Introduction
Spinal cord injury (SCI) is a serious neurological
and pathological disease, which directly damages spinal cord
parenchyma and the associated spinal nerves. There are ~12,000 new
cases of SCI annually in the USA (1), the majority of which are caused by
vehicle accidents, falls or violence (2). Secondary complications, which occur
in the endocrinologic, cardiovascular, gastrointestinal,
musculoskeletal and respiratory systems, are common in cases of SCI
(3), which often lead to high
morbidity and mortality rates. At present, the primary therapeutic
methods for SCI include drugs (4),
hypothermia therapy (5), stem cell
transplantation (2) and
immunotherapy (6). Despite
considerable improvement in therapeutic methods of SCI in previous
decades, the long-term outcomes of patients with SCI remain poor.
Thus, it is important to identify and implement novel and more
effective therapeutic strategies for patients with SCI.
Hyperglycemia, often caused by diabetes mellitus or
non-diabetic hyperglycemia, is considered to be a potentially
life-threatening clinical condition. Food diet, genetic factors,
obesity and drugs may induce hyperglycemia. Increasing evidence has
shown that hyperglycemia can impair several systems, including the
cardiovascular system (7),
auditory system (8), urinary
system (9) and visual system
(10). Meng et al (11) revealed that hyperglycemia can lead
to neuroinflammation by activating the NLR family pyrin domain
containing 1 inflammasome, which may be a mechanism of
diabetes-associated neural injury in diabetic rats. Dai et
al (12) demonstrated that
neuropeptide FF-amide peptide precursor (NPFF) contributed to
diabetic nerve injury recovery through NPFF receptor 2, and
neuropeptide FF was a potential neuroregenerative factor for nerve
injury associated with hyperglycemia. Hyperglycemia is an important
indicator of health in patients with SCI (13); however, to date, the underlying
association between hyperglycemia and SCI remains to be fully
elucidated.
To our knowledge, there is no consensus on the
treatment of patients with SCI with hyperglycemia. In previous
years, studies have showed that mitochondria are important in nerve
cell death following contusive SCI, which primarily regulate cell
apoptosis through the mitochondrial permeability transition pore
(mPTP), a non-specific giant channel (14). Therefore, inhibiting mPTP channels
can prevent and reduce cell apoptosis. Cyclosporin A (CsA), an 11
amino acid cyclic peptide separated from Tolypocladium
inflatumin culture medium, is regarded as an immunosuppressant.
CsA can inhibit T lymphocytes through the following two pathways:
i) CsA inhibits the generation of interleukin (IL)-2 (15); ii) CsA inhibits generation of the
IL-2 receptor (16). CsA has been
widely used in the treatment of various diseases, including
aplastic anemia (17), nephritic
syndrome (18), rheumatoid
arthritis (19) and psoriasis
(20). Studies have shown that CsA
inhibits cyclophilin-D (Cyp-D), and then prevents the formation of
mPTP (21). CsA can effectively
reduce cerebral ischemic injury aggravated by hyperglycemia
(22). However, to date, whether
CsA can also reduce the severity of SCI aggravated by hyperglycemia
remains to be elucidated.
The present study aimed to focus on the cell
apoptotic pathway associated with mitochondria in the spinal cord,
which may be used to clarify the underlying mechanism of CsA in the
treatment of SCI with hyperglycemia. The results may provide a
potential therapeutic drug and effective strategy for the treatment
of patients with SCI and hyperglycemia.
Materials and methods
Animals and groups
A total of 40 adult female healthy SPF
Sprague-Dawley rats, weighing 220–240 g, were obtained from
Shanghai Laboratory Animal Center Co., Ltd. (Shanghai, China).
Animal experimental trials were approved by the Animal Use and
Ethics Committee of Ningxia Medical University (Yinchuan, China).
The 40 rats were randomly allocated into four groups: Sham group,
(n=10), in which rats underwent laminectomy only; SCI group (n=10),
in which rats underwent SCI only; SCI+hyperglycemia group (n=10),
in which rats received streptozotocin (STZ) prior to SCI; and
SCI+hyperglycemia+CsA group (n=10), in which rats received STZ
prior to SCI, and CsA following SCI.
All animals were allowed to adapt to the environment
for 3 weeks prior to the trial. The rats of the SCI+hyperglycemia
group and SCI+hyperglycemia+CsA group were intraperitoneally
injected with STZ (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) at 30 mg/kg body weight dissolved in citrate buffer (pH
4.5) every day to induce hyperglycemia. The rats were considered to
have hyperglycemia if their blood glucose levels were maintained at
>16.8 mmol/l 48 h following STZ injection. The rats were
maintained in ambient temperature (20–25°C) with a 12:12 dark/light
cycle and were provided with free access to food and ultrapure
water. All attempts were made to reduce the number of animals used
in the present study and to minimize their suffering.
