | 47.3 |
<0.001a |
| Turk | 26.9 | 53.8
(42.9–67.9) | 38.4 (25–50) | 7.6 (1.8–14.3) | 19.9 |
<0.001a |
| Caspian | 17.1 | 75.0
(59.4–87.5) | 15.6
(3.1–28.1) | 9.3 (0.0–21.9) | 25.1 |
<0.001a |
| Lure | 46.1 | 15.3
(0.0–38.5) | 76.9
(53.8–100.0) | 7.6 (0.0–23.1) | 11.2 | 0.004a |
| Kurd | 21.4 | 64.2
(35.7–85.7) | 28.5 (7.1–50) | 7.1 (0.0–21.4) | 7.0 | 0.030a |
| Total
population | 27.1 | 54.2 (48.3-
60.1) | 37.6
(31.7–43.5) | 8.1 (5.2–11.4) | 88.7 |
<0.001a |
RFLP and SSCP for CYP2C19*3
The prevalence of the A/A homozygous variant (PM) of
the CYP2C19*3 was undetectable among different ethnicities,
indicating the absence of this genotype within the Iranian
population, while homozygous G/G (EM) and heterozygous G/A (IM)
frequencies were 96.4 and 3.57%, respectively (Table I). The frequency of the G/A variant
(IM) of the CYP2C19*3 allele was significantly high among the Fars
population (χ2=93.633; P<0.001), while Lures had the
low frequency (P<0.001) in comparison with other ethnicities.
However, the frequency of the heterozygous CYP2C19*3 G/A variant in
Caspians was similar to that of Kurds. Furthermore, the frequency
of the wild-type G/G variant (EM) of the CYP2C19*3 was
significantly highest in Lures (χ2=69.055; P<0.001),
and lowest in Fars (P<0.001) in comparison with other
ethnicities.
RFLP and SSCP for CYP2C19*17
The frequency of the C/C (EM), C/T (IM) and T/T
[ultra-rapid metabolizer (UM)] variants of CYP2C19*17 were 54.24,
37.64 and 8.12%, respectively, in the total population (Table I). The frequency of the homozygous
T/T variant (UM) of the CYP2C19*17 allele was significantly highest
among the Caspians (P<0.001), while Kurds had the lowest
frequency (P<0.001) in comparison with rest of Iranian
ethnicities; the Lures and Turks demonstrated a similar percentage,
which was close to that of Kurds. Additionally, the frequency of
