Introduction
MicroRNAs (miRs) are small endogenous non-coding RNA
molecules that regulate essential mechanisms for cardiovascular
development and function, including cell growth, differentiation,
proliferation and apoptosis (1–3).
Dysregulated expression of specific microRNAs leads to the
reactivation of fetal genes, left ventricular (LV) remodeling and
cardiac hypertrophy in the failing heart (1,4). As
circulating microRNAs are stable and likely reflect myocardial
damage and ventricular remodeling, they may be useful as diagnostic
or prognostic biomarkers for cardiovascular disorders, including
heart failure (HF) (4–6).
Studies have focused on the potential application of
circulating microRNAs in the diagnosis of HF (6). Their prognostic value was evaluated
in clinically stable (7,8) and decompensated (7) outpatients with HF, and in patients
with HF who improved from New York Heart Association (NYHA) class
IV to class III (9). A number of
investigators have additionally reported on the association between
circulating miR levels and short-term outcomes in patients with
acute decompensated HF (ADHF) (10–13).
Based on findings reported by Qiang et al (8), Fukushima et al (9), Fan et al (14), Goren et al (15) and Tijsen et al (16), and considering that episodes of
ADHF are associated with transient increases in the blood levels of
markers of cardiac myocyte injury, extracellular matrix turnover,
and inflammation (17–19), possibly reflecting an acceleration
of pathological myocardial remodeling (20), it was decided to measure the plasma
levels of miR-21, −126 and −423-5p in patients with ADHF at the
time of hospital admission to determine whether they alter during
clinical improvement. The present study additionally investigated
whether the levels of these three miRs were associated with
all-cause mortality for a longer follow-up period (up to 48
months).
Materials and methods
Study population
A total of 60 adult patients (age ≥18 years) with
ADHF admitted to the emergency room of the Hospital de Clínicas de
Porto Alegre (HCPA; Porto Alegre, Brazil) between May 2011 and June
2012 were prospectively enrolled within 24 h of admission.
Inclusion criteria were a previous diagnosis of HF and impaired LV
systolic function with a LV ejection fraction (LVEF) ≤45%, defined
by transthoracic echocardiography. The diagnosis of ADHF with
volume overload was defined clinically by the presence of worsening
symptoms of dyspnea, paroxysmal nocturnal dyspnea, and/or orthopnea
in conjunction with clinical signs of circulatory congestion
(elevated jugular venous pressure, hepatojugular reflux,
hepatomegaly and/or peripheral edema). Exclusion criteria were as
follows: Pregnancy, dialysis, moderate to severe aortic stenosis,
active malignancy, significant renal (creatinine >265.2 µmol/l)
or hepatic (cirrhosis or active hepatitis) dysfunction, and
moderate to severe rheumatic disease. Patients with concomitant
acute coronary syndromes within the previous three months or severe
hemodynamic instability requiring intravenous vasoactive drugs were
excluded. In addition, patients for whom it was not possible to
collect at least one additional blood sample after admission (n=12)
were excluded.
The present study additionally included 17 healthy
controls without LV dysfunction from the Hemotherapy Division of
HCPA between February 2013 and February 2015 to constitute a
reference group for the determination of the relative expression
levels of miRs (mean age, 59±10 years; 12 males; 16 Caucasians)
(21). The Research Ethics
Committee of HCPA approved the research protocol and all subjects
provided written informed consent (Institutional Review Board no.
0000921; study nos. 11-0016 and 12–0084).
Data and sample collection
Following enrollment, demographic, clinical history,
comorbidity, echocardiographic, electrocardiographic and laboratory
data were collected by reviewing the patient records. To measure
the plasma levels of B-type natriuretic peptide (BNP) and miRs,
blood samples were collected within the first 24 h of admission at
emergency, at the time of hospital discharge, and a number of weeks
post-discharge (a mean of 4±2 months). The blood sample at the
third time point was collected during an outpatient visit as part
of routine clinical care from those who had returned to a chronic
stable compensated state (clinical compensation). The criteria for
chronic stable compensation were a lack of evidence of volume
overload by clinical examination, no current need for diuretic
adjustment and no hospital admission for ADHF within the previous
two months.
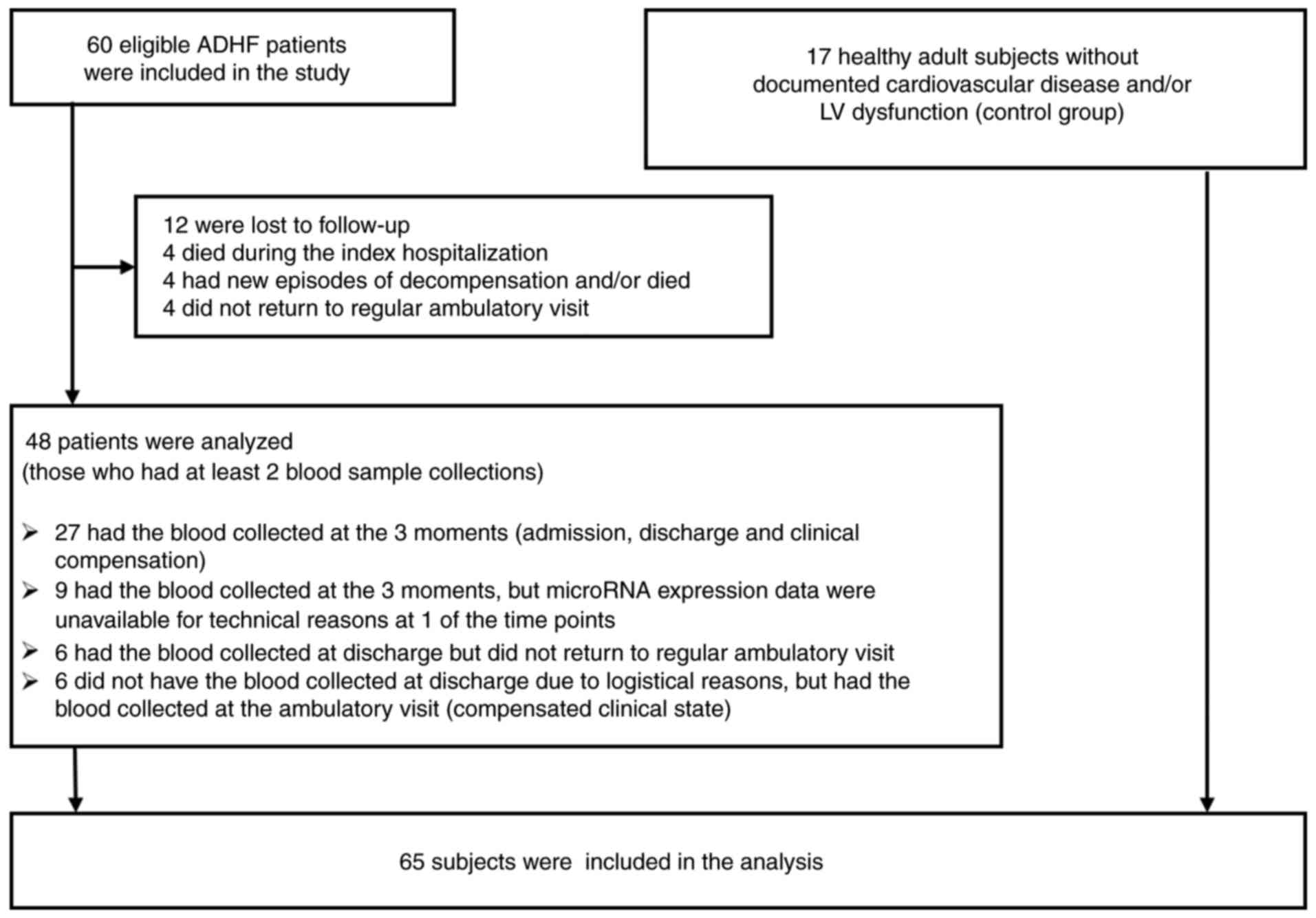
Of the 60 patients admitted to emergency service and
initially included in the study, 42 had the second sample collected
at the time of hospital discharge and 41 had the third sample
collected a number of weeks after discharge, in the chronic
compensated state. In total, 48 patients had at least two blood
samples collected. Among them, 27 had the blood samples collected
at the three time points. The reasons for missing patients during
the follow-up included logistical problems collecting blood samples
at discharge, new episodes of HF decompensation, lack of attendance
at regular consultations, and mortality. Therefore, the findings
reported in the present study are the result of the data analysis
of 48 patients from whom at least two blood samples were taken
(Fig. 1).
