Introduction
The mortality and paralysis rate of spinal cord
injury (SCI) remains remarkably high, but an effective treatment is
still required and SCI pathology is still unclear (1). SCI pathology includes initial injury
and secondary injury of spinal cord neurons. Secondary injury after
SCI is considered to be an important mechanism of SCI progress,
including spinal cord neurons inflammation, apoptosis, death,
demyelination, and axonal degeneration (2). Furthermore, secondary injury is
considered to be a key cause of clinical symptoms of SCI, therefore
treatment and prevention of secondary injury to improve the
prognosis of SCI is of great significance (1). Reactive oxygen species (ROS) and
oxidative stress have been thought to be the main cause for the
secondary injury with the apoptosis and death of spinal cord
neurons, leading to the SCI progression (3). Taken together, alleviation of ROS and
oxidative stress in spinal cord neuron might be an alternative
method to treat the SCI (4). In
this paper, the primary spinal cord neurons were exposed to
H2O2 to mimic the oxidative stress confronted
by the cells after SCI.
Pterostilbene is a natural plant antitoxin that
exists in a variety of plants, including blueberries and grapes
(5). Importantly, pterostilbene is
an analog of resveratrol which has been acknowledged as an
anti-oxidative stress and anti-tumor drug (5). Pterostilbene has a variety of
pharmacological effects, including anticancer, anti-inflammatory
response, anti-oxidative stress and anti-diabetes. Compared with
the resveratrol, pterostilbene has a characteristic of better oral
absorption and metabolic property (5). In the nervous system, pterostilbene
can protect central neuronal cells from the damage caused by
ischemia and hypoxia (6). Oral
pterostilbene can relieve the injury of neuronal cells caused by
ischemia-reperfusion (7); however,
the effect of pterostilbene on spinal neuron cells has not been
reported.
Autophagy, a self-protecting mechanism, maintains
cellular homeostasis by fusing with lysosomes to degrade malformed
proteins and damaged organelles (8,9).
Under stress conditions, moderately activated autophagy is
important for the survival and adaptation of spinal cord neurons
(10). It has been reported that
autophagy could prevent spinal cord neurons from apoptosis induced
by oxidative stress (11);
however, the effect of autophagy on ROS production remains unclear
in spinal cord neuron. Given the role of autophagy in ROS
production in other cells, we hypothesize that autophagy could
inhibit ROS production and apoptosis in spinal cord neuron.
Furthermore, it has been reported that pterostilbene could activate
autophagy in human oral cancer cells and vascular endothelial cells
(12,13); however no related reports have been
reported in spinal cord neurons.
In this study, primary spinal cord neurons were
cultured and treated with pterostilbene, and the effects of
pterostilbene on neuronal autophagy, ROS production and apoptosis,
as well as the involvement of autophagy in the ROS production and
apoptosis, were investigated.
Materials and methods
The animal protocols in this study were approved by
the Animal Care and Use Committee of Affiliated Hospital of Hebei
University of Engineering (Handan, China). The animal experiments
were performed according to Guidance Suggestions for the Care and
Use of Laboratory Animals, which were formulated by the Ministry of
Science and Technology of China.
Reagents and antibodies
Primary antibodies of neuron specific enolase (NSE),
light chain 3 (LC3)b, Beclin-1, p62, ATG5 and β-actin were
purchased from Abcam (Cambridge, MA, USA); 2′,7′-dichlorofluorescin
diacetate (DCFDA) Staining kit, western blotting, secondary
antibody labeled with Alexa Fluor-555,
4′,6-diamidino-2-phenylindole (DAPI) and protein extraction kits
were bought from Beyotime Institute of Biotechnology (Haimen,
China); Cell culture reagents, including Neurobasal-A, fetal bovine
serum (FBS) and 0.25% trypsin, were obtained from Thermo; Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan); MitoSOX Red Staining Kit was
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Culture of primary spinal cord
neurons
Primary spinal cord neurons culture was carried out
according to a published literature (14). Briefly, spinal cord tissues were
isolated from embryos of E16 Sprague-Dawley (SD) rats under a
microscopy, and spinal meninges and dorsal root ganglias were
separated from spinal cords under aseptic conditions. After washing
with PBS three times, the spinal cord was digested with 0.25%
trypsin at 37°C for 30 min. After digestion, cell suspension was
filtered through a 200 mesh sieve. The filtered cells were seeded
on plates with Neurobasal-A medium (Thermo Fisher Scientific,
Inc.), which was added with 2% B-27, 2 mM glutamine, 1 µM
cytarabine and 50 ng/ml nerve growth factor (R&D Systems, Inc.,
Minneapolis, MN, USA).
Experimental design
When analyzing pterostilbene cytotoxicity, spinal
cord neurons were treated with 1, 5, 10, 20, 40, 60 and 80 µM
pterostilbene for 24 and 48 h. When analyzing effects of
pterostilbene on autophagy in normal spinal cord neurons, neurons
were treated with 1, 5, 10 and 20 µM pterostilbene for 24 h. In
order to investigate whether pterostilbene could activate autophagy
and regulate ROS production in the neurons under oxidative stress,
cells were divided into 4 groups: Control, pterostilbene,
H2O2, pterostilbene +
H2O2. 10 µM H2O2 were
added 1 h prior to pterostilbene with 24 h treatment. In order to
investigate the involvement in of autophagy in the regulation of
ROS production, cells were divided into 4 groups: Control,
pterostilbene + H2O2, pterostilbene +
H2O2 + ATG5 siRNA, Control siRNA. In order to
investigate the involvement of autophagy in the regulation of
apoptosis percentage, cells were divided into 6 groups: Control,
H2O2, pterostilbene, pterostilbene +
H2O2, pterostilbene +
H2O2 + ATG5 siRNA, Control siRNA.
Cell immunofluorescence
Primary spinal cord neurons were fixed with 4%
paraformaldehyde in 24-well plates for 10 min and treated with 0.2%
Triton X-100 for 15 min. Then cells were incubated with 5% goat
serum for 30 min and NSE primary antibody (1:100) overnight at 4°C.