Spinal cord injury modeling
The rats were anesthetized with 1.25% halothane in
an oxygen/nitrous oxide (30/70%) gas mixture. The dorsal skin was
incised and the soft tissue was dissected, following which a
laminectomy was performed carefully at the T10 vertebrae, and the
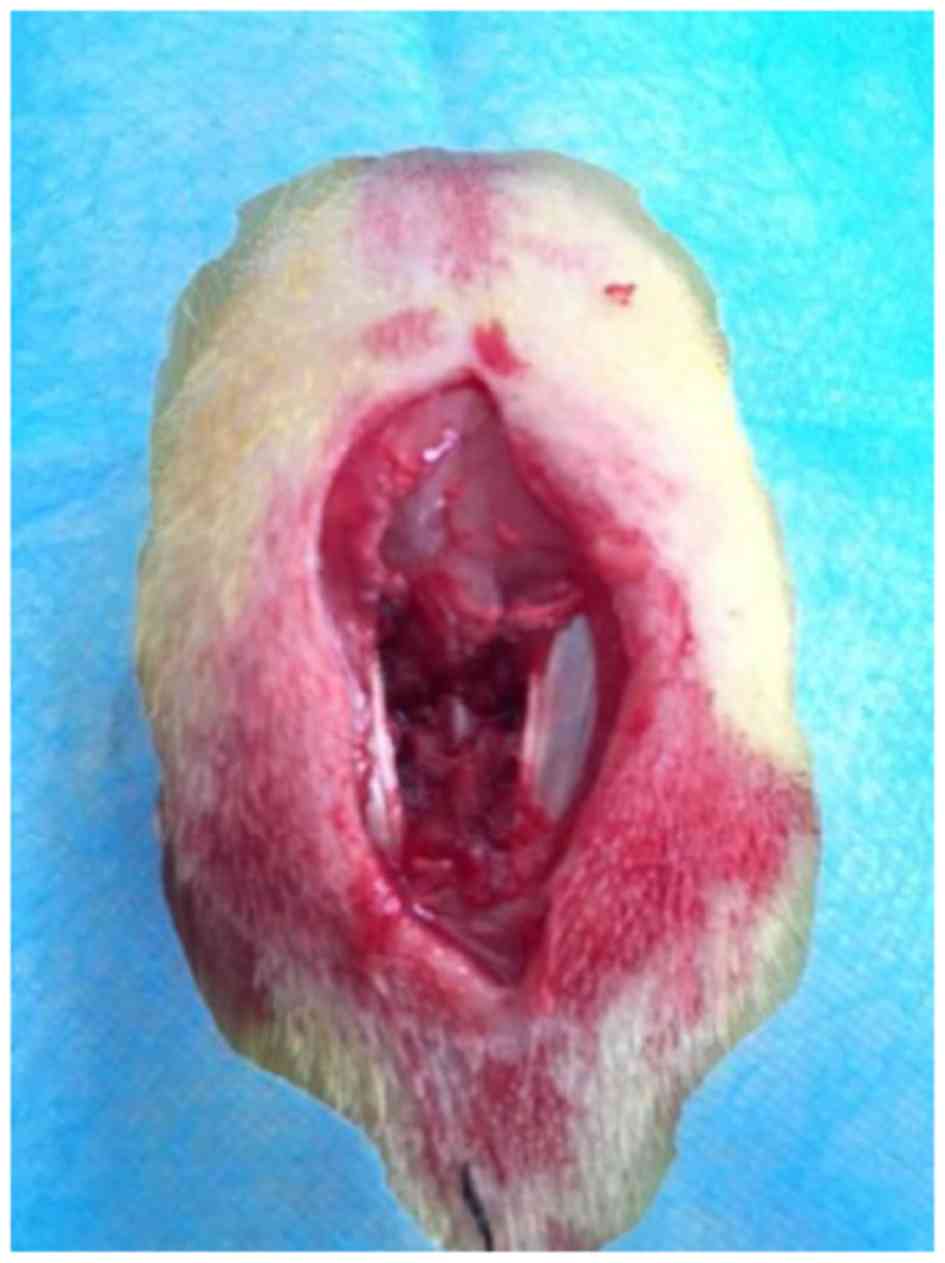
vertebral column was completely exposed (Fig. 1). Contusion injury was performed in
accordance with Allen's method (23). A weight of 10 g was dropped from a
height of 5 cm onto the exposed spinal cord, and the impounder
remained for 20 sec following withdrawal to produce a moderate
contusion. Bacitracin ointment (Qualitest Pharmaceuticals,
Huntsville, AL, USA) was used to avoid infections, and another 5 ml
of normal saline solution was injected subcutaneously. The animals
were allowed to recover on a water-circulating heating pad at 37°C
(Gaymar Industries, Orchard Park, NY, USA). The animals in the Sham
group received only a laminectomy for 1 min. Following contusive
SCI, twice daily bladder expression was performed until bladder
function had recovered. Two of the hyperglycemia rats died within
the first week following contusive SCI. The SCI+hyperglycemia+CsA
group rats were subcutaneously injected with CsA (4 mg/kg body
weight; Novartis, Basal, Switzerland) every day. According to the
trial, the rats were sacrificed using an overdose of pentobarbital
sodium on day 7, with the exception of 18 rats, which were used to
assess locomotion recovery.
Neurological function assessment
The Basso, Beattie, Bresnahan (BBB) locomotor rating
scale (24) was used to detect the
recovery of motor function following contusive SCI. A score of 0
was considered to indicate no spontaneous mobility, whereas a score
of 21 was considered to indicate complete mobility. The ability of
an animal to maintain postural stability was assessed using
Rivlin's inclined plane test (25). The maximum angle at which the rats
were able to maintain a constant position on an oblique board for
at least 5 sec was considered the critical value and was recorded.
Two independent examiners who were blinded to treatment observed
the rats prior to injury, and at 1, 3, 7, 14 and 21 days
post-injury.
Flow cytometric analysis of cell
apoptosis
Several 5-µm-thick spinal cord sections containing
the contusion sites were isolated. A single-cell suspension was
obtained by trypsinization, and the rate of apoptosis was measured
using an Annexin V Apoptosis Detection kit according the
manufacturer's protocol. Finally, the apoptotic cells were examined
and quantified using flow cytometry (BD Biosciences, San Diego, CA,
USA).
ELISA analysis
Total proteins from the spinal cord tissues were
extracted in RIPA lysis buffer (Beyotime, Haimen, China) containing
protease inhibitor phenylmethanesulfonyl fluoride (Beyotime). A BCA
protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used to assess protein concentrations. The concentrations
of interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) were
measured using respective ELISA kits according to the
manufacturer's protocol, and analyzed using a microplate reader
(Dynex Technologies, Chantilly, CA, USA). The values are expressed
as pg/ml.