Blood sample preparation and
measurement of BNP and miR levels
All laboratory analyses were performed by
investigators blinded to clinical data. Blood samples were
collected in EDTA-containing tubes and plasma was isolated by
centrifugation at 453 × g for 15 min at 4°C within 1 h following
collection. Plasma samples were frozen at −70°C until the assays
were performed and subjected to one freeze-thaw cycle. Levels of
BNP were measured using a commercially available immunoassay kit
(Advia Centaur BNP System; Siemens AG, Munich, Bavaria, Germany;
cat. no. 02816138).
miRs were isolated from 495 µl plasma using the
mirVana PARIS kit with enrichment for small RNAs (Ambion; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Following protein
denaturation, 50 pM synthetic miR-39 from Caenorhabditis
elegans (cel-miR-39; Qiagen, Inc., Valencia, CA, USA) was
spiked-in (fixed volume of 5 µl) to plasma samples to control for
technical variations throughout the RNA isolation and quantitative
procedures. Total RNA concentration was determined by
spectrophotometry (NanoDrop 1000; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA).
Plasma levels of miR-21, −126 and −423-5p were
measured by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) on the 7500 Real Time PCR System (Life
Technologies; Thermo Fisher Scientific, Inc.). Reverse
transcription reactions were performed using a TaqMan®
miR RT kit (Life Technologies; Thermo Fisher Scientific, Inc.). The
reaction mixtures for cDNA synthesis were incubated for 30 min at
16°C, 30 min at 42°C, and 5 min at 85°C, and then held at 4°C. The
PCR amplification reactions were run in triplicate using
pre-designed TaqMan® MicroRNA Assays, containing
specific primers and probes, according to the manufacturer's
protocol (Life Technologies; Thermo Fisher Scientific, Inc.; cat.
no. 4427975, assay ID nos. 000200, 000397, 002228 and 002340 for
cel-miR-39, hsa-miR-21, hsa-miR-126 and hsa-miR-423-5p,
respectively). The PCR conditions were 95°C for 10 min followed by
40 cycles of amplification (95°C for 15 sec and 60°C for 60 sec).
Relative expression levels for each microRNA were estimated using
the 2−ΔΔCq method (22)
using cel-miR-39 as the reference gene and healthy controls as the
reference group.
Study end points
Primarily, the present study assessed whether the
expression of miR-21, miR-126 and miR-423-5p differed according to
the (de)compensated state of patients with HF by comparing the
plasma levels among the three time points. Secondly, the present
study evaluated the correlation between plasma levels of BNP and
miRs. Thirdly, the association between the selected miRs with
hospital readmission and all-cause mortality within 24 and 48
months was evaluated. These outcomes were verified by reviewing the
hospital registry or by telephone contact. Survival data were last
updated in August 2016.
Statistical analysis
Categorical variables are reported as the absolute
numbers and percentages. Continuous variables are expressed as the
mean ± standard deviation, mean ± standard error of the mean, or as
the median (25th and 75th percentile). Plasma levels of BNP and
miRs at admission, at discharge and following clinical compensation
were compared by the generalized estimation equation, and P-values
were adjusted for pairwise comparisons by Bonferroni correction. To
compare the microRNA levels of healthy controls with those
estimated for patients with HF at each time point, the
Kruskal-Wallis test was used followed by Dunn's post-hoc test. The
Shapiro-Wilk test was used to verify whether continuous variables
had a normal distribution. For the comparison of means between two
groups and for the correlation analyses, the values of non-normally
distributed variables were log-transformed or converted to the
square root. Differences between quantitative variables across
binary clinical categories were assessed using the Student's t-test
or the Mann-Whitney test for the variables that were unable to be
normalized (sodium, number of hospital readmissions, and Δ-miR,
corresponding to the alteration in miR levels at clinical
compensation compared with admission). Categorical variables were
compared between groups of patients with the χ2 test or
Fisher's exact test.
Kaplan-Meier survival analysis was performed to
evaluate the association between BNP and miRs with all-cause
mortality within 24 and 48 months, by grouping the patients in two
categories according to whether they had levels above or below the
median. Survival curves were constructed considering the period
between the date of the first admission and the last registry of
follow-up or mortality, and were compared using the log-rank test.
Statistical analyses were performed with the use of the SPSS
statistical package (version 18.0) for Windows (SPSS, Inc.,
Chicago, IL, USA) or GraphPad Prism (version 6.01) for Windows
(GraphPad Software Inc., La Jolla, CA, USA). Two-tailed P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline characteristics of study
population
The baseline characteristics of the 48 patients with
ADHF included in the present study are presented in Table I. The majority of patients were
middle-aged Caucasian males with severe LV dysfunction and
non-ischemic etiology (hypertensive, alcoholic, valvular or
myocarditis). Upon admission for ADHF, all patients were diagnosed
as NYHA class III or IV. The primary causes of the acute
decompensation episodes were infection (50%) and poor adherence to
treatment (35%). The presence of comorbidities, including
hypertension, ischemic heart disease and diabetes mellitus, were
additionally prevalent. At discharge, all patients were taking
diuretics and the majority were taking beta-blockers,
angiotensin-converting enzyme inhibitors and digoxin (Table I). Patients were hospitalized for a
median length of 7 days (ranging between 1 and 38 days). Patients
who succumbed within the 24-month follow-up exhibited a similar
baseline profile compared with that observed in those who remained
alive during this period; the only difference between the two
groups of patients was the frequency of atrial fibrillation, which
was more prevalent among those who died (Table I).
 | Table I.Baseline characteristics of patients
with acute decompensated heart failure. |
Table I.
