Introduction
Bladder cancer is the most common urological tumor
and ranks as the leading cause of death in patients with urinary
tract disease (1). Despite the
development of treatment strategies, within the first year after
TURP (transurethral resection of prostate) treatment of NMIBC
(non-muscle invasive bladder cancer), the probability of disease
recurrence ranges from 15 to 60% and the probability of disease
progression at 5 years ranges from 7 to 40% (2). Furthermore, MIBC has a higher rate of
progressing to distant, life-threatening metastases than NMIBC and
is still the major cause of low survival rate. The high metastatic
rate of MIBC has always been the main obstacle in clinical
treatment (3). Improved treatment
requires a detailed understanding of urothelial carcinoma
pathogenesis and molecular biology. Therefore, what we urgently
need is the suppression of bladder cancer invasion and metastasis
and the development of an adjuvant therapy for bladder cancer.
Aminopeptidase N (APN) is also known as the cell
surface molecule CD13. It is involved in various cellular
processes, including cell cycle control, cell differentiation, cell
motility, angiogenesis, cellular attachment and invasion/metastasis
of various malignancies, including bladder cancer (4). APN/CD13 is overexpressed in several
cancers, including pancreatic cancer, non-small cell lung cancer
and colon cancer. In the cancers mentioned above, APN levels
positively correlate with tumor metastasis (5). Although the correlation between APN
expression and invasion/metastasis of cancer is increasingly clear,
little is known about the role of APN/CD13 in the invasion and
metastatic ability of bladder cancer cells.
Ubenimex has been exploited as an adjuvant therapy
to enhance the effects of antitumor treatments after surgery. It
has been widely used in the treatment of leukemia, non-small cell
lung cancer, gastric cancer and cervical cancer (6–8). Our
previous study demonstrated that ubenimex functions as an antitumor
drug in prostate cancer cells. We also demonstrated that the
inhibition of APN activity and the resulting induction of cell
death plays a key role during this process (9). Apoptosis, or caspase-dependent cell
death, is a type of programmed cell death (PCD) that can dispose of
damaged cells (10). Autophagy, or
caspase-independent cell death, also plays a crucial role in tumor
cell death (11). Akt is a
classical signaling pathway that participates in the regulation of
tumor cell apoptotic and autophagic cell death (12). Thus, we propose that ubenimex
triggers mixed PCD in bladder cancer. In this study, we determine
the effects of ubenimex on bladder cancer cells and the potential
underlying mechanisms.
Materials and methods
cell culture
The RT112 and 5637 cell lines were purchased from
the Cell Bank of the Chinese Academy of Sciences. Cells were
maintained in RPMI-1640 medium (BioInd, Kibbutz Beit Haemek,
Israel) supplemented with 1% penicillin, streptomycin and 10% fetal
bovine serum (FBS; BioInd). The cells were incubated at 37°C in a
humidified atmosphere with 5% CO2.
CCK-8 cytotoxicity assay
RT112 and 5637 cells in an exponential phase of
growth were harvested and seeded into 96-well plates at a density
of 15,000 cells/well (RT112) and 10,000 cells/well (5637) in
RPMI-1640 medium supplemented with different concentrations of
ubenimex or/and 3MA (control, 0.1 mg/ml, 0.25 mg/ml, 0.5 mg/ml, 1
mg/ml, 1 mg/ml+3MA). Another treatment groups were divided into
four parts (control, 0.5 mg/ml UB, Akt stimulator, 0.5 mg/ml UB+Akt
stimulator (SC-79, melonepharma, Dalian, China). After 16, 24, 36
and 48 h of culture, 10 µl CCK-8 solution (CCK-8 cytotoxicity assay
kit; EnoGene Biotech, Co., Ltd., Nanjing, China) was added into
each well. The plates were then incubated for an additional 4 h at
37°C, and the absorbance was determined using a microplate reader
(EL340; Bio-Tek Instruments, Hopkinton, MA, USA) at 450 nm.
Wound healing migration assays
5637 cells were plated in 6-well culture plates and
grown to 90% confluency. Next, a sterile P200 pipette tip was used
to create a scratch across the monolayer. Cell debris was removed
by washing with phosphate-buffered saline (PBS), and the cells were
cultured in RPMI-1640 medium without FBS supplemented with
different concentrations of ubenimex. The area of the scratch was
measured at 16 and 24 h. Quantification was performed by measuring
the area of cell migration at different time points compared to the
scratched area at 0 h.
Matrigel invasion assay and matrigel
migration assay
Invasion assays were performed using transwell
chambers and 5637 cells were used. Control untreated cells or cells
treated with ubenimex (0.5 or 1 mg/ml for 16 h) were trypsinized,
and 1×105 cells were plated in the upper wells in
serum-free medium, while medium with 10% FBS was added to the lower
well as a stimulus. After 20 h of incubation, the cells on the
Matrigel side of the chambers were removed using a cotton swab. The
inserts were fixed in methanol and stained using hematoxylin and
eosin (H&E) staining. The number of invading cells attached to
the other side of the inserts was quantified under a light
microscope using 6 random fields at a magnification of ×200. The
experiment was performed in triplicate. 5637 was chosen to form the
invasion assay. In addition, invasion assays were performed using
transwell chambers that were pre-coated with 40 µl of 1 mg/ml
Matrigel matrix (BD Biosciences, Franklin Lakes, NJ, USA, USA). We
controlled each group contains 2.5×105 cells for plated
in the upper wells, the remaining steps followed as Matrigel
migration assay.
Acridine orange (AO)/ethidium bromide
(EB) double staining
Cells were cultured in 6-well plates for 24 h, and
were treated with different doses of ubenimex (0, 0.5 and 1 mg/ml)
for 16 h. After the indicated treatment times, AO/EB operating
fluid was mixed reagent A, reagent B, and reagent C at a certain
rate of 1:1:8. Each sample was added 2 ul AO/EB operating fluid,
discarded the supernatant after low speed centrifugal (500 g/min),
resuspend cells in AO/EB dilution buffer at a concentration of
5×106 cells/ml, then add 1 ul AO/EB operating fluid to
25 ul cell suspension. Next, the cells were observed under a
fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Western blot analysis
To determine APN, LC-3, Beclin 1, Akt/P-Akt, P62,
C-caspase-3/P-caspase-3 levels, proteins were extracted from the
cells by suspension in radioimmunoprecipitation assay (RIPA)
buffer. Samples were centrifuged at 12,000 rpm at 4°C for 30 min,
and the supernatants were recovered for analysis. The protein
concentrations were determined using the Bradford protein method
and the bicinchoninic acid (BCA) protein assay kit (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Protein (40 µg) was
electrophoresed on a pre-cast bis-Tris polyacrylamide gel (12%),
and then transferred onto a polyvinylidene difluoride (PVDF)
membrane. Membranes were blotted with rabbit anti-APN (1:1,000),
rabbit anti-LC3B (1:500), rabbit anti-beclin 1 (1:1,000), rabbit
anti-Akt (1:1,000), rabbit anti-p-Akt (1:1,000), rabbit anti-P62
(1:1,000), rabbit anti-Caspase-3 (1:1,000) (all from Abcam,
Cambridge, MA, USA) and mouse anti-actin (1:1,000; ProteinTech
Group, Inc., Chicago, IL, USA), followed by horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:5,000; ZsBio, Beijing,
China). Immunoblots were visualized using enhanced
chemiluminescence (LAS-4000).
