Introduction
Cervical cancer is the third most common cancer in
women worldwide (1,2), and >85% of the cervical cancer
burden is in developing countries (2,3).
Metastasis is one of the primary causes of treatment failure and
mortality in women diagnosed with cervical cancer, indicating that
the inhibition of metastasis serves a pivotal role in improving the
survival and cure rate.
Accumulating studies have reported the association
between serine proteases, and tumor invasion and metastasis.
Urokinase plasminogen activator (uPA) is an important serine
protease (4), which serves a role
in the extracellular matrix degradation process, in addition to
being associated with cell division, adhesion and migration. uPA
receptor (uPAR) is a high affinity receptor for uPA on the cell
surface (5), and may activate uPA
and localize to the cell surface to provide a local concentration
mechanism between cells and the junction of the cell and matrix,
thus creating a suitable environment for uPA-mediated proteolysis
in uPAR-expressing tumor cells (6). The level of expression of uPA and
uPAR in invasive cervical cancer tissues is increased compared with
normal cervical tissues (7). The
same phenomenon may be observed with matriptase, a newly-identified
type II serine transmembrane protease, and 72 kDa type IV
collagenase (MMP-2), a subtype of the matrix metalloproteinase
(MMP) family (8–11). Additionally, matriptase is able to
hydrolyze single-stranded pro-uPA to form an active double-stranded
structure. Activated uPA converts plasminogen into plasmin, which
in turn converts pro-MMPase to MMPs (including MMP-2), resulting in
proteolysis of the extracellular matrix. Subsequently, a cascade of
protein cleavage reactions occurs, promoting tumor growth and
angiogenesis, in addition to accelerating extracellular matrix
degradation (12). Thus,
inhibition of tumor invasion and metastasis via suppression of the
uPA system in tumor cells has become the focus of studies.
The mechanism underlying the invasion and metastasis
induced by overexpression of matriptase, uPA, uPAR and MMP-2 in
cervical cancer remains to be elucidated. Additionally, the
intricate network of the uPA system remains unclear, and has become
a leading area of research and development in cervical cancer.
Amiloride, as a synthetic inhibitor of uPA, serves a role in tumor
invasion and metastasis prevention by inhibiting the proteolytic
catalytic activity of the extracellular area of uPA, in a
competitive and selective manner (13,14).
The purpose of the present study was to investigate the effects of
amiloride on the invasion and metastasis of human cervical cancer
cells in vitro.
Materials and methods
Cell culture
The human cervical cancer cell line HeLa was
obtained from the Laboratory of Gynecologic Oncology of Fujian
Provincial Maternity and Children Hospital, Affiliated Hospital of
Fujian Medical University (Fujian, China). All cells were cultured
in 90% Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin, and 1% streptomycin (100 IU/ml) in a 37°C incubator
with 5% CO2.
Drug treatment
The amiloride was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) and prepared in 100% dimethyl
sulfoxide (DMSO). Prior to treatment, the HeLa cervical cancer
cells were seeded in 6-well plates at a density of 1×107
cells/well and cultured in 3 ml serum-free DMEM for 12 h to achieve
adherence. For the dose-dependent study, five groups were set up;
four groups were treated with final concentrations of 50, 100, 150
or 200 µmol/l amiloride, respectively, for 24 h, and one group
treated only with DMSO was used as a control. For the
time-dependent study, cells were cultured with 150 µmol/l amiloride
for different time periods (2, 4 or 8 h). Similarly, prior to the
cellular scratch assay and Transwell chamber assay, cells were
incubated with final concentrations of 50, 100 or 150 µmol/l
amiloride for 24 h, or incubated with 150 µmol/l amiloride for
different time periods (6, 12 or 24 h).
Detection of mRNA expression levels of
matriptase, uPA, uPAR and MMP-2 by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Samples of 1 µg DNase
I-treated RNA were reverse-transcribed to cDNA using the reverse
transcription system A3500 (Promega Corporation, Madison, WI, USA).
The PCR primer sets were synthesized by Takara Biotechnology Co.,
Ltd. (Dalian, China) and were as follows: GAPDH sense,
5′-GAAGGTGAAGGTCGGAGTC-3′ and antisense,
5′-GAAGATGGTGATGGGATTTC-3′; matriptase sense,
5′-GGGACACACCCAGTATGGAGG-3′ and antisense,
5′-CCGGAATCACCCTGGCAGGA-3′; uPA sense, 5′-AGAATTCACCACCATCGAGA-3′
and antisense, 5′-ATCAGCTTCAACAGTCAT-3′; uPAR sense,
5′-GAGCTGGTGGAGAAAAGCTG-3′ and antisense,
5′-TGTTGCAGCATTTCAGGAAG-3′; and MMP-2 sense,
5′-AGATCTTCTTCTTCAAGGAGACCGGTT-3′ and antisense,
5′-GGCTGGTCAGTGGCTTGGGGTA-3′. The thermocycling conditions of qPCR
were as follows: 95°C for 15 sec, 45 cycles of denaturation at 95°C
for 5 sec, annealing at 60°C for 20 sec, 95°C for 1 min and then
cooled to 55°C. The relative levels of matriptase, uPA, uPAR and
MMP-2 mRNA was quantified using the 2−ΔΔCq method
(15) and normalized to GAPDH
expression. Following qPCR analysis, the PCR products were also
electrophoresed on 2% agarose gel stained with ethidium
bromide.
ELISA analysis for uPA and MMP-2
quantification
The protein expression quantification for uPA and
MMP-2 was performed using human uPA and MMP-2 ELISA kits (cat. nos.
