Introduction
With the increase of patients with end-stage liver
disease requiring liver transplantation, the transplantation
community has attempted to expand donor numbers using donation
after cardiac death (DCD). Currently, DCD is a primary source of
transplanted livers and a means of reducing the transplant waiting
list. However, ischemia-reperfusion (I/R) injury is the most common
cause of primary graft dysfunction (10–30% of grafts) and primary
graft nonfunction (5% of grafts) for all liver transplants. Primary
graft nonfuction results in 81% of retransplantation during the
first week following surgery (1).
Therefore, limiting the extent of I/R injury would improve the
outcome for patients undergoing liver resection, particularly for
recipients of DCD and fatty donor livers. However, the exact
molecular mechanisms underlying I/R injury-associated innate immune
cell activation remain to be fully elucidated.
Heme oxygenase (HO) is an enzyme specialized in
degrading heme and is assembled with biliverdin, carbon monoxide
and free iron (2,3). The upregulation of heme oxygenase 1
(HO-1) may provide protection from cellular stress following
ischemia and inflammation, inhibiting the damage caused by heme and
exerting anti-inflammatory and anti-apoptotic effects. HO-1
overexpression is cytoprotective in transplant models of hepatic
I/R injury, with recipients exhibiting improved liver architecture
and function, increased survival, and reduced inflammation
(4). However, certain studies have
suggested that these effects may be limited to a narrow window of
HO-1 concentration (5,6).
Cobalt-protoporphyrin (CoPP) is considered to be the
most effective metalloporphyrin inducer of HO-1 (1,7,8). The
present study investigated the anti-apoptotic mechanisms underlying
HO-1-induced cytoprotection using CoPP and the HO-1 inhibitor
zinc-protoporphyrin (ZnPP) (9) in
a mouse model of liver I/R injury.
Materials and methods
Animals and experimental model
Fifteen C57BL/6J mice (weight, 25–30 g) were
purchased from the Laboratory Animal Center of the Academy of
Military Medical Sciences (Beijing, China) and were housed in the
animal facilities of Tianjin Medical University (Tianjin, China) at
23–25°C and 45–55% humidity with a 12 h light/dark cycle. Mice had
free access to food and water. All mice were handled according to
the Guidelines for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MD, USA) (10). Mice were denied solid food for ~12
h and liquid food for ~4 h prior to surgery. Liver I/R injury was
induced in mice as previously described (11), following anesthesia with an
intraperitoneal injection of 4% chloral hydrate (10 mg/100 g).
Following a midline laparotomy, all structures in the portal triad
(hepatic artery, portal vein and bile duct) were occluded with a
vascular traumatic clamp. The blood supply to the liver was
occluded for 60 min followed by 2 h reperfusion. Μice were
scarified after reperfusion with an overdose of anesthetic. Mice
were randomly assigned to five groups (n=3/group): i) Control
(sham-operated); ii) I/R; iii) CoPP pretreatment, in which mice
received an intraperitoneal injection of 5 mg/kg body weight CoPP
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 48 h prior to I/R;
iv) CoPP and ZnPP pretreatment, in which mice received an
intraperitoneal injection of CoPP 48 h prior to I/R and an
intraperitoneal injection of 15 mg/kg body weight ZnPP
(Sigma-Aldrich; Merck KGaA) 24 h prior to I/R; and v) ZnPP
pretreatment, in which mice received an intraperitoneal injection
of 15 mg/kg body weight ZnPP 48 h prior to I/R.
Aspartate transaminase (AST) and
alanine aminotransferase (ALT) detection
Serum was collected from all mice from the inferior
vena cava at 2 h following reperfusion. The levels of AST and ALT
in serum was determined using the following commercial kits:
Aspartate Aminotransferase (AST) reagent OSR6509 and Alanine
Aminotransferase (ALT) reagent OSR6607 (Beckman Coulter, Inc.,
Brea, CA, USA), according to the manufacturer's protocol.
Histological examination
Liver samples harvested from mice were fixed in
formalin for 24 h, embedded in paraffin and sectioned (4-µm thick).
Sections were stained with hematoxylin and eosin (H&E) to
detect histological alterations. H&E staining was analyzed
under a light microscope by two pathologists.
Western blotting
Liver tissues (20 mg) were lysed with
radioimmunoprecipitation assay buffer (Beijing SolarBio Science
& Technology Co., Ltd., Beijing, China) and the proteins were
extracted from the lysates following centrifugation at 13,363 × g,
4°C for 15 min. Protein concentration was determined using a
Bicinchoninic Acid Protein assay kit (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Liver proteins (40 µg/lane)
were separated on 10% SDS-PAGE gels and transferred onto
polyvinylidene difluoride membranes at 250 mA for 2 h. Non-specific
proteins were blocked by incubating membranes in 5% non-fat milk
powder. Membranes were incubated with the following primary
antibodies overnight at 4°C: Mouse monoclonal anti-β-actin
(1:1,000, cat no. 3700S), mouse mAb anti-B-cell lymphoma 2
[(Bcl-2); 1:200; cat no. 15071S] (both from Cell Signaling
Technology, Inc., Danvers, MA, USA) and mouse monoclonal anti-HO-1
(1:500; ab13248; Abcam, Cambridge, MA, USA). Membranes were
subsequently washed with TBS containing Tween-20 (TBST) three times
for 10 min each time and incubated with anti-mouse IgG, horseradish
peroxidase-conjugated secondary antibodies (1:500, cat no. 7076P2;
Cell Signaling Technology, Inc.) for 2 h at room temperature.
