Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by polyarticularsynovitis (1). Its autoantigen is not organ-specific,
but a common component of many organs and tissues, such as nucleus,
mitochondria and so on, so it can cause the damage of multiple
organs and multiple tissues (2).
Epidemiological survey shows that in China, the incidence rate of
RA is 0.32–0.38%, while in Europe, the United States and Africa, it
is up to 0.5–2.0%. Its pathogenesis is complex, influenced by
genetic and environmental factors (3). As the patients with RA have high
disability rate, it brings a great financial burden to the patients
themselves and the whole community, which has becomethe hot topic
during the last 10 years for domestic and foreign scholars
(3). The basic pathologic changes
of RA are synovial cell hyperplasia, thickening of lining layer,
infiltration of multiple inflammatory cells, pannus formation and
destruction of cartilage and bone tissue, eventually leading to
joint deformity and loss of function (4).
RA is related to the signal transduction pathways of
multiple cytokines. In recent years, it has been found that
phosphoinositide 3 kinase (PI3K)/protein kinase B (PKB) signaling
pathway (PI3K/Akt pathway) is involved in a very rapid signal
transduction system from the membrane to the nuclear and its
downstream pathways Bad, caspase and nuclear factor (NF)-κB are
important pathways affecting osteoarthritis (5). Recent studies have shown that the
signal transduction pathway ofkinase plays an important role in the
process of chondrocyte apoptosis (5,6).
PI3K/Akt/mTOR signal pathway plays an important role
in the proliferation and survivalof lymphocyte (7). However, the literature has shown that
PI3K/Akt/mTOR signal transduction pathway isstudied in the
pathological mechanisms and treatment methods of malignant tumors.
mTOR and PI3K signals play an important role in regulation of B
lymphocyte morphology, metabolic activity, and cell cycle
progression (8).
Gamboge is adry resinsecreted by Garcinia plant,
Garcinia hanbaryi Hook. f. It is produced mainly in
Cambodia, India, Thailand and Vietnam, as well as Guangdong and
Hainan in China (9). It has been
documented for a long time in our traditional medicine, as Garcinia
cold, Pickle, Sim, astringent, toxic, with the functions of
breaking blood, detoxifying, stopping bleeding and killing insects,
which has been used for the treatment of crewels, scrofulaceum, and
carbuncle, since ancient times (10). It contains gambogic acid,
neogambogic acid, allogambogic acid, morellin, isomorellin,
morellic acid, isomorellic acid and so on, in which gambogic acid
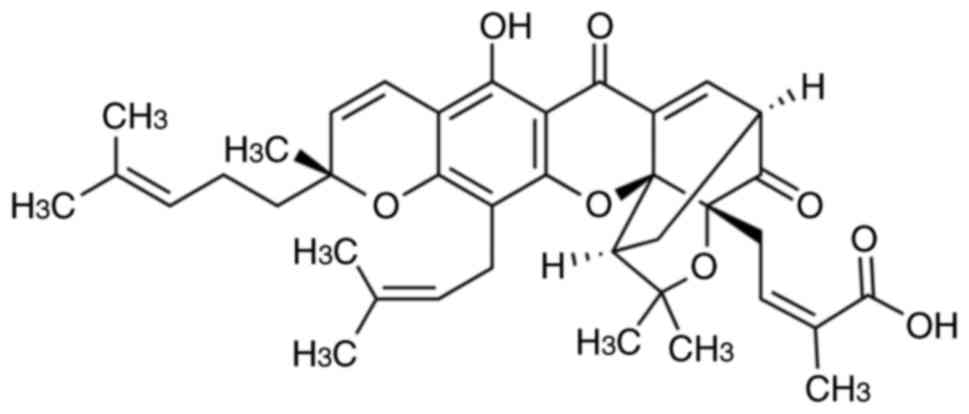
is the main active ingredient. Gambogic acid (Fig. 1) has anti-inflammatory,
antioxidation and anticancer effects (11,12).
Ma et al (13) showed that
gambogic acid inhibits osteoclast formation and ovariectomy-induced
osteoporosis. The purpose of this study is for the first time to
elucidate the anti-inflammatory and antiproliferative effects of
gambogic acid on arthritis and the possible mechanisms.
Materials and methods
Animals and treatment
Collagen-induced arthritis in mice was a common RA
model using collagen II. Male DBA/1 mice (5-week-old) was purchased
from Wenzhou Laboratory Animal Center (Wenzhou, China) and kept in
specific pathogen-free conditions in Animal Center of Wenzhou
Medical University. This study gained ethical approval from Chinese
Traditional Medicine (Wenzhou, China). Arthritis was induced and
emulsified in complete Freund's adjuvant and injected intradermally
into the tail with 200 µg of collagen II (14). Meanwhile, in treatment with
gambogic acid group, mice were treatment with 2 mg/kg/3 day
(intraperitoneal injection) for 28 days (15). All mice were randomly assigned into
three groups: Sham group, RA model group and GA group; amount of
very group is 10 mice.
ELISA analyzing
Serum from very mice after treatment with gambogic
acid at 28 days was collected and centrifuged at 1,500 g for 10
min. Serum was used to analyze inflammation factors [tumor necrosis
factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-18 concentrations]
using ELISA kits (Nanjing Jiancheng Biology Engineering Institute,
Nanjing, China).
Then, tissue extracts were prepared from very mice
after treatment with gambogic acid at 28 days and extracted using
RIPA assay. Tissue extracts were prepared after treatment with
gambogic acid at 28 days and extracted using RIPA assay. Protein
quantified was measured using BCA assay. Briefly, proteins were
used to analyze caspase-3 and caspase-9 activity using Commercial
ELISA kits (Nanjing Jiancheng Biology Engineering Institute).