Cell apoptosis analysis by flow
cytometer
Apoptosis was measured by Annexin V-FITC/PI dual
staining. Cells were cultured in a petri dish for 36 h, and then
were treated with different doses of ubenimex (0, 0.5 and 1 mg/ml)
for 16 h. After the indicated treatment times (16 h), the cells in
an exponential phase of growth were harvested. We divided RT112 and
5637 cells into control group, PE group, 7-AAD group and treatment
group. Then wash cells twice with cold PBS and resuspend cells in
1X Binding buffer at a concentration of 1×106 cells/ml,
transfer 100 µl of the solution (1×105 cells) to a 5 ml
culture tube. Add 5 µl of PE Annexin V and 5 µl 7-AAD. Gently
vortex the cells and incubate for 15 min at RT (25°C) in the dark.
Add 400 µl of 1X Binding buffer to each tube. Finally, the samples
were analyzed by flow cytometry within 1 h on a FACSCalibur (BD
Biosciences, Franklin Lakes, NJ, USA).
Trypan blue staining
The cells were cultured in six-well plates for 24 h,
which is divided into four groups (control, 0.5 mg/ml UB, 0.5 mg/ml
UB+3MA, 0.5 mg/ml UB+Akt stimulator). The groups were treated for
24 h. After the indicated treatment times, cell suspension and 0.4%
trypan blue solution mixed with 9:1. In three min, with the
counting plate, live cells and dead cells were counted. Cell
vitality were measured under the following formula: Living cell
rate (%) = living cell total/(total number of living cells + dead
cells) × 100%.
Statistical analysis
The data were statistically analyzed with Student's
t-test, χ2 or Fisher's exact tests using SPSS version
19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Ubenimex inhibits APN expression in
bladder cancer cells
We investigated the expression of APN/CD13 and the
involvement of the Akt signaling pathway in bladder cancer cells
following treatment with ubenimex by western blot analysis
(Fig. 1). Both RT112 and 5637
cells were treated with ubenimex for 16 h. Inhibition of APN was
significant and correlated with ubenimex dosage. Thus, we concluded
that ubenimex functions as an APN inhibitor in bladder cancer
cells. Therefore, the findings underline that ubenimex functions as
an APN inhibitor in bladder cancer cells.
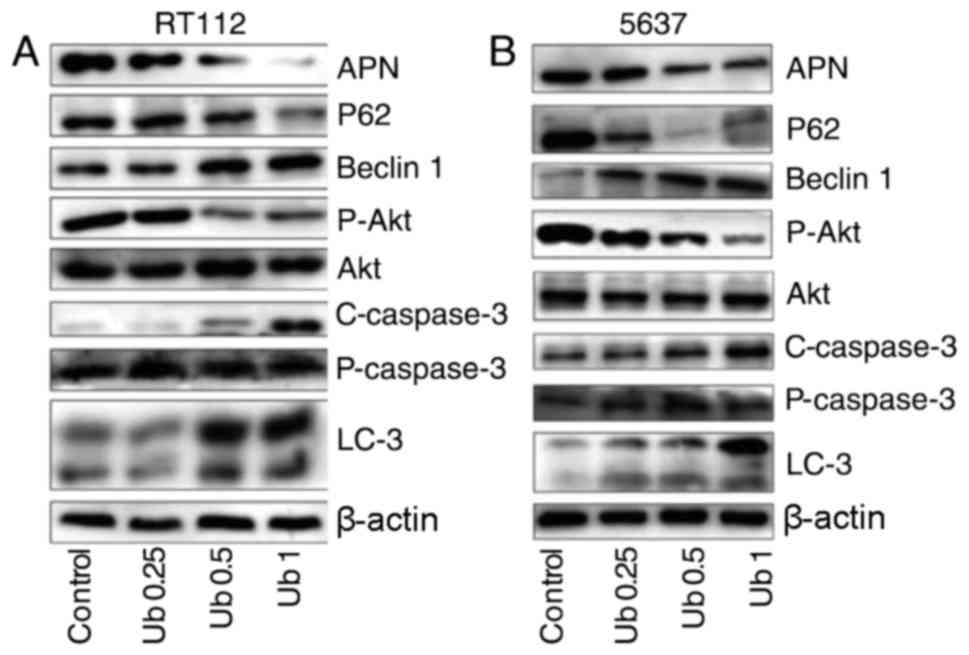 | Figure 1.Ubenimex promotes cell death and
inhibits the expression of APN and Akt in bladder cancer cells. (A
and B) Western blotting showing the expression of APN, LC-3, Beclin
1, P62, P-Akt, Akt, C-caspase-3, and P-caspase-3 in RT112 and 5637
cells after treatment for 16 h with ubenimex (0.25, 0.5 or 1
mg/ml). β-actin is shown as a loading control. Levels of LC-3,
Beclin 1 and P62 changed in a dose-dependent manner, with a trend
mirroring that of autophagy levels. Western blotting of P-Akt and
Akt expression in RT112 and 5637 cells after treatment for 16 h
with ubenimex (0.25, 0.5 or 1 mg/ml). Data are expressed as the
mean ± standard deviation of 3 independent experiments.