Following incubation, the membranes were washed with TBST three
times for 10 min each time. Enhanced Chemiluminescence (EMD
Millipore, Billerica, MA, USA) was performed according to the
manufacturer's protocol. Western blots were quantified using Image
Studio Digits 4.0 (LI-COR C-DiGit Blot Scanner; LI-COR Biosciences,
Lincoln, NE, USA) and expression of proteins were normalized
against β-actin expression.
Immunohistochemistry
Paraffin-embedded liver sections (4-µm thick) were
dewaxed using xylene and hydrated through graded ethanol. Antigen
retrieval was performed at 95°C for 15 min using citric acid buffer
(10 mM citric acid, pH 6.0) in a microwave. Non-specific proteins
were blocked using 3% H2O2. Sections were
subsequently incubated with a rabbit polyclonal anti-activated
caspase-3 antibody (1:100; cat no. 9662S; Cell Signaling
Technology, Inc.) at 4°C overnight and incubated with a horseradish
peroxidase-conjugated goat anti-rat polyclonal secondary antibody
(dilution, 1:100; cat no. ab6721; Abcam) at 25°C for 1 h. Color was
developed using 3,3-diaminobenzidine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and sections were counterstained with
hematoxylin. Sections were observed under a light microscope, and
four fields per slide were randomly selected under ×400
magnification for evaluation of caspase-3 expression using
Image-Pro Plus version 7.0 Image Analysis software (Media
Cybernetics, Inc., Rockville, MD, USA).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL assay (Roche Diagnostics, Indianapolis, IN,
USA) was performed according to the manufacturer's protocol.
Positive controls were obtained by incubating paraffin-embedded
liver sections (4-µm thickness) with 1,500 U/ml DNase to induce DNA
strand breaks prior to labeling. Sections incubated with labeling
solution not containing terminal transferase served as a negative
control (12). Apoptotic cells
were imaged under a fluorescence microscope (Nikon Corporation,
Tokyo, Japan). For each section, three non-overlapping fields of
view (magnification, ×400) were randomly selected. The percentage
of apoptotic cells in each field was calculated as follows:
(Apoptotic cell number/total cell number) ×100 (13).
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA) was used
to perform statistical analyses. Unpaired Student's t-test was used