Western blot analysis
Tissue extracts were prepared after treatment with
gambogic acid at 28 days and extracted using RIPA assay. Protein
quantified was measured using BCA assay. Briefly, proteins were
size fractionated by 8–12% sodium dodecyl sulfate polyacrylamide
gel electrophoresis and then transferred to a polyvinylidene
difluoride membrane. The following antibodies were employed with
matrix metalloproteinases (MMP)-2, MMP-9, NF-κB, phosphorylated
(p-)p38, tissue inhibitors of matrix metalloproteases-1 (TIMP-1),
PI3K, p-Akt and GAPDH (Santa Cruz Biotechnology Inc., Milan, Italy)
at 4°C overnight. Secondary anti-mouse IgG peroxidase conjugate was
used to incubate for 1 h at 37°C. Detection was carried out with
ECL Fast Pico (ECL-1002; Immunological Sciences, Rome, Italy) and
quantified the ImageJ software system and Alliance LD (Uvitec,
Cambridge, UK).
Statistical analysis
The data of statistical analysis are presented as
mean ± SD and were compared by two-way analysis of variance with a
Sidak's multiple comparison test post hoc. P-value <0.05
considered to be statistically significant.
Results
Gambogic acid decreases arthritis
scores in RA mice
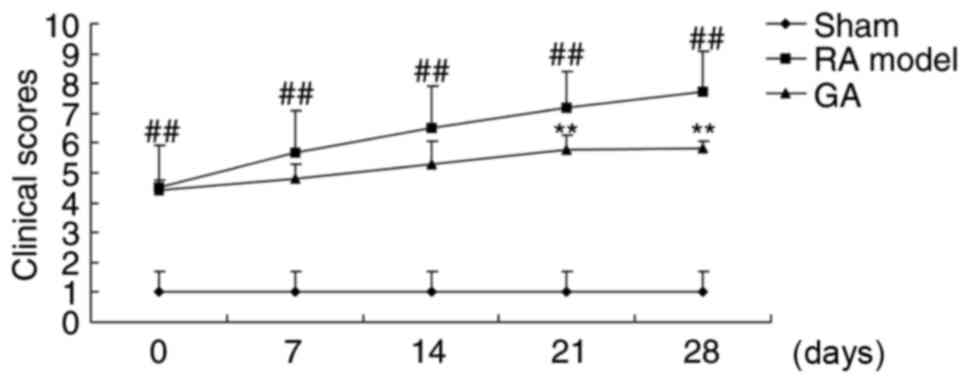
To examine whether the effects of gambogic acid on
arthritis, we recorded arthritis scores in RA mice by gambogic
acid. As showed in Fig. 1, a
significant increase of arthritis scores in RA model mice. However,
gambogic acid inhibited arthritis scores at 21 or 28 days in RA