Western blot analysis
To determine the relative protein expression levels
of Cyp-D and apoptosis-inducing factor (AIF), equal amounts of
protein (40 µg) from each group were separated by SDS-PAGE (12%
gel) and then transferred onto a PVDF membrane (Millipore,
Billerica, MA, USA). Targeted proteins were detected by incubation
overnight at 4°C with primary antibodies against Cyp-D (dilution,
1:500; cat. no. PA5-31061; Invitrogen; Thermo Fisher Scientific,
Inc.) and AIF (dilution, 1:200; cat. no. 5318; Cell Signaling
Technology, Inc., Danvers, MA, USA), followed by incubation for 1 h
at 37°C with horseradish peroxidase-conjugated secondary antibody
(dilution, 1:500; cat. no. 29-0382-76; GE Healthcare, Chicago, IL,
USA). Signals were visualized using ECL Advance reagent (Beyotime
Institute of Biotechnology) and quantified using ImageJ 2.0
software (National Institutes of Health, Bethesda, MD, USA). The
expression levels of target protein were determined following
normalization to individual β-actin levels.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM SPSS, Armonk, NY, USA) and GraphPad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). All data are
presented as the mean ± standard deviation. Unpaired Student's
t-test was used to compare the experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight and blood glucose
As shown in Table
I, prior to STZ injection, no significant differences in weight
or blood glucose were observed among the four groups of rats. At 4
weeks post-STZ injection, prior to SCI, the, rats of the
SCI+hyperglycemia group and SCI+hyperglycemia+CsA group had
significantly lower body weights and higher blood glucose levels
(P<0.05), compared with the rats of the Sham group and SCI
group, indicating that the rats exhibited hyperglycemia.
 | Table I.Body weights and blood glucose levels
of rats. |
Table I.
Body weights and blood glucose levels
of rats.
| Group | Body weight (g) | Blood glucose
(mmol/l) |
|---|
| Pre-STZ
injection |
|
|
| Sham | 233±6 | 4.2±0.5 |
|
SCI | 232±6 | 4.1±0.5 |
|
SCI+hyperglycemia | 231±6 | 4.2±0.6 |
|
SCI+hyperglycemia+CsA | 233±6 | 4.3±0.6 |
| 4 weeks post-STZ
injection/pre-SCI |
|
|
|
Sham | 233±6 | 4.2±0.6 |
|
SCI | 232±6 | 4.2±0.5 |
|
SCI+hyperglycemia | 209±15a |
24.3±4.9a |
|
SCI+hyperglycemia+CsA | 211±16b |
23.8±5.0a |
Neurological function assessment
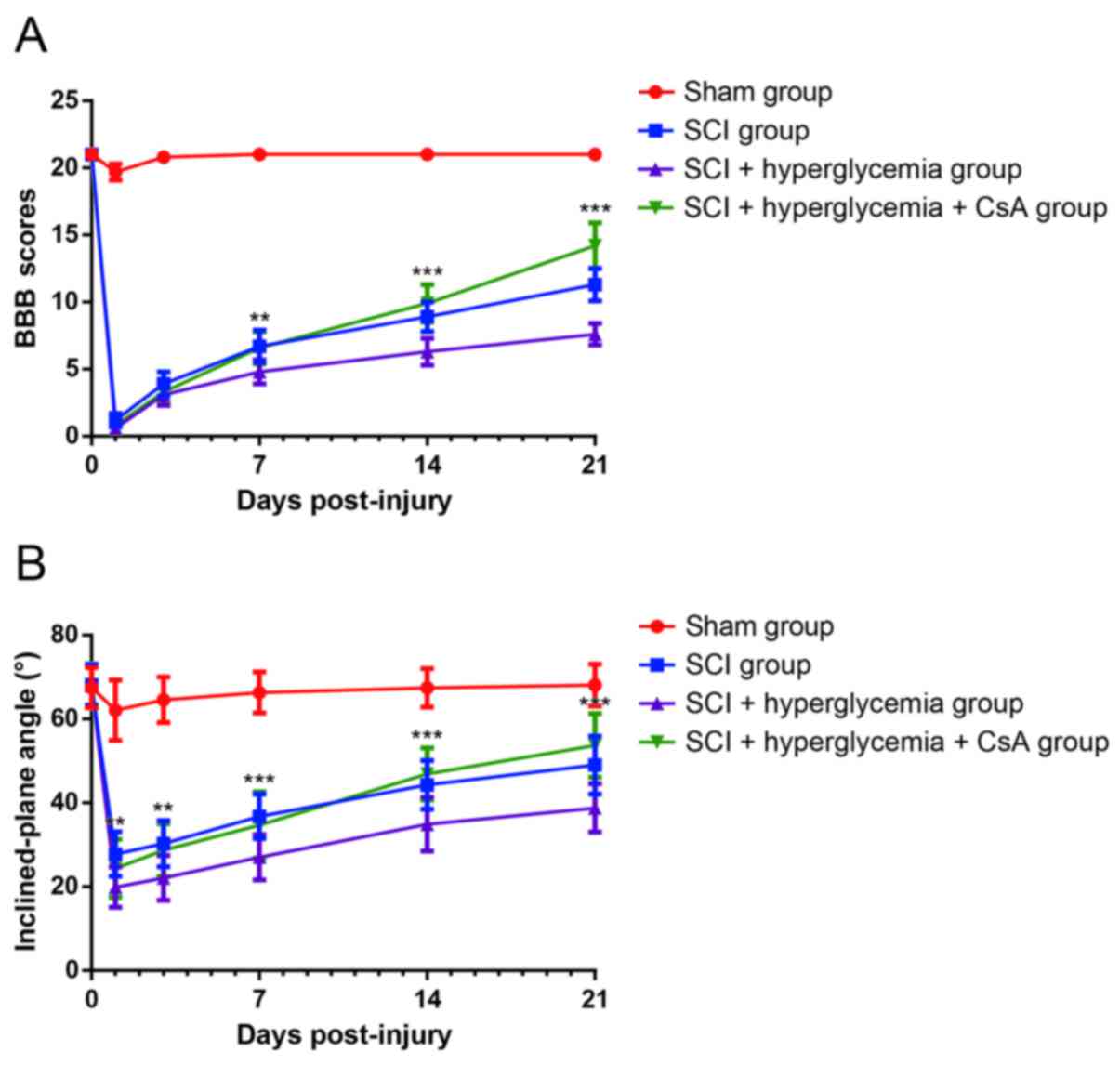
As shown in Fig.