C-caspase-3, cleaved-caspase-3; P-caspase-3, pro-caspase-3. |
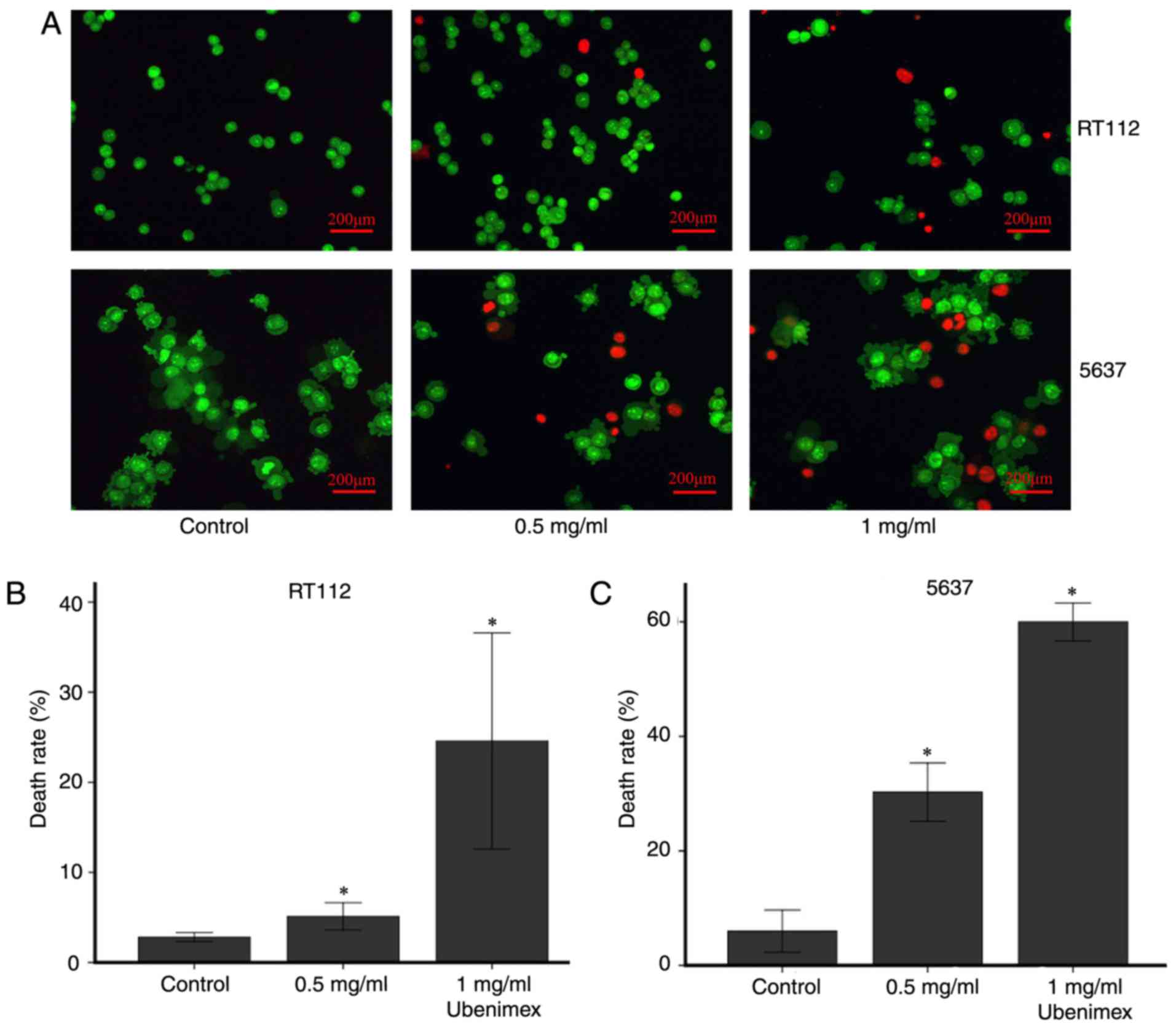
Ubenimex induces autophagic cell death
in bladder cancer cells
AO-EB staining and western blot analyses were used
to examine the level of autophagy in bladder cancer cells.
According to the results of a CCK8 cytotoxicity assay, we used
cells treated for 16 h with ubenimex for study. In addition, we
observed that ubenimex increased the expression of LC3B and Beclin
1 and decreased the expression of P62 in both RT112 and 5637 cells
(Fig. 1). This finding indicates
that a higher level of autophagic cell death was induced by higher
doses of drug. Moreover, AO-EB staining can be a reflection of the
extent of DNA damage, which indicates autophagic cell death
(13). AO staining levels vary
depending on ubenimex dosage in the bladder cancer cells: Staining
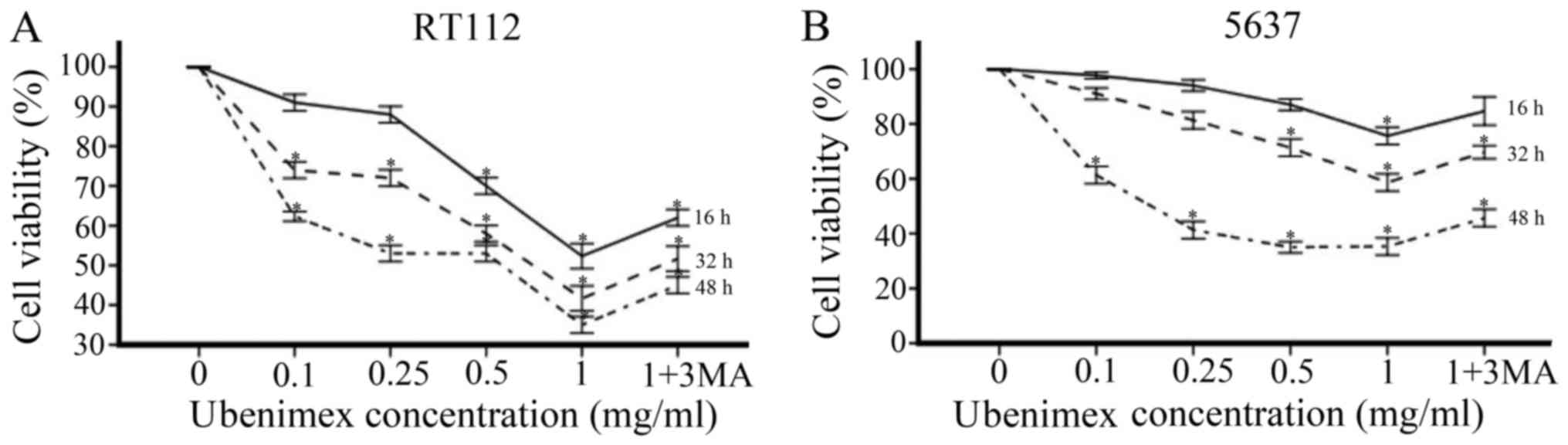
levels increased with the dose of ubenimex (Fig. 2). In addition, combined treatment
of ubenimex and 3-methyladenine (an inhibitor of autophagy)
increased viability compared with ubenimex treatment alone
(Fig. 3A and B). Therefore, the
treatment of ubenimex was shown to induce autophagic cell death in
bladder cancer cells.