mice (Fig. 2).
Gambogic acid decreases TNF-α, IL-1β,
IL-6 and IL-18 concentrations in RA mice
To investigate the anti-inflammation effects of
gambogic acid on RA, TNF-α, IL-1β, IL-6 and IL-18 concentrations
were measured using ELISA KITS. Fig.
3 showed that TNF-α, IL-1β, IL-6 and IL-18 concentrations in RA
model mice were significantly increased, compared with control
group. These inflammation factors were significantly reduced in RA
mice by gambogic acid for 28 days (Fig. 3).
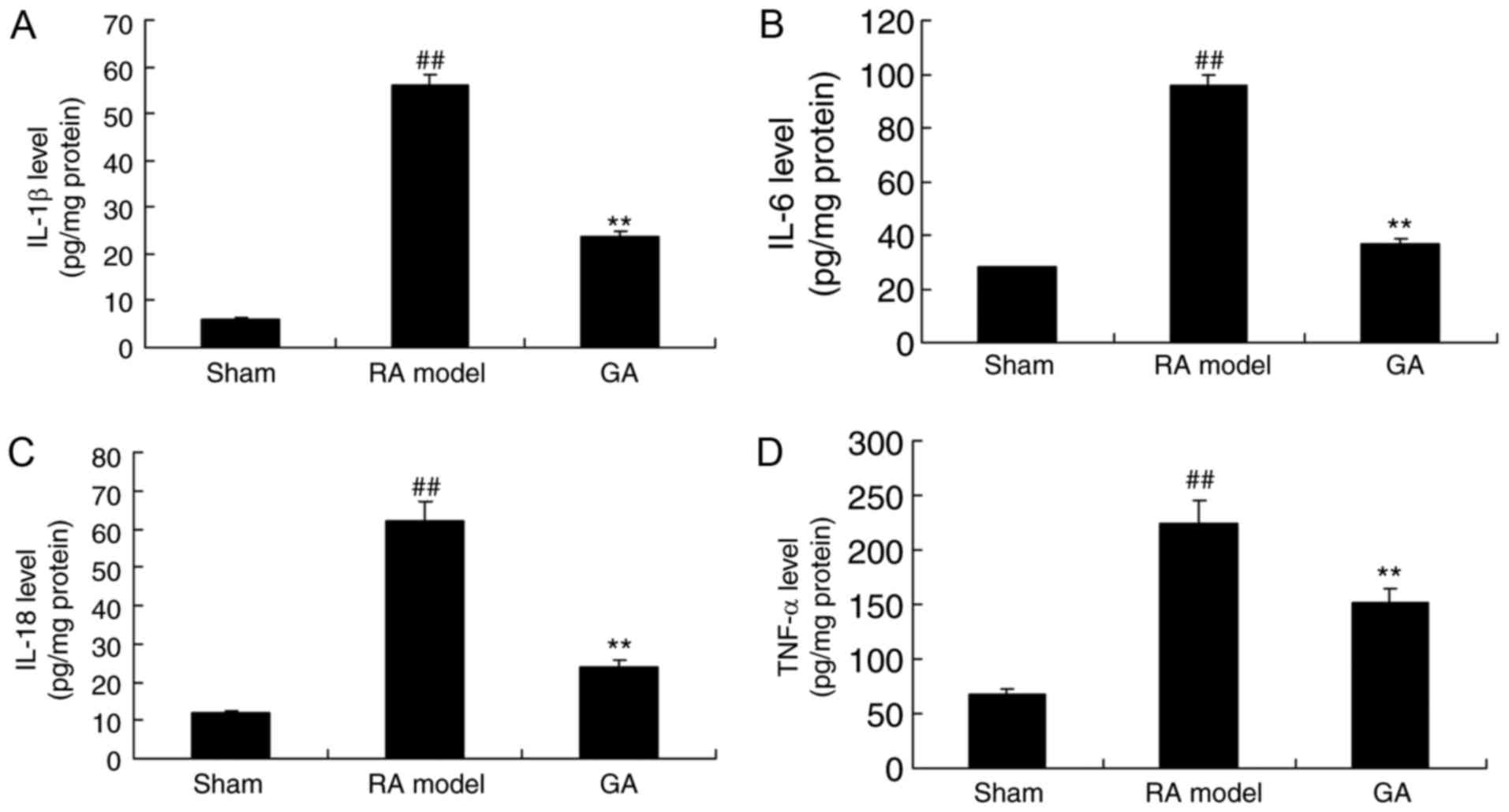 | Figure 3.Gambogic acid decreased TNF-α, IL-1β,
IL-6 and IL-18 concentrations in RA mice. Gambogic acid decreased
(A) IL-1β, (B) IL-6, (C) IL-18 and (D) TNF-α, concentrations in RA
mice. ##P<0.01 compared with Sham, **P<0.01
compared with RA model. TNF, tumor necrosis factor; IL,
interleukin; RA, rheumatoid arthritis; Sham, sham control group; RA
model, RA model group; GA, gambogic acid group. |
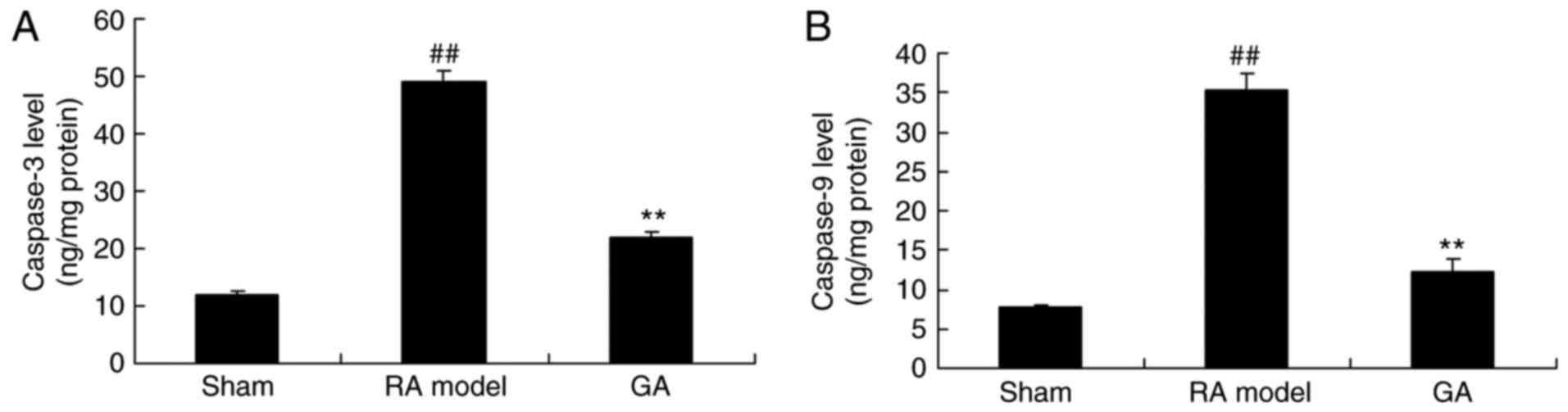
Gambogic acid decreases caspase-3 and
caspase-9 activity in RA mice
To assess the involvement of caspases for cell
apoptosis of RA treated by gambogic acid, caspase-3 and caspase-9
activity were analyzed using ELISA kits. As showed that caspase-3
and caspase-9 activity of RA model was higher than those of control
group (Fig. 4). The high level of
caspase-3 and caspase-9 activity in RA model reduced by gambogic
acid, compared with RA model group (Fig. 4).