SEA140Hu and SEA100Hu; Cloud-Clone Corp., Wuhan, China), according
to the manufacturer's instructions. Supernatant obtained from the
cell culture with different concentrations of amiloride (0, 50,
100, 150 or 200 µmol/l), or different treatment durations (0, 2, 4
or 8 h), were harvested and centrifuged at 12,000 × g at 4°C for 10
min. The results of the reaction were measured at 450 nm, using an
automated microplate spectrophotometer (RT-6100; Rayto Life and
Analytical Sciences Co., Ltd., Shenzhen, China). Total protein was
quantified in pg/ml. The results were calculated using the standard
curves created in each assay. The ELISAs were performed in a
blinded manner and in triplicate.
Cellular scratch assay
The horizontal migration of cells was assessed via a
scratch assay (16). Cells were
seeded at a density of 5.0×105 cells/well and observed
with an inverted microscope at 0, 6, 12 and 24 h post-scratch.
Image ProExpress C software 5.1 (Olympus Corporation, Tokyo, Japan)
was used to measure the alteration in cell distance between the
scratches. The average horizontal migration distance was calculated
using the following formula: Width0 h -
Widthpost-scratching.
Transwell chamber assay
The cellular invasive capacity was determined using
a Matrigel invasion chamber assay, as previously reported (17). Cells were seeded at a density of
5.0×105 cells/well. The number of cells on the underside
of the filter was determined by counting cells in five random
fields from three filters for each treatment, at ×200 magnification
with an inverted microscope (Olympus Corporation).
Statistical analysis
All experiments were performed in triplicate.
Statistical analysis was performed using the average results of
three repeated experiments under identical conditions. Numerical
data are presented as the mean ± standard deviation. A one-way
analysis of variance was performed for multiple comparisons of
groups, which was followed by the Fisher's least significant
difference post hoc test, and associated parameters were further
analyzed using the Pearson's correlation test. Data were analyzed
using SPSS software 19.0 for Windows (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Dose-dependent mRNA expression of uPA,