to compare differences between two groups, whereas one-way analysis
of variance was performed to compare multiple groups, followed by a
Tukeys test. P<0.05 was considered to indicate a statistically
significant difference.
Results
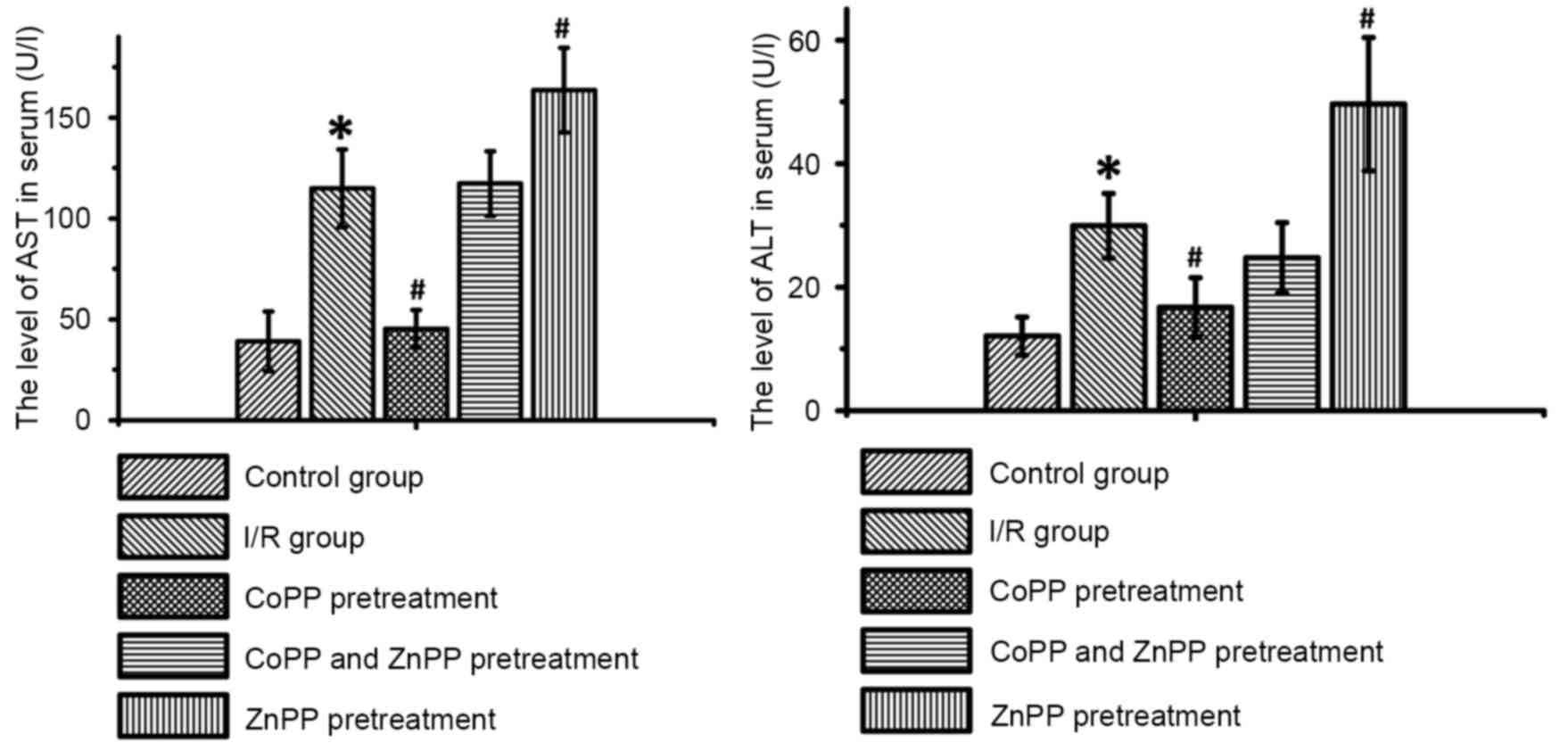
CoPP pretreatment decreases serum
levels of AST and ALT following I/R
As presented in Table
I and Fig. 1, serum levels of
liver enzymes in the sham-operated group remained in the normal
range (ALT: 0–20 U/l, AST: 0–40 U/l). Following I/R, the levels of
AST and ALT were significantly increased compared with the
sham-operated group (P<0.05); CoPP pretreatment significantly
inhibited this increase (P<0.05). ZnPP pretreatment increased
the serum levels of AST and ALT compared with the I/R only group
(P<0.05); however, there were no significant differences in AST
and ALT levels between the CoPP and ZnPP pretreated group and the
I/R only group.
 | Table I.Serum levels of liver enzyme AST and
ALT following I/R injury. |
Table I.
Serum levels of liver enzyme AST and
ALT following I/R injury.
| Group | AST (IU/l) | ALT (IU/l) |
|---|
| Control | 39.11±14.73 | 12.08±3.07 |
| I/R |
114.93±19.22a |
29.94±5.21a |
| I/R with CoPP
pretreatment |
45.35±9.35b |
16.75±4.80b |
| I/R with CoPP and
ZnPP pretreatment | 117.35±16.02 | 24.74±5.70 |
| I/R with ZnPP
pretreatment |
163.66±20.97b |
49.63±10.81b |
CoPP pretreatment alleviates liver
injury following I/R
To investigate histopathological alterations
following I/R, H&E staining was performed. The liver cells in
the control sham-operated group were arranged in plates surrounding
the sinusoids (Fig. 2A). In the
I/R group, hepatocyte swelling, cytoplasm rarefaction, spotty
necrosis scattering in hepatic lobules and inflammatory cell
infiltration in portal duct areas was observed (Fig. 2B). CoPP pretreatment alleviated
cellular swelling and the cellular morphology was not visibly
different from the control group (Fig.
2C). The liver cells of the CoPP and ZnPP pretreatment group
had an irregular arrangement and loss of the portal duct (Fig. 2D). Hydropic degeneration, an
increase in cell size, irregular arrangement and a clear
cytoplasmic shape of liver cells was observed in the ZnPP
pretreatment group, and the liver sinusoid appeared narrow
(Fig. 2E).