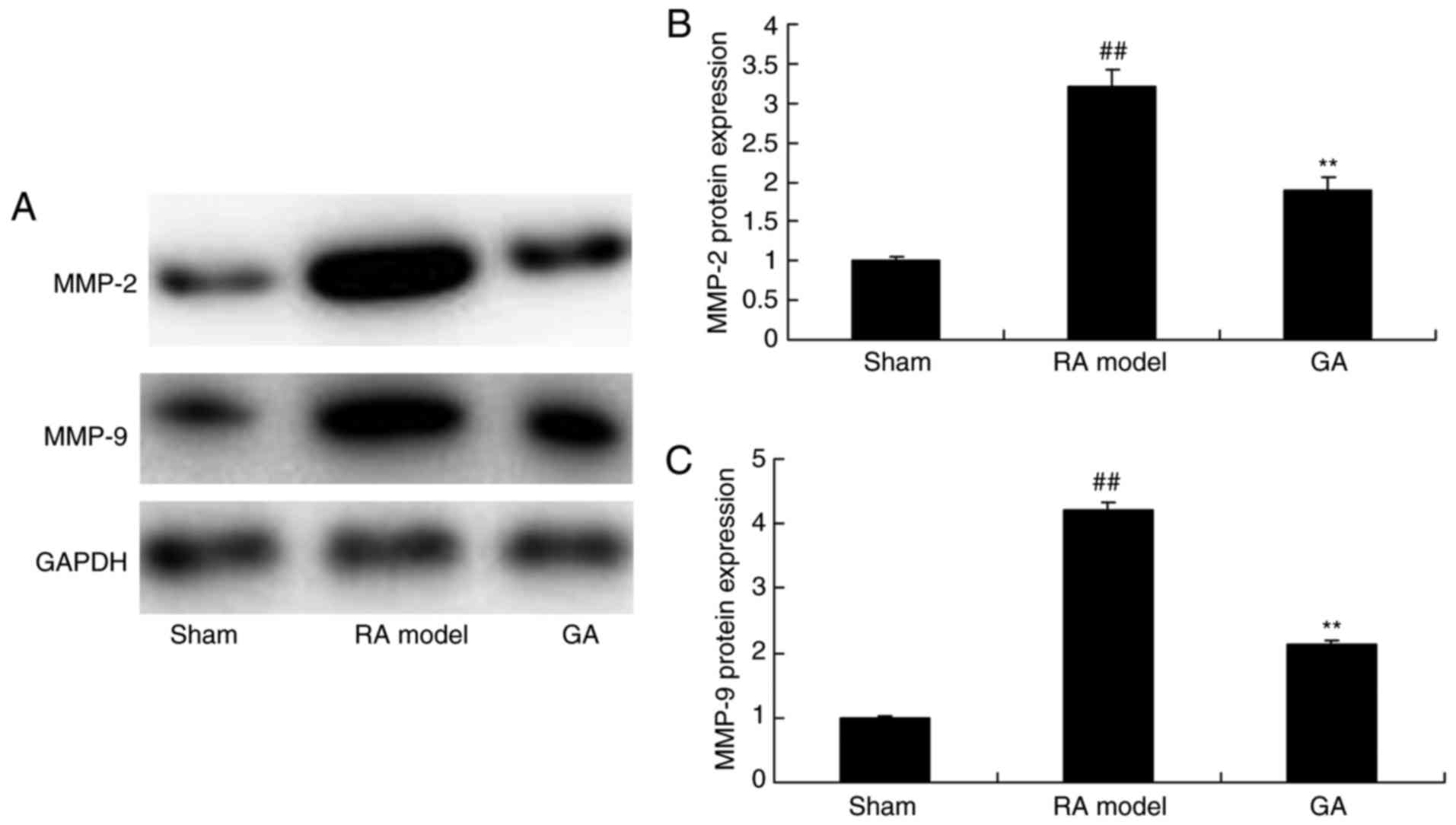
Gambogic acid decreases MMP-2 and
MMP-9 protein expression in RA mice
Then, we analyzed MMP-2 and MMP-9 protein expression
treated by gambogic acid. The result of Fig. 5 showed that RA induced MMP-2 and
MMP-9 protein expression in mice model, compared with control
group. Certainly, gambogic acid significantly inhibited MMP-2 and
MMP-9 protein expression in RA model group, compared with RA model
group (Fig. 5).
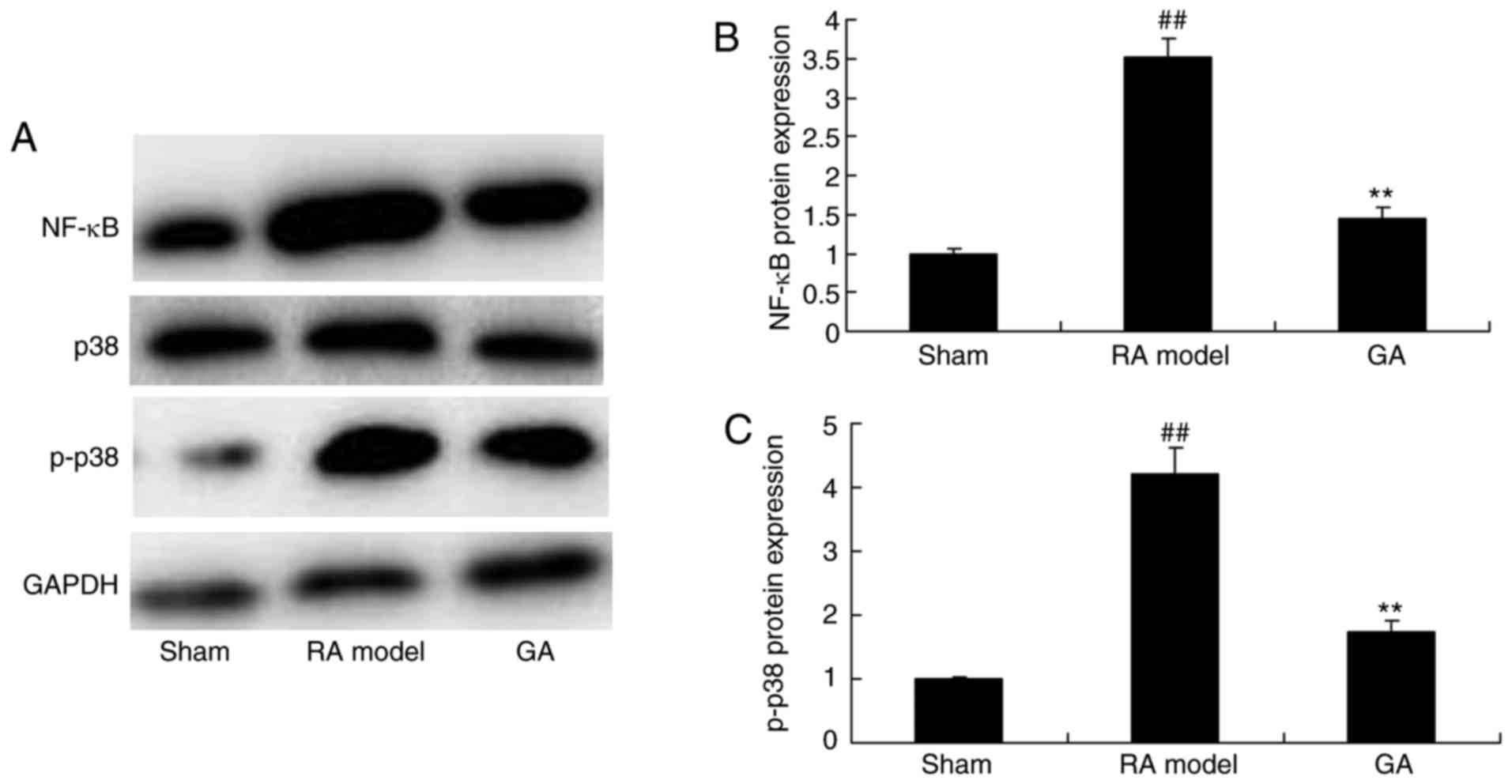
Gambogic acid decreases NF-κB and
p-p38 protein expression in RA mice
To understand the anti-inflammation mechanism of
gambogic acid on RA, NF-κB and p-p38 protein expression were
measured using western blot analysis. As showed in Fig. 6, NF-κB and p-p38 protein expression
were significantly induced in RA, model group, compared with
control group. Treatment with gambogic acid significantly
suppressed NF-κB and p-p38 protein expression in RA mice, exhibited
that gambogic acid inhibited inflammation in RA mice through NF-κB
and p-p38 protein expression (Fig.
6).
Gambogic acid increases PI3K/Akt
singnaling pathway in RA mice
Furthermore, to investigate the anti-apoptosis
mechanism of gambogic acid on RA, TIMP-1 and PI3K/Akt singnaling
pathway were selected and analyzed in this study. Fig. 7 showed that TIMP-1 and PI3K/Akt
singnaling pathway were suppressed in RA model group, compared with
control group. Gambogic acid could promote TIMP-1 and PI3K/Akt
singnaling pathway in RA mice. These data illuminated that gambogic
acid alleviated cell apoptosis in RA through TIMP-1 and PI3K/Akt
singnaling pathway.