Ubenimex inhibits the proliferation of
bladder cancer cell lines
To assess the effects of ubenimex on the
proliferation of bladder cancer cells, we used the CCK8 cell
proliferation assay and measured viability after 16, 32, and 48 h
(Fig. 3A and B). RT112 and 5637
cells were treated with ubenimex as mentioned previously. Both
bladder cancer cell lines demonstrated a prominent, dose-dependent
decrease in cell viability with ubenimex treatment, though the
effect was more obvious in the RT112 cell line. In summary,
proliferation of bladder cancer cell lines was markedly decreased
after treatment with ubenimex.
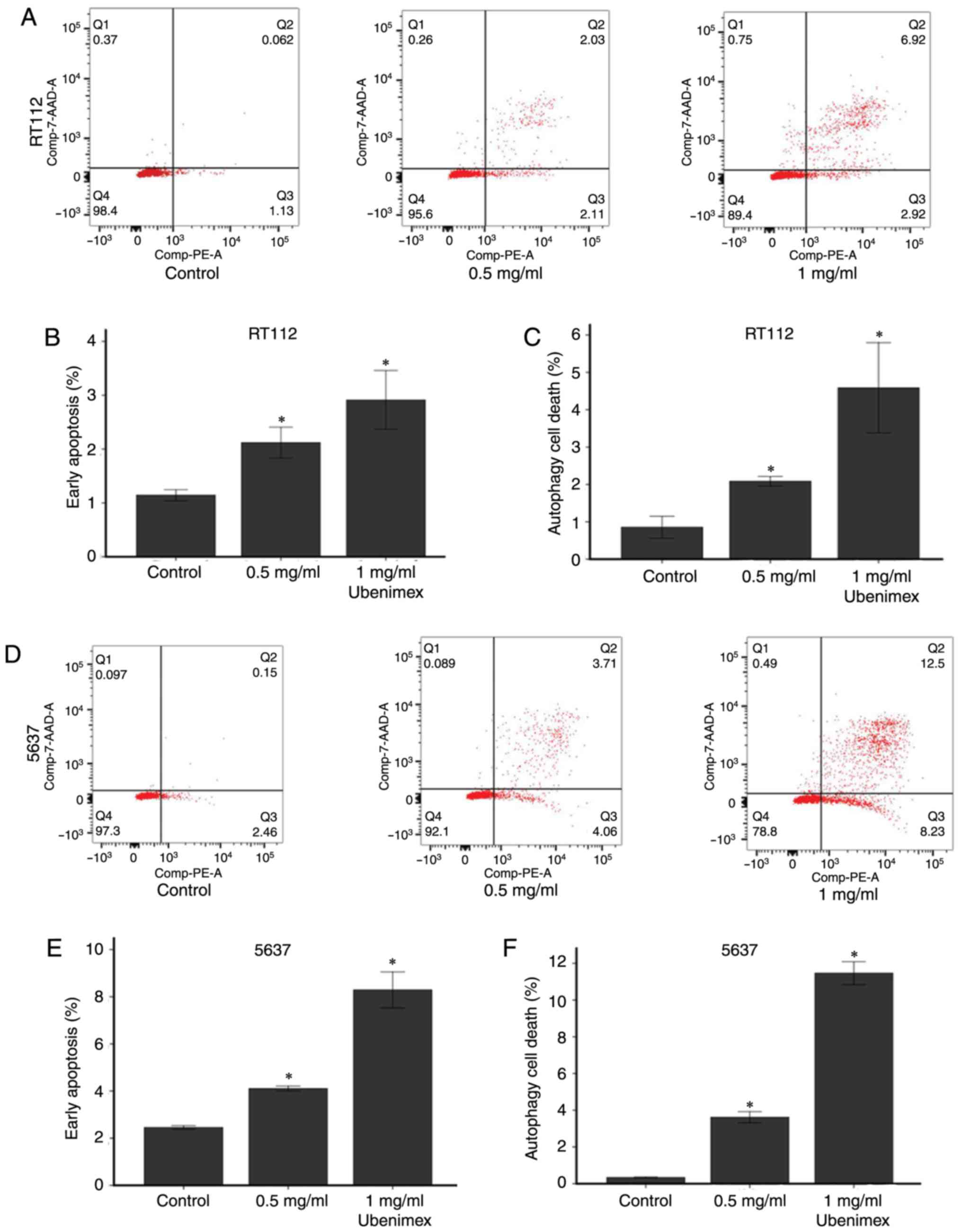
Ubenimex induces apoptosis in bladder
cancer cells
Western blot analyses and flow cytometry were used
to examine apoptosis levels in the bladder cancer cell lines.
Ubenimex induced apoptosis in a significantly higher percentage of
treated cells compared to the control groups. For further
experiments, we treated bladder cancer cells with ubenimex for a 16
h period. Next, we demonstrated that ubenimex enhanced the
expression of caspase-3 in bladder cancer cells. Flow cytometry was
used to assess the proportion of cells undergoing early apoptosis
and autophagy in RT112 and 5637 cells with the treatment of
ubenimex (Fig. 4). The
quantitative results showed that ubenimex significantly induced
early apoptosis and autophagic cell death in a dose-dependent
manner in RT112 and 5637 cells. It also had shown a steady
increasing trend with the increased dose of ubenimex.