2A, the BBB scores of the rats in Sham group were similar to
the normal values at each time point. Compared with the rats in the
Sham group, the rats with SCI showed lower BBB scores at each time
point, and the differences were statistically significant
(P<0.001), indicating severe impairment of neurological
function. The BBB scores of the SCI rats with hyperglycemia at 21
days remained <10, whereas the rats treated with CsA had scores
above 10 points, and the differences were statistically significant
(P<0.001). As shown in Fig. 2B,
following contusive SCI, the inclined-plate angles of the rats were
decreased, compared with those in the Sham group at each time
point, and the differences were statistically significant
(P<0.001). The inclined-plate angles of the rats treated with
CsA increased to significantly higher angles, compared with those
of rats in the SCI+hyperglycemia group at 14 and 21 days
post-injury (P<0.001). These results suggested that
hyperglycemia exaggerated the neurological function of the rats
following contusive SCI, and that CsA accelerated the recovery of
neurological function.
Flow cytometric analysis of apoptotic
cells
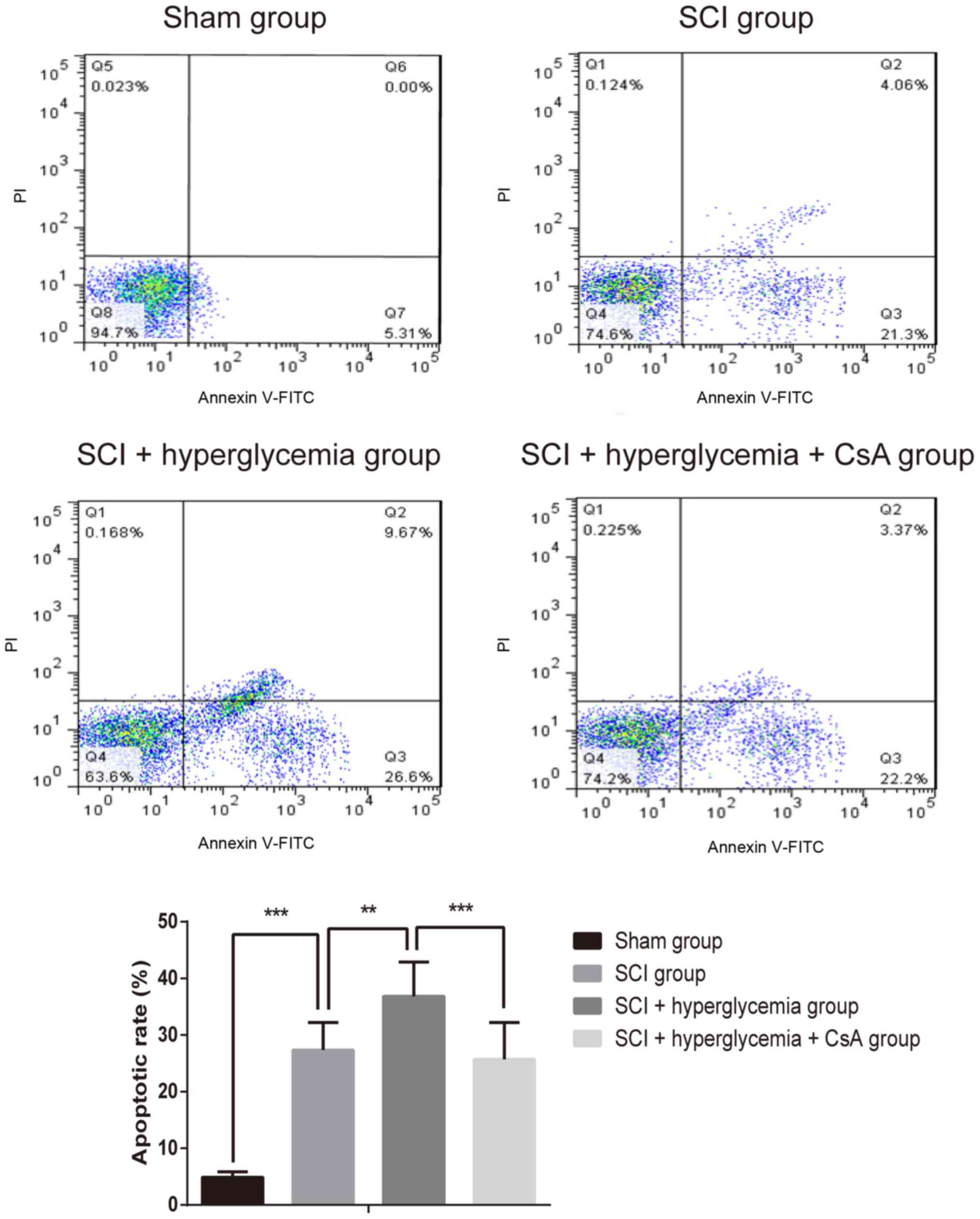
As shown in Fig. 3,
in the Sham group, almost no apoptotic cells were observed.