After washing, cells were incubated with Alexa Fluor-555-labeled
secondary antibody for 2 h at room temperature and stained with
DAPI (4′,6-diamidino-2-phenylindole) for 5 min. Finally, neurons
were observed under fluorescence microscopy (Olympus Corporation,
Tokyo, Japan).
CCK-8 analysis
A total of 5,000 cells were cultured in each well of
96-wells and treated as the experimental design described. At 24
and 48 h after treatment, the previous medium was replaced with 100
µl fresh medium, and 10 µl CCK-8 reagent was added to each well.
Then, neurons were incubated in a 37°C incubator for 1 h, and OD
value was obtained in a microplate reader at 450 nm. The results
were normalized by the absorbance of control cells.
Western blotting
Total protein was extracted from neurons by using
RIPA contained 1% PMSF. A bicinchoninic acid (BCA) method was used
to determine protein concentration. Then the protein was separated
by SDS-PAGE and transferred to Polyvinylidene Fluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were incubated with 5% milk for 2 h and then incubated
with LC3b, Beclin-1, p62 and ATG5 primary antibody (1:1,000
dilution) overnight at 4°C. After washing three times with TBST,
the membranes were incubated secondary antibody labeled with
horseradish peroxidase at 37°C for 2 h and exposed to an enhanced
chemiluminescence detection system (PerkinElmer, Inc., Waltham, MA,
USA) with ECL plus regent (Thermo Fisher Scientific, Inc.).
Semi-quantitative analysis of protein bands was performed using an
AlphaEaseFC 4.0 software.
Green fluorescent protein (GFP)-LC3
transfection
Spinal cord neurons were cultured on a glass bottom
dish with 2 ml medium overnight. When neurons were approximately
50–70% confluent, cells were transfected with GFP-LC3 adenovirus
with 100 multiplicity of infection (MOI) and 1010/ml
titer. The adenovirus was purchased from Han Heng Biology
(Shanghai, China). The volume and number of virus were 20 µl and
2×108 respectively, which was calculated according to
the 100 MOI and 1–2×106 cells in each six-well plate.
According to manufacturer's protocol, the adenovirus was diluted
with 1 ml serum-free medium, and cells were cultured with the 1 ml
medium and incubated in a 37°C incubator for 2 h. Then, the
serum-free medium was replaced with 2 ml normal medium. After
culture overnight, cells were observed under fluorescence
microscopy to detect transfection efficiency. After treatments
described in the experimental design, autophagosomes were observed
using a laser confocal microscopy (Leica TCS SP8; Leica
Microsystems GmbH, Wetzlar, Germany).
DCFDA staining
DCFDA is a classical method for analyzing total
intracellular ROS levels. Neurons were treated with the
above-mentioned treatments and incubated with 10 µM DCFDA dye in a
dish with glass bottom at 37°C for 20 min. The cells were observed
under confocal microscopy or digested with 0.25% trypsin followed
by semi-quantitative analysis of fluorescence intensity using a
flow cytometer (FACSCaliber; BD Biosciences, Heidelberg, Germany)
at excitation wavelength of 488 nm and emission wavelength of 525
nm.
MitoSOX red staining
MitoSOX Red is a new type staining for detecting
mitochondrial ROS, which emits red fluorescence when oxidized by
mitochondrial superoxide anion (O2−). 50 µg MitoSOX Red
solution was dissolved in 13 µl DMSO to prepare a 5 mM stock
solution. Spinal cord neurons were treated with 5 µM MitoSOX Red
solution at 37°C for 15 min, washed with PBS and replaced with
fresh Neurobasal-A medium. Finally, cells were observed under
confocal microscopy (Leica TCS SP8; Leica Microsystems GmbH).
ATG5 siNRA transfection
ATG5 siRNA sequence was designed based on a previous
reference (19), which was shown
in Table I and synthesized by
Gemma Co., Ltd. (Shanghai, China). Neurons number was counted after
digestion, and 2×105 cells were added to each well in a
6-well plate and cultured overnight. Transfection was performed
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to Invitrogen's protocol. Western
blotting was used to analyze the success of silence. At 48 h after
transfection, cells were treated according to the experimental
design. Non-specific siRNA was used as a control RNA.
 | Table I.Sequence of ATG5 siRNA. |
Table I.
Sequence of ATG5 siRNA.
| Primer | Direction | Sequence 5′-3′ |
|---|
| Atg5 | Sense |
GGCCUUUCAUUCAGAAGCUTT |
|
| Antisense |
AGCUUCUGAAUGAAAGGCCTT |
| Negative | Sense |
UUCUCCGAACGUGUCACGUTT |
| Control | Antisense |
ACGUGACACGUUCGGAGAATT |
TUNEL staining
Spinal cord neurons were fixed with 4%
paraformaldehyde for 30 min and incubated with 0.1% Triton X-100
for 2 min, followed by the incubation with in situ cell
death detection kit (Roche, Hertfordshire, UK) for 60 min. Neurons
treated without the terminal transfer were used as negative
controls; Neurons treated with 2 U/ml DNase were used as positive
controls. Then, cells were stained with DPAI for 5 min and observed
under fluorescence microscopy. Apoptosis percentage was calculated
according to the ratio of green nuclei to blue nuclei in 6 fields
(×200) each group.