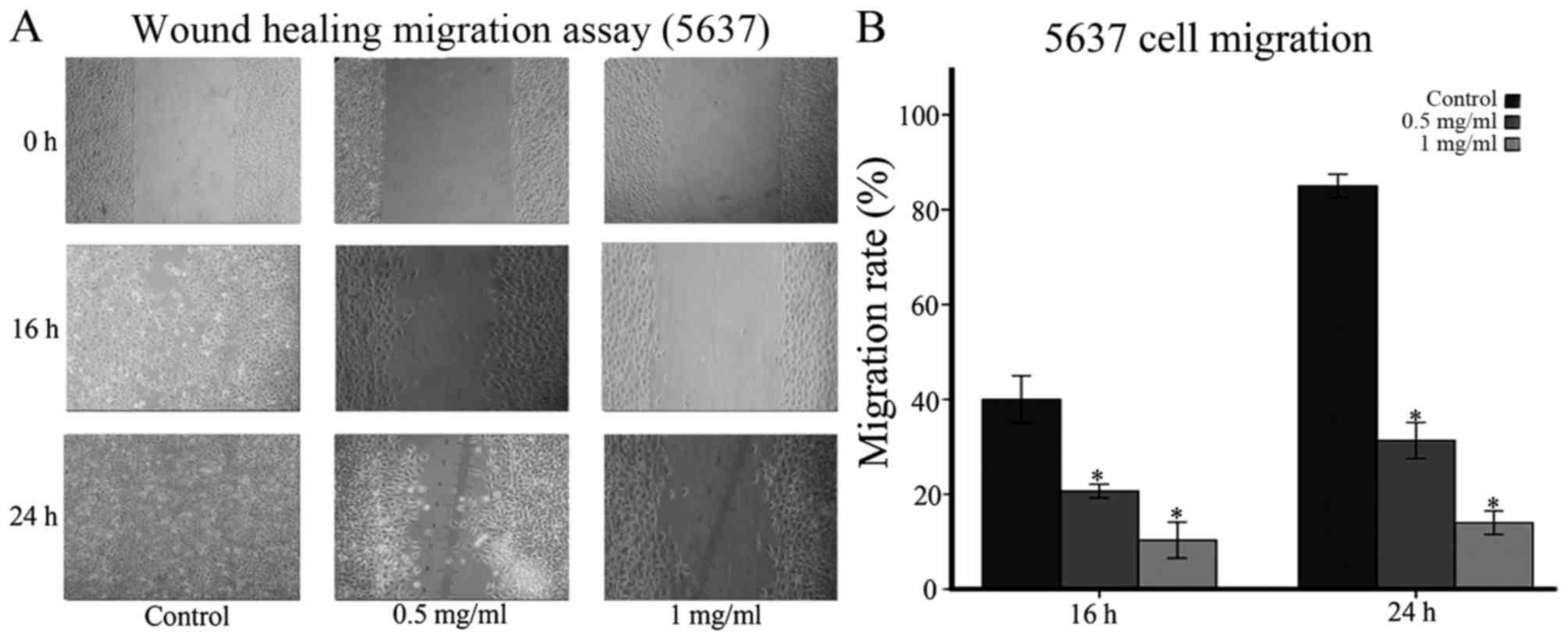
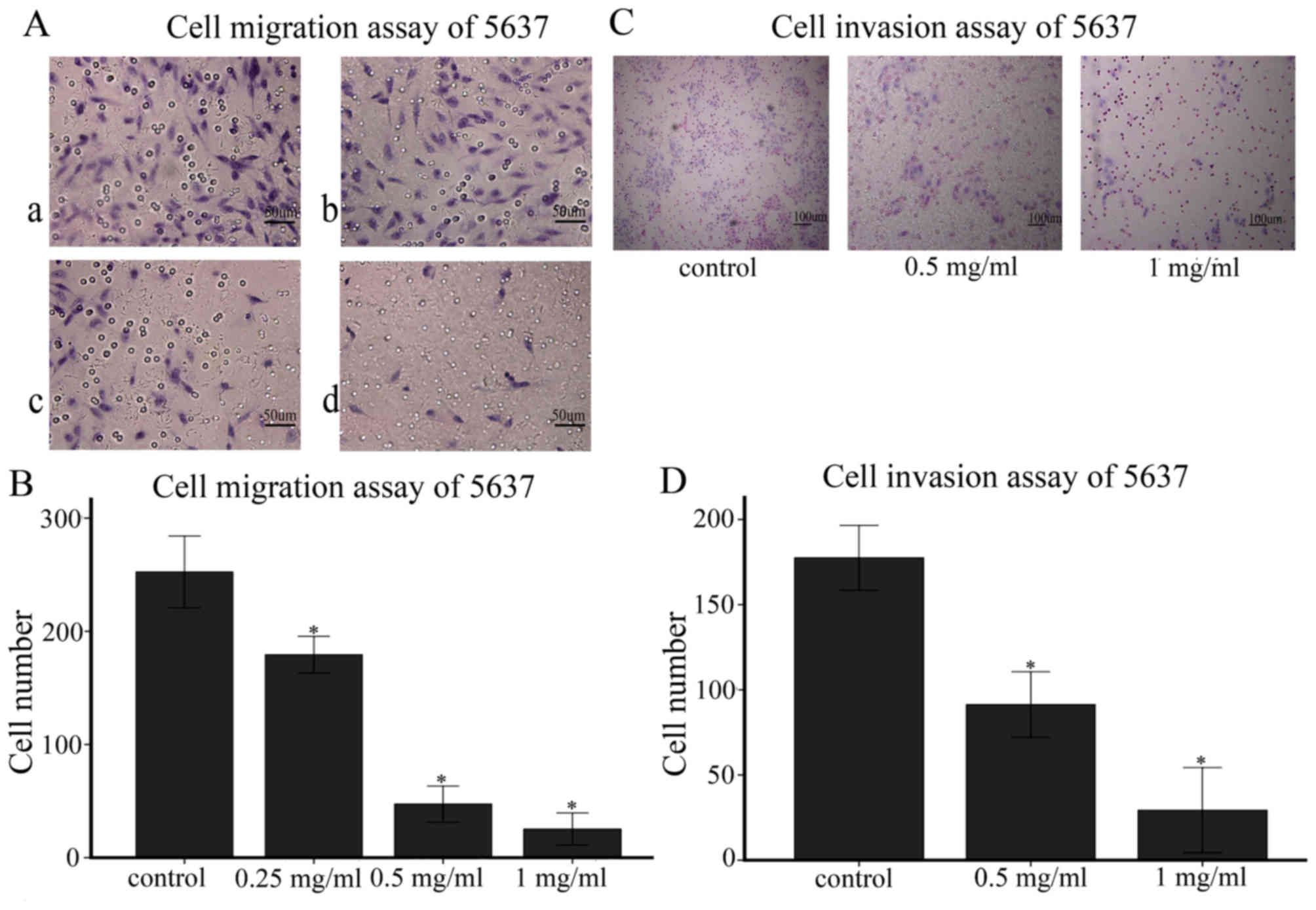
Ubenimex inhibits migration and
invasion in bladder cancer cell lines
As indicated in Figs.
5 and 6, the migration and
invasion capacity of 5637 cells using wound-healing migration and
transwell assays were assessed, respectively. We expected that the
decreased migratory capacity of the 5637 cells was due to ubenimex
treatment after 16 or more h of exposure (Figs. 5A and 6A). Obviously, the increased dose of
ubenimex also plays a key role in inhibiting the migration ability
of bladder cancer cells. Furthermore, we investigated the effect of
ubenimex on the invasive activity of 5637 cells using a transwell
cell-culture chamber coated with Matrigel. As shown in (Fig. 5C), the invasion ability of 5637
cells was strongly suppressed by ubenimex. Thus, we demonstrated
that ubenimex treatment suppressed the migration and invasion of
bladder cancer cells.
Ubenimex treatment effects involve the
Akt signaling pathway
Previous studies have shown that the Akt signaling
pathway is involved in the regulation of autophagy and apoptosis
(12). Stress is capable of
activating the Akt signal transduction pathway in tumor cells,
stimulating cell-protective mechanisms (14,15).