the heterozygous C/T variant (IM) of the CYP2C19*17 allele among
was the highest the Lures (P=0.004), but Caspians had the lowest
frequency in comparison with other Iranian ethnicities (P<0.001;
Table I; Fig. 4).
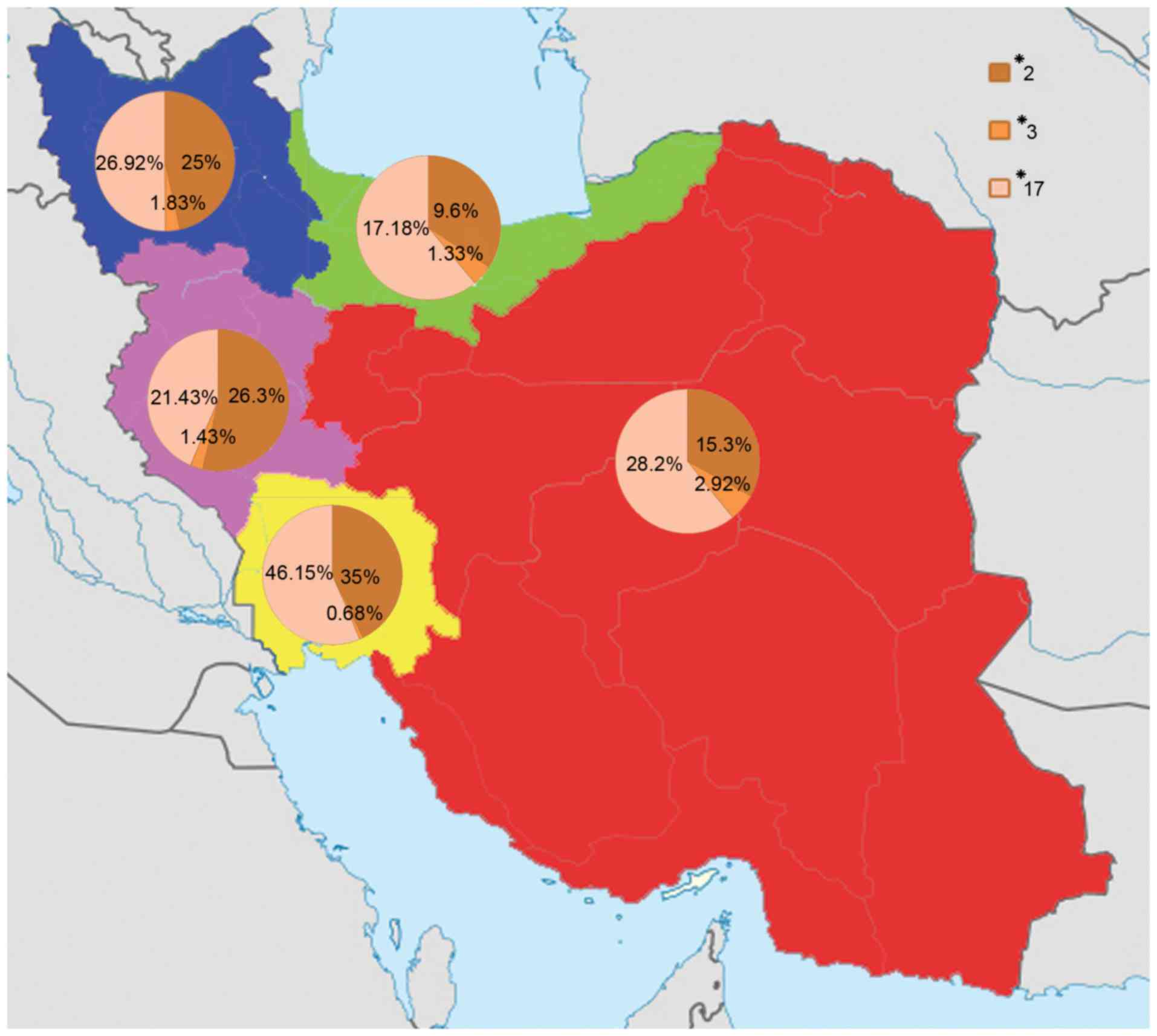 | Figure 4.The CYP2C19*2, *3 and *17 alleles
distribution among different Iranian ethnicities including Fars,
Turk, Caspian, Lure and Kurd populations. The highest frequency of
the CYP2C19*2 allele was found in Lure individuals (35%) and the
lowest frequency was found in Caspian individuals (9.6%). The
highest frequency of the CYP2C19*3 allele was found in Fars
individuals (2.92%) and the lowest frequency was found in Lure
individuals (0.68%). The highest frequency of the CYP2C19*17 allele
was found in Lure individuals (46.15%) and the lowest frequency was
found in Caspian individuals (17.18%). The red, blue, green, yellow
and pink colors indicate the Fars, Turk, Caspian, Lure and Kurd
regions. CYP2C19, cytochrome P450 2C19. |
Discussion
The present study provides comprehensive data
regarding distribution of the allelic and genotypic frequency of
the CYP2C19 among Iranian population of different ethnicities. The
variant alleles of the CYP2C19 family including CYP2C19*2,
CYP2C19*3 and CYP2C19*17 are associated with variation in adverse
drug reactions among populations according to race and ethnic
backgrounds. Many of these polymorphic genes encode inactive
enzymes that may cause adverse drug reactions among individuals
because of their poor metabolic activities (12). CYP2C19 is the main factor for the
metabolism of drugs, including omeprazole, lansoprazole,
imipramine, propranolol, mephenytoin, chloroguanide, hexabarbitone,
diazepam, proguanil and certain antidepressants (1,5).
Polymorphisms in CYP2C19 may produce non-functional alleles and
therefore no enzyme activity to metabolize these drugs correctly.