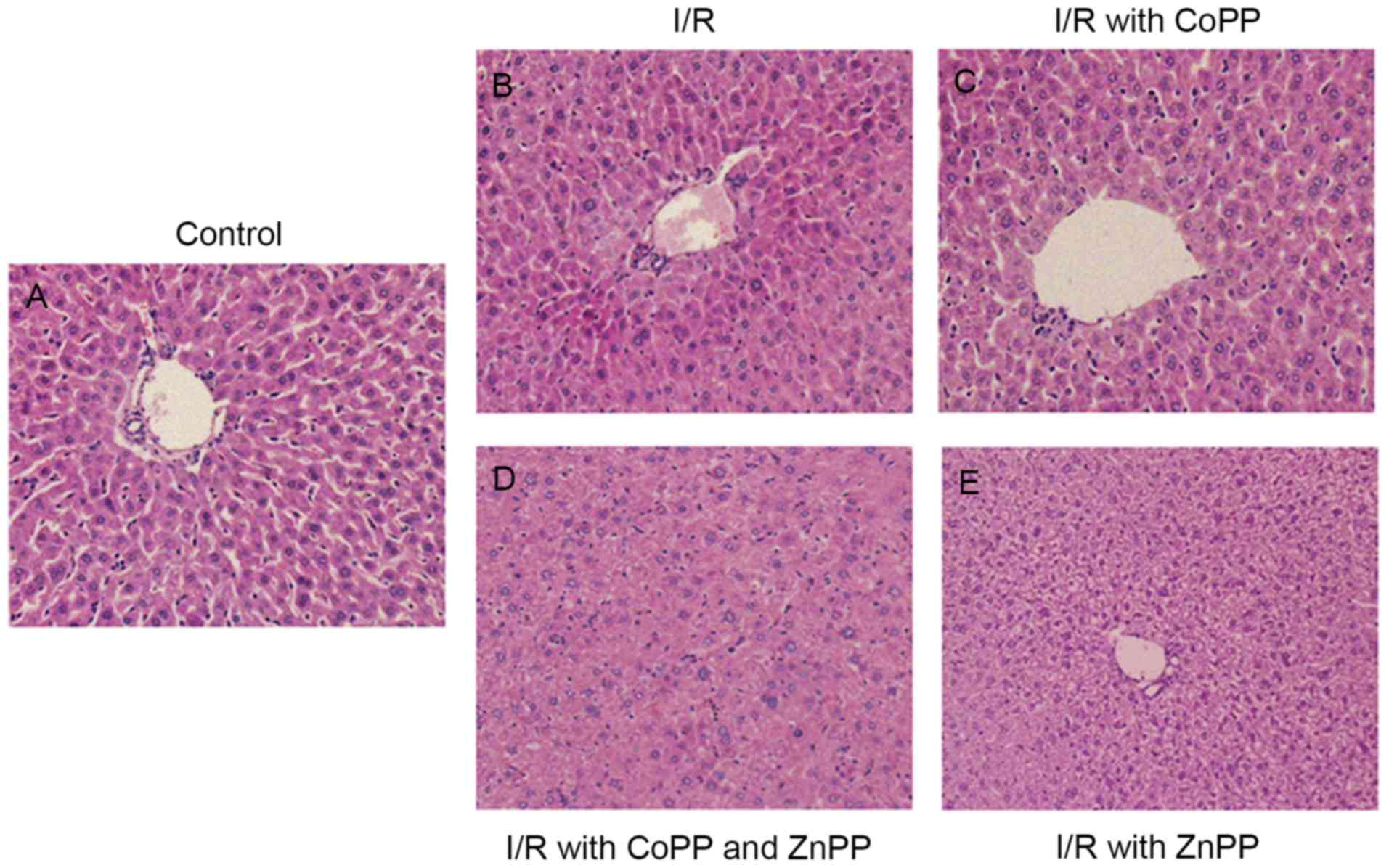 | Figure 2.Hematoxylin and eosin staining of
liver sections following I/R injury. Liver tissues were obtained
from mice following liver I/R injury, in the absence or presence of
pretreatment with CoPP and/or ZnPP. (A) Control (sham-operated)
group, with liver cells are arranged in plates surrounding the
sinusoids. (B) I/R group, with hepatocyte swelling, cytoplasm
rarefaction, spotty necrosis scattering in hepatic lobules and
inflammatory cell infiltration in portal duct areas. (C) I/R with
CoPP pretreatment group, with cellular swelling alleviated and
cellular morphology that appeared no different to the control
group. (D) I/R with CoPP and ZnPP pretreatment group, with
irregular arrangement and loss of portal duct. (E) I/R with ZnPP
pretreatment group, with hydropic degeneration, irregular
arrangement and narrow liver sinusoid. Original magnification,
×100. I/R, ischemia-reperfusion; CoPP, cobalt-protoporphyrin; ZnPP,
zinc-protoporphyrin. |
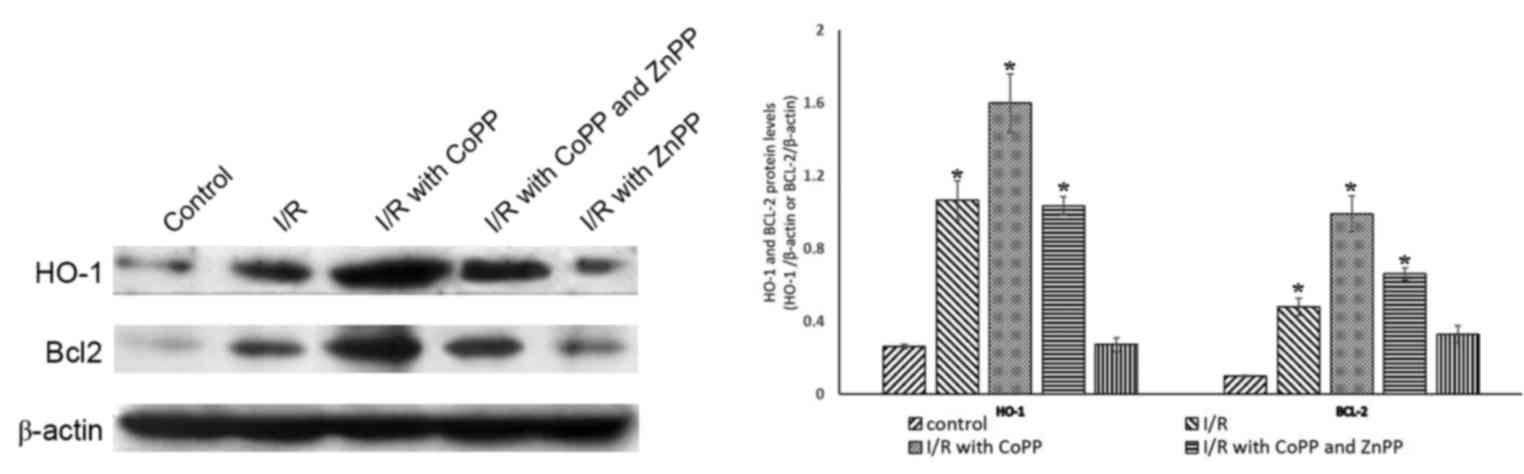
CoPP pretreatment promotes the
expression of Bcl-2 following I/R