MMP-2, matriptase and uPAR following treatment with amiloride
Following incubation with various concentrations of
amiloride (50, 100 and 200 µmol/l) for 24 h, there were no
significant differences in matriptase mRNA expression between in
HeLa cells treated with different concentrations of amiloride and
the control group. A similar result was observed with the mRNA
expression of uPAR in the HeLa cells following treatment with
amiloride (matriptase, F=0.282, P=0.837; uPAR, F=0.106, P=0.954;
Fig. 1A and B). However, following
incubation with different concentrations of amiloride (50, 100, 150
and 200 µmol/l) for 24 h, the mRNA expression levels of uPA and
MMP-2 were significantly downregulated compared with the control
group (uPA, F=42.639, P<0.01; MMP-2, F=77.357, P<0.01). With
the concentration of amiloride increasing, the mRNA expression
levels of uPA and MMP-2 exhibited a gradual steady decrease.
However, there was no significant difference between the 150 µmol/l
group and the 200 µmol/l group (uPA, P=0.413; MMP-2, P=0.588;
Fig. 1C and D).
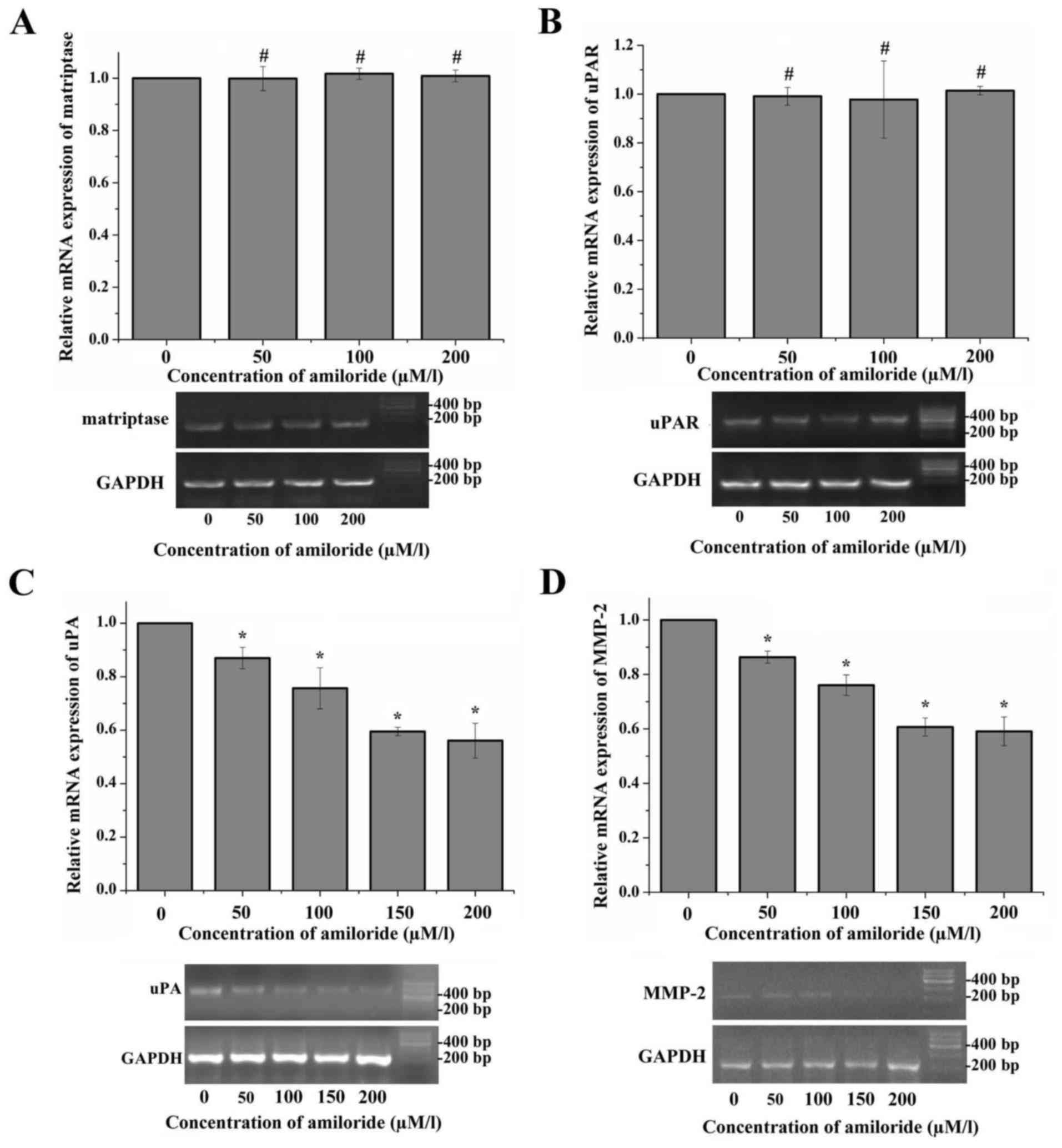 | Figure 1.Expression of matriptase, uPA, uPAR
and MMP-2 mRNA in HeLa cells treated with amiloride. The relative
mRNA expression levels of matriptase, uPA, uPAR and MMP-2 were
detected in HeLa cells treated with different concentrations of
amiloride using RT-qPCR. The images of the gels are representative
of the electrophoresed RT-qPCR products on 2% agarose gel stained
with ethidium bromide. The 0 µmol/l concentration of amiloride
group was set as the negative control. (A) No statistically
significant differences were observed between the mRNA expression
levels of matriptase in HeLa cells cultured with various
concentrations of amiloride. (B) No statistically significant
differences were observed between the mRNA expression levels of
uPAR in HeLa cells cultured with various concentrations of
amiloride. (C) Amiloride significantly inhibited the mRNA
expression level of uPA in HeLa cells in a concentration-dependent
manner, and there was no significant difference in the levels
between the 150 µmol/l group and the 200 µmol/l group. (D)
Amiloride significantly inhibited the mRNA expression level of