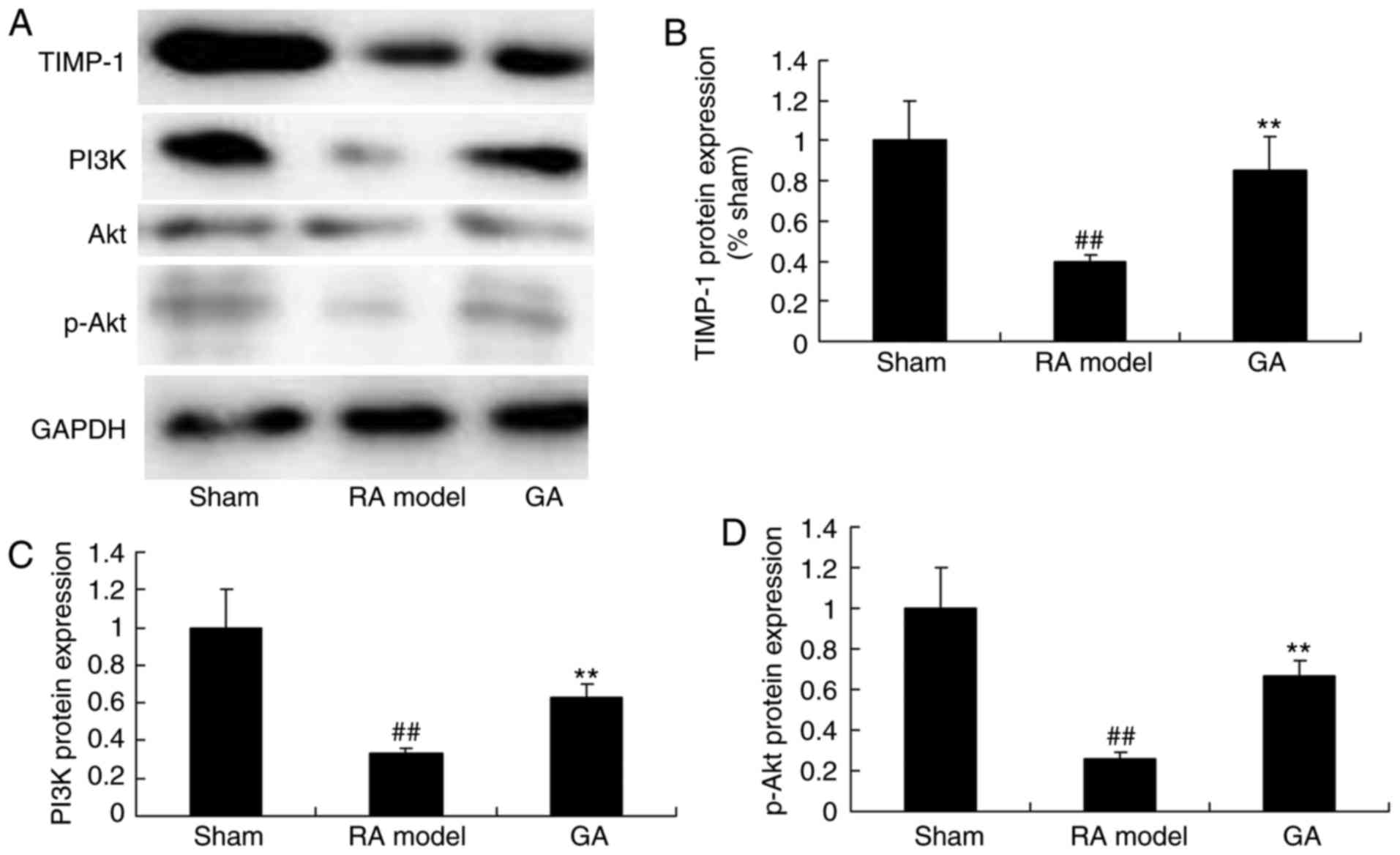 | Figure 7.Gambogic acid increased TIMP-1,
PI3K/Akt singnaling pathway in RA mice. Gambogic acid decreased
TIMP-1, PI3K and p-Akt protein expression by (A) western blot
assays and (B, C and D) statistical analysis of TIMP-1, PI3K and
p-Akt protein expression in RA mice. ##P<0.01
compared with Sham, **P<0.01 compared with RA model. TIMP-1,
tissue inhibitor of matrix metalloprotease-1; PI3K,
phosphoinositide 3-kinase; RA, rheumatoid arthritis; p-,
phosphorylated; Sham, sham control group; RA model, RA model group;
GA, gambogic acid group. |
Discussion
RA is a systemic inflammatory autoimmune disease
related to the involvement of the surrounding joints, which is
characterized by inflammation of the synovial tissue and
progressive destruction of the joints (16). Cytokine TNF-α plays an important
role in synovial inflammation and bone destruction of RA (17). It is suggested that TNF-α induces
synovial cells to produce the expressions of many inflammatory
cytokines (such as IL-6 and IL-8) and collagenase (MMP), which is a
key factor for the persistence and progression of RA (18). In our study, gambogic acid
inhibited arthritis scores, and TNF-α, IL-1β, IL-6 and IL-18
concentrations in RA mice. Wen et al (15) suggested that gambogic acid exhibits
anti-psoriatic efficacy through inhibition of inflammation.
NF-κB signal pathway plays an important role in the
inflammatory process of RA (19).
The transcription factor, NF-κB may play an important role in the
pathogenesis of infectious diseases and autoimmune diseases by
regulating the expression of various cytokines and cell adhesion