Statistical analysis
The differences between different groups were
detected using one-way ANOVA analysis with SPSS15 software (SPSS,
Inc., Chicago, IL, USA). If the difference is statistically
significant, a LSD method was used to analyze the differences
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of primary spinal cord
neurons and the effect of pterostilbene on cell viability
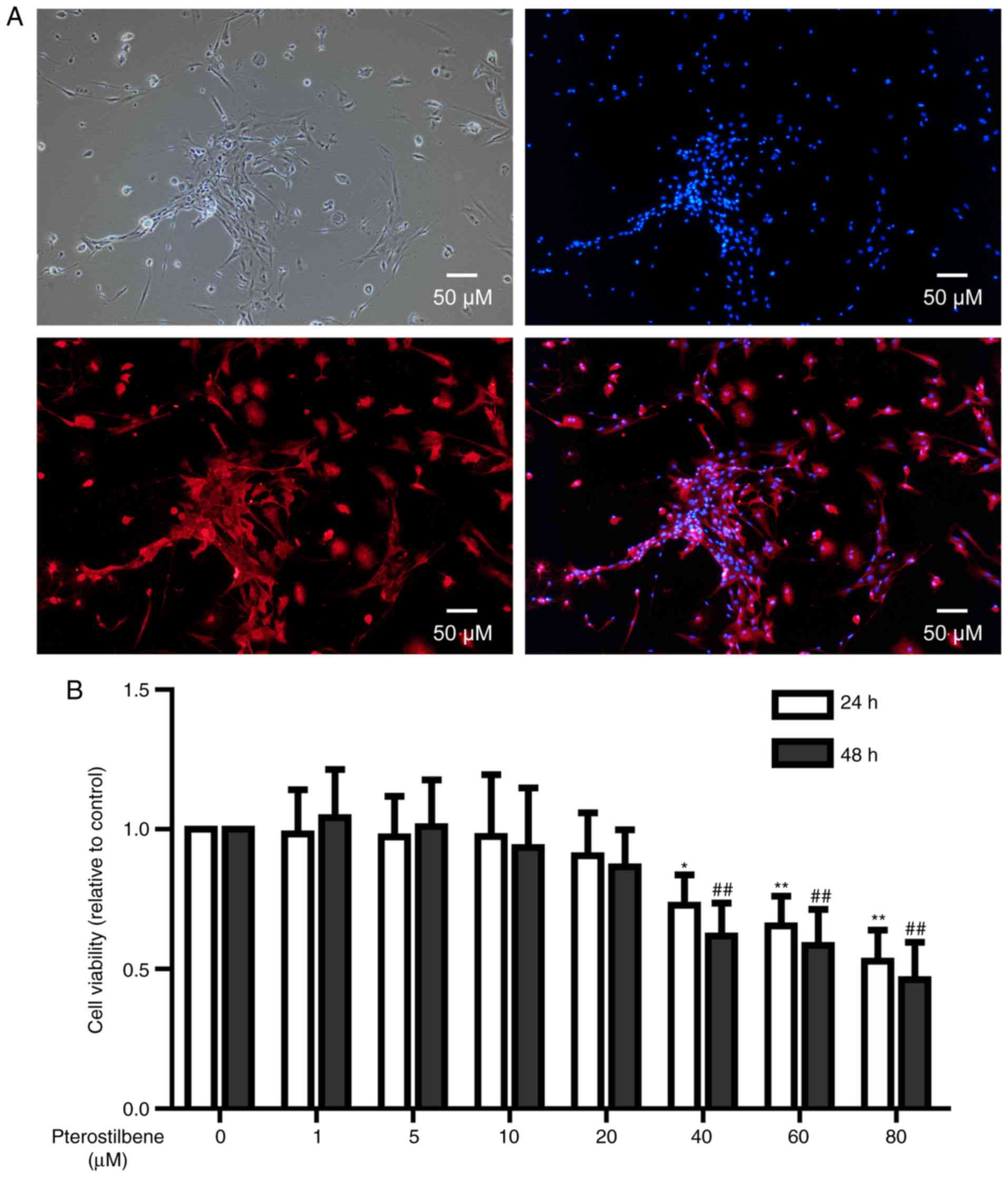
Under a phase-contrast microscopy, primary spinal
cord neurons with long and spindle morphology were observed.
Typical cells had long neurites on both sides. Some neurites showed
bifurcation and intertwined into a network (Fig. 1A). NSE immunofluorescence showed
that neuronal cell cytoplasm was rich in red, demonstrating a high
expression of NSE protein (Fig.
1A).
In order to investigate the cytotoxicity of
pterostilbene on spinal cord neurons, CCK-8 test was used to
analyze the viability of neurons treated with 0–80 µM pterostilbene
for 24 and 48 h. After 24 and 48 h treatment, 40, 60 and 80 µM
pterostilbene significantly inhibited the viability of neuronal
cells (P<0.05, Fig. 1B);
however, 1, 5, 10 and 20 µM pterostilbene had no influences on the
cell viability (Fig. 1B).
Therefore, 20 µM pterostilbene with 24 h treatment was used in the
following experiments.
Pterostilbene activated autophagy and
inhibited mechanistic target of rapamycin (mTOR) pathway in spinal
cord neurons
Western blotting was used to analyze the expression
levels of LC3-II, Beclin-1 and p62 in spinal cord neurons. A
dose-dependent increase in LC3-II/β-actin and Beclin-1/β-actin
expression was observed in pterostilbene-treated cells (P<0.05,
Fig. 2A-C). Because p62 forms
protein aggregate degraded by autophagy, it was reasonable the p62
expression was decreased gradually by the pterostilbene in a
dose-dependent manner, demonstrating a stimulatory role of
pterostilbene on autophagic flux in spinal cord neurons (P<0.05,
Fig. 2A and D).