MMP-2 in HeLa cells in a concentration-dependent manner, and there
was no significant difference in the levels between the 150 µmol/l
group and the 200 µmol/l group. *P<0.05, #P>0.05
vs. 0 µmol/l group. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; uPA, urokinase plasminogen activator;
uPAR, urokinase plasminogen activator receptor; MMP-2, 72 kDa type
IV collagenase. |
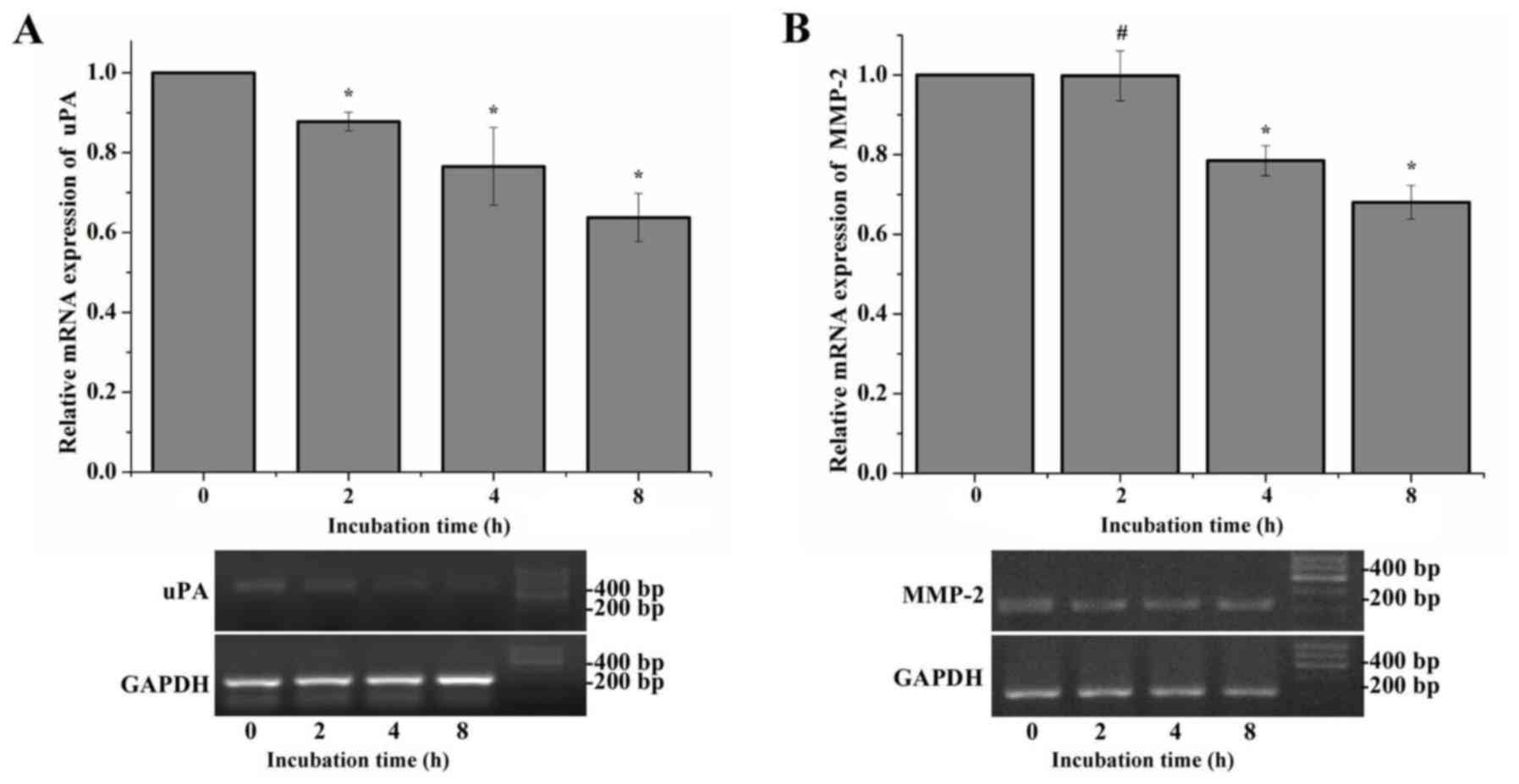
Time-dependent mRNA expression of uPA,
MMP-2, matriptase and uPAR following treatment with amiloride
The expression of uPA and MMP-2 was adjusted by
amiloride in a dose-dependent manner, which was not observed with
the expression of matriptase and uPAR. A time-dependent study was
additionally performed to analyze the mRNA expression of uPA and
MMP-2 following treatment with amiloride. Compared with the control
group, the mRNA expression levels of uPA in HeLa cells treated with
150 µmol/l amiloride exhibited a significant time-dependent
decrease following incubation for 2–8 h (F=21.042, P<0.01;
Fig. 2A). Notably, in the first 2
h of treatment, the mRNA expression of MMP-2 was not observed to be
significantly different between the cells treated with 150 µmol/l
amiloride and the control cells (P=0.958). A total of 4 h following
the start of treatment, a decrease in MMP-2 mRNA expression was
observed in the HeLa cells, which was dependent on the incubation
time compared with the control group (F=42.575, P<0.01; Fig. 2B). These results suggested that
amiloride may inhibit the mRNA expression level of uPA and MMP-2 in
a time-dependent manner.
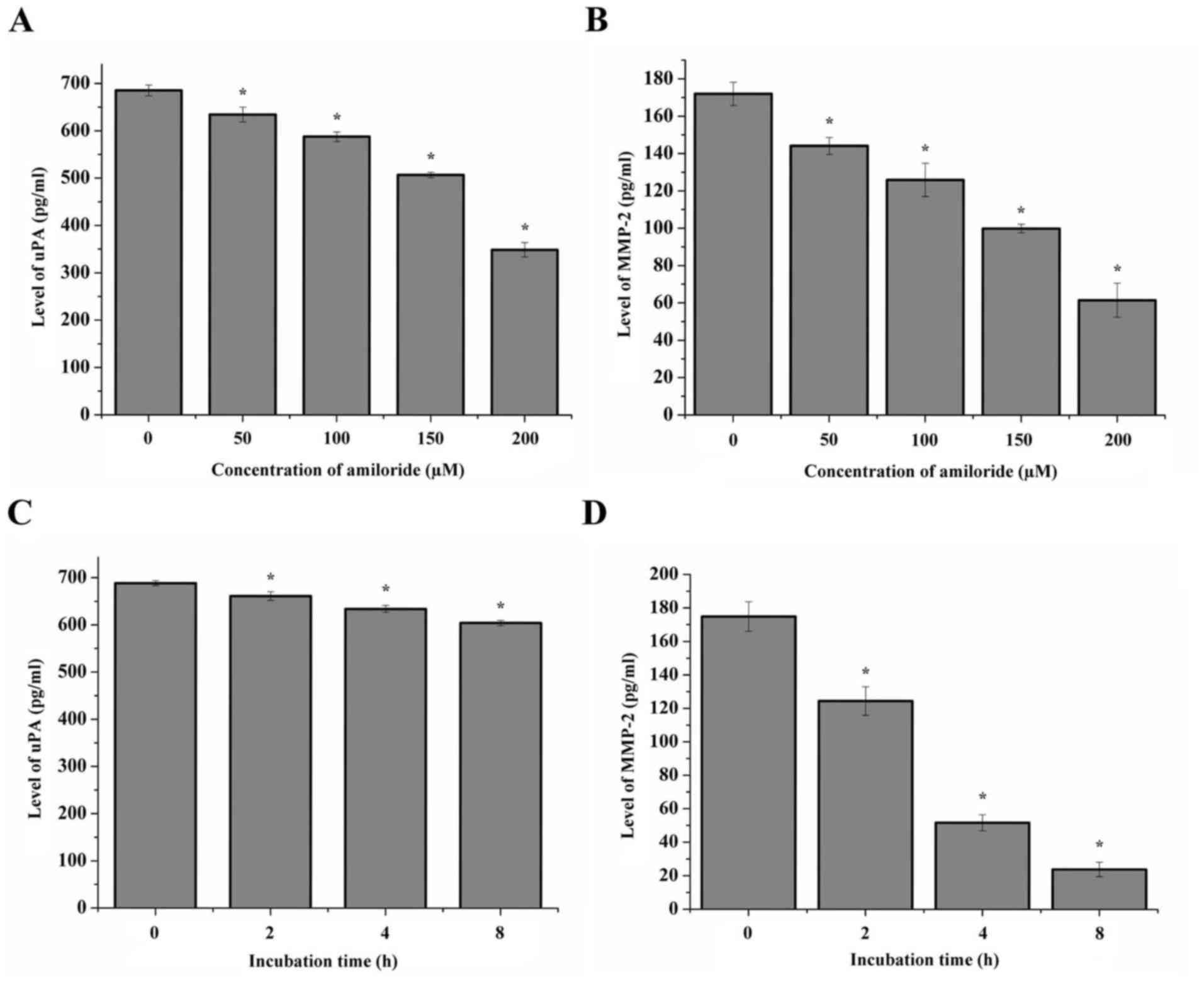
Protein expression of uPA and MMP-2
following treatment with amiloride
The protein expression of uPA and MMP-2 in HeLa
cells treated with different concentrations of amiloride was
quantitatively detected using ELISA, and the data are presented in
Fig. 3. The results indicated a
decrease in uPA and MMP-2 mRNA in the cells following treatment
with 50, 100 or 200 µmol/l amiloride, when compared with the
control group (P<0.05; Fig. 3A and
B). Similarly, the protein expression levels of uPA and MMP-2
in the HeLa cells treated with 150 µmol/l amiloride decreased with
the prolonged treatment duration (0, 2, 4 and 8 h) (P<0.05;
Fig. 3C and D).