The most important alleles, CYP2C19*2, CYP2C19*3 and CYP2C19*17,
carry polymorphisms at 681 G>A in exon 5, 636 G>A in exon 4
and −806 C>T in the 5′ flanking region, respectively (3,13,14).
CYP2C19*2 results in a splicing defect, CYP2C19*3 in a premature
stop codon and CYP2C19*17 in increased gene transcription (13,14).
CYP2C19*2 is the most common allele among the Asian
population and its prevalence is varies in different region of
Asia. The CYP2C19*2 allele was observed with a frequency of 21.4%
in the Iranian population, which is higher than the Swedish (14.4%)
(15), German (15%) (16), Ethiopian (13.6%) (17) and Zimbabwean (13.1%) (18) populations, while not as high as
reported in other populations, including Japanese (23%) (19) and Chinese-Taiwanese (32%) (19). The population with most frequent
detection of the CYP2C19*2 allele in the previous reports that were
examined was Filipino (39%; Table
II). The prevalence of the CYP2C19*2 increases steeply from
Western Asia and Iran to India, reaching its maximum (>75%) in
Melanesian populations (20).
 | Table II.Comparison of allele frequencies of
CYP2C19*2 and CYP2C19*3 reported from different populations. |
Table II.
Comparison of allele frequencies of
CYP2C19*2 and CYP2C19*3 reported from different populations.
| Author, year | Population | NA | *2 frequency
(%) | *3 frequency
(%) | Method | (Refs.) |
|---|
| Dehbozorgi et
al, 2017 | Iranian | 1916 | 21.4 | 1.7 | PCR-RFLP, PCR-SSCP
and sequencing | Present study |
| Jurima-Romet et
al, 1996 | Canadian
(Inuit) | 304 | 11 | 0 | PCR-RFLP | (21) |
| Bathum et
al, 1998 | Danish | 478 | 16.1 | 0 | Oligonucleotide
ligation assay | (22) |
| Kurzawski et
al, 2006 | Polish | 250 | 11.6 | ND |
| (29) |
| Rudberg et
al, 2008 | Norwegian | 664 | 18.1 | 0.6 | PCR-RFLP | (28) |
| Yamada et
al, 1998 | Swedish | 166 | 14.4 | 0.7 | PCR-RFLP | (15) |
| Brockmöller et
al, 1995 | German | 280 | 15 | 0 | PCR-RFLP | (16) |
| Ruas et al,
1997 | Portuguese | 306 | 13 | 0 | PCR-RFLP | (30) |
| Hoskins et
al, 1998 | Australian | 198 | 14.6 | 0 | PCR-RFLP | (31) |
| Goldstein et
al, 1997 | Saudi Arabia | 194 | 15 | 0 | PCR-RFLP | (19) |
| Goldstein et
al, 1997 | Japanese | 106 | 23 | 10.4 | PCR-RFLP | (19) |
| Herrlin et
al, 1998 | Korean | 206 | 20.9 | 11.6 | PCR-SSCP | (23) |
| Goldstein et
al, 1997 |
Chinese-Taiwanese | 236 | 32 | 5.5 | PCR-RFLP | (19) |
| Goldstein et
al, 1997 | Filipino | 104 | 39 | 7.7 | PCR-RFLP | (19) |
| Lamba et al,
2000 | North Indian | 242 | 30 | 0 | PCR-RFLP | (32) |
| Hamdy et al,
2002 | Egyptians | 494 | 11 | 0.2 | AFNCRAS | (1) |
| Herrlin et
al, 1998 |
Bantu-Tanzanian | 502 | 17.9 | 0.6 | PCR-SSCP | (23) |
| Persson et
al, 1996 | Ethiopian | 228 | 13.6 | 1.8 | PCR-RFLP | (17) |
| Dandara et
al, 2001 | Venda | 304 | 21.7 | 0 | PCR-RFLP | (18) |
| Dandara et
al, 2001 | Zimbabwean | 336 | 13.1 | 0 | PCR-RFLP | (18) |
| Goldstein et
al, 1997 |
African-Americans | 216 | 25 | 0 | PCR-RFLP | (19) |
CYP2C19 is a clinically important enzyme in
metabolism of different drugs, including antiplatelet drug,
clopidogrel. Among individuals treated with clopidogrel, carriers
of CYP2C19*2, CYP2C19 reduced-function alleles, exhibited markedly
lower levels of the active metabolite of the drug, decreased
platelet inhibition, and a higher rate of subsequent cardiovascular
events, compared with non-carriers. However, the CYP2C19*17 allele
is reported significantly related with an increased response to
clopidogrel and high risk of bleeding. Thus, identification and
functional analysis of CYP2C19 genetic polymorphisms are important
for improving the understanding of safer drug therapy (2).