To investigate the association between the protein
expression levels of HO-1 and cell death, HO-1 and Bcl-2 were
detected by western blotting; β-actin served as an internal
control. Compared with the control group, the protein expression
levels of HO-1 were greater in the I/R group; this increase was
inhibited by ZnPP pretreatment and further increased by CoPP
pretreatment. The expression of Bcl2 followed a similar pattern
(Fig. 3).
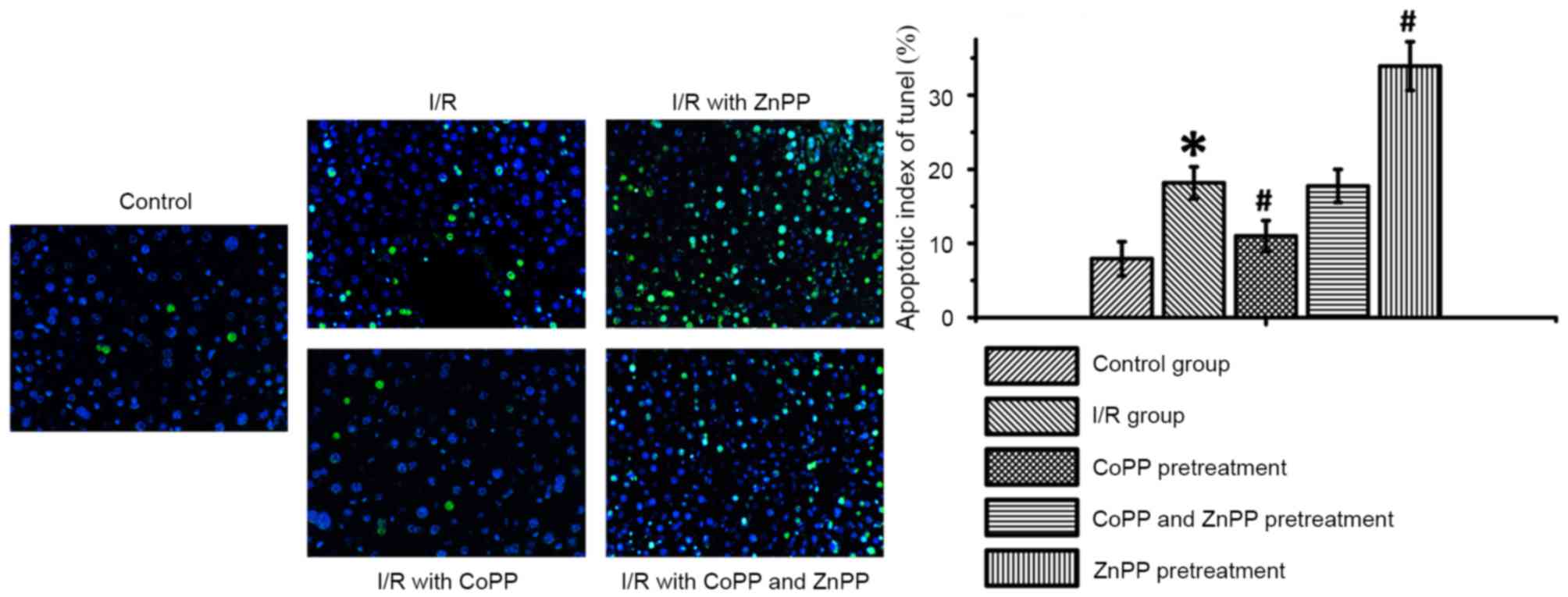
CoPP pretreatment inhibits apoptosis
following I/R
To investigate the role of HO-1 in apoptosis a TUNEL
assay was performed. In the CoPP pretreatment group, the percentage
of apoptotic cells in the liver was reduced compared with the I/R
group. The percentage of apoptotic cells in the CoPP and ZnPP
pretreatment group was increased compared with the CoPP treatment
group. The greatest percentage of apoptotic cells was observed in
the ZnPP pretreatment group (P<0.05; Table II and Fig. 4).
 | Table II.Apoptosis index following I/R, as
determined by terminal deoxynucleotidyl transferase dUTP nick end
labeling. |
Table II.