As mentioned above, to evaluate the involvement of the Akt
signaling pathway in the effects of ubenimex treatment, we
performed a western blotting to detect the expression of protein
phosphorylation. High doses of ubenimex treatment decreased the
phosphorylation of Akt compared with the control group. In order to
assess the involvement of Akt, we introduced Akt stimulator into
treated group to assess the changing trend of Akt (Fig. 7). Both trypan blue staining and
CCK-8 cytotoxicity assay were used to estimate the rate of cell
death or cell viability after the introduction of Akt-stimulator.
Upon Akt activation, cell death decreased and a distinct cell
viability change was noticed. (Fig.
7A, B and D). We speculated that ubenimex plays a role as an
Akt agonist. These findings suggest that ubenimex strongly induces
both apoptotic and autophagic cell death.
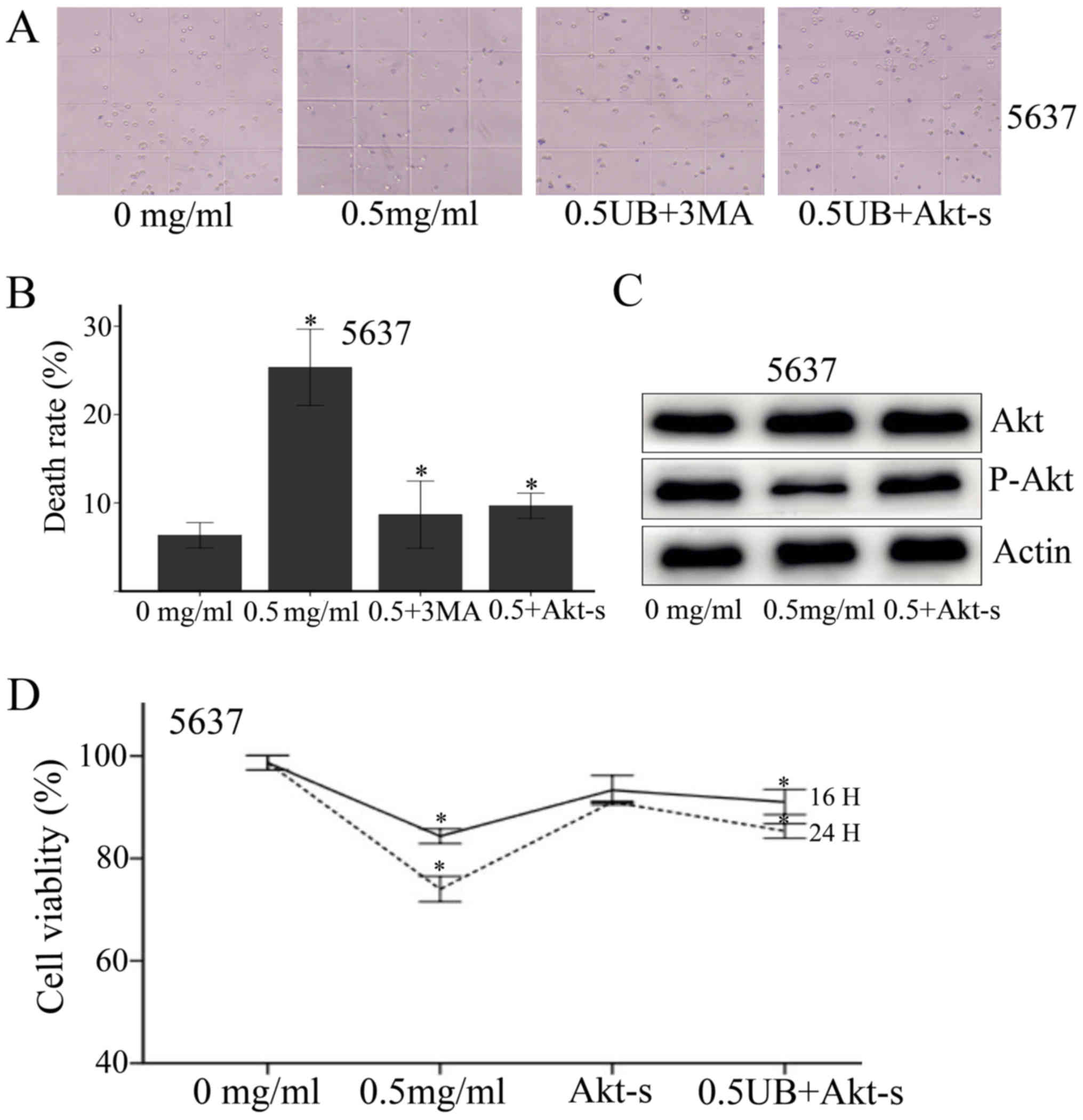 | Figure 7.Trypan blue staining was performed to
determine the role of Akt stimulator. (A) The cells are divided
into four groups (control, 0.5 mg/ml UB, 0.5 UB+3MA, 0.5 UB+10
ug/ml Akt stimulator). (B) Ubenimex promoted cell death as
expected. Furthermore, 3MA and Akt stimulator reversed the toxic
effects of ubenimex, significantly increasing the cell survival
rate above that of the ubenimex treatment group. *P<0.05, for
cells treated with 0.5 UB+Akt stimulator and 0.5 UB+3MA vs. 0.5
mg/ml ubenimex treatment alone. CCK-8 cytotoxicity assay was
performed to determine the role of Akt stimulator. The cells are
divided into four groups (control, 0.5 mg/ml UB, Akt stimulator,
0.5 UB+Akt stimulator). After 16 h and 24 h culture of 5637 cells
with the indicated concentrations of ubenimex and Akt stimulator
(10 ug/ml), (D) CCK-8 cytotoxicity assays revealed that cell
viability increased with Akt stimulator treatment compared with the
0.5 mg/ml ubenimex treatment group. (C) Western blotting confirmed
this result. *P<0.05, for cells treated with 0.5 mg/ml UB+Akt
stimulator vs. 0.5 mg/ml ubenimex treatment alone. Data are
expressed as the mean ± standard deviation of 3 independent
experiments. UB, ubenimex; 3MA, 3-Methyladenine; Akt-s, Akt
stimulator. |
Discussion
In our previous and current studies, we found
ubenimex is sufficient to induce prostate cancer cells and bladder
cancer cells death, and at the same time the level of autophagy
increased. With the help of literature review, we found that
ubenimex have suppressive effect on proliferation of leukemic cell
line (16). The process contained
the induction of apoptosis by ubenimex (17). Ubenimex also could inhibit cell
proliferation, migration and invasion in renal cell carcinoma,
which of the effect is autophagy-associated (18). Furthermore, ubenimex
synergistically enhances the effects of anticancer drugs in
hepatocellular carcinoma, osteosarcoma cell lines and human
tumor-derived cell lines (19–21).