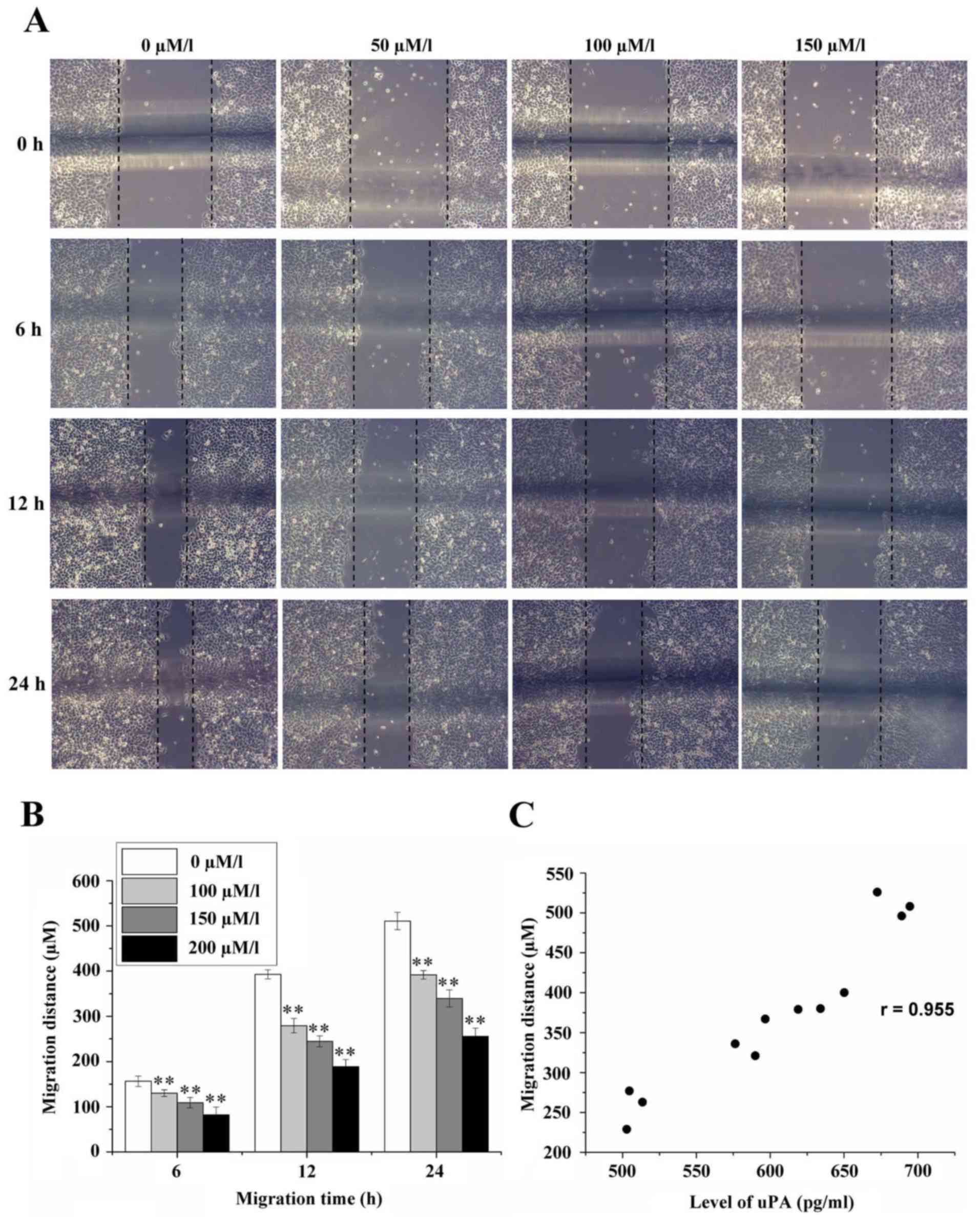
Effect of amiloride on the migration
of HeLa cells
The migration distances of HeLa cells at the time
points of 6, 12 and 24 h were 156.44±11.35, 392.89±9.93 and
510.67±19.05 µm, respectively, in the control group without
amiloride, as determined by cellular scratch assay. When the
concentration of amiloride was 50 µmol/l, the migration distances
of HeLa cells at 6, 12 and 24 h were 130.11±7.39, 279.33±15.90 and
391.78±9.56 µm, respectively. When the concentration of amiloride
was 100 µmol/l, the migration distances of HeLa cells at 6, 12 and
24 h were 109.11±11.60, 244.56±12.24 and 339.78±18.86 µm,
respectively. When the concentration of amiloride was 150 µmol/l,
the migration distances of HeLa cells at 6, 12 and 24 h were
82.00±17.69, 188.78±15.53 and 256.00±18.06 µm, respectively. All
distances in the intervention groups were significantly decrease
compared with the control group (0 µmol/l) (F=56.893, 360.000 and
360.038, respectively; P<0.01; Fig.