Baseline characteristics of patients
with acute decompensated heart failure.
|
|
| 24-month
survival |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | All patients
(n=48) | Survived
(n=37) | Succumbed
(n=11) | P-value |
|---|
| Age, years | 62±13 | 61±14 | 64±10 | 0.508 |
| Male | 33 (69%) | 25 (68%) | 8 (73%) | >0.999 |
| Caucasian | 41 (85%) | 32 (86%) | 9 (82%) | 0.653 |
| Heart failure
etiology |
|
|
|
|
|
Ischemic | 18 (38%) | 14 (38%) | 4 (36%) | 0.643 |
|
Hypertensive | 17 (35%) | 11 (30%) | 6 (54%) |
|
|
Alcoholic | 9 (19%) | 8 (22%) | 1 (9%) |
|
| Clinical conditions
at admission |
|
|
|
|
| NYHA
functional class III/IV | 15
(31%); | 11
(30%); | 4
(36%); | 0.720 |
|
| 33 (69%) | 26 (70%) | 7 (64%) |
|
| Heart
rate, bpm | 95±20 | 95±20 | 94±20 | 0.852 |
|
Systolic blood pressure,
mmHg | 130±28 | 133±29 | 122±24 | 0.271 |
|
Diastolic blood pressure,
mmHg | 85±17 | 87±16 | 79±18 | 0.193 |
| Comorbidities |
|
|
|
|
|
Hypertension | 36 (75%) | 27 (73%) | 9 (82%) | 0.705 |
|
Previous myocardial
infarction | 17 (35%) | 14 (38%) | 3 (27%) | 0.723 |
|
Diabetes mellitus | 24 (50%) | 17 (46%) | 7 (64%) | 0.492 |
| Chronic
renal insufficiency | 11 (23%) | 8
(22%) | 3 (27%) | 0.697 |
|
COPD | 18 (38%) | 14 (38%) | 4 (36%) | >0.999 |
|
Smoking, past or current | 33 (69%) | 25 (68%) | 8 (73%) | >0.999 |
| Alcohol
abuse, past or current | 23 (48%) | 18 (49%) | 5 (46%) | >0.999 |
| Echocardiographic
data |
|
|
|
|
| LV
ejection fraction, % | 26±8 | 25±8 | 26±10 | 0.932 |
| LV
end-systolic diameter, cm | 5.7±0.9 | 5.8±0.9 | 5.6±1.1 | 0.696 |
| LV
end-diastolic diameter, cm | 6.5±0.9 | 6.5±0.8 | 6.4±1.0 | 0.564 |
| Electrocardiogram
data |
|
|
|
|
| Atrial
fibrillation | 19 (40%) | 11 (30%) | 8 (73%) | 0.016 |
|
Interventricular block | 19 (40%) | 15 (40%) | 4 (36%) | >0.999 |
| QRS
duration, msec | 120±26 | 122±24 | 115±32 | 0.451 |
| Laboratory
data |
|
|
|
|
| BNP at
admission, pg/ml | 659
(472–1,255) | 667
(480–1,333) | 566
(374–1,075) | 0.593 |
|
Creatinine, µmol/l | 115±35 | 112±42 | 114±33 | 0.752 |
| Urea,
mmol/l | 20.3±8.4 | 19.3±8.4 | 23.4±8.2 | 0.136 |
| Sodium,
mmol/l | 140±4 | 139±4 | 140±5 | 0.394 |
|
Potassium, mmol/l | 4.2±0.4 | 4.2±0.4 | 4.2±0.5 | 0.987 |
| Medications at
discharge |
|
|
|
|
|
β-blockers | 38 (79%) | 29 (78%) | 9 (82%) | >0.999 |
| ACE
inhibitors | 39 (81%) | 31 (84%) | 8 (73%) | 0.409 |
|
Digoxin | 42 (88%) | 32 (89%) | 9 (82%) | 0.609 |
|
Vasodilators | 24 (50%) | 18 (49%) | 6 (54%) | >0.999 |
Plasma levels of BNP and miRs
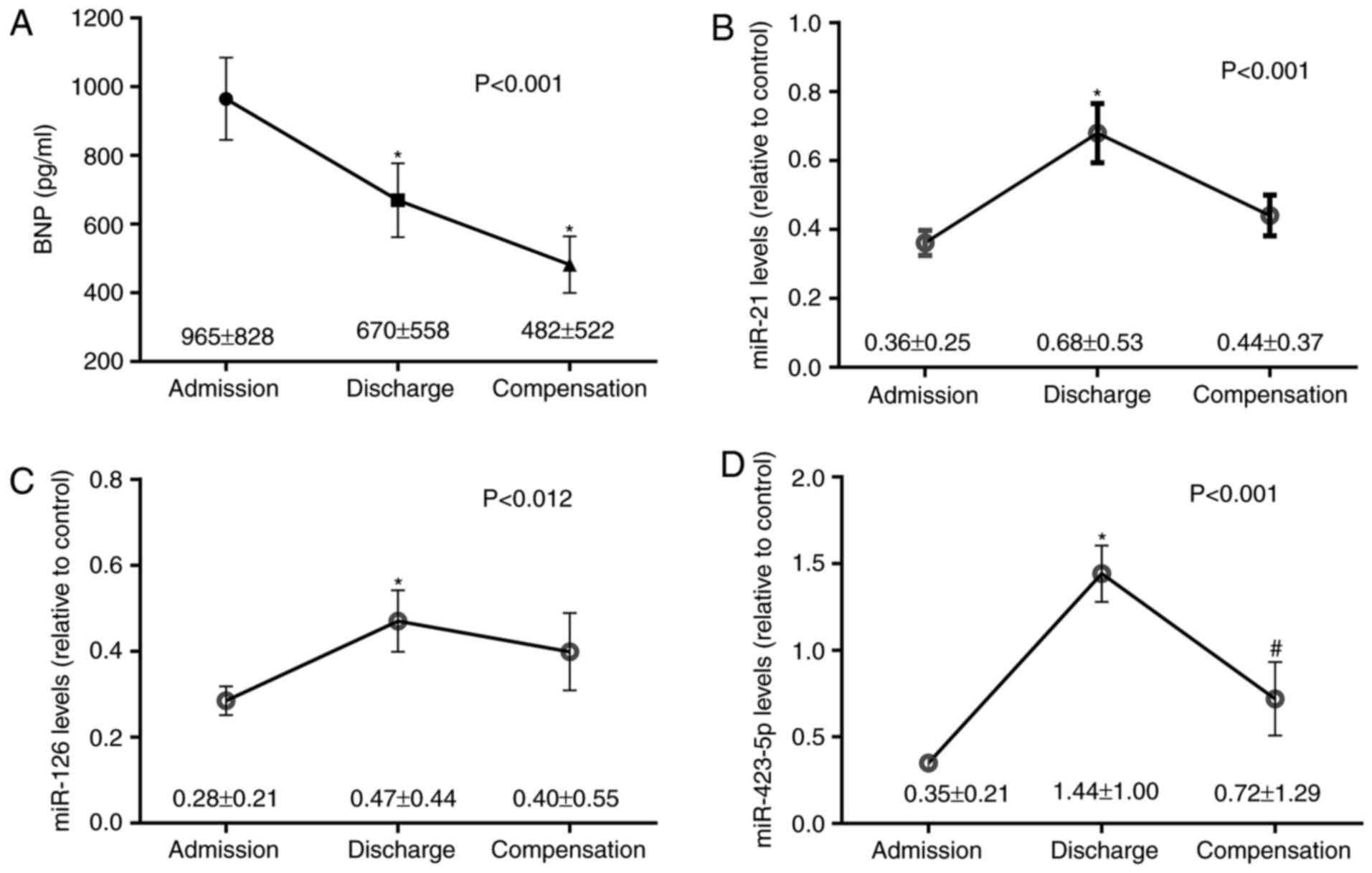
Fig. 2 illustrates
the plasma levels of BNP, miR-21, miR-126 and miR-423-5p in
patients with ADHF at the time of admission, discharge, and
following clinical compensation. As hypothesized, BNP levels
decreased between admission and discharge [Bonferroni-corrected
P-value (Pc)<0.001] and compensation
(Pc=0.003) (Fig.