molecules (6). The activated
transcription factor, NF-κB, binds to the κB site in the promoter
region of the corresponding inflammatory mediator target gene,
leading to the over-expression of the inflammatory mediator gene
(20). In recent years, we have
found that NF-κB is activated in RA synovial tissue abnormally, and
the NF-κB pathway in RA synovial tissue and synovial fluid
regulates the expression of many inflammatory cytokines, such as
cytokines (TNF-α, IL-1, IL-6, IL-2, IL-12, IFN-γ), adhesion
molecules (VCAM-1, ICAM-1), chemokines (IL-8, MIP-1α, MCP-1,
TIMP-1), and MMPs (MMP-1, MMP-3, MMP-13) (21). And we also found that treatment
with gambogic acid significantly suppressed NF-κB and MMP-2 and
MMP-9 protein expression in RA mice. Tang et al (11) suggested that gambogic acid inhibits
the growth of ovarian cancer tumors through RELA/NF-κB p65 (p65)
activity. Qi et al (22)
indicated that gambogic acid suppressed lung metastasis of human
breast carcinoma cell through protein kinase C (PKC) mediated
MMP-2/9 expression inhibition.
p38 mitogen-activated protein kinase plays an
important role in normal cartilage cell physiology and pathogenesis
of osteoarthritis (23). P38 can
regulate the proliferation and survival of chondrocytes, balance
the extracellular matrix metabolism, and play a key role in the
pathogenesis of osteoarthritis cartilage degeneration induced by
MMPs and proinflammatory factors (24). Our results indicated that gambogic
acid significantly suppressed p-p38 protein expression in RA mice.
Ma et al (13) demonstrated
that gambogic acid inhibits osteoclast formation in p38 and Akt
signalling pathways.