Then we assumed that ubenimex may have a general effect on
different cancer types.
Based on the decrease in APN expression, ubenimex
could be a potential treatment for the suppression of metastasis
and invasion in bladder cancer cells. APN expression has been
confirmed to be involved in various cellular processes, including
cellular attachment, motility and invasion/metastasis of various
malignancies (22). The inhibition
of APN expression results in a significant decrease in the
metastasis of T24 cell lines (4).
High APN expression has been considered an indicator of metastasis
and poor prognosis in bladder cancer, as well as in lung, pancreas
and colon cancers (5,23,24).
Moreover, a previous study revealed that ubenimex could inhibit
metastasis and invasion of PC-3 cells and that this effect was
related to the inhibition of APN activity (9). Thus, we analyzed ubenimex-treated
RT112 and 5637 cells by western blotting and found that the
expression of APN was significantly decreased compared to the
control group. Furthermore, we showed by Matrigel invasion,
Matrigel migration and wound-healing migration assays that the
migratory and invasion capacity of 5637 cells was decreased with
ubenimex treatment. Thus, we conclude that ubenimex functions as an
APN inhibitor to inhibit metastasis and invasion in bladder cancer
cells. In several cancer cell lines, the mechanism of overexpress
or knock-down APN has been explored in multimolecular process
regulating cell migration (25,26).
To a certain extent, these prove that there exist relations between
reduced expression of APN and decreased migration/invasion.
However, in bladder cancer cells, this paper didn't go deep enough
on it, and this part will be gradually improved in our future
research.
Autophagy is a dynamic recycling system which relies
on lysosomal proteolysis to maintain cellular homeostasis. However,
excessive autophagy can lead to cell death (27). Autophagy directly takes part in
many types of physiological processes, including metabolism, stress
responses, and cell death pathways in cancer cells (28). However, the roles of autophagy in
bladder cancer need to be further clarified. In our previous study,
we showed that autophagic cell death in prostate cells could be
induced by ubenimex (9). In the
current study, we demonstrate similar effects in bladder cancer
cells. This is the first attempt to study effects of ubenimex
treatment on the regulation of autophagy in bladder cancer cells.
In our study, we used western blotting to examine the level of
autophagy in this context, while AO/EB staining was used to show
the amount of cell death related to the doses of ubenimex. Positive
results indicated that ubenimex induces autophagic cell death in
bladder cancer cells and that this induction occurred in a
dose-dependent manner. Furthermore, as both the CCK-8 cell
cytotoxicity assay and AO/EB staining demonstrated a correlation
between cell death and dosage, we considered that autophagic cell
death may participate in the cell death program. As it turned out,
3MA (3-Methyladenine, an inhibitor of autophagy) partially reversed
the beneficial effects induced by ubenimex: Cell death was
significantly reduced when compared to the ubenimex only treatment
group. Thus, we maintain that ubenimex induces autophagic cell
death in bladder cancer cells.
Apoptosis plays an extensively investigated role
whereby cells undergo a caspase-dependent ‘self-killing’ mechanism.
The activation of catabolic enzymes leads to rapid demolition of
cellular structures and organelles, eliminating damaged or aged
cells and organelles (10). In our
study, the apoptosis of bladder cancer cells was assessed by
Annexin V-PE/7AAD staining and western blotting. Analysis of flow
cytometric results showed that apoptosis of RT112 and 5637 cells
increased with ubenimex treatment. Western blotting also
demonstrated that caspase-3 was increased in this context. Thus, we
detected a mixed phenotype of apoptosis and autophagic cell death
in response to ubenimex treatment in bladder cancer cells. However,
further study is necessary to elucidate the mechanisms of apoptosis
and autophagy activation in bladder cancer. A literature review
showed many examples of a complicated relationship between
apoptosis and autophagy (10).
The Akt signaling pathway plays a crucial role in
the regulation of both apoptosis and autophagy, involving many
growth factors and receptors (29,30).
The activation of the Akt pathway acts as a survival signal,
preventing cells from undergoing apoptosis (31). In oral cancer and colorectal
cancer, the induction of autophagy and apoptosis is associated with
the disruption of Akt signaling (32,33).
Previous literature indicates that some signal transduction
pathways triggered by common cellular stresses can trigger both
autophagic cell death and apoptosis (10). In our present study, the addition
of ubenimex significantly induced apoptotic and autophagic cell
death accompanied by a decrease in Akt expression. It was
demonstrated that ubenimex might activate mixed PCD concurrent with
the inhibition of the Akt signaling pathway.
We expect that additional clinical insights may be
obtained from bladder cancer cells. They are valuable in the study
of early postoperative bladder irrigation after treatment of a
partial cystectomy as well as transurethral resection in
early-stage bladder cancer. Experiments in vitro may be a
fairly good approximation of postoperative bladder irrigation
because bladder cancer cells and drugs make reciprocal contacts in
this environment. Combined infusions of ubenimex and BCG or
mitomycin may be more practical in the clinic. Ubenimex has already
been used in medical treatments, but its role as an adjuvant
therapy for the treatment of bladder cancer has not yet been
studied.
Overall, the present study demonstrated that high
expression of APN plays a key role in the cell proliferation,
migration and invasion of bladder cancer cells. Further, we
demonstrated that ubenimex could be an excellent inhibitor of APN.
The enhancement of autophagy and apoptosis is associated with the
inhibition of the Akt signaling pathway. In conclusion, ubenimex
could be a desirable adjuvant therapy for the treatment of bladder
cancer.
Acknowledgements
The present study was funded by the National Natural
Science Foundation (nos. 81400575 and 81602227), the Science and
Technology Development Plan Project of Shandong Province (nos.
2014GSF118144 and 2015GSF118055), the Shandong Provincial Natural
Science Foundation (no. ZR2017MH091), the source of our financial
support.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abufaraj M, Shariat SF, Haitel A, Moschini
M, Foerster B, Chłosta P, Gust K, Babjuk M, Briganti A, Karakiewicz
PI and Albrecht W: Prognostic role of N-cadherin expression in
patients with non-muscle-invasive bladder cancer. Urol Oncol.
35:264–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CK, Chen Z, Fang J, Xu A, Zhang W and
Wang Z: H19-derived miR-675 contributes to bladder cancer cell
proliferation by regulating p53 activation. Tumor Biol. 37:263–270.