2A). The levels of miR-21 almost doubled between the time of
admission and discharge (1.9-fold-change; Pc=0.001),
decreasing following clinical compensation (0.65-fold-change in
relation to discharge; Pc=0.05) (Fig. 2B). The levels of miR-126
additionally increased between admission and discharge
(1.7-fold-change; Pc=0.016) (Fig. 2C). miR-423-5p expression exhibited
the most prominent change, increasing between admission and
discharge (4.1-fold-change; Pc<0.001), and decreasing
following clinical compensation (0.5-fold-change in relation to
discharge; Pc=0.017) (Fig.
2D).
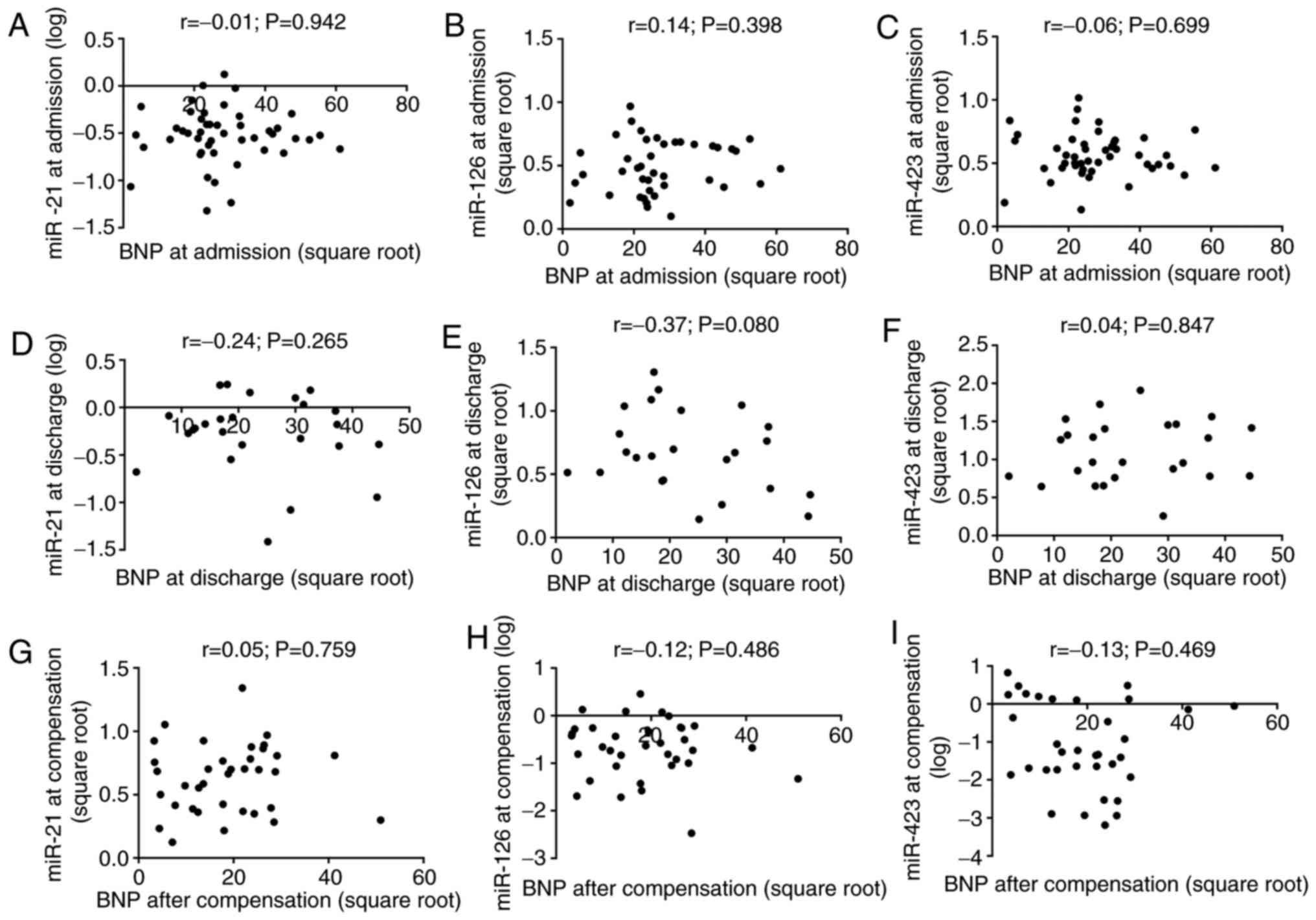
As presented in Fig.
3, there was no correlation between the plasma levels of BNP
and the three microRNAs at the time of admission and discharge, and
following clinical compensation. The levels of miR-21 and miR-126
were directly correlated over time. At admission, miR-21 was
positively correlated with miR-423-5p, whereas miR-126 was
inversely correlated with miR-423-5p following clinical
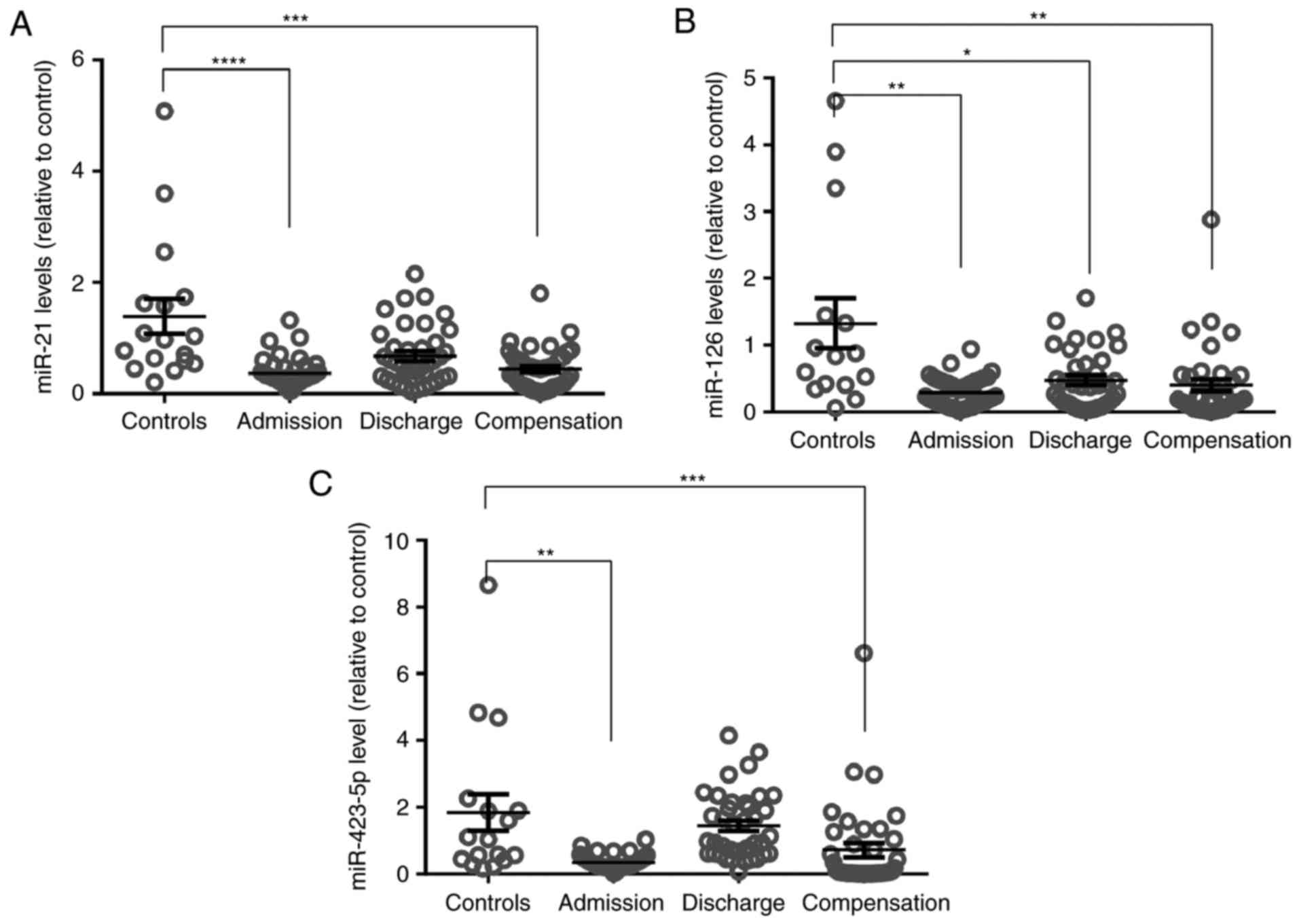
compensation (Table II). In
addition, healthy controls had higher plasma microRNA levels in
comparison with ADHF patients at admission and after clinical
compensation (Fig. 4).