Furthermore, the CYP2C19*3 allele was observed with
a prevalence of 1.7% among the Iranian population in the present
study, which is higher than the previously reported frequency in
the Canadian (0%) (21) and Danish
(0%) (22) populations, but not as
high as reported in other populations, including Japanese (10.4%)
(19) and Korean (11.6%) (23), and was demonstrated to be of an
approximately similar frequency to the Ethiopian population (1.8%;
Table II) (17).
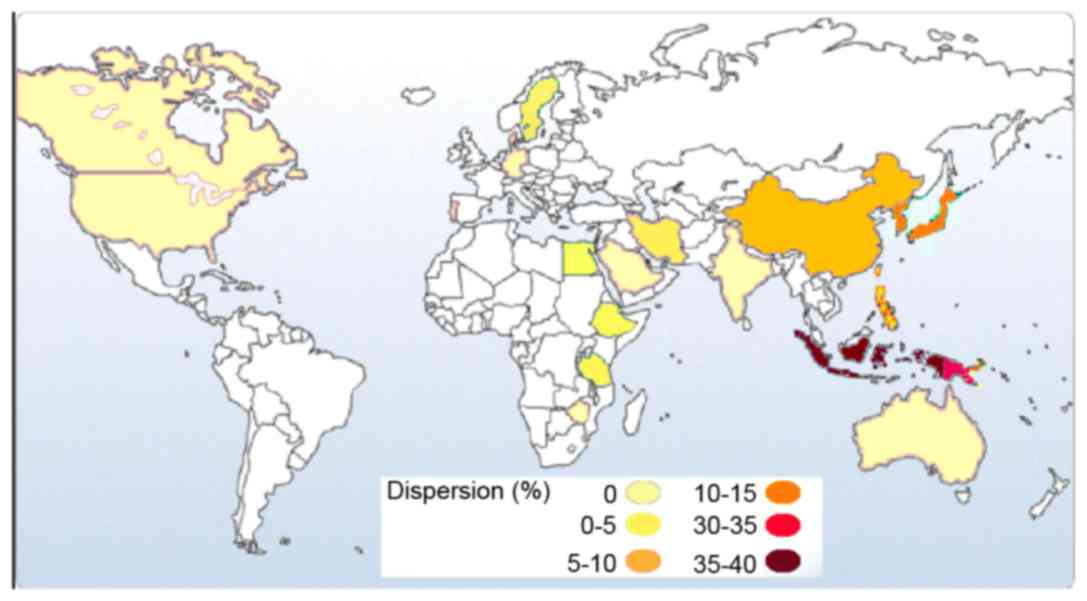
The frequency of CYP2C19*3 as an Asian mutation
(1) increases from the west to the
east of Asia (Table II; Fig. 5). According to previous studies,
the highest frequency of the CYP2C19*3 allele was reported in the
Indonesian populations of South-East Asia (37%) (24), followed by the Iruna population
from New Guinea (34%; Fig. 5)
(24). These results suggest that
the prediction of the CYP2C19*3 allele is necessary in drug
research and therapy, and the effects of the CYP2C19*3 allele on
drug metabolism should be investigated, because side effects of
drugs are comparatively common among these countries (25,26).
In fact, individuals with CYP2C19*3 (A/A) genotype have no ability
to metabolize drugs completely, consequently resulting in
incomplete and poor metabolism of the drugs, drug accumulation in
the blood and drug toxicity.