4A and B). Correlation analysis demonstrated that there was a
positive correlation between cell migration distance and the
expression level of uPA (r=0.955, P<0.01; Fig. 4C).
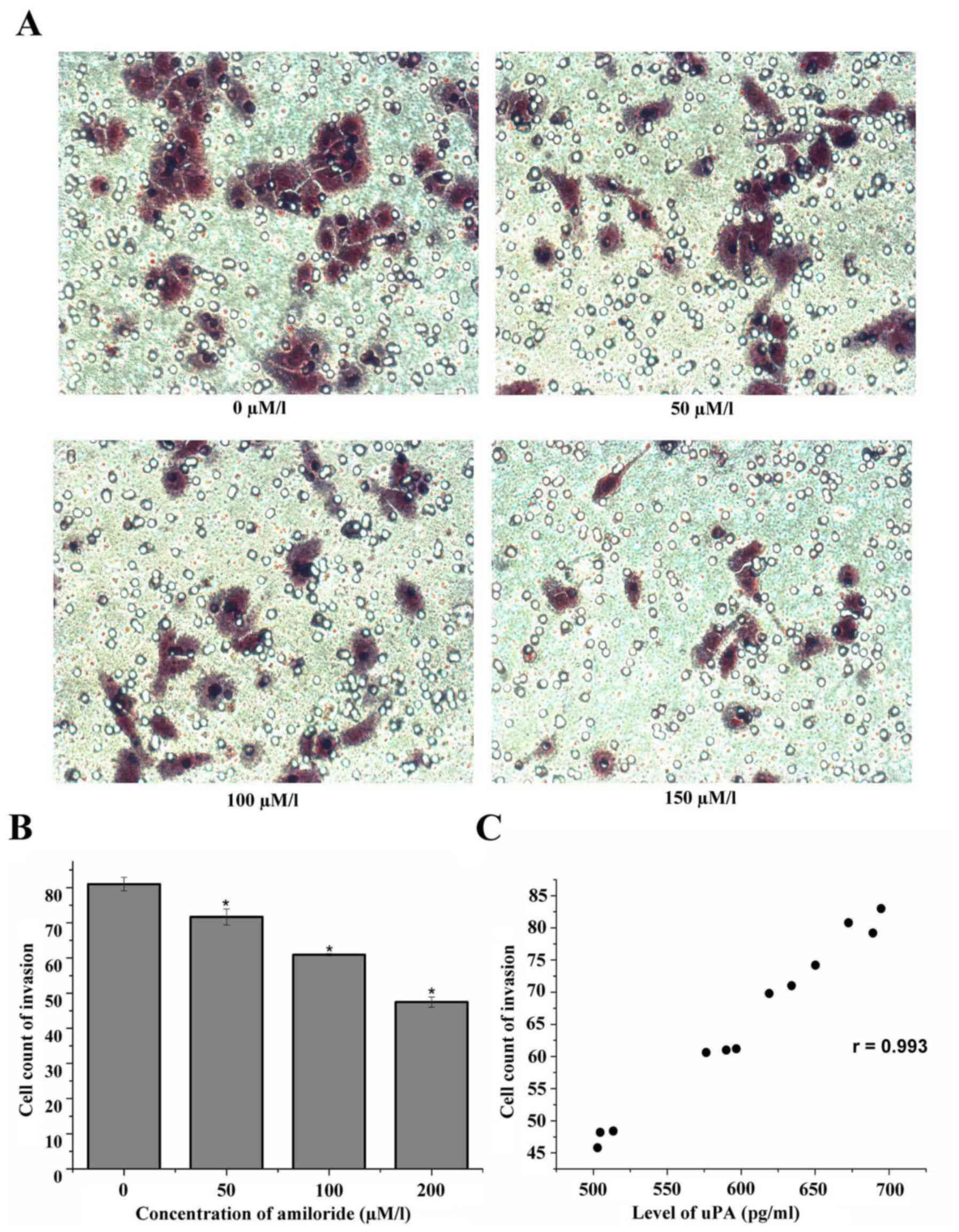
Effect of amiloride on the invasion of
HeLa cells
The HeLa cells were cultured with 0, 50, 100 and 150
µmol/l amiloride for 24 h. The results of the cell invasion assay
demonstrated that the number of HeLa cells that passed through the
membrane was significantly decreased as the amiloride concentration
increased: Control group (0 µmol/l), 81.00±1.91; 50 µmol/l
amiloride, 71.67±2.27; 100 µmol/l amiloride, 60.93±0.31; and 150
µmol/l amiloride, 47.47±1.45. There was a negative association
between the number of cells penetrating the membrane and the
concentration of amiloride. Amiloride concentrations of 50, 100 and
150 µmol/l decreased the number of membrane-penetrating cells; this
result was statistically significant compared with the control
group (F=226.95, P<0.01; Fig. 5A
and B). Additionally, there was a positive correlation between
the number of membrane-penetrating cells and the expression level
of uPA (r=0.993, P<0.01; Fig.
5C).
Discussion
The invasion and metastasis of malignant tumors,
processes primarily regulated by the expression of proteolytic
enzymes, is one of the principal causes of treatment failure and
mortality in patients diagnosed with cancer. Studies have reported
that tumor cell infiltration and metastatic capacity are closely
associated with the degree of protease production (18). Tumor cells produce a large number
of proteolytic enzyme degradation matrices to facilitate the
migration of tumor cells (19). In
malignant tumor tissues and cells, there are four principal types
of proteolytic enzyme: Serine proteases, cysteine proteases,
aspartic acid proteases and MMPs. uPA is an important type of
serine protease that is able to stimulate the production of plasmin
and plasmin-dependent MMPs (20).