 | Table II.Pearson correlation coefficients for
the relative expression levels of miRs in patients with acute
decompensated heart failure. |
Table II.
Pearson correlation coefficients for
the relative expression levels of miRs in patients with acute
decompensated heart failure.
| Time point | miR-126 | miR-423-5p |
|---|
| At admission |
|
|
|
miR-21 | r=0.54;
P<0.001 | r=0.57;
P<0.001 |
|
miR-126 | – | r=−0.02;
P=0.913 |
| At discharge |
|
|
|
miR-21 | r=0.76;
P<0.001 | r=0.25;
P=0.126 |
|
miR-126 | – | r=0.02;
P=0.901 |
| Following clinical
compensation |
|
|
|
miR-21 | r=0.70;
P<0.001 | r=−0.26;
P=0.115 |
|
miR-126 | – | r=−0.44;
P=0.008 |
Association between miR levels, and
hospital readmission and all-cause mortality
During the 4-year period following the initial
admission, 38 patients (79.2%) were rehospitalized at least once
and 21 patients (43.8%) succumbed. One-half of the patients had up
to 36 months of follow-up treatment (ranging between 2 and 63
months). The number of hospital readmissions experienced by each
patient varied between one (n=7) and 42 (n=1); almost 60% of the
patients had up to three readmissions. In relation to all-cause
mortality, the cumulative rates were, respectively, 23 and 44% at
24 and 48 months following initial admission.
A proportion of the patients had levels of BNP
(65%), miR-21 (43%), miR-126 (52%) and miR-423-5p (66%) which
reduced between admission and clinical compensation, while the
remaining had exhibited increased levels between these two time
points (Δ-BNP/Δ-miR). The alteration in the plasma levels of BNP
and miRs from initial admission to clinical compensation was not
significantly different between patients who succumbed during the
follow-up and those who remained alive (Table III). However, the levels of
miR-21 and miR-126 at the time of clinical compensation were
associated with all-cause mortality within 24 months following the
initial admission. Notably, no patient with levels of miR-21 or
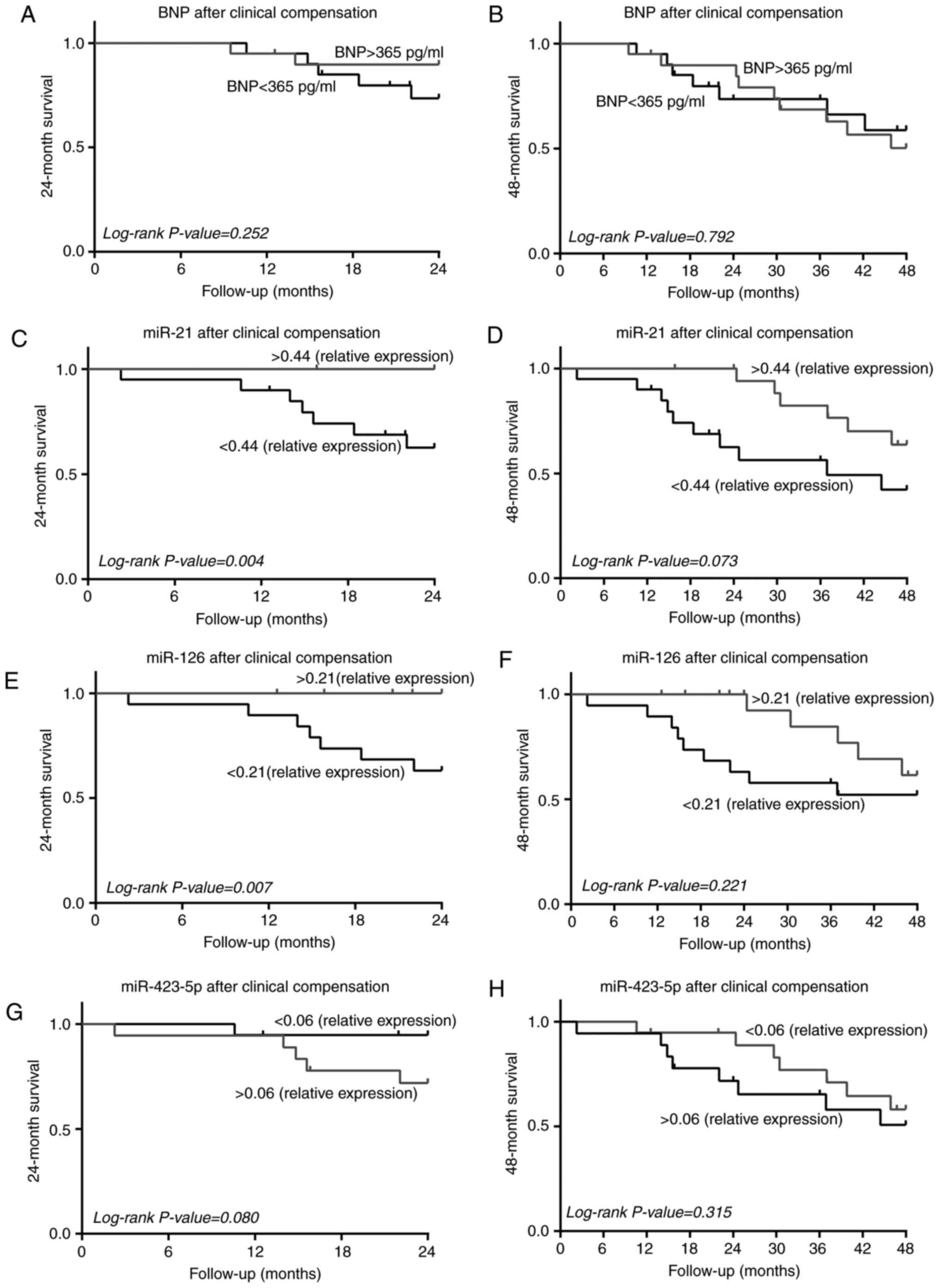
miR-126 above the median succumbed (Fig. 5). Patients who succumbed within 24
months more frequently exhibited atrial fibrillation compared with
those who remained alive (Table
I), and patients with atrial fibrillation had 55% lower miR-21
levels at the time of clinical compensation compared with those
with sinus rhythm (P=0.008).
 | Table III.Delta values of BNP and microRNAs
according to all-cause mortality. |
Table III.