In the current study, the CYP2C19*17 allele was
identified with a prevalence of 27.1% among the Iranian population,
which was higher than most countries included in Table III, including the Danish (20.1%)
(27) and Norwegian (22%)
(28) population, and was similar
in frequency to the Polish population (27.2%) (29).
 | Table III.Comparison of allele frequencies of
CYP2C19*17 reported from different populations. |
Table III.
Comparison of allele frequencies of
CYP2C19*17 reported from different populations.
| Author, year | Population | NA | *17 frequency
(%) | Method | (Refs.) |
|---|
| Dehbozorgi et
al, 2017 | Iranian | 542 | 27.1 | PCR-RFLP, PCR-SSCP
and sequencing | Present study |
| Pedersen et
al, 2010 | Danish | 552 | 20.1 | Quantitative
PCR | (27) |
| Kurzawski et
al, 2006 | Polish | 250 | 27.2 | PCR-RFLP | (29) |
| Rudberg et
al, 2008 | Norwegian | 664 | 22 | PCR-RFLP | (28) |
| Ramsjö et
al, 2010 | Swedish | 370 | 20 | Quantitative
PCR | (33) |
| Sugimoto et
al, 2008 | Japanese | 530 | 1.3 | PCR-RFLP | (14) |
| Kim et al,
2010 | Korean | 542 | 1.5 | Multiplex
pyrosequencing | (34) |
| Chen et al,
2008 |
Chinese-Taiwanese | 800 | 1.2 | Direct
sequencing | (35) |
|
| Egyptians | 494 | ND |
|
|
| Sim et al,
2006 | Ethiopian | 380 | 17.9 | Sequencing | (10) |
| Kearns et
al, 2010 |
African-Americans | 228 | 21 | PCR-RFLP | (36) |
In summary, the determination of the allelic and
genotypic frequencies of the CYP2C19 gene among different
ethnicities may provide data to be used to personalize treatments
in order to improve the efficiency of the therapeutic outcomes and
decrease the appearance of adverse effects, and therefore,
facilitate the advancement of personalized medicine. Genotyping of
the CYP2C19*2, *3 and *17 alleles among different ethnicities
within the Iranian population, including Fars, Turks, Caspians,
Lures and Kurds is an important for avoiding the side effect of
drugs and drug interactions, and may lead to improved survival and
decreased drug modality risk.
Acknowledgements
This work was supported by Dr M. Houshmand (National
Institute of Genetic Engineering and Biotechnology, Tehran, Iran),
to whom we give our grateful appreciation.
References
|
1
|
Hamdy SI, Hiratsuka M, Narahara K,
El-Enany M, Moursi N, Ahmed MS and Mizugaki M: Allele and genotype
frequencies of polymorphic cytochromes P450 (CYP2C9, CYP2C19,
CYP2E1) and dihydropyrimidine dehydrogenase (DPYD) in the Egyptian
population. Br J Clin Pharmacol. 53:596–603. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, An N, Wang H, Gao Y, Liu D, Bian
T, Zhu J and Chen C: Evaluation of the effects of 20 nonsynonymous
single nucleotide polymorphisms of CYP2C19 on S-mephenytoin
4′-hydroxylation and omeprazole 5′-hydroxylation. Drug Metab
Dispos. 39:830–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaudhry A, Kochhar R and Kohli K: Genetic
polymorphism of CYP2C19 & therapeutic response to proton pump
inhibitors. Indian J Med Res. 127:521–530. 2008.PubMed/NCBI
|
|
4
|
Yin T and Miyata T: Pharmacogenomics of
clopidogrel: Evidence and perspectives. Thromb Res. 128:307–316.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blaisdell J, Mohrenweiser H, Jackson J,
Ferguson S, Coulter S, Chanas B, Xi T, Ghanayem B and Goldstein JA:
Identification and functional characterization of new potentially
defective alleles of human CYP2C19. Pharmacogenetics. 12:703–711.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buzoianu AD, Trifa AP, Popp RA, Militaru
MS, Militaru CF, Bocşan CI, Farcaş MF and Pop IV: Screening for
CYP2C19*2, *3 and *4 gene variants in a Romanian population study
group. Farmacia. 58:806–818. 2010.
|
|
7
|
Beitelshees AL, Horenstein RB, Vesely MR,
Mehra MR and Shuldiner AR: Pharmacogenetics and clopidogrel
response in patients undergoing percutaneous coronary
interventions. Clin Pharmacol Ther. 89:455–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scott SA, Sangkuhl K, Gardner EE, Stein
CM, Hulot JS, Johnson JA, Roden DM, Klein TE and Shuldiner AR;
Clinical Pharmacogenetics Implementation Consortium, : Clinical
Pharmacogenetics Implementation Consortium guidelines for
cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy.