Apoptosis index following I/R, as
determined by terminal deoxynucleotidyl transferase dUTP nick end
labeling.
| Group | Apoptotic index
(%) |
|---|
| Control | 7.92±2.31 |
| I/R |
18.17±2.15a |
| I/R with CoPP
pretreatment |
11.00±2.09b |
| I/R with CoPP and
ZnPP pretreatment | 17.75±2.22 |
| I/R with ZnPP
pretreatment |
33.92±3.26b |
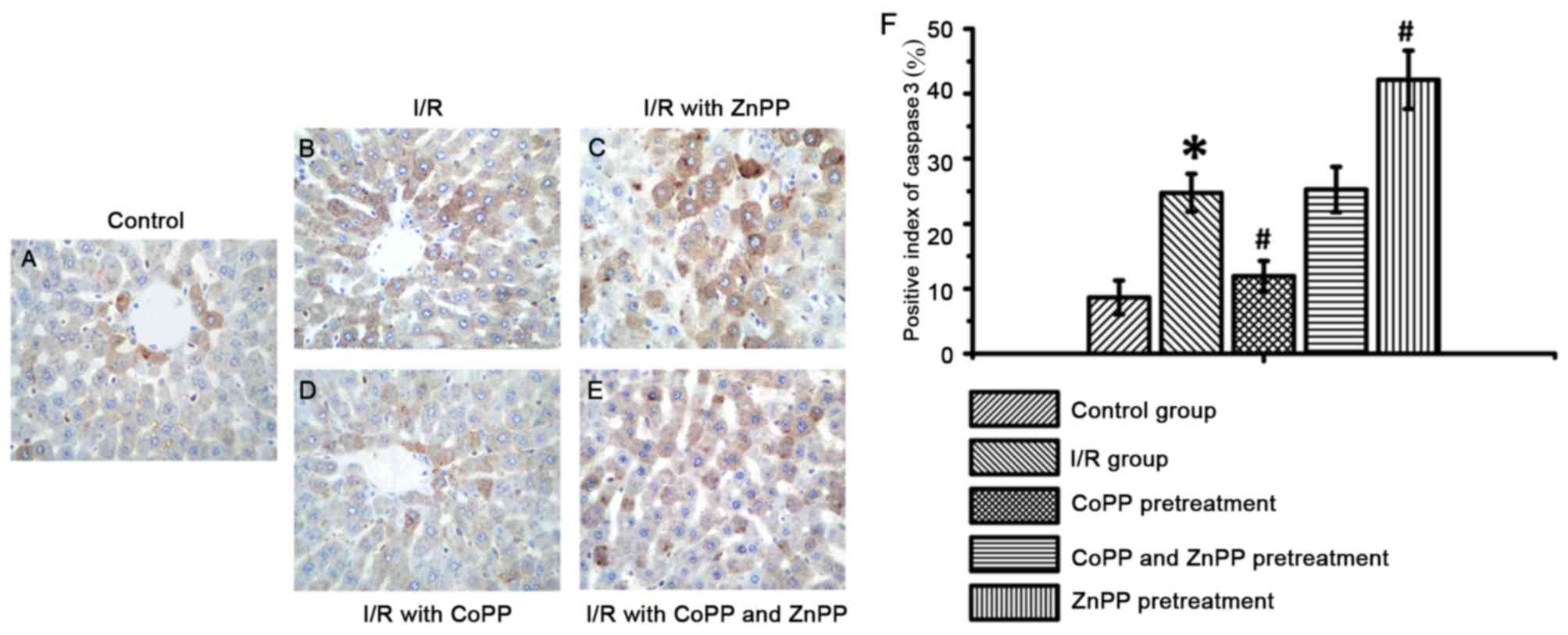
CoPP pretreatment inhibits caspase-3
expression following I/R
To assess the role of HO-1 in apoptosis, the
expression of caspase-3 was detected by immunohistochemistry.
Caspase-3 expression is a marker of apoptotic cell death (14); therefore, apoptotic cells were
identified by detecting caspase-3 expression by
immunohistochemistry. Caspase-3 staining was greater in the I/R
group compared with the control group (P<0.05); this increase
was attenuated by CoPP pretreatment and enhanced by ZnPP
pretreatment (P<0.05, Table
III and Fig. 5). These results
indicated that HO-1 may prevent cells from damage and death by
inhibiting the expression of caspase-3.
 | Table III.Caspase-3 expression following I/R, as
detected by immunohistochemistry. |
Table III.