MMP-2 has been observed to be highly expressed in various solid
tumors, including those in cervical cancer, and is associated with
tumorigenesis and prognosis (21–23).
Matriptase is a novel serine protease and the upstream regulator of
the uPA system (21). It was
isolated from the human breast cancer cell line T-47D and milk by
Lin et al (24) and is
briefly activated in normal cells under specific controllable
conditions, although it is consistently activated in cancer cells.
uPA, MMP-2, and matriptase have been demonstrated to be
overexpressed in cervical cancer cells, while the same phenomenon
was not observed in normal cervical cells (7,8,20,25).
Tumor treatment has been advanced by the study of
the mechanism of action of uPA series factors. Considering that the
modulation of uPA was predicted to regulate tumor invasion and
metastasis (26,27), further studies were performed.
Following this, the regulation of uPA transcription by amiloride
was revealed in numerous studies (28,29).
Klinghofer et al (30)
reported the inhibitory effects of amiloride, B428 and other
amidine-based urokinases on the human uPA gene, and that the murine
uPA gene was unable to be inhibited. While certain studies have
supported the role of the balance of calcium and other ions as an
underlying mechanism for the anticancer properties of amiloride, it
is clear that a number of the anticancer effects may arise either
independently or synergistically via inhibition of uPA (31,32).
In the present study, an experiment was performed to demonstrate
the effects of different concentrations of the uPA synthetic
inhibitor amiloride on a human cervical cancer cell line (HeLa)
in vitro. The results demonstrated that amiloride
significantly inhibited the mRNA expression level of uPA in HeLa
cells in a concentration- and time-dependent manner, whereas the
mRNA expression level of uPA reached a plateau when the
concentration of amiloride reached 150 µmol/l. Increasing drug
concentrations were unable to inhibit the mRNA expression of uPA,
which may be associated with the saturation effect of drugs on the
uPA system. It was additionally demonstrated that the exposure of
HeLa cells to amiloride resulted in a significant decrease in the
mRNA expression level of MMP-2 following the period of decline in
the mRNA expression level of uPA. The possible reason is that the
mRNA expression level of uPA may be inhibited by amiloride,
followed by inhibition of plasmin activation, leading to a decrease
in MMP-2 activation. In the quantitative ELISA, as hypothesized,
the levels of uPA and MMP-2 were demonstrated to be downregulated
with the increase in concentration of amiloride and treatment
duration. The results of the present study additionally
demonstrated that there was no significant inhibitory effect of
amiloride on the mRNA expression levels of matriptase and uPAR.
The cellular scratch assay was performed to
investigate the migratory ability of HeLa cells. The results
demonstrated a concentration-dependent effect, whereby amiloride
significantly suppressed the migration of HeLa cells; the migration
distance of the cells was significantly reduced and wound healing
time was prolonged. There was a negative correlation between the
concentration of amiloride and the protein expression level of uPA
in HeLa cells. Therefore, amiloride was able to downregulate the
mRNA expression of uPA in HeLa cells, and suppress the migration of
human cervical cancer cells, leading to inhibition of tumor
infiltration and growth towards the surrounding tissue.
The Transwell chamber assay was performed to
investigate the alteration in invasiveness of HeLa cells following
culturing with amiloride. The results suggested that amiloride had
anti-invasive effects on HeLa cells in a concentration-dependent
manner, which are negatively correlated with the mRNA expression
level of uPA in those cells. Therefore, the present study confirmed
that amiloride was able to downregulate the mRNA expression of uPA
in HeLa cells and suppress the invasion and metastasis of human
cervical cancer cells in a concentration-dependent manner.
In conclusion, uPA may be associated with cervical
cancer invasion and metastasis-associated genes. The present study
partly revealed the association between the uPA system and the
behavior of human cervical cancer cells by examining the
association between amiloride and mRNA expression levels of
matriptase, uPA, uPAR and MMP-2, in addition to the effect of
amiloride on the migration and invasion of HeLa cells. The results
of the present study further confirmed that amiloride, a type of
synthetic uPA inhibitor, may serve a role in the inhibition of
tumor invasion by suppressing the mRNA expression level of uPA, and
its antitumor role in cervical cancer merits investigation in
further studies.
Acknowledgements
The present study was supported in part by grant no.
2009-CXB-33 from the Medical Foundation for Innovation of Fujian
Province of China, and grant no. 2007 (170) from the Advanced
Program of National Ministry of Personnel for the Returned Overseas
Chinese Scholars. The funders had no role in study design, data
collection, data analysis, data interpretation or writing of this
study. The authors had full access to the data and were responsible
for the final decision to submit.
References
|
1
|
de Sanjosé S, Diaz M, Castellsagué X,
Clifford G, Bruni L, Muñoz N and Bosch FX: Worldwide prevalence and
genotype distribution of cervical human papillomavirus DNA in women
with normal cytology: A meta-analysis. Lancet Infect Dis.
7:453–459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN, 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sahasrabuddhe VV, Parham GP, Mwanahamuntu
MH and Vermund SH: Cervical cancer prevention in low- and
middle-income countries: Feasible, affordable, essential. Cancer
Prev Res (Phila). 5:11–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hildenbrand R, Allgayer H, Marx A and
Stroebel P: Modulators of the urokinase-type plasminogen activation
system for cancer. Expert Opin Invest Drugs. 19:641–652. 2010.