Clin Pharmacol Ther. 90:328–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gawrońska-Szklarz B, Adamiak-Giera U,
Wyska E, Kurzawski M, Gornik W, Kaldonska M and Drozdzik M: CYP2C19
polymorphism affects single-dose pharmacokinetics of oral
pantoprazole in healthy volunteers. Eur J Clin Pharmacol.
68:1267–1274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sim SC, Risinger C, Dahl ML, Aklillu E,
Christensen M, Bertilsson L and Ingelman-Sundberg M: A common novel
CYP2C19 gene variant causes ultrarapid drug metabolism relevant for
the drug response to proton pump inhibitors and antidepressants.
Clin Pharmacol Ther. 79:103–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikebuchi J, Yamada M, Ogura Y, Yamamoto Y,
Nishimura A, Nishi K, Yamada K and Irizawa Y: Individual difference
in drug metabolism and disposition: Toxicological significance of
genotypes and phenotypes of S-mephenytoin 4′-hydroxylase (CYP2C19).
Int Congress Series. 1239:589–592. 2003. View Article : Google Scholar
|
|
12
|
Shastry BS: Pharmacogenetics and the
concept of individualized medicine. Pharmacogenomics J. 6:16–21.
2005. View Article : Google Scholar
|
|
13
|
Nakamoto K, Kidd JR, Jenison RD, Klaassen
CD, Wan YJ, Kidd KK and Zhong XB: Genotyping and haplotyping of
CYP2C19 functional alleles on thin-film biosensor chips.
Pharmacogenet Genomics. 17:103–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugimoto K, Uno T, Yamazaki H and Tateishi
T: Limited frequency of the CYP2C19*17 allele and its minor role in
a Japanese population. Br J Clin Pharmacol. 65:437–439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada H, Dahl ML, Lannfelt L, Viitanen M,
Winblad B and Sjöqvist F: CYP2D6 and CYP2C19 genotypes in an
elderly Swedish population. Eur J Clin Pharmacol. 54:479–481. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brockmöller JJ, Rost K, Gross D, Schenkel
A and Roots I: Phenotyping of CYP2C19 with enantiospecific
HPLC-quantification of R- and S-mephenytoin and comparison with the
intron4/exon5 G->A-splice site mutation. Pharmacogenetics.
5:80–88. 1995.PubMed/NCBI
|
|
17
|
Persson I, Aklillu E, Rodrigues F,
Bertilsson L and Ingelman-Sundberg M: S-mephenytoin hydroxylation
phenotype and CYP2C19 genotype among Ethiopians. Pharmacogenetics.
6:521–526. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dandara C, Masimirembwa CM, Magimba A,
Sayi J, Kaaya S, Sommers DK, Snyman JR and Hasler JA: Genetic
polymorphism of CYP2D6 and CYP2C19 in east-and southern African
populations including psychiatric patients. Eur J Clin Pharmacol.
57:11–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldstein JA, Ishizaki T, Chiba K, de
Morais SM, Bell D, Krahn PM and Evans DA: Frequencies of the
defective CYP2C19 alleles responsible for the mephenytoin poor
metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian
and American black populations. Pharmacogenetics. 7:59–64. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sistonen J, Fuselli S, Palo JU, Chauhan N,
Padh H and Sajantila A: Pharmacogenetic variation at CYP2C9,
CYP2C19, and CYP2D6 at global and microgeographic scales.