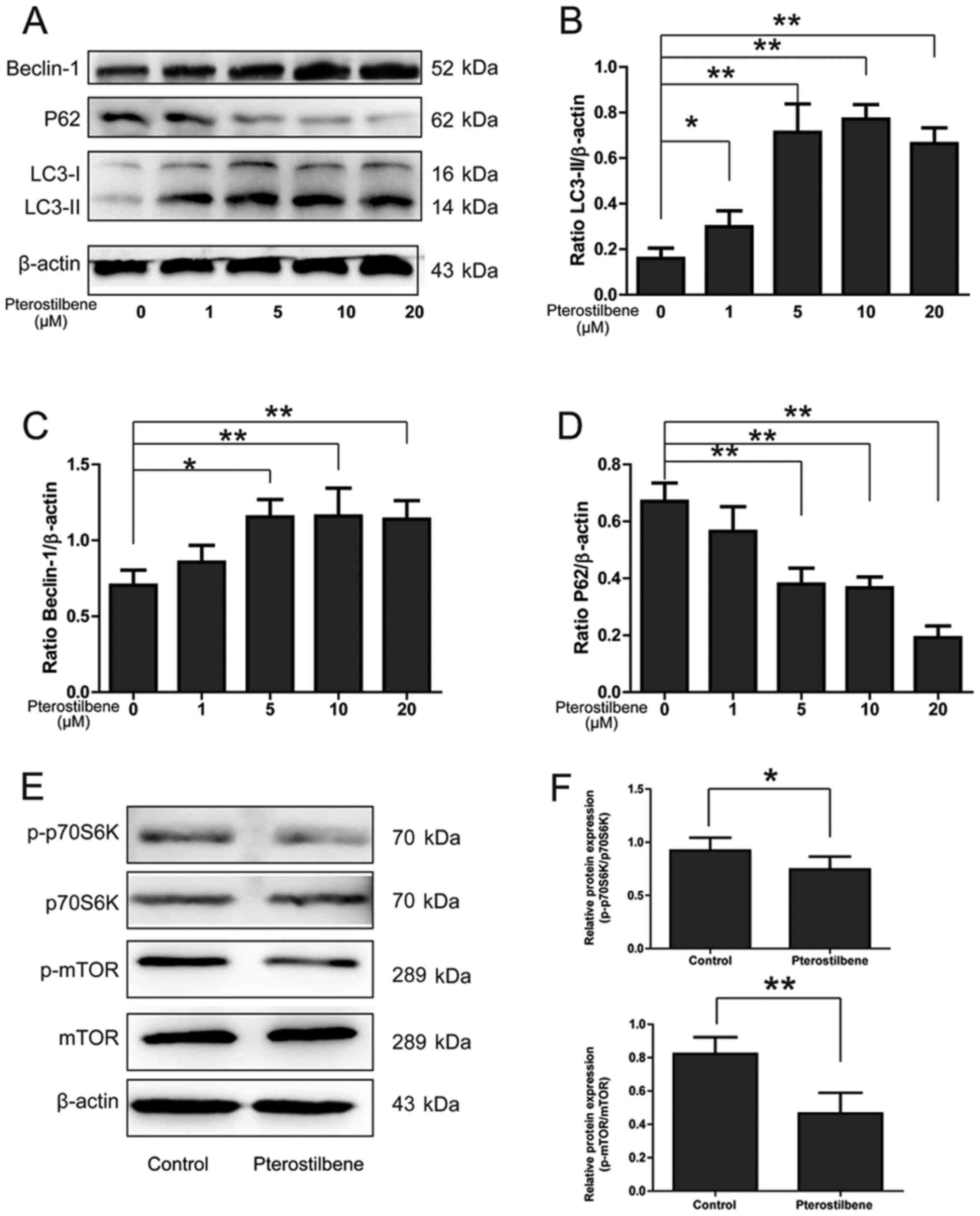 | Figure 2.The effect of pterostilbene on the
expression of LC3, Beclin-1, p62 and mTOR pathway in spinal cord
neurons. (A) Expression of LC3-II, Beclin-1 and p62 in spinal cord
neurons. (B) Semi-quantity analysis of LC3-II/β-actin expression.
(C) Semi-quantity analysis of Beclin-1/β-actin expression. (D)
Semi-quantity analysis of p62/β-actin expression, *P<0.05,
**P<0.01, compared between groups, n=6. (E) Expression of
p-p70S6K, p70S6K, p-mTOR and mTOR in spinal cord neurons. (F)
Semi-quantity analysis of p-p70S6K/p70S6K, p-mTOR/mTOR expression,
*P<0.05, **P<0.01, compared between groups, n=6. LC3, light
chain 3; mTOR, mechanistic target of rapamycin. |
mTOR pathway, a classic signaling pathway involving
in the autophagic activation, was investigated by Western blotting.
As shown in Fig. 2E and F,
pterostilbene significantly inhibited the expression of
p-p70S6K/p70S6K and p-mTOR/mTOR in spinal cord neurons
(P<0.05).
Pterostilbene activated autophagy in
spinal cord neurons treated with H2O2
In order to further investigate whether
pterostilbene could activate autophagy in spinal cord neurons under
oxidative stress, cells were treated with 10 µM
H2O2 1 h prior to pterostilbene.
Pterostilbene significantly increased the level of LC3-II/β-actin
in the H2O2-treated spinal cord neurons
(P<0.05, Fig. 3A and B).
GFP-LC3 assay showed that pterostilbene significantly enhanced the
number of LC3-positive green dots in one cell and the number of
LC3-positive cells (Fig. 3C).
Furthermore, pterostilbene combined with H2O2
also increased the number of LC3-positive cells compared with
H2O2 alone (Fig.
3C), indicating that pterostilbene could activate autophagy in
the spinal cord neurons under oxidative stress.