However, the numbers of apoptotic cells in the spinal cord were
significantly increased following contusive SCI (P<0.001),
indicating increased apoptotic activity of cells in the spinal
cords. Additionally, the number of apoptotic cells in the
SCI+hyperglycemia group was higher, compared with that in the SCI
group (P=0.002), and the number of apoptotic cells in the
SCI+hyperglycemia+CsA group was significantly lower, compared with
that of the SCI+hyperglycemia group (P<0.001). These results
suggested that hyperglycemia exaggerated the apoptosis of spinal
cord cells following contusive SCI, and that CsA inhibited this
apoptosis.
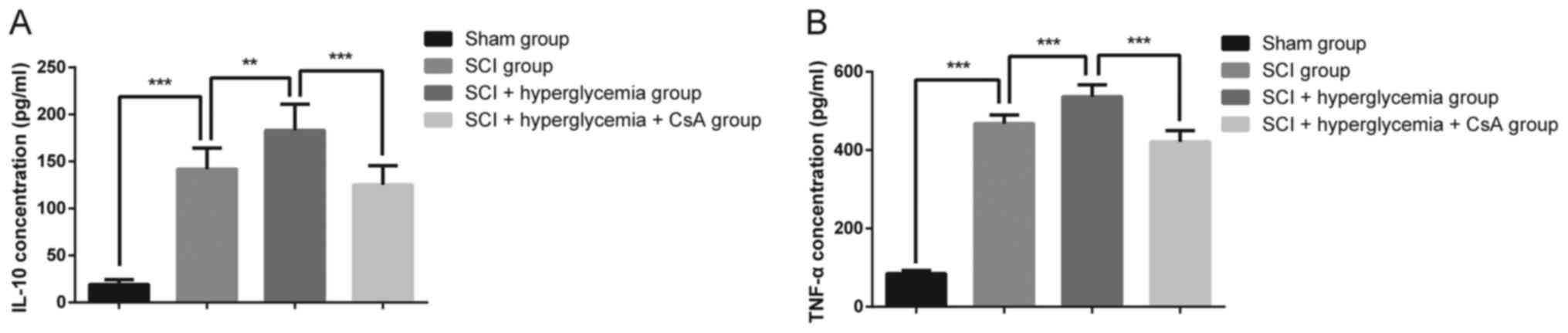
ELISA analysis of IL-10 and TNF-α
As shown in Fig. 4A and
B, according to the results of ELISA analysis, the rats with
SCI exhibited a significant increase in the expression levels of
IL-10 and TNF-α, compared with those in the Sham group
(P<0.001). In addition, the expression levels of IL-10 and TNF-α
in the rats in the SCI+hyperglycemia group were higher, compared
with those in the rats in the SCI group (P=0.003 and P<0.001,
respectively), whereas the expression levels of IL-10 and TNF-α in
the rats in the SCI+hyperglycemia+CsA group were significantly
lower, compared with those in the SCI+hyperglycemia group
(P<0.001). These results suggested that hyperglycemia
exaggerated the inflammation in the spinal cord following contusive
SCI, and that CsA alleviated the inflammation in the spinal
cord.
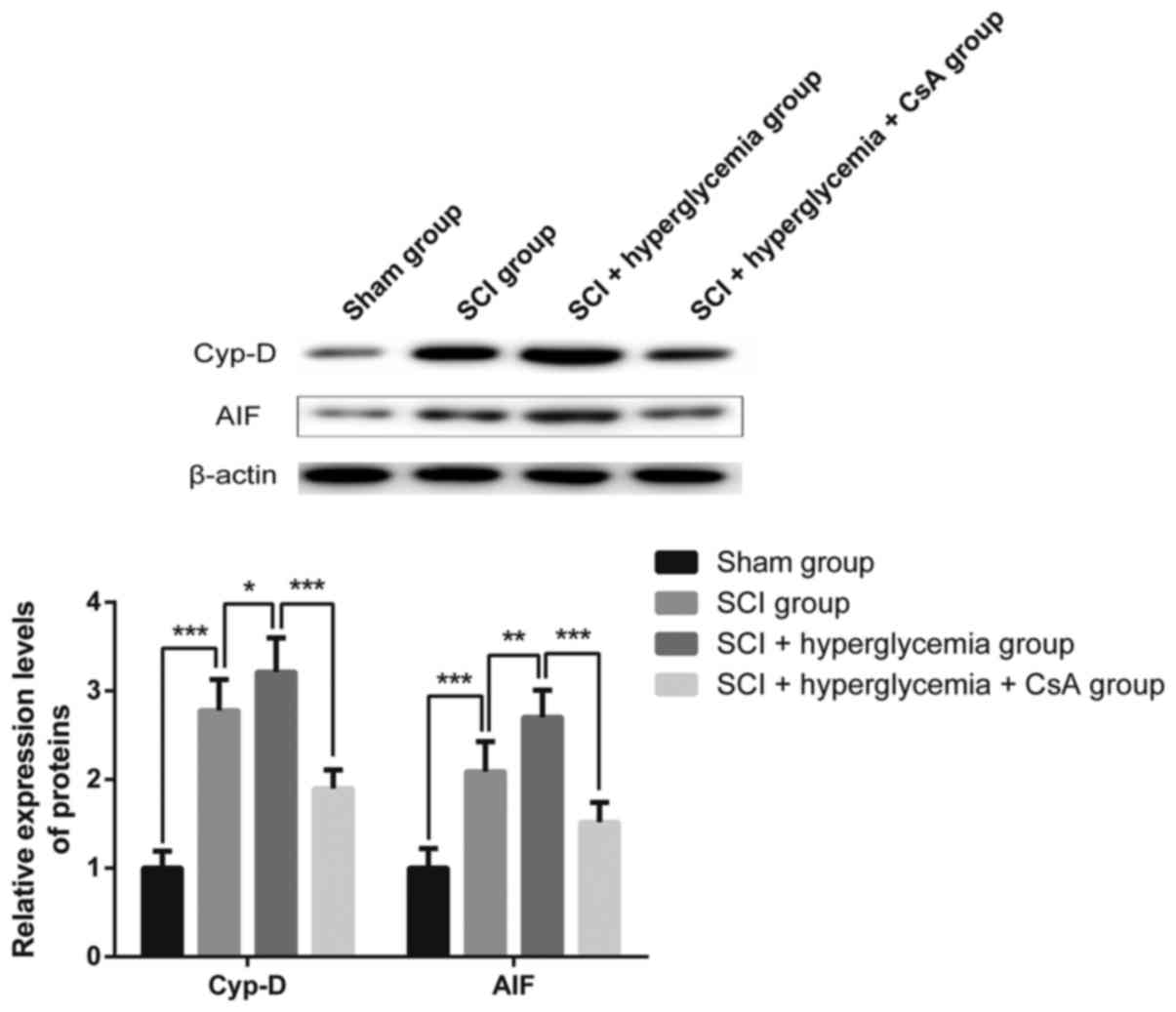
Western blot analysis of the protein
expression of Cyp-D and AIF
As shown in Fig. 5,
according to the results of the western blot analysis, compared
with the rats of the Sham group, the rats with SCI exhibited
increased protein expression levels of Cyp-D and AIF in the spinal
cord (P<0.001). The protein levels of Cyp-D and AIF in the
spinal cords of rats in the SCI+hyperglycemia group were
significantly higher, compared with those of rats in the SCI group
(P=0.026 and P=0.001, respectively), whereas the protein expression
levels of Cyp-D and AIF in the spinal cords of rats in the
SCI+hyperglycemia+CsA group were lower, compared with those in the
SCI+hyperglycemia group (P<0.001). These results indicated that
hyperglycemia exaggerated the impairment of mitochondrial function
in the spinal cord cells following contusive SCI, and that CsA
inhibited the impairment of mitochondrial function.
Discussion
The pathogenesis of SCI can usually be divided into
two stages, primary injury and secondary injury (26). Through the establishment of SCI rat
models, the present study aimed to reveal the therapeutic effects
of CsA on SCI of rats with hyperglycemia and to identify a novel
method to treat SCI with hyperglycemia. The results showed that
hyperglycemia aggravated the secondary injury of SCI, whereas that
CsA improved the motor capacity of rats and reduced inflammation in
the spinal cord following contusive SCI. This indicated that CsA
effectively alleviated SCI impairment aggravated by
hyperglycemia.
As is already known, the main clinical
manifestations of SCI are motor and sensory disorders with a series
of serious complications (27,28).
The BBB locomotor rating scale and Rivlin's inclined plane test are
commonly used methods, which can evaluate changes in the motor
capacity of experimental animals. In the present study, the results
showed that the BBB scores and inclined-plate angles were decreased
in the SCI rats. In addition, the BBB scores and inclined-plate
angles were significantly decreased in the SCI rats with
hyperglycemia, whereas CsA partially improved the decreased BBB
scores and inclined-plate angles. These results indicated that the
SCI rat models were established successfully, and that CsA improved
the neurological function of the SCI rats with hyperglycemia.