The abnormal mechanism of chondrocyte apoptosis has
not been elucidated and may be related to many signal transduction
pathways, in which PI3K/AKT signal transduction pathway has been
considered as an important pathway of chondrocyte apoptosis
(25). PI3K is a membrane protein
that can directly or indirectly activate the downstream factor AKT
by accepting the afferent signals of tyrosine kinase receptors,
cytokine receptors, CD19, BCR, GPCR, etc. on the membrane (26). The biological effects of AKT
include protein synthesis, anti-apoptosis, blocking the cytoplasm,
regulating cell growth cycle, glucose metabolism (glycolysis,
glucose conversion and glycogen synthesis) and neurodegeneration
(27). The anti-apoptotic effect
of AKT is realized by the following three ways: Playing
anti-apoptotic effectby XIAP factor; inhibition of Bax, Bcl-2, Bim
and FOX01-induced apoptosis; isolation of Bad (14-3-3) in the
cytoplasm (27). PI3K/Akt signal
is involved in the regulation of B lymphocyte proliferation and
differentiation by the activation of mTOR, and mTOR is a target
molecule for rapamycin, which plays a critical role in regulating
cell growth and proliferation (28). mTOR signal affects cell cycle, cell
growth and cell proliferation. In many human cancers, the absence
of some important tumor suppressors (PTEN, TSC1/2, LKB1) in mTOR
signal pathway, cell mutations or the gene amplification in PI3CA
(P110α subtype) and abnormalities caused by Akt activation
mutations, ultimately lead to cell proliferation, cell survival and
cell self-phagocytosis inhibition (8,29).
Our data also showed that gambogic acid could promote PI3K/Akt
singnaling pathway in RA mice. Wang et al (30) identified that gambogic acid
suppresses multiple myeloma cell growth through PI3K/Akt singnaling
pathway.
In summary, this study reveals that gambogic acid
prevents inflammation and apoptosis in RA model mice through NF-κB
p65/MMP-2/9 and PI3K/Akt singnaling pathway. These findings may be
valuable in the further exploration of gambogic acid in therapy for
RA.
References
|
1
|
Bijlsma JW, Welsing PM, Woodworth TG,
Middelink LM, Pethö-Schramm A, Bernasconi C, Borm ME, Wortel CH,
Ter Borg EJ, Jahangier ZN, et al: Early rheumatoid arthritis
treated with tocilizumab, methotrexate, or their combination
(U-Act-Early): A multicentre, randomised, double-blind,
double-dummy, strategy trial. Lancet. 388:343–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sellam J, Marion-Thore S, Dumont F,
Jacques S, Garchon HJ, Rouanet S, Taoufik Y, Hendel-Chavez H,
Sibilia J, Tebib J, et al: Use of whole-blood transcriptomic
profiling to highlight several pathophysiologic pathways associated
with response to rituximab in patients with rheumatoid arthritis:
Data from a randomized, controlled, open-label trial. Arthritis
Rheumatol. 66:2015–2025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka Y, Kawai S, Takeuchi T, Yamamoto K
and Miyasaka N: Prevention of joint destruction by tacrolimus in
patients with early rheumatoid arthritis: A post hoc analysis of a
double-blind, randomized, placebo-controlled study. Mod Rheumatol.
23:1045–1052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li R, Zhao JX, Su Y, He J, Chen LN, Gu F,
Zhao C, Deng XR, Zhou W, Hao YJ, et al: High remission and low
relapse with prolonged intensive DMARD therapy in rheumatoid
arthritis (PRINT): A multicenter randomized clinical trial.
Medicine (Baltimore). 95:e39682016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan
X, Xu Z and Zhang P: Cucurbitacin E inhibits TNF-α-induced
inflammatory cytokine production in human synoviocyte MH7A cells
via suppression of PI3K/Akt/NF-κB pathways. Int Immunopharmacol.
29:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho ML, Ju JH, Kim KW, Moon YM, Lee SY,
Min SY, Cho YG, Kim HS, Park KS, Yoon CH, et al: Cyclosporine A
inhibits IL-15-induced IL-17 production in CD4+ T cells via
down-regulation of PI3K/Akt and NF-kappaB. Immunol Lett. 108:88–96.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv Q, Zhu XY, Xia YF, Dai Y and Wei ZF:
Tetrandrine inhibits migration and invasion of rheumatoid arthritis
fibroblast-like synoviocytes through down-regulating the
expressions of Rac1, Cdc42, and RhoA GTPases and activation of the
PI3K/Akt and JNK signaling pathways. Chin J Nat Med. 13:831–841.
2015.PubMed/NCBI
|
|
8
|
Kim TH, Choi SJ, Lee YH, Song GG and Ji
JD: Combined therapeutic application of mTOR inhibitor and vitamin
D(3) for inflammatory bone destruction of rheumatoid arthritis. Med
Hypotheses. 79:757–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Yi Y, Chen J, Sun Y, Guo Q, Zheng
Z and Song S: Gambogic acid inhibits Hsp90 and deregulates
TNF-α/NF-κB in HeLa cells. Biochem Biophys Res Commun. 403:282–287.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palempalli UD, Gandhi U, Kalantari P,
Vunta H, Arner RJ, Narayan V, Ravindran A and Prabhu KS: Gambogic
acid covalently modifies IkappaB kinase-beta subunit to mediate
suppression of lipopolysaccharide-induced activation of NF-kappaB
in macrophages. Biochem J. 419:401–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang Q, Lu M, Zhou H, Chen D and Liu L:
Gambogic acid inhibits the growth of ovarian cancer tumors by
regulating p65 activity. Oncol Lett. 13:384–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geng J, Xiao S, Zheng Z, Song S and Zhang
L: Gambogic acid protects from endotoxin shock by suppressing
pro-inflammatory factors in vivo and in vitro. Inflamm Res.