2016. View Article : Google Scholar
|
|
4
|
Liu J, Xu R and Zhao X: Mechanisms for
effect of osthole on inhibiting the growth and invasion of bladder
cancer cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 41:345–352.
2016.(In Chinese). PubMed/NCBI
|
|
5
|
Hashida H, Takabayashi A, Kanai M, Adachi
M, Kondo K, Kohno N, Yamaoka Y and Miyake M: Aminopeptidase N is
involved in cell motility and angiogenesis: Its clinical
significance in human colon cancer. Gastroenterology. 122:376–386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mina-Osorio P: The moonlighting enzyme
CD13: Old and new functions to target. Trends Mol Med. 14:361–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishii K, Usui S, Sugimura Y, Yoshida S,
Hioki T, Tatematsu M, Yamamoto H and Hirano K: Aminopeptidase N
regulated by zinc in human prostate participates in tumor cell
invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fontijn D, Duyndam MC, van Berkel MP,
Yuana Y, Shapiro LH, Pinedo HM, Broxterman HJ and Boven E:
CD13/aminopeptidase N overexpression by basic fibroblast growth
factor mediates enhanced invasiveness of 1F6 human melanoma cells.
Br J Cancer. 94:1627–1636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Niu Z, Jia Y, Cui M, Han L, Zhang
Y, Liu Z, Bi D and Liu S: Ubenimex inhibits cell proliferation,
migration and invasion by inhibiting the expression of APN and
inducing autophagic cell death in prostate cancer cells. Oncol Rep.
35:2121–2130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horita H, Frankel AE and Thorburn A: Acute
myeloid leukemia-targeted toxin activates both apoptotic and
necroptotic death mechanisms. PLoS One. 3:e39092008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan CH, Horng CT, Lee CF, Chiang NN, Tsai
FJ, Lu CC, Chiang JH, Hsu YM, Yang JS and Chen FA: Epigallocatechin
gallate sensitizes cisplatin-resistant oral cancer CAR cell
apoptosis and autophagy through stimulating AKT/STAT3 pathway and
suppressing multidrug resistance 1 signaling. Environ Toxicol.
32:845–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mujtaba SF, Dwivedi A, Yadav N, Ch R,
Kushwaha HN, Mudiam MK, Singh G and Ray RS: Superoxide mediated
photomodification and DNA damage induced apoptosis by
Benz(a)anthracene via mitochondrial mediated pathway. J Photochem
Photobiol B. 142:92–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu
B, Pan S, Dong X, Tan G, Wei Z, et al: Inhibition of Akt reverses
the acquired resistance to sorafenib by switching protective
autophagy to autophagic cell death in hepatocellular carcinoma. Mol
Cancer Ther. 13:1589–1598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan J, Liu T, Mei L, Li J, Gong K, Yu C
and Li W: Synergistic antitumour activity of sorafenib in
combination with tetrandrine is mediated by reactive oxygen species
(ROS)/Akt signaling. Br J Cancer. 109:342–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsukamoto H, Shibata K, Kajiyama H,
Terauchi M, Nawa A and Kikkawa F: Aminopeptidase N (APN)/CD13
inhibitor, Ubenimex, enhances radiation sensitivity in human
cervical cancer. BMC Cancer. 8:742008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sekine K, Fujii H and Abe F: Induction of
apoptosis by bestatin (ubenimex) in human leukemic cell lines.
Leukemia. 13:729–734. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Xie F, Wang H, Liu Z, Liu X, Sun L
and Niu Z: Ubenimex inhibits cell proliferation, migration and
invasion in renal cell carcinoma: The effect is
autophagy-associated. Oncol Rep. 33:1372–1380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang W, Gao B, Xu G, Weng D, Xie M and
Qian Y: Possible contribution of aminopeptidase N (APN/CD13) to
migration and invasion of human osteosarcoma cell lines. Int J
Oncol. 45:2475–2485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Wang X, Hou J, Huang Y, Zhang Y and
Xu W: Enhanced anticancer activity of 5-FU in combination with
Bestatin: Evidence in human tumor-derived cell lines and an H22
tumor-bearing mouse. Drug Discov Ther. 9:45–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamashita M, Wada H, Eguchi H, Ogawa H,
Yamada D, Noda T, Asaoka T, Kawamoto K, Gotoh K, Umeshita K, et al:
A CD13 inhibitor, ubenimex, synergistically enhances the effects of
anticancer drugs in hepatocellular carcinoma. Int J Oncol.
49:89–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nohara S, Kato K, Fujiwara D, Sakuragi N,
Yanagihara K, Iwanuma Y and Kajiyama Y: Aminopeptidase N (APN/CD13)
as a target molecule for scirrhous gastric cancer. Clin Res Hepatol
Gastroenterol. 40:494–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tokuhara T, Hattori N, Ishida H, Hirai T,
Higashiyama M, Kodama K and Miyake M: Clinical significance of
aminopeptidase N in non-small cell lung cancer. Clin Cancer Res.
12:3971–3978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pang L, Zhang N, Xia Y, Wang D, Wang G and
Meng X: Serum APN/CD13 as a novel diagnostic and prognostic
biomarker of pancreatic cancer. Oncotarget. 7:77854–77864. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kehlen A, Lendeckel U, Dralle H, Langner J
and Hoang-Vu C: Biological significance of aminopeptidase N/CD13 in
thyroid carcinomas. Cancer Res. 63:8500–8506. 2003.PubMed/NCBI
|
|
26
|
Wang SN, Yang SF, Tsai HH, Lee KT and Yeh
YT: Increased adiponectin associated with poor survival in
hepatocellular carcinoma. J Gastroenterol. 49:1342–1351. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang ZJ, Chee CE, Huang S and Sinicrope F:
Autophagy modulation for cancer therapy. Cancer Biol Ther.
11:169–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa
T, Aoki H, Mills GB and Kondo S: Synergistic augmentation of
rapamycin-induced autophagy in malignant glioma cells by
phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer
Res. 65:3336–3346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, Wu J, Shi H, Wang Z, Zhang G, Cao
Y, Jiang C and Ding Y: Hepatic stellate cell coculture enables
sorafenib resistance in Huh7 cells through HGF/c-Met/Akt and
Jak2/Stat3 pathways. Biomed Res Int. 2014:7649812014.PubMed/NCBI
|
|
31
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Liu Y, Wang M, Qian Y, Dai X, Zhu
Y, Chen J, Guo S and Hisamitsu T: Celastrus orbiculatus extract
triggers apoptosis and autophagy via PI3K/Akt/mTOR inhibition in
human colorectal cancer cells. Oncol Lett. 12:3771–3778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|