Previous studies have found that inflammatory
cytokines and inflammatory response in the spinal cord are closely
correlated with the pathogenesis of SCI (29). For example, interleukin-4 (IL-4) is
an anti-inflammatory cytokine, which is crucial in regulating acute
macrophage activation and confining secondary cavity formation
following contusive SCI (30).
Fenn et al (31) showed
that IL-4 reprogrammed active microglia to a phenotype promoting
improved neurite growth, which was impaired with age, associated
with reduced functional recovery following contusive SCI. IL-10 is
also an anti-inflammatory cytokine, which can reduce
pain-associated behaviors following contusive SCI (32). In addition, Lin et al
(33) demonstrated that
downregulation of IL-1l significantly decreased the expression of
TNF-α, and had therapeutic benefits by alleviating the inflammatory
response and improving the recovery of SCI. This indicated that
inhibiting TNF-α may contribute to recovery from SCI. To date,
whether hyperglycemia can aggravate the severity of SCI remains
unclear. In the present study, the expression levels of IL-10 and
TNF-α were significantly higher in SCI rats with hyperglycemia.
These results demonstrated that hyperglycemia aggravated the
severity and inflammatory response of SCI. At present, there are no
effective therapies for SCI due to the frequent occurrence of
secondary injury, including edema, ischemia, excitotoxicity,
inflammation, oxidative damage and apoptotic cell death (34).
Mitochondria, a primary structure responsible for
generating ATP, are important in maintaining the normal function
and growth of eukaryotic cells. When mitochondrial damage occurs,
the cells lack energy supply due to the inability to produce
sufficient ATP, which leads to cell dysfunction and even necrosis.
Mitochondrial membrane perturbations are also involved in calcium
overloading, the opening of mPTP channels and the release of
apoptotic mediators into the cytoplasm (35). mPTP channels consist predominantly
of three proteins, Cyp-D, adenine nucleotide translocator 1 and the
voltage-dependent anion channel. mPTP channels are induced to open
by pro-apoptotic stimuli, which cause loss of mitochondrial
integrity and allow the release of several molecules into the
cytoplasm (36). Mitochondria
function as Ca2+ ‘sinks’, taking up excess
Ca2+ to maintain Ca2+ levels in the cytosol,
and mitochondrial function is severely impaired following contusive
SCI (34). AIF is also a
mitochondrial protein, which can exert a pro-apoptotic effect. It
is released from mitochondria and translocates to nuclei, followed
by the induction of chromatin condensation and DNA degradation
during cell apoptosis (37). To
date, previous studies have indicated that CsA has a therapeutic
effect on a number of diseases through various pathways, including
reducing the proportion of CD4(+) T cells and the expression level
of interferon-i for the treatment of aplastic anemia (38). Guan et al (39) demonstrated that CsA has a
protective effect in improving mitochondrial function through
inhibiting mitofusin proteins, which are located on the outside of
mitochondrial membranes and regulate mitochondrial fusion. In the
present study, the number of apoptotic cells, and the protein
levels of Cyp-D and AIF in the spinal cord were higher in the SCI
rats with hyperglycemia, indicating that hyperglycemia accelerated
the apoptosis of spinal cord cells following contusive SCI. CsA
reduced the apoptosis of spinal cord cells and increased the
protein expression levels of Cyp-D and AIF. Consistently, Kim et
al (40) showed that
CsA-mediated Cyp-D inhibition, which protected retinal ganglion
cells against mitochondrial dysfunction, providing therapeutic
potential for ischemic injury. These results demonstrated that CsA
effectively reduced the SCI impairment aggravated by
hyperglycemia.