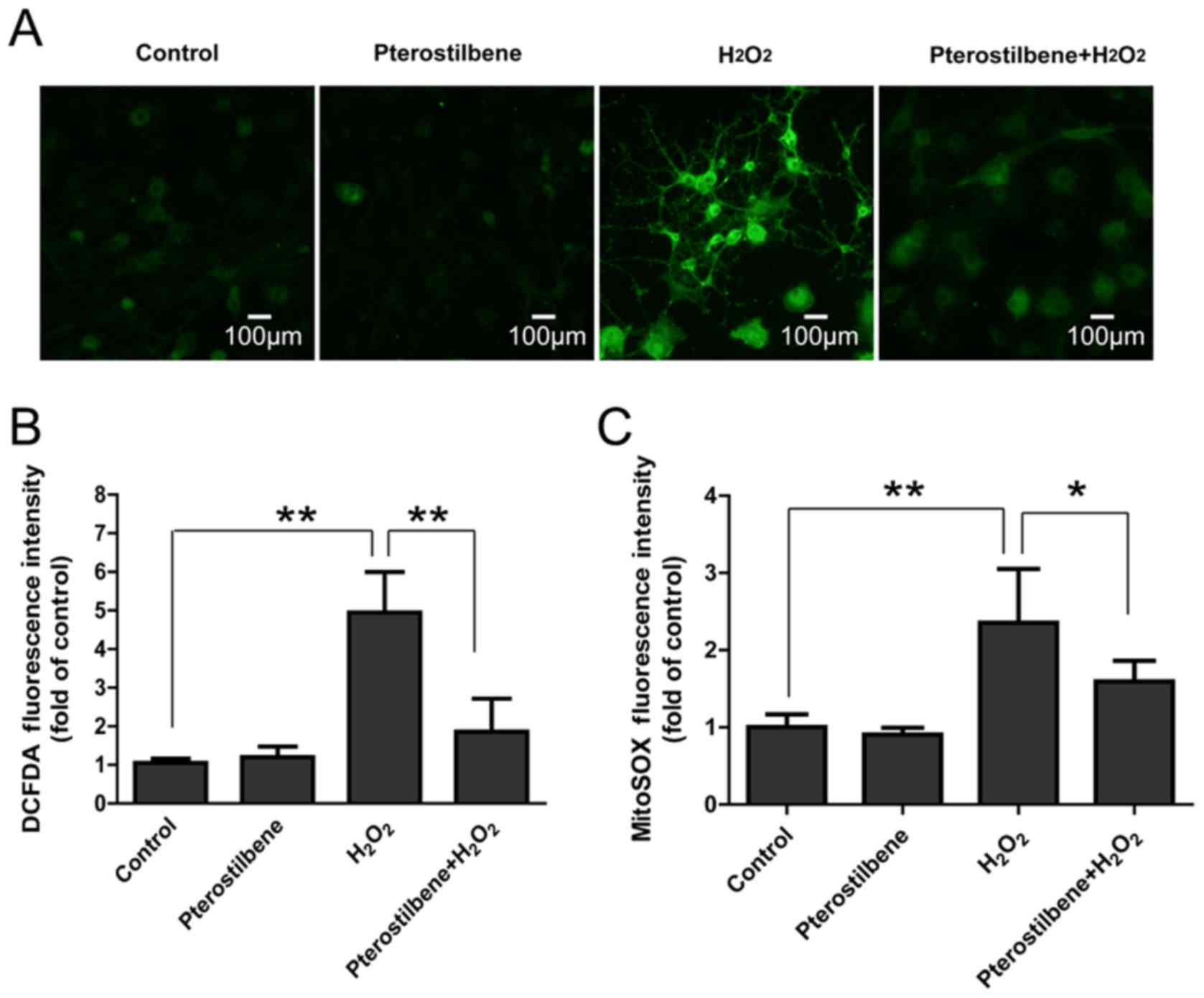
Pterostilbene inhibits ROS production
in spinal cord neurons treated with H2O2
DCFDA and MitoSOX staining were used to analyze the
total ROS and mitochondrial ROS production in spinal cord neurons.
It was not surprising that 10 µM H2O2
significantly increased fluorescence intensity of DCFDA and MitoSOX
in spinal cord neurons; however, pterostilbene significantly
attenuated the increase in ROS production (P<0.05, Fig. 4), suggesting that pterostilbene
could inhibited the intracellular ROS production induced by
H2O2.
The involvement of autophagy in the
inhibitory effect of pterostilbene on ROS production in spinal cord
neurons
ATG5, a key participator of the autophagic process,
was silenced by ATG5 siRNA transfection to explore the involvement
of autophagy in the inhibitory effect of pterostilbene on ROS
production. 48 h after ATG5 siRNA transfection, the ATG5 expression
was significantly inhibited (P<0.05, Fig. 5A and C). Western blotting showed
that ATG5 siRNA significantly inhibited the expression of
LC3-II/β-actin, demonstrating the decline of autophagy (P<0.05,
Fig. 5B and D). DCFDA and MitoSOX
staining showed that ATG5 siRNA significantly increased the ROS
production in cells treated with pterostilbene combined with
H2O2, suggesting that inhibition of autophagy
reversed the protective effect of pterostilbene against the ROS
production in spinal cord neurons (Fig. 5E and F).