Caspase-3 expression following I/R, as
detected by immunohistochemistry.
| Group | Positive index
(%) |
|---|
| Control | 8.67±2.61 |
| I/R |
24.75±2.93a |
| I/R with CoPP
pretreatment |
11.92±2.35b |
| I/R with CoPP and
ZnPP pretreatment | 25.25±3.50 |
| I/R with ZnPP
pretreatment |
42.17±4.47b |
Discussion
The results of the present study demonstrated that
CoPP pretreatment was associated with the attenuation of injury
induced by hepatic I/R, whereas this injury was exacerbated by ZnPP
pretreatment. Results of western blotting and TUNEL assays
indicated that the CoPP pretreatment group had greater protein
expression levels of HO-1 and Bcl-2, and a reduced percentage of
apoptotic cells. In addition, liver cell injury was increased
following ZnPP pretreatment. Furthermore, the protein expression
levels of HO-1 were decreased following pretreatment with ZnPP. The
results of the present study suggested that preconditioning with
CoPP may have a protective effect and attenuate I/R injury in liver
transplantation through induction of HO-1 expression.
Preconditioning with ZnPP downregulated HO-1 expression and
aggravated cell injury. Therefore, HO-1 expression may serve an
important role in the protection of cells against I/R injury.
Previous studies have identified that mice lacking
HO-1 (Hmox−/−) are more susceptible to I/R
injury compared with Hmox−/+ and Hmox+/+
animals, indicating that HO-1 may serve a protective role in I/R
injury (3,15,16).
A further study revealed that HO-1 was the most sensitive index of
stress, and knockdown of HO-1 expression by small interfering
(si)RNA may promote cell death (17).
Apoptosis serves an important role in hepatic I/R
injury; the underlying mechanisms may involve death receptors
including Fas and tumor necrosis factor α, and/or mitochondrial
dysfunction induced by cellular stress (1). The pro-apoptotic Bcl-2 and caspase
families are the primary signaling pathways. The metabolic products
of heme degraded by HO-1, ferrous iron, biliverdin and carbon
monoxide, are considered vital to protect cells from inflammation,
apoptosis and oxidative stress (18,19).
Ferrous iron released by HO-1 may induce expression of the
Fe2+ sequestering protein ferritin, which may inhibit
apoptosis to protect endothelial and liver cells (20). In addition, carbon monoxide may
significantly decrease mRNA expression levels of the pro-apoptotic
Bcl-2-associated X protein and promote the expression of Bcl-2
(21,22).
The induction of the caspase cascade results in cell
death. Caspase-3 is the ‘effector’ protease of the apoptosis
cascade and serves an important role in programmed cell death
(23). Caspase-3 knockdown by
siRNA decreased cell damage and improved cell survival (24). In the present study, CoPP
pretreatment decreased caspase-3 expression and the degree of cell
death compared with I/R alone. Therefore, high expression levels of
HO-1 induced by CoPP may inhibit caspase-3 expression and promote
Bcl-2 expression to protect cells from I/R-induced injury and
apoptosis.
Liver transplantation is an important treatment for
patients with hepatocellular carcinoma (25). I/R injury is an inevitable
consequence of organ transplantation and limits long-term survival
(26). I/R injury was described by
Jennings et al (27) in
1960 as damage that occurred when blood supply returned to tissue
following a period of ischemia. The findings of the present study
suggested that apoptosis is important in I/R injury, and that CoPP
may protect liver cells by upregulating HO-1 expression. CoPP may
therefore be a potential therapeutic agent for the treatment of
ischemic diseases.
References
|
1
|
Ben-Ari Z, Issan Y, Katz Y, Sultan M,
Safran M, Michal LS, Nader GA, Kornowski R, Grief F, Pappo O and
Hochhauser E: Induction of heme oxygenase-1 protects mouse liver
from apoptotic ischemia/reperfusion injury. Apoptosis. 18:547–555.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maines MD: The heme oxygenase system: A
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang CF, Wang ZY and Li JY: Dual
protective role of HO-1 in transplanted liver grafts: A review of
experimental and clinical studies. World J Gastroenterol.
17:3101–3108. 2011.PubMed/NCBI
|
|
4
|
Liu B and Qian JM: Cytoprotective role of
heme oxygenase-1 in liver ischemia reperfusion injury. Int J Clin
Exp Med. 8:19867–19873. 2015.PubMed/NCBI
|
|
5
|
Lai IR, Ma MC, Chen CF and Chang KJ: The
protective role of heme oxygenase-1 on the liver after hypoxic
preconditioning in rats. Transplantation. 77:1004–1008. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun N, Eum HA and Lee SM: Protective role
of heme oxygenase-1 against liver damage caused by hepatic ischemia
and reperfusion in rats. Antioxid Redox Signal. 13:1503–1512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin HY, Tsai CH, Lin C, Yeh WL, Tsai CF,
Chang PC, Wu LH and Lu DY: Cobalt protoporphyrin upregulates
cyclooxygenase-2 expression through a heme oxygenase-independent
mechanism. Mol Neurobiol. 53:4497–4508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang HF, Zeng Z, Wang KH, Zhang HY, Wang
S, Zhou WX, Wang ZB, Xu WG and Duan J: Heme oxygenase-1 protects
rat liver against warm ischemia/reperfusion injury via
TLR2/TLR4-triggered signaling pathways. World J Gastroenterol.