In conclusion, the results of the present study
showed that hyperglycemia aggravated the severity of SCI, and that
CsA effectively reduce the SCI aggravated by hyperglycemia. These
results provide a novel and relatively more effective potential
therapeutic strategy for patients with SCI and hyperglycemia.
However, the present study had several limitations, including
lacking of in vitro experiments and no determination of the
expression levels of IL-10 and TNF-α at each time point. Future
investigations aim to examine whether CsA has a relative effect on
spinal cord nerve cells in vitro and determine its specific
mechanisms.
Acknowledgements
This study was funded by the Natural Science
Foundation of China (grant no. 81360196) and the Natural Science
Foundation of Ningxia (grant no. NZ15157).
References
|
1
|
Saunders LL, Clarke A, Tate DG,
Forchheimer M and Krause JS: Lifetime prevalence of chronic health
conditions among persons with spinal cord injury. Arch Phys Med
Rehabil. 96:673–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falnikar A, Li K and Lepore AC:
Therapeutically targeting astrocytes with stem and progenitor cell
transplantation following traumatic spinal cord injury. Brain Res.
1619:91–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gorman PH: The review of systems in spinal
cord injury and dysfunction. Continuum (Minneap Minn). 17:630–634.
2011.PubMed/NCBI
|
|
4
|
Kabu S, Gao Y, Kwon BK and Labhasetwar V:
Drug delivery, cell-based therapies, and tissue engineering
approaches for spinal cord injury. J Control Release. 219:141–154.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J and Pearse DD: Therapeutic
hypothermia in spinal cord injury: The status of its use and open
questions. Int J Mol Sci. 16:16848–16879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YT, Lu XM, Chen KT, Shu YH and Qiu
CH: Immunotherapy strategies for spinal cord injury. Curr Pharm
Biotechnol. 16:492–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquier J, Hoarau-Véchot J, Fakhro K,
Rafii A and Abi Khalil C: Epigenetics and Cardiovascular Disease in
Diabetes. Curr Diab Rep. 15:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Helzner EP and Contrera KJ: Type 2
diabetes and hearing impairment. Curr Diab Rep. 16:32016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendez CE, Der Mesropian PJ, Mathew RO and
Slawski B: Hyperglycemia and acute kidney injury during the
perioperative period. Curr Diab Rep. 16:102016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Usuelli V and La Rocca E: Novel
therapeutic approaches for diabetic nephropathy and retinopathy.
Pharmacol Res. 98:39–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng XF, Wang XL, Tian XJ, Yang ZH, Chu
GP, Zhang J, Li M, Shi J and Zhang C: Nod-like receptor protein 1
inflammasome mediates neuron injury under high glucose. Mol
Neurobiol. 49:673–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai Y, Zhao X, Chen P, Yu Y, Wang Y and
Xie L: Neuropeptide FF promotes recovery of corneal nerve injury
associated with hyperglycemia. Inves Ophthalmol Vis Sci.
56:7754–7765. 2015. View Article : Google Scholar
|
|
13
|
Wang YH, Chen SY, Wang TD, Hwang BS, Huang
TS and Su TC: The relationships among serum glucose, albumin
concentrations and carotid atherosclerosis in men with spinal cord
injury. Atherosclerosis. 206:528–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Readnower RD, Pandya JD, McEwen ML, Pauly
JR, Springer JE and Sullivan PG: Post-injury administration of the
mitochondrial permeability transition pore inhibitor, NIM811, is
neuroprotective and improves cognition after traumatic brain injury
in rats. J Neurotrauma. 28:1845–1853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YX, Zhu KY, Liu YL and Jiang DF:
Effects of dietary conjugated linoleic acids on cellular immune
response of piglets after cyclosporin A injection. Animal.
10:1660–1665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Gowelli HM, Helmy MW, Ali RM and El-Mas
MM: Celecoxib offsets the negative renal influences of cyclosporine
via modulation of the TGF-β1/IL-2/COX-2/endothelin ET(B) receptor
cascade. Toxicol Appl Pharmacol. 275:88–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dao AT, Yamazaki H, Takamatsu H, Sugimori
C, Katagiri T, Maruyama H, Zaimoku Y, Maruyama K, Ly TQ, Espinoza L
and Nakao S: Cyclosporine restores hematopoietic function by
compensating for decreased Tregs in patients with pure red cell
aplasia and acquired aplastic anemia. Ann Hematol. 95:771–781.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Working Group For National Survey On
Status Of Diagnosis And Treatment Of Childhood Renal Diseases, .
Multicenter survey of diagnostic and therapeutic status in Chinese
childhood patients with steroid-sensitive,
relaping/steroid-dependent nephrotic syndrome. Zhonghua Er Ke Za
Zhi. 52:194–200. 2014.(In Chinese). PubMed/NCBI
|
|
19
|
Hashimoto A, Kanisawa Y, Fujimi A,
Nakajima C, Hayasaka N, Yamada S, Okuda T, Minami S, Yamauchi N,
Iwasaki S, et al: Thrombocytopenia and anemia with anti-c-mpl
antibodies effectively treated with cyclosporine in a patient with
rheumatoid arthritis and chronic renal failure. Intern Med.