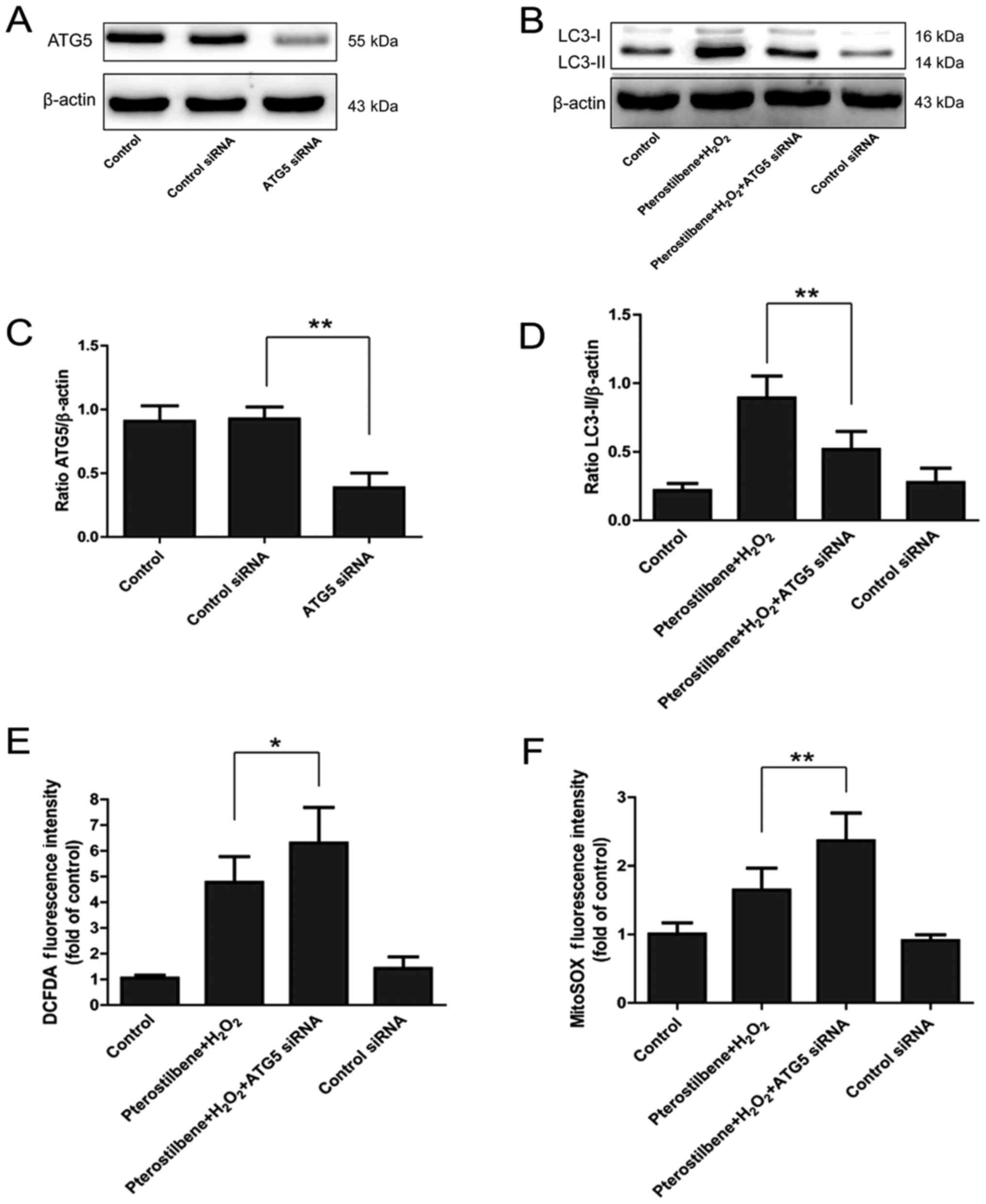 | Figure 5.The effect of autophagic inhibition
on the ROS production regulated by the pterostilbene in spinal cord
neurons. (A) ATG5 expression after ATG5 siRNA transfection. (B)
LC3-II expression after ATG5 silence. (C) Semi-quantity analysis of
the ATG5/β-actin expression, **P<0.01, compared with each group,
n=6. (D) Semi-quantity analysis of LC3-II/β-actin expression,
**P<0.01, compared with each group, n=6. (E) DCFDA staining
after ATG5 siRNA, *P<0.05, compared with each group, n=6. (F)
MitoSOX staining after ATG5 siRNA, **P<0.01, compared with each
group, n=6. ROS, reactive oxygen species; LC3, light chain 3. |
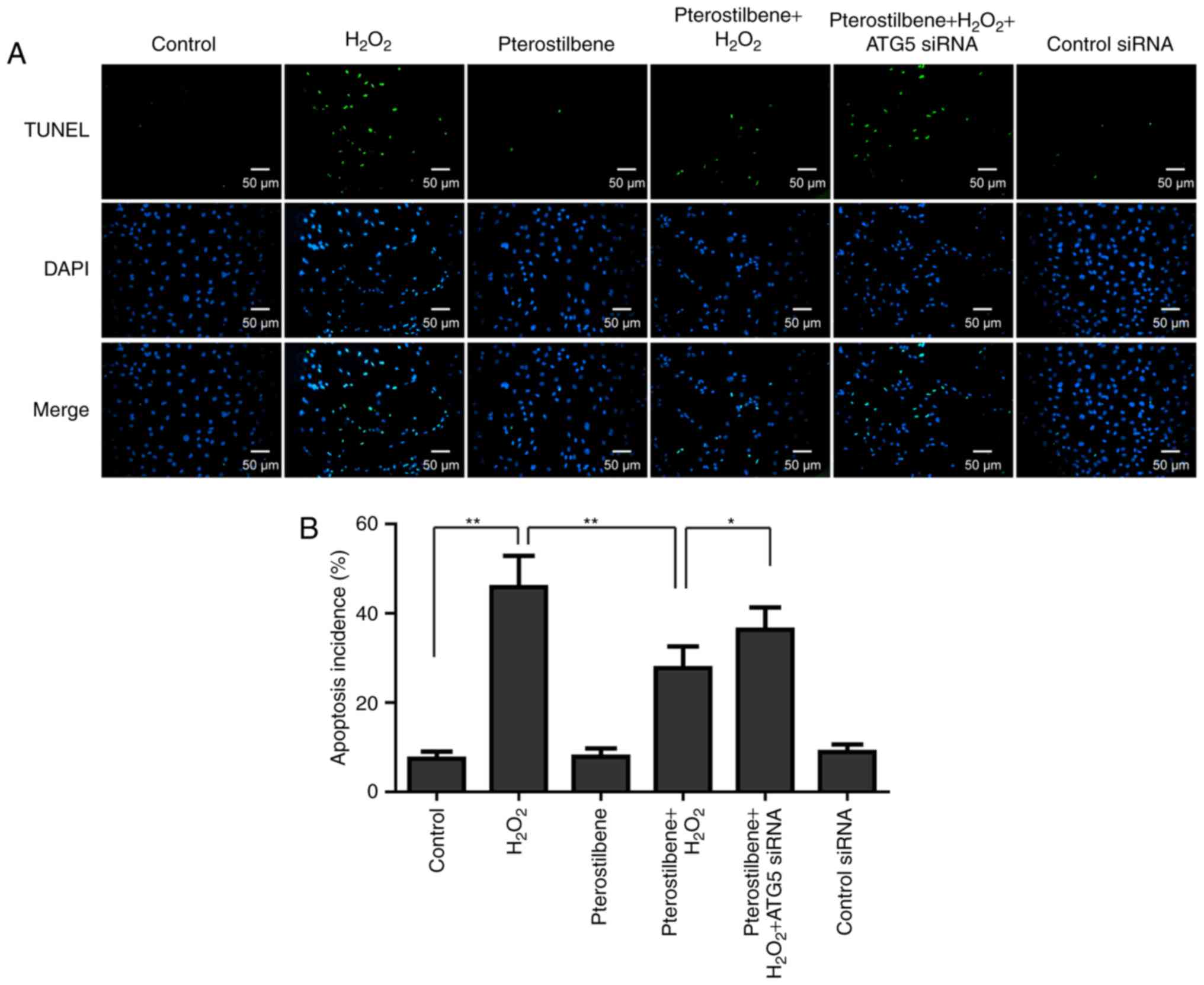
Effects of pterostilbene and autophagy
on apoptosis of spinal cord neurons under oxidative stress
Since apoptosis has been demonstrated as a main
mechanism of spinal cord neuronal death induced by oxidative
stress, the effect of pterostilbene on apoptosis was also
investigated by TUNEL staining. The TUNEL staining showed that
apoptosis incidence was induced by 10 µM
H2O2; however pterostilbene significantly
inhibited the apoptosis incidence in the
H2O2-treated cells (P<0.05, Fig. 6). ATG5 siRNA significantly
increased the apoptosis incidence in the cells treated with
pterostilbene combined with H2O2 (P<0.05,
Fig. 6), suggesting that autophagy
inhibiton reversed the inhibitory effect of pterostilbene on
apoptosis.