Delta values of BNP and microRNAs
according to all-cause mortality.
| All-cause
mortality | Δ-BNP | Δ-miR-21 | Δ-miR-126 | Δ-miR-423-5p |
|---|
| Within 24
months |
|
|
|
|
| Yes
(n=8) | −328
(−1605-19) | −0.05
(−0.17–0.15) | −0.06
(−0.19–0.01) | 0.01
(−0.21–1.11) |
| No
(n=32) | −347
(−935-208) | 0.11
(−0.16–0.33) | 0.07
(−0.10–0.38) | −0.17
(−0.30–0.57) |
|
P-value | 0.710 | 0.507 | 0.146 | 0.284 |
| Within 48
months |
|
|
|
|
| Yes
(n=18) | −238
(−1104-276) | 0.11
(−0.15–0.47) | −0.05
(−0.13–0.34) | −0.15
(−0.35–0.75) |
| No
(n=22) | −433
(−1025-15) | 0.04
(−0.16–0.29) | 0.07
(−0.10–0.24) | −0.16
(−0.23–0.78) |
|
P-value | 0.664 | 0.465 | 0.515 | 0.643 |
In addition, patients who had increased levels of
miR-21 following clinical compensation remained
rehospitalization-free for a longer time compared with those in
whom the levels of miR-21 were below the median (mean period of 362
and 181 days, respectively; P=0.034, for the first hospital
readmission following the day of blood collection at the outpatient
clinic). The same finding was observed for the levels of miR-126
(mean period of 423 and 177 days, respectively; P=0.028). In
addition, patients whose levels of miR-423-5p increased between
admission and clinical compensation experienced fewer hospital
readmissions in the 24-month period following the time of clinical
compensation compared with those who had decreased levels (median
of 0 and 2, respectively; P=0.040).
Discussion
The results of the present study demonstrated that
the plasma concentrations of miR-21, −126 and −423-5p altered
during the clinical improvement of patients with HF admitted for an
episode of acute decompensation, and were associated with
rehospitalization and all-cause mortality. A number of studies have
demonstrated that circulating miR levels in patients with stable or
acute decompensated HF differ from those in control subjects,
supporting the concept that they may be used as biomarkers for HF
diagnosis (6,8,14).
The first evidence suggesting that circulating miRs may reflect HF
severity was predominantly based on the observed correlations of
the circulating miR levels with BNP/N-terminal prohormone BNP
and/or other prognostic clinical parameters, including NYHA
functional class and LVEF (9,15,16,23).
Recently, miR levels were observed to be associated with
rehospitalization and mortality in patients with acute HF (7,11–13).
In the majority of these previous studies, circulating levels of
miRs were measured at one or more time points, at most, within the
first 7 days of the hospital admission, and the prognostic outcomes
were evaluated after a short- or mid-term follow-up (7,12,13).
The increase observed in the levels of miR-21 and
miR-423-5p between hospital admission and discharge in the present
study appears to contrast with the findings of previous studies
into the clinical diagnosis of HF, in which the levels of miR-21
and miR-423-5p were increased in patients with HF compared with
controls (6,14). Based on this pattern, it may be
hypothesized that the miR levels may be reduced during the clinical
recovery of patients with ADHF. However, the results of the present
study are in accordance with what is known about the
pathophysiology of ADHF (17–19),
with experimental evidence of the pathways regulated by miRs
(1–4,24),
and with previous clinical studies into the prognosis of ADHF that
investigated circulating levels of miR-21, miR-126 and miR-423-5p
(11,12). These findings suggested that
patients considered clinically stable in a routine visit to the
outpatient clinic, although exhibiting low levels of miR-21,
miR-126 or miR-423-5p, may be more likely to experience new
episodes of decompensation in the short-term.
miR-21 is expressed in cardiomyocytes, fibroblasts
and endothelial cells, wherein it regulates apoptosis, fibrosis,
and cell proliferation and migration, respectively (1). In the failing heart, overexpression
of miR-21 in cardiac fibroblasts upregulates the RAC-α
serine/threonine-protein kinase and mitogen-activated protein
kinase signaling pathways, leading to fibroblast proliferation and
fibrosis (1,24,25),
which in turn impairs ventricular function (1). However, miR-21 inhibits cellular
apoptosis (1,24,26).
During myocardial ischemia, miR-21 is acutely downregulated within
the ischemic zone, and replenishing its expression reduces the
infarct size and delays the development of HF (26). These results were corroborated by
experiments in cardiac myocytes, in which the overexpression of
miR-21 was demonstrated to inhibit hypoxia-induced apoptosis, while
the knockdown of miR-21 enhanced oxidative stress-induced apoptosis
(1,24). As the LV remodeling process
involves myocyte loss by apoptosis (27), it may be hypothesized that in the
initial clinical recovery (between admission and the time of
hospital discharge), the increased levels of miR-21 observed in the
present study contributed to protecting patients from acute myocyte
loss during the episode of clinical decompensation.
In the present study, patients with high levels of
miR-21 had an improved prognosis compared with those with reduced
levels. In relation to rehospitalization, the results of the
present study are consistent with those reported by Seronde et
al (12), who assessed the
plasma levels of miR-21, miR-126 and miR-423-5p in patients with
acute HF. Levels of miR-21 were similar among patients with acute
dyspnea (acute HF and non-acute HF) and patients with stable
chronic HF. A subgroup of patients with acute HF had their miR
levels measured at the time of admission and 5 days subsequently.
Although the levels of miR-21 remained unaltered, they were
decreased in patients who were readmitted in the year following the
initial hospitalization compared with patients who were not. In
relation to mortality, plasma levels of miR-21 were similar between
patients who survived and patients who succumbed within the 1 year
of follow-up. In the genome-wide expression study reported by
Cakmak et al (7), plasma
levels of miR-21 in patients with systolic HF were not associated
with rehospitalization or cardiovascular mortality at 6 months of
follow-up. In the present study, however, patients who succumbed
during a 24-month follow-up period had ~2-fold lower plasma levels
of miR-21 at the time of clinical compensation and more frequently
exhibited atrial fibrillation compared with those who survived. The
present results are in accordance with the findings reported by
McManus et al (28) in a
study population composed predominantly of middle-aged Caucasian
men. The authors observed that subjects with atrial fibrillation
had 2.1-fold lower plasma levels of miR-21 compared with controls,
and that miR-21 expression increased by 3.4-fold following catheter
ablation (28).
The endothelial cell-enriched miR-126 is highly
expressed in the heart (26),
where it maintains endothelial cell homeostasis and vascular
integrity by enhancing angiogenesis. miR-126 is able to inhibit
inflammation and atherosclerosis, leading to the recruitment of
progenitor cells to repair the endothelial cells. In addition to
its intracellular action, miR-126 exerts paracrine effects through
the release of miR-126-containing microparticles, apoptotic bodies
or exosomes (1,2). In patients with HF, impaired
angiogenesis leads to alterations in the extracellular matrix that
contribute to the LV remodeling process (27). As miR-126 is essential for
ischemia-induced angiogenesis (1,2),
patients with HF may be expected to have lower circulating miR-126
levels. Indeed, it has been demonstrated that the circulating
levels of miR-126 are decreased in patients with ischemic systolic
HF compared with control subjects (9,15),
in addition to in patients with acute HF compared with those with
stable HF (12). In 10 patients
with HF, the plasma concentrations of miR-126 were assessed twice
(when the patients were at NYHA functional class IV and when they
improved to class III). As the clinical condition improved, plasma
concentrations of miR-126 were upregulated (9). In rats with hypertension-induced HF,
the plasma levels of miR-126 were increased in response to
therapeutic treatment (29). In
relation to prognosis, low levels of miR-126 were associated with
readmission in the year following the initial hospitalization in
acute HF (12), and with increased
risk of cardiovascular death in patients with chronic HF of
ischemic etiology followed-up for 24 months (8). These findings are similar to those
obtained in the present study, as the levels of miR-126 increased
between the time of initial admission and discharge, and patients
with low levels of miR-126 had a worse prognosis compared with
those with high levels.