62:165–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Ma Y, Liu X, Chen S, Liu C, Qin A
and Fan S: Gambogic acid inhibits osteoclast formation and
ovariectomy-induced osteoporosis by suppressing the JNK, p38 and
Akt signalling pathways. Biochem J. 469:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito H, Noda K, Yoshida K, Otani K, Yoshiga
M, Oto Y, Saito S and Kurosaka D: Prokineticin 2 antagonist, PKRA7
suppresses arthritis in mice with collagen-induced arthritis. BMC
Musculoskelet Disord. 17:3872016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen J, Pei H, Wang X, Xie C, Li S, Huang
L, Qiu N, Wang W, Cheng X and Chen L: Gambogic acid exhibits
anti-psoriatic efficacy through inhibition of angiogenesis and
inflammation. J Dermatol Sci. 74:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lal P, Su Z, Holweg CT, Silverman GJ,
Schwartzman S, Kelman A, Read S, Spaniolo G, Monroe JG, Behrens TW
and Townsend MJ: Inflammation and autoantibody markers identify
rheumatoid arthritis patients with enhanced clinical benefit
following rituximab treatment. Arthritis Rheum. 63:3681–3691. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nair SC, Welsing PM, Jacobs JW, van Laar
JM, Rensen WH, Ardine de Wit G, Bijlsma JW and Lafeber FP: Economic
evaluation of a tight-control treatment strategy using an imaging
device (handscan) for monitoring joint inflammation in early
rheumatoid arthritis. Clin Exp Rheumatol. 33:831–838.
2015.PubMed/NCBI
|
|
18
|
Kullich WC, Mur E, Aglas F, Niksic F and
Czerwenka C: Inhibitory effects of leflunomide therapy on the
activity of matrixmetalloproteinase-9 and the release of cartilage
oligomeric matrix protein in patients with rheumatoid arthritis.
Clin Exp Rheumatol. 24:155–160. 2006.PubMed/NCBI
|
|
19
|
Min HK, Kim SM, Baek SY, Woo JW, Park JS,
Cho ML, Lee J, Kwok SK, Kim SW and Park SH: Anthocyanin extracted
from black soybean seed coats prevents autoimmune arthritis by
suppressing the development of Th17 cells and synthesis of
proinflammatory cytokines by such cells, via inhibition of NF-κB.
PLoS One. 10:e01382012015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XZ, Fan J, Qi K, Liu SP, Xu WD, Gao Y,
Gu XD, Li J, Bai CG, Shi YQ, et al: Dishevelled2 promotes apoptosis
and inhibits inflammatory cytokine secretion in rheumatoid
arthritis fibroblast-like synoviocytes through crosstalk with the
NF-κB pathway. Oncotarget. 8:12649–12663. 2017.PubMed/NCBI
|
|
21
|
Li Y, Wang LM, Xu JZ, Tian K, Gu CX and Li
ZF: Gastrodia elata attenuates inflammatory response by inhibiting
the NF-κB pathway in rheumatoid arthritis fibroblast-like
synoviocytes. Biomed Pharmacother. 85:177–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi Q, Gu H, Yang Y, Lu N, Zhao J, Liu W,
Ling H, You QD, Wang X and Guo Q: Involvement of matrix
metalloproteinase 2 and 9 in gambogic acid induced suppression of
MDA-MB-435 human breast carcinoma cell lung metastasis. J Mol Med
(Berl). 86:1367–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li CH, Xu LL, Zhao JX, Sun L, Yao ZQ, Deng
XL, Liu R, Yang L, Xing R and Liu XY: CXCL16 upregulates RANKL
expression in rheumatoid arthritis synovial fibroblasts through the
JAK2/STAT3 and p38/MAPK signaling pathway. Inflamm Res. 65:193–202.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HR, Park MK, Cho ML, Kim KW, Oh HJ,
Park JS, Heo YM, Lee SH, Kim HY and Park SH: Induction of
macrophage migration inhibitory factor in ConA-stimulated
rheumatoid arthritis synovial fibroblasts through the P38 map
kinase-dependent signaling pathway. Korean J Intern Med.
25:317–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han W, Xiong Y, Li Y, Fang W, Ma Y, Liu L,
Li F and Zhu X: Anti-arthritic effects of clematichinenoside (AR-6)
on PI3K/Akt signaling pathway and TNF-α associated with
collagen-induced arthritis. Pharm Biol. 51:13–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Pan YF, Xue YQ, Fang LK, Guo XH,
Guo X, Liu M, Mo BY, Yang MR, Liu F, et al: uPAR promotes
tumor-like biologic behaviors of fibroblast-like synoviocytes
through PI3K/Akt signaling pathway in patients with rheumatoid
arthritis. Cell Mol Immunol. Jan 16–2017.(Epub ahead of print).
|
|
27
|
Yuan FL, Xu RS, Jiang DL, He XL, Su Q, Jin
C and Li X: Leonurine hydrochloride inhibits osteoclastogenesis and
prevents osteoporosis associated with estrogen deficiency by
inhibiting the NF-κB and PI3K/Akt signaling pathways. Bone.
75:128–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malemud CJ: The PI3K/Akt/PTEN/mTOR
pathway: A fruitful target for inducing cell death in rheumatoid
arthritis? Future Med Chem. 7:1137–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitra A, Raychaudhuri SK and Raychaudhuri
SP: IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR
signaling cascade. Cytokine. 60:38–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Zhang W, Guo L, Bao W, Jin N, Liu
R, Liu P, Wang Y, Guo Q and Chen B: Gambogic acid suppresses
hypoxia-induced hypoxia-inducible factor-1α/vascular endothelial
growth factor expression via inhibiting phosphatidylinositol
3-kinase/Akt/mammalian target protein of rapamycin pathway in
multiple myeloma cells. Cancer Sci. 105:1063–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|