21:2937–2948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi X, Yao W, Xia H, Jin Y, Li X, Cai J
and Hei Z: Elevation of HO-1 expression mitigates intestinal
ischemia-reperfusion injury and restores tight junction function in
a rat liver transplantation model. Oxid Med Cell Longev.
2015:9860752015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guide for the Care and Use of Laboratory
Animals. The National Academies Press; Washington, DC: 2011,
PubMed/NCBI
|
|
11
|
Selzner N, Selzner M, Jochum W and Clavien
PA: Ischemic preconditioning protects the steatotic mouse liver
against reperfusion injury: An ATP dependent mechanism. J Hepatol.
39:55–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhardt SU, Weiss JB, Smolka C,
Maxeiner J, Pankratz F, Bemtgen X, Kustermann M, Thiele JR, Schmidt
Y, Bjoern Stark G, et al: MicroRNA-155 aggravates
ischemia-reperfusion injury by modulation of inflammatory cell
recruitment and the respiratory oxidative burst. Basic Res Cardiol.
110:322015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fortes GB, Alves LS, de Oliveira R, Dutra
FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher
M, Golenbock D, et al: Heme induces programmed necrosis on
macrophages through autocrine TNF and ROS production. Blood.
119:2368–2375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Devey L, Ferenbach D, Mohr E, Sangster K,
Bellamy CO, Hughes J and Wigmore SJ: Tissue-resident macrophages
protect the liver from ischemia reperfusion injury via a heme
oxygenase-1-dependent mechanism. Mol Ther. 17:65–72. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Wang J, Li Y, Fan C, Jiang S, Zhao
L, Di S, Xin Z, Wang B, Wu G, et al: HO-1 signaling activation by
pterostilbene treatment attenuates mitochondrial oxidative damage
induced by cerebral ischemia reperfusion injury. Mol Neurobiol.
53:2339–2353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stocker R, Yamamoto Y, McDonagh AF, Glazer
AN and Ames BN: Bilirubin is an antioxidant of possible
physiological importance. Science. 235:1043–1046. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harder Y, Amon M, Schramm R, Rücker M,
Scheuer C, Pittet B, Erni D and Menger MD: Ischemia-induced
up-regulation of heme oxygenase-1 protects from apoptotic cell
death and tissue necrosis. J Surg Res. 150:293–303. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berberat PO, Katori M, Kaczmarek E,
Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K,
Csizmadia E, et al: Heavy chain ferritin acts as an antiapoptotic
gene that protects livers from ischemia-reperfusion injury. FASEB
J. 17:1724–1726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han HH, Lim YM, Park SW, Lee SJ, Rhie JW
and Lee JH: Improved skin flap survival in venous
ischemia-reperfusion injury with the use of adipose-derived stem
cells. Microsurgery. 35:645–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakao A, Kimizuka K, Stolz DB, Neto JS,
Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA,
Otterbein LE and Murase N: Carbon monoxide inhalation protects rat
intestinal grafts from ischemia/reperfusion injury. Am J Pathol.
163:1587–1598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Contreras JL, Vilatoba M, Eckstein C,
Bilbao G, Anthony Thompson J and Eckhoff DE: Caspase-8 and
caspase-3 small interfering RNA decreases ischemia/reperfusion
injury to the liver in mice. Surgery. 136:390–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hua P, Liu LB, Liu JL, Wang M, Jiang HQ,
Zeng K, Yang YQ and Yang SR: Inhibition of apoptosis by knockdown
of caspase-3 with siRNA in rat bone marrow mesenchymal stem cells.
Exp Biol Med (Maywood). 238:991–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cotterell AH and Fisher RA:
Ischemia/reperfusion injury and hepatocellular carcinoma recurrence
after liver transplantation: Cancer at WIT's end? Dig Dis Sci.
60:2579–2580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dare AJ, Logan A, Prime TA, Rogatti S,
Goddard M, Bolton EM, Bradley JA, Pettigrew GJ, Murphy MP and
Saeb-Parsy K: The mitochondria-targeted anti-oxidant MitoQ
decreases ischemia-reperfusion injury in a murine syngeneic heart
transplant model. J Heart Lung Transplant. 34:1471–1480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jennings RB, Sommers HM, Smyth GA, Flack
HA and Linn H: Myocardial necrosis induced by temporary occlusion
of a coronary artery in the dog. Arch Pathol. 70:68–78.
1960.PubMed/NCBI
|