Discussion
The secondary injuries after severe SCI are composed
of ischemia-reperfusion injury, inflammation, edema, oxidative
stress induced by ROS over-expression, glutamate-induced
cytotoxicity, accumulation of intracellular calcium, cell apoptosis
and necrosis (15). Glutamate
accumulation in cells after SCI leads to the influx of
intracellular calcium, and mitochondrial membrane permeability
transition (MPT) induced by the absorption of intracellular calcium
results in the over-production of ROS, leading to the oxidative
stress in spinal cord neurons (15,16).
Oxidative stress contributes to the complex secondary injury
cascades, therefore, antioxidants might be helpful to alleviate the
SCI damage by maintaining the oxidative/antioxidative balance
(15).
Resveratrol, a well-acknowledged anti-oxidant, can
relieve the progression of various diseases, such as cardiovascular
diseases and osteoarthritis, but the poor bioavailability of
resveratrol impedes the clinical translation (17). By contrast, pterostilbene, a
resveratrol analogue, has a smaller molecule and longer half-life
with a relatively high bioavailability (5). Therefore, pterostilbene aroused more
attention from researchers compared with resveratrol. Pterostilbene
not only inhibited endothelial cell apoptosis induced by oxidative
stress (18), but also activated
autophagy of endothelial cells through the AMPKα1-mTOR signaling
pathway (12). In a variety of
tumor cells, pterostilbene could also activate autophagy and exert
anticancer effect (13). Although
pterostilbene has certain cytotoxicity to tumor cells, we first
confirmed that less than 20 µM pterostilben had no cytotoxic effect
on primary spinal cord neurons, providing a basis for the following
researches. In the paper, we found that pterostilbene activated
autophagy in spinal cord neurons by inhibiting mTOR pathway, which
was demonstrated by the LC3-I, Beclin-1 and p62 western blotting
and GFP-LC3 assay. The promontory effect of pterostilbene on
autophagy was further demonstrated in the
H2O2-treated cells. In addition,
pterostilbene inhibited the increase in ROS production induced by
H2O2, and the inhibition of autophagy could
reverse the protection, indicating the involvement of autophagy
activation. Finally, the inhibitory effect of pterostilbene on
apoptosis through autophagy activation was also demonstrated by
using the TUNEL assay. Taken together, these founds might provide a
theoretical and experimental basis for future clinical treatment of
SCI injury.
Spinal cord neuron is a cell type with high
demanding of energy, therefore the number of mitochondria in the
cytoplasm is larger than other cells in the body. In addition,
energy generation depends on the mitochondria, leading to the
mitochondrial ROS production in cells under oxidative stress
(19). On the other hand, ROS
production disturbs the energy synthesis in mitochondria and causes
apoptosis and necrosis (20). In
this paper, H2O2 was used to simulate the
oxidative stress, and DCFDA and MitoSOX staining demonstrated the
increase in ROS production induced by H2O2.
H2O2 causes the injury of mitochondria,
nucleic acids and proteins via a Fenton reaction (20), which might be a mechanism of
apoptosis under oxidative stress.
Oxidative stress is caused by an imbalance in
production and clearance of intracellular ROS and active nitrogen.
Recently, it has been reported that pterostilbene could inhibit the
production of ROS in a variety of cells. In neuronal HT22 cells of
mouse hippocampus, pterostilbene inhibits glutamate and high
glucose-induced ROS by Nrf2 (NF-E2)-related factor 2 signaling
pathway (21,22). In this study, we validated that
pterostilbene inhibited H2O2-induced ROS
production and confirmed the role of pterostilbene in antioxidant
stress in spinal cord neurons.
Recently, accumulating evidences have suggested that
autophagy is an essential cellular antioxidant pathway. In neural
stem cell, defect of autophagy resulted in a rise in ROS production
(23). Furthermore, autophagy has
been reported to regulate the nuclear translocation of Nrf2 which
is a transcription factor of anti-oxidant genes (24). Therefore, Nrf2 might be a mechanism
by which autphagy inhibits the ROS production. Given the
relationship between autophagy and oxidative stress, autophagy
might be a promising mechanism involved in the effect of
pterostilbene on ROS production in spinal cord neurons. In this
paper, autophagy inhibition by ATG5 silencing reversed the
protection of pterostilbene against on ROS production,
demonstrating the participation of autophagy in the regulation;
however, the downstream of autophagy required further
researches.
Cell apoptosis is also a major secondary
pathological change of SCI. Autophagy and apoptosis have crosstalks
in spinal cord neurons (25).
Resveratrol could inhibit apoptosis and activate autophagy via
SIRT1/AMPK signaling pathway after SCI in rats (25). Electroacupuncture preconditioning
and postconditioning could reduce apoptosis induced by spinal cord
ischemia reperfusion via activating autophagy (26). In the paper, autophagy inhibition
by ATG5 silencing increased apoptosis perecentage detected by TUNEL
assay, demonstrating the involvement of autophagy in the effect of
pterostilbene on apoptosis.
In summary, 20 µM pterostilbene is not cytotoxic to
the primary spinal cord neurons and increases autophagy levels
under normal and oxidative stress condotions. mTOR pathway was
inhibited by pterostilbene. Pterostilbene inhibits ROS production
and apoptosis induced by H2O2, but autophagy
inhibition by ATG5 silencing reverses the protection of
pterostilbene against ROS production and apoptosis in spinal cord
neurons. Taken together, pterostilbene inhibits ROS production and
apoptosis in spinal cord neurons by activating autophagy via mTOR
pathway.