55:683–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar R, Dogra S, Amarji B, Singh B, Kumar
S, Sharma, Vinay K, Mahajan R and Katare OP: Efficacy of novel
topical liposomal formulation of cyclosporine in mild to moderate
stable plaque psoriasis: A randomized clinical trial. JAMA
Dermatol. 152:807–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sánchez JA, Alfonso A, Leirós M, Alonso E,
Rateb ME, Jaspars M, Houssen WE, Ebel R and Botana LM: Spongionella
secondary metabolites regulate store operated calcium entry
modulating mitochondrial functioning in SH-SY5Y neuroblastoma
cells. Cell Physiol Biochem. 37:779–792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai Y, Sun Q, Zhang X, Hu Y, Zhou M and
Shi J: Cyclosporin A ameliorates early brain injury after
subarachnoid hemorrhage through inhibition of a Nur77 dependent
apoptosis pathway. Brain Res. 1556:67–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yacoub A, Hajec MC, Stanger R, Wan W,
Young H and Mathern BE: Neuroprotective effects of perflurocarbon
(oxycyte) after contusive spinal cord injury. J Neurotrauma.
31:256–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotraum. 12:1–21. 1995. View Article : Google Scholar
|
|
25
|
Han X, Yang N, Xu Y, Zhu J, Chen Z, Liu Z,
Dang G and Song C: Simvastatin treatment improves functional
recovery after experimental spinal cord injury by upregulating the
expression of BDNF and GDNF. Neurosci Lett. 487:255–259. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou KL, Zhou YF, Wu K, Tian NF, Wu YS,
Wang YL, Chen DH, Zhou B, Wang XY, Xu HZ and Zhang XL: Stimulation
of autophagy promotes functional recovery in diabetic rats with
spinal cord injury. Sci Re. 5:171302015.
|
|
27
|
Grabher P, Callaghan MF, Ashburner J,
Weiskopf N, Thompson AJ, Curt A and Freund P: Tracking sensory
system atrophy and outcome prediction in spinal cord injury. Ann
Neurol. 78:751–761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alexanian AR, Svendsen CN, Crowe MJ and
Kurpad SN: Transplantation of human glial-restricted neural
precursors into injured spinal cord promotes functional and sensory
recovery without causing allodynia. Cytotherapy. 13:61–68. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ni H, Jin W, Yuan B, Zhu T, Wang J, Jiang
J, Liang W and Ma Z: Curcumin inhibits the increase of labile zinc
and the expression of inflammatory cytokines after traumatic spinal
cord injury in rats. J Surg Res. 187:646–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SI, Jeong SR, Kang YM, Han DH, Jin BK,
Namgung U and Kim BG: Endogenous expression of interleukin-4
regulates macrophage activation and confines cavity formation after
traumatic spinal cord injury. J Neurosci Res. 88:2409–2419.
2010.PubMed/NCBI
|
|
31
|
Fenn AM, Hall JC, Gensel JC, Popovich PG
and Godbout JP: IL-4 signaling drives a unique arginase+/IL-1β+
microglia phenotype and recruits macrophages to the inflammatory
CNS: Consequences of age-related deficits in IL-4Rα after traumatic
spinal cord injury. J Neurosci. 34:8904–8917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lau D, Harte SE, Morrow TJ, Wang S, Mata M
and Fink DJ: Herpes simplex virus vector-mediated expression of
interleukin-10 reduces below-level central neuropathic pain after
spinal cord injury. Neurorehabil Neural Repair. 26:889–897. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin WP, Lin JH, Cai B, Shi JX, Li WJ,
Choudhury GR, Wu SQ, Wu JZ, Wu HP and Ke QF: Effect of
adenovirus-mediated RNA interference of IL-1β expression on spinal
cord injury in rats. Spinal Cord. 54:778–784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McEwen ML, Sullivan PG, Rabchevsky AG and
Springer JE: Targeting mitochondrial function for the treatment of
acute spinal cord injury. Neurotherapeutics. 8:168–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
White CR, Giordano S and Anantharamaiah
GM: High-density lipoprotein, mitochondrial dysfunction and cell
survival mechanisms. Chemistry and physics of lipids. 199:161–169.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fu M, Shi W, Li Z and Liu H: Activation of
mPTP-dependent mitochondrial apoptosis pathway by a novel pan HDAC
inhibitor resminostat in hepatocellular carcinoma cells. Biochem
Biophys Res Commun. 477:527–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Scott AJ, Wilkinson AS and Wilkinson JC:
Basal metabolic state governs AIF-dependent growth support in
pancreatic cancer cells. BMC Cancer. 16:2862016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu S, Wang X, Lu Y, Xiao J, Liang J,
Zhong X and Chen Y: The combined use of cytokine-induced killer
cells and cyclosporine a for the treatment of aplastic anemia in a
mouse model. J Interferon Cytokine Res. 35:401–410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guan N, Ren YL, Liu XY, Zhang Y, Pei P,
Zhu SN and Fan Q: Protective role of cyclosporine A and minocycline
on mitochondrial disequilibrium-related podocyte injury and
proteinuria occurrence induced by adriamycin. Nephrol Dial
Transplant. 30:957–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SY, Shim MS, Kim KY, Weinreb RN,
Wheeler LA and Ju WK: Inhibition of cyclophilin D by cyclosporin A
promotes retinal ganglion cell survival by preventing mitochondrial
alteration in ischemic injury. Cell Death Dis. 5:e11052014.
View Article : Google Scholar : PubMed/NCBI
|