A study by Tijsen et al (16) demonstrated that the plasma levels
of miR-423-5p were able to be used to distinguish patients with HF
from patients with dyspnea attributable to other causes and healthy
controls. Similar results were observed in different populations,
in which circulating miR-423-5p levels were increased in patients
with HF compared with healthy subjects (14,15,21,30–32).
A previous study demonstrated a positive transcoronary gradient of
plasma miR-423-5p in stable outpatients with HF, whereas a negative
gradient was identified in subjects without structural cardiac
disease, thereby suggesting that miR-423-5p may be of cardiac
origin (33). Recently, the
function of miR-423-5p began to be elucidated. Transfection
experiments demonstrated that overexpression of miR-423-5p
inhibited the proliferation and induced the apoptosis of human
cardiomyoblasts (30) and murine
cardiomyocytes via upregulation of p53 and caspase-3 (31).
However, in the context of the prognosis of acute HF
in humans, decreased expression of miR-423-5p appears to be
associated with the severity of HF and poor outcomes. In the report
by Seronde et al (12),
patients with acute dyspnea (with or without acute HF) had lower
levels of miR-423-5p compared with those with stable HF. The levels
upon admission of this miR were lower in patients who were
readmitted to the hospital in the year following the initial
hospitalization, compared with the patients who were not. In this
same cohort, plasma levels of miR-423-5p were similar between
patients who survived and those who succumbed within the 1 year of
follow-up. In another cohort of patients with acute HF from the
same study; however, low admission levels of miR-423-5p were
associated with an increased risk of mortality (12). Similarly, circulating levels of 12
miRs, including miR-423-5p, were observed to be lower in acute HF
(from three different cohorts) compared with chronic HF, acute
exacerbation of chronic obstructive pulmonary disease and healthy
controls (11). In one of the
cohorts of acute HF, the levels of miRs were evaluated at four time
points (at admission, and at 24 h, 48 h and 7 days following
admission). In another cohort, their levels were measured at
discharge and 6 months post-hospitalization. The lowest levels of
miR-423-5p were observed in the period between admission and
discharge. The levels increased and converged at 6 months towards
the levels observed in patients with chronic HF and healthy
controls. Additionally, further-decreasing levels of miR-423-5p
within 48 h following hospital admission for acute HF were
predictive of 180-day mortality (11). The results of the present study
corroborated these previous studies (11,12),
as plasma levels of miR-423-5p increased from the time of admission
until discharge, decreasing following clinical compensation. In the
following 24 months, patients with low levels of miR-423 had a
worse prognosis in terms of rehospitalization compared with those
with high levels. These findings led to the hypothesis that
miR-423-5p may reflect additional cardiomyocyte death occurring
subsequent to multiple episodes of decompensation.
The findings of the present study and previous
reports (7,11,12)
support the hypothesis that miR-21, −126, and −423-5p may be useful
as prognostic biomarkers of ADHF. Although the origin and the
function of circulating miRs are not completely understood, an
increasing number of studies have demonstrated that miRs are more
than by-products of cellular injury, being actively secreted by
cells and exerting paracrine effects (34,35).
However, it is important to understand that levels of plasma miRs
fluctuate according to HF clinical status (stable chronic HF, ADHF
admission, ADHF discharge and early compensated period), a concept
that has not been considered in previous studies. In addition,
pre-clinical studies in HF models revealed the potential of
microRNA-based therapies to improve cardiac function, increase
survival rates and reduce cardiac remodeling (34–36).
Overall, this evidence suggested that circulating levels of miRs
may help to identify patients who are at risk of poor outcomes and
may even guide pharmacological therapy. However, findings from
previous studies (7,11–13)
and from the present cohort are insufficient to establish a cause
and effect association. Consequently, it is too early to determine
whether alterations in the expression of miRs during episodes of
decompensation and clinical recovery are a consequence or a trigger
of the functional and cellular alterations that occur in the
failing myocardium.
The results of the present study may be interpreted
in the light of certain logistical limitations. It was not possible
to collect three blood samples from all patients included in the
study, thus limiting the sample size and consequently the power of
the observed associations. For the same reason, it was not possible
to determine whether the associations detected were independent of
clinical covariates, such as atrial fibrillation. Additionally, the
selection of the three miRs investigated in the present study was
based on intellectual choice rather than on genome-wide gene
expression profiling. When the present study was designed, few
reports had been published on circulating miRs in decompensated HF
(9,16). Among the miRs investigated in
cardiovascular disorders, miR-21, miR-126 and miR-423-5p were
recurrently observed to be dysregulated in atherosclerosis, acute
coronary syndromes and HF (4–6).
These miRs were selected as they are associated with cardiac
myocyte injury, extracellular matrix turnover and inflammation
(1–4,24),
all of which are characteristic of HF decompensation (17–19).
Despite these limitations, the present analysis is novel in the
context of assessing the association between circulating miRs and
the prognosis of ADHF in three different clinical scenarios in the
same patients followed-up for a long period.
In conclusion, in the present prospective cohort
study of patients admitted with ADHF, plasma levels of miR-21,
miR-126 and miR-423-5p altered over time with clinical improvement,
and low levels of these miRs were associated with poor outcomes
(rehospitalization and all-cause mortality). The results of the
present study confirm and expand the results from previous studies
into ADHF, supporting the concept that plasma levels of miRs alter
with HF progression and may have prognostic value.
Acknowledgements
The present study was supported by the Conselho
Nacional de Desenvolvimento Científico e Tecnológico (CNPq,
Brasília, Brazil; grant no. 478093/2011-0) and the Fundo de
Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto
Alegre (Porto Alegre, Brazil; grant no. 11-0016). Dr Rohde received
a research scholarship from CNPq (grant no. 308808/2013-4). The
authors would like to thank Dr Fernanda Alves and Ms. Leticia
Orlandin for assisting with sample and data collection.