References
|
1
|
Chen HC, Fong TH, Lee AW and Chiu WT:
Autophagy is activated in injured neurons and inhibited by
methylprednisolone after experimental spinal cord injury. Spine
(Phila Pa 1976). 37:470–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang JF, Li Y, Song JN and Pang HG: Role
of hydrogen sulfide in secondary neuronal injury. Neurochem Int.
64:37–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia Z, Zhu H, Li J, Wang X, Misra H and Li
Y: Oxidative stress in spinal cord injury and antioxidant-based
intervention. Spinal Cord. 50:264–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu D, Liu J, Sun D and Wen J: The time
course of hydroxyl radical formation following spinal cord injury:
The possible role of the iron-catalyzed Haber-Weiss reaction. J
Neurotrauma. 21:805–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Remsberg CM, Yáñez JA, Ohgami Y,
Vega-Villa KR, Rimando AM and Davies NM: Pharmacometrics of
pterostilbene: Preclinical pharmacokinetics and metabolism,
anticancer, antiinflammatory, antioxidant and analgesic activity.
Phytother Res. 22:169–179. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Song T, Yang L, Wang X, Yang C and
Jiang Y: Neuroprotective actions of pterostilbene on
hypoxic-ischemic brain damage in neonatal rats through upregulation
of heme oxygenase-1. Int J Dev Neurosci. 54:22–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Zhang XM, Ma A, Zhang YL, Chen YY,
Zhou H, Li WJ and Jin X: Orally administrated pterostilbene
attenuates acute cerebral ischemia-reperfusion injury in a dose-
and time-dependent manner in mice. Pharmacol Biochem Behav.
135:199–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang LB, Lee S, Wang Y, Xu QT, Meng DH
and Zhang J: Adipose-derived stem cells induce autophagic
activation and inhibit catabolic response to pro-inflammatory
cytokines in rat chondrocytes. Osteoarthritis Cartilage.
24:1071–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Yuan F, Yin X and Dong J:
Responses and adaptations of intervertebral disc cells to
microenvironmental stress: A possible central role of autophagy in
the adaptive mechanism. Connect Tissue Res. 55:311–321. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su M, Guan H, Zhang F, Gao Y, Teng X and
Yang W: HDAC6 regulates the chaperone-mediated autophagy to prevent
oxidative damage in injured neurons after experimental spinal cord
injury. Oxid Med Cell Longev. 2016:72637362016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Cui L, Zhou G, Jing H, Guo Y and
Sun W: Pterostilbene, a natural small-molecular compound, promotes
cytoprotective macroautophagy in vascular endothelial cells. J Nutr
Biochem. 24:903–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ko CP, Lin CW, Chen MK, Yang SF, Chiou HL
and Hsieh MJ: Pterostilbene induce autophagy on human oral cancer
cells through modulation of Akt and mitogen-activated protein
kinase pathway. Oral Oncol. 51:593–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anderson KN, Potter AC, Piccenna LG, Quah
AK, Davies KE and Cheema SS: Isolation and culture of motor neurons
from the newborn mouse spinal cord. Brain Res Brain Res Protoc.
12:132–136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fatima G, Sharma VP, Das SK and Mahdi AA:
Oxidative stress and antioxidative parameters in patients with
spinal cord injury: Implications in the pathogenesis of disease.
Spinal Cord. 53:3–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McEwen ML, Sullivan PG and Springer JE:
Pretreatment with the cyclosporin derivative, NIM811, improves the
function of synaptic mitochondria following spinal cord contusion
in rats. J Neurotrauma. 24:613–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subramanian L, Youssef S, Bhattacharya S,
Kenealey J, Polans AS and van Ginkel PR: Resveratrol: Challenges in
translation to the clinic-a critical discussion. Clin Cancer Res.
16:5942–5948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Zhou G, Song W, Tan X, Guo Y,
Zhou B, Jing H, Zhao S and Chen L: Pterostilbene protects vascular
endothelial cells against oxidized low-density lipoprotein-induced
apoptosis in vitro and in vivo. Apoptosis. 17:25–36. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hall ED: Lipid peroxidation. Adv Neurol.
71:247–258. 1996.PubMed/NCBI
|
|
20
|
Fariss MW, Chan CB, Patel M, Van Houten B
and Orrenius S: Role of mitochondria in toxic oxidative stress. Mol
Interv. 5:94–111. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Liu H, Yue L, Li X, Zhao L, Yang
X, Wang X, Yang Y and Qu Y: Neuroprotective effects of
pterostilbene against oxidative stress injury: Involvement of
nuclear factor erythroid 2-related factor 2 pathway. Brain Res.
1643:70–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Fan C, Wang B, Ma Z, Wang D, Gong
B, Di S, Jiang S, Li Y, Li T, et al: Pterostilbene attenuates high
glucose-induced oxidative injury in hippocampal neuronal cells by
activating nuclear factor erythroid 2-related factor 2. Biochim
Biophys Acta. 1863:827–837. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Liang CC, Bian ZC, Zhu Y and Guan
JL: FIP200 is required for maintenance and differentiation of
postnatal neural stem cells. Nature Neurosci. 16:532–542. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taguchi K, Fujikawa N, Komatsu M, Ishii T,
Unno M, Akaike T, Motohashi H and Yamamoto M: Keap1 degradation by
autophagy for the maintenance of redox homeostasis. Proc Natl Acad
Sci USA. 109:pp. 13561–13566. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao H, Chen S, Gao K, Zhou Z, Wang C,
Shen Z, Guo Y, Li Z, Wan Z, Liu C and Mei X: Resveratrol protects
against spinal cord injury by activating autophagy and inhibiting
apoptosis mediated by the SIRT1/AMPK signaling pathway.
Neuroscience. 348:241–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang B, Qin M, Li Y, Li X, Tan W, Zhang Y
and Ma H: Electroacupuncture preconditioning and postconditioning
inhibit apoptosis and neuroinflammation induced by spinal cord
ischemia reperfusion injury through enhancing autophagy in rats.
Neurosci Lett. 642:136–141. 2017. View Article : Google Scholar : PubMed/NCBI
|