View Article : Google Scholar
|
|
5
|
Blasi F and Sidenius N: The urokinase
receptor: Focused cell surface proteolysis, cell adhesion and
signaling. FEBS Lett. 584:1923–1930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mekkawy AH, Morris DL and Pourgholami MH:
Urokinase plasminogen activator system as a potential target for
cancer therapy. Future Oncol. 5:1487–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daneri-Navarro A, Macias-Lopez G,
Oceguera-Villanueva A, Del Toro-Arreola S, Bravo-cuellar A,
Perez-montfort R and Orbach-arbouys S: Urokinase-type plasminogen
activator and plasminogen activator inhibitors (PAI-1 and PAI-2) in
extracts of invasive cervical carcinoma and precursor lesions. Eur
J Cancer. 34:566–569. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santin AD, Cane' S, Bellone S, Bignotti E,
Palmieri M, De Las Casas LE, Anfossi S, Roman JJ, O'Brien T and
Pecorelli S: The novel serine protease tumor-associated
differentially expressed gene-15 (matriptase/MT-SP1) is highly
overexpressed in cervical carcinoma. Cancer. 98:1898–1904. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe H: Extracellular
matrix-regulation of cancer invasion and metastasis. Gan To Kagaku
Ryoho. 37:2058–2061. 2010.PubMed/NCBI
|
|
10
|
Webb SL, Sanders AJ, Mason MD and Jiang
WG: Type II transmembrane serine protease (TTSP) deregulation in
cancer. Front Biosci. 16:539–552. 2011. View Article : Google Scholar
|
|
11
|
Zitka O, Kukacka J, Krizkova S, Huska D,
Adam V, Masarik M, Prusa R and Kizek R: Matrix metalloproteinases.
Curr Med Chem. 17:3751–3768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
List K: Matriptase: A culprit in cancer?
Future Oncol. 5:97–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jankun J and Skrzypczakjankun E: Molecular
basis of specific inhibition of urokinase plasminogen activator by
amiloride. Cancer Biochem Biophys. 17:109–123. 1999.PubMed/NCBI
|
|
14
|
Vassalli JD and Belin D: Amiloride
selectively inhibits the urokinase-type plasminogen activator. FEBS
Lett. 214:187–191. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa
P, Gui Y and Cai Z: Synthetic miRNA-Mowers targeting miR-183-96-182
cluster or miR-210 inhibit growth and migration and induce
apoptosis in bladder cancer cells. PLoS One. 7:e522802012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi S, Song Y, Peng Y, Wang H, Long H, Yu
X, Li Z, Fang L, Wu A, Luo W, et al: ZEB2 mediates multiple
pathways regulating cell proliferation, migration, invasion and
apoptosis in glioma. PLoS One. 7:e388422012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones JL and Walker RA: Control of matrix
metalloproteinase activity in cancer. J Pathol. 183:377–399. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lijnen HR: Matrix metalloproteinases and
cellular fibrinolytic activity. Biochemistry. 67:92–98.
2002.PubMed/NCBI
|
|
21
|
Overall CM and Kleifeld O: Tumour
microenvironment-opinion: Validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nature Rev
Cancer. 6:227–239. 2006. View
Article : Google Scholar
|
|
22
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (Review). Int J Oncol.
34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baltazarrodriguez LM, Anayaventura A,
Andradesoto M, Monrroy-Guizar EA, Bautista-Lam JR, Jonguitud-Olguin
G, Cepeda-Lopez FR, Centeno-Aguilar VA, Gonzalez-Hernandez NA,
Soriano-Hernández AD, et al: Polymorphism in the matrix
metalloproteinase-2 gene promoter is associated with cervical
neoplasm risk in Mexican women. Biochem Genet. 46:137–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CY, Tseng IC, Chou FP, Su SF, Chen YW,
Johnson MD and Dickson RB: Zymogen activation, inhibition and
ectodomain shedding of matriptase. Front Biosci. 13:621–635. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JW, Song S, Choi JJ, Lee SJ, Kim BG,
Park CS, Lee JH, Lin CY, Dickson RB and Bae DS: Increased
expression of matriptase is associated with histopathologic grades
of cervical neoplasia. Hum Pathol. 36:626–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danø K, Andreasen PA, Grøndahl-Hansen J,
Kristensen P, Nielsen LS and Skriver L: Plasminogen activators,
tissue degradation and cancer. Adv Cancer Res. 44:139–266. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saksela O and Rifkin DB: Cell-associated
plasminogen activation: regulation and physiological functions.
Annu Rev Cell Biol. 4:93–126. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Jones CJ and Dang J: Human
urokinase receptor expression is inhibited by amiloride and induced
by tumor necrosis factor and phorbol ester in colon cancer cells.
FEBS Lett. 353:138–142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Dang J, Liang X and Doe WF:
Amiloride modulates urokinase gene expression at both transcription
and post-transcription levels in human colon cancer cells. Clin Exp
Metastasis. 13:196–202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klinghofer V, Stewart K, Mcgonigal T,
Smith R, Sarthy A, Nienaber V, Butler C, Dorwin S, Richardson P,
Weitzberg M, et al: Species specificity of amidine-based urokinase
inhibitors. Biochemistry. 40:9125–9131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park KS, Poburko D, Wollheim CB and
Demaurex N: Amiloride derivatives induce apoptosis by depleting ER
Ca(2+) stores in vascular endothelial cells. Br J Pharmacol.
156:1296–1304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matthews H, Ranson M and Kelso MJ:
Anti-tumour/metastasis effects of the potassium-sparing diuretic
amiloride: An orally active anti-cancer drug waiting for its
call-of-duty? Int J Cancer. 129:2051–2061. 2011. View Article : Google Scholar : PubMed/NCBI
|