Glossary
Abbreviations
Abbreviations:
|
HF
|
heart failure
|
|
miR
|
microRNA
|
|
BNP
|
B-type natriuretic peptide
|
|
LV
|
left ventricular
|
|
NYHA
|
New York Heart Association
|
|
ADHF
|
acute decompensated heart failure
|
|
LVEF
|
left ventricular ejection fraction
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
HCPA
|
Hospital de Clínicas de Porto
Alegre
|
References
|
1
|
Fang YC and Yeh CH: Role of microRNAs in
vascular remodeling. Curr Mol Med. 15:684–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Small EM and Olson EN: Pervasive roles of
microRNAs in cardiovascular biology. Nature. 469:336–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou J, Dong X, Zhou Q, Wang H, Qian Y,
Tian W, Ma D and Li X: MicroRNA expression profiling of heart
tissue during fetal development. Int J Mol Med. 33:1250–1260. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papageorgiou N, Tslamandris S, Giolis A
and Tousoulis D: MicroRNAs in cardiovascular disease: Perspectives
and reality. Cardiol Rev. 24:110–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navickas R, Gal D, Laucevičius A,
Taparauskaitė A, Zdanytė M and Holvoet P: Identifying circulating
microRNAs as biomarkers of cardiovascular disease: A systematic
review. Cardiovasc Res. 111:322–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romaine SP, Tomaszewski M, Condorelli G
and Samani NJ: MicroRNAs in cardiovascular disease: An introduction
for clinicians. Heart. 101:921–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cakmak HA, Coskunpinar E, Ikitimur B,
Barman HA, Karadag B, Tiryakioglu NO, Kahraman K and Vural VA: The
prognostic value of circulating microRNAs in heart failure:
Preliminary results from a genome-wide expression study. J
Cardiovasc Med (Hagestown). 16:431–437. 2015. View Article : Google Scholar
|
|
8
|
Qiang L, Hong L, Ningfu W, Huaihong C and
Jing W: Expression of miR-126 and miR-508-5p in endothelial
progenitor cells is associated with the prognosis of chronic heart
failure patients. Int J Cardiol. 168:2082–2088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukushima Y, Nakanishi M, Nonogi H, Goto Y
and Iwai N: Assessment of plasma miRNAs in congestive heart
failure. Circ J. 75:336–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruno N, Ter Maaten JM, Ovchinnikova ES,
Vegter EL, Valente MA, van der Meer P, de Boer RA, van der Harst P,
Schmitter D, Metra M, et al: MicroRNAs relate to early worsening of
renal function in patients with acute heart failure. Int J Cardiol.
203:564–569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ovchinnikova ES, Schmitter D, Vegter EL,
Ter Maaten JM, Valente MA, Liu LC, van der Harst P, Pinto YM, de
Boer RA, Meyer S, et al: Signature of circulating microRNAs in
patients with acute heart failure. Eur J Heart Fail. 18:414–423.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seronde MF, Vausort M, Gayat E, Goretti E,
Ng LL, Squire IB, Vodovar N, Sadoune M, Samuel JL, Thum T, et al:
Circulating microRNAs and outcome in patients with acute heart
failure. PLoS One. 10:e01422372015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao J, Gao R, Bei Y, Zhou Q, Zhou Y,
Zhang H, Jin M, Wei S, Wang K, Xu X, et al: Circulating miR-30d
predicts survival in patients with acute heart failure. Cell
Physiol Biochem. 41:865–874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan KL, Zhang HF, Shen J, Zhang Q and Li
XL: Circulating microRNAs levels in Chinese heart failure patients
caused by dilated cardiomyopathy. Indian Heart J. 65:12–16. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goren Y, Kushnir M, Zafrir B, Tabak S,
Lewis BS and Amir O: Serum levels of microRNAs in patients with
heart failure. Eur J Heart Fail. 14:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tijsen AJ, Creemers EE, Moerland PD, de
Windt LJ, van der Wal AC, Kok WE and Pinto YM: MiR423-5p as a
circulating biomarker for heart failure. Circ Res. 106:1035–1039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biolo A, Fisch M, Balog J, Chao T, Schulze
PC, Ooi H, Siwik D and Colucci WS: Episodes of acute heart failure
syndrome are associated with increased levels of troponin and
extracellular matrix markers. Circ Heart Fail. 3:44–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato Y, Yamada T, Taniguchi R, Nagai K,
Makiyama T, Okada H, Kataoka K, Ito H, Matsumori A, Sasayama S and
Takatsu Y: Persistently increased serum concentrations of cardiac
troponin T in patients with idiopathic dilated cardiomyopathy are
predictive of adverse outcomes. Circulation. 103:369–374. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schulze PC, Biolo A, Gopal D, Shahzad K,
Balog J, Fish M, Siwik D and Colucci WS: Dynamics in insulin
resistance and plasma levels of adipokines in patients with acute
decompensated and chronic stable heart failure. J Cardiac Fail.
17:1004–1011. 2011. View Article : Google Scholar
|
|
20
|
DeAguero JL, McKown EN, Zhang L, Keirsey
J, Fischer EG, Samedi VG, Canan BD, Kilic A, Janssen PM and Delfín
DA: Altered protein levels in the isolated extracellular matrix of
failing human hearts with dilated cardiomyopathy. Cardiovasc
Pathol. 26:12–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomé JG, Mendoza MR, Cheuiche AV, La
Porta VL, Silvello D, Dos Santos KG, Andrades ME, Clausell N, Rohde
LE and Biolo A: Circulating microRNAs in obese and lean heart
failure patients: A case-control study with computational target
prediction analysis. Gene. 574:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Endo K, Naito Y, Ji X, Nakanishi M,
Noguchi T, Goto Y, Nonogi H, Ma X, Weng H, Hirokawa G, et al:
MicroRNA 210 as a biomarker for congestive heart failure. Biol
Pharm Bull. 36:48–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Liew OW, Richards AM and Chen YT:
Overview of microRNAs in cardiac hypertrophy, fibrosis, and
apoptosis. Int J Mol Sci. 17:E7492016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu R-H, Ning B, Ma X-E, Gong WM and Jia
TH: Regulatory roles of microRNA-21 in fibrosis through interaction
with diverse pathways (Review). Mol Med Rep. 13:2359–2366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdellatif M: Differential expression of
microRNAs in different disease states. Circ Res. 110:638–650. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Topkara VK and Mann DL: Clinical
applications of miRNAs in cardiac remodeling and heart failure. Per
Med. 7:531–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McManus DD, Tanriverdi K, Lin H, Esa N,
Kinno M, Mandapati D, Tam S, Okike ON, Ellinor PT, Keaney JF Jr, et
al: Plasma microRNAs are associated with atrial fibrillation and
change after catheter ablation (the miRhythm study). Heart Rhythm.
12:3–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dickinson BA, Semus HM, Montgomery RL,
Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG and
van Rooij E: Plasma microRNAs serve as biomarkers of therapeutic
efficacy and disease progression in hypertension-induced heart
failure. Eur J Heart Fail. 15:650–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q, Yu Q, Na R and Liu B: MiR-423-5p
inhibits human cardiomyoblast proliferation and induces cell
apoptosis by targeting Gab 1. Int J Clin Exp Pathol. 9:8953–8962.
2016.
|
|
31
|
Luo P, He T, Jiang R and Li G:
MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in
cardiomyocytes. Mol Med Rep. 12:1163–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olivieri F, Antonicelli R, Lorenzi M,
D'Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La
Sala L, Galeazzi R, et al: Diagnostic potential of circulating
miR-499-5p in elderly patients with acute non ST-elevation
myocardial infarction. Int J Cardiol. 167:531–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goldraich LA, Martinelli NC, Matte U,
Cohen C, Andrades M, Pimentel M, Biolo A, Clausell N and Rohde LE:
Transcoronary gradient of plasma microRNA 423–5p in heart failure:
Evidence of altered myocardial expression. Biomarkers. 19:135–141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vegter EL, van der Meer P, de Windt LJ,
Pinto YM and Voors AA: MicroRNAs in heart failure: From biomarker
to target for therapy. Eur J Heart Fail. 18:457–468. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Viereck J, Bang C, Foinquinos A and Thum
T: Regulatory RNAs and paracrine networks in the heart. Cardiovasc
Res. 102:290–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caroli A, Cardillo MT, Galea R and
Biasucci LML: Potential therapeutic role of microRNAs in ischemic
heart disease. J Cardiol. 61:315–320. 2013. View Article : Google Scholar : PubMed/NCBI
|