Pharmacogenet Genomics. 19:170–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jurima-Romet M, Goldstein JA, LeBelle M,
Aubin RA, Foster BC, Walop W and Rode A: CYP2C19 genotyping and
associated mephenytoin hydroxylation polymorphism in a Canadian
Inuit population. Pharmacogenetics. 6:329–339. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bathum L, Andersen-Ranberg K, Boldsen J,
Brøsen K and Jeune B: Genotypes for the cytochrome P450 enzymes
CYP2D6 and CYP2C19 in human longevitY. Role of CYP2D6 and CYP2C19
in longevity. Eur J Clin Pharmacol. 54:427–430. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrlin K, Massele AY, Jande M, Alm C,
Tybring G, Abdi YA, Wennerholm A, Johansson I, Dahl ML, Bertilsson
L and Gustafsson LL: Bantu Tanzanians have a decreased capacity to
metabolize omeprazole and mephenytoin in relation to their CYP2C19
genotype. Clin Pharmacol Ther. 64:391–401. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu HL, Woad KJ, Woodfield DG and Helsby
NA: A high incidence of polymorphic CYP2C19 variants in archival
blood samples from Papua New Guinea. Hum Genomics. 3:17–23. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blendon RJ, Schoen C, DesRoches C, Osborn
R and Zapert K: Common concerns amid diverse systems: Health care
experiences in five countries. Health Aff (Millwood). 22:106–121.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindley CM, Tully MP, Paramsothy V and
Tallis RC: Inappropriate medication is a major cause of adverse
drug reactions in elderly patients. Age and Ageing. 21:294–300.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pedersen RS, Brasch-Andersen C, Sim SC,
Bergmann TK, Halling J, Petersen MS, Weihe P, Edvardsen H,
Kristensen VN, Brøsen K and Ingelman-Sundberg M: Linkage
disequilibrium between the CYP2C19*17 allele and wildtype CYP2C8
and CYP2C9 alleles: Identification of CYP2C haplotypes in healthy
Nordic populations. Eur J Clin Pharmacol. 66:1199–1205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rudberg I, Mohebi B, Hermann M, Refsum H
and Molden E: Impact of the ultrarapid CYP2C19*17 allele on serum
concentration of escitalopram in psychiatric patients. Clin
Pharmacol Ther. 83:322–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurzawski M, Gawrońska-Szklarz B,
Wrześniewska J, Siuda A, Starzyńska T and Droździk M: Effect of
CYP2C19*17 gene variant on Helicobacter pylori eradication in
peptic ulcer patients. Eur J Clin Pharmacol. 62:877–880. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruas JL and Lechner MC: Allele frequency
of CYP2C19 in a Portuguese population. Pharmacogenetics. 7:333–335.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoskins JM, Shenfield GM and Gross AS:
Relationship between proguanil metabolic ratio and CYP2C19 genotype
in a Caucasian population. Br J Clin Pharmacol. 46:499–504. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lamba JK, Dhiman RK and Kohli KK: CYP2C19
genetic mutations in North Indians. Clin Pharmacol Ther.
68:328–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramsjö M, Aklillu E, Bohman L,
Ingelman-Sundberg M, Roh HK and Bertilsson L: CYP2C19 activity
comparison between Swedes and Koreans: Effect of genotype, sex,
oral contraceptive use, and smoking. Eur J Clin Pharmacol.
66:871–877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim KA, Song WK, Kim KR and Park JY:
Assessment of CYP2C19 genetic polymorphisms in a Korean population
using a simultaneous multiplex pyrosequencing method to
simultaneously detect the CYP2C19*2, CYP2C19*3, and CYP2C19*17
alleles. J Clin Pharm Ther. 35:697–703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Qin S, Xie J, Tang J, Yang L, Shen
W, Zhao X, Du J, He G and Feng G: Genetic polymorphism analysis of
CYP2C19 in Chinese Han populations from different geographic areas
of mainland China. Pharmacogenomics. 9:691–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kearns GL, Leeder JS and Gaedigk A: Impact
of the CYP2C19*17 allele on the pharmacokinetics of omeprazole and
pantoprazole in children: Evidence for a differential effect. Drug
Metab Dispos. 38:894–897. 2010. View Article : Google Scholar : PubMed/NCBI
|