Introduction
Serum levels of insulin-like growth factor-binding
protein-2 (IGFBP-2) are significantly increased in, and have been
correlated with, tumor progression in a number of different
cancers, including colon (1),
ovarian (2,3), lung (4), and prostate (5,6). In
addition, the levels of IGFBP-2 in the serum and tumors of breast
cancer patients are significantly elevated (7,8), and
tumor IGFBP-2 expression correlates with malignancy (7). IGFBP-2 has also been shown to
modulate cell adhesion and migration (9) in an IGF-independent manner, but the
exact nature of its role in breast cancer is not clear. IGFBP-2
contains an arginine-glycine-aspartic(RGD) integrin-recognition
sequence near its C-terminus. Accordingly, the biological effects
of this protein are reported to be mediated by its ability to bind
to integrin receptors such integrin-α5β1, thereby facilitating
activation of downstream signaling pathways (9,10).
Our previous work has demonstrated that adipocytes surrounding
breast cancer cells may secrete IGFBP-2 and promote breast cancer
metastasis (11). We have also
found that IGFBP-2 expression was elevated in both cancer cells and
adipocytes in patients presenting with metastatic breast disease.
In this study, we have designed an adeno-associated virus (AAV)
construct containing a small interfering RNA to IGFBP-2 to examine
the function of this protein in MDA-MB-468 cells. These cells are a
model of triple-negative
(ER−/PR−/HER2−) breast cancer and
have high basal levels of IGFBP-2 expression.
AAVs are attractive candidates for the creation of
viral vectors for gene therapy, as these viruses are non-pathogenic
and less immunogenic compared to other gene therapy vectors
(12–15). AAVs are non-enveloped parvoviruses
that measure approximately 22 nm in diameter and have been used in
many clinical trials for cancer treatment (16). In addition, Alam et al have
found that although AAV2 is capable of infecting normal human
mammary epithelial cells (nHMECs), it is unable to express Rep
proteins or undergo active replication in this cell type (17). Therefore, AAV2 maybe a useful
candidate for breast cancer gene therapy. Moreover, Alam et
al have also found that infection with wild-type
adeno-associated virus type2 (AAV2) inhibited proliferation of
breast cancer cell lines representing both weakly (MCF-7 and
MDA-MB-468) and highly invasive (MDA-MB-231) cancer types (17). Therefore, the present study
evaluated the effects of a recombinant AAV2(rAAV2) encoding an
shRNA to human IGFBP-2 (rAAV2-shRNA-hIGFBP-2) on phenotypes of
MDA-MB-468 breast cancer cells. We show that administration of
rAAV2-shRNA-hIGFBP-2 resulted in down-regulation of IGFBP-2 in
vitro and inhibited MDA-MB-468 breast cancer cell
proliferation. Since paclitaxel is a commonly used drug to treat
human breast cancer patients with metastasis and the drug
resistance has limited its use, our tests show that
rAAV2-shRNA-hIGFBP-2 could enhance the effect of paclitaxel at the
same concentration. We also demonstrate that MCF10A cells are
resistant to rAAV2-shRNA-scramble infection and in vivo
injection of rAAV2-shRNA-hIGFBP-2 inhibits the growth of tumor
xenografts derived from MDA-MB-468 cells. Finally, we show that
rAAV2-shRNA-hIGFBP-2 can reduce the invasive potential of
MDA-MB-468 cells to some extent.
In breast cancer, autocrine or paracrine IGFBP-2
signaling may be an important event during metastasis and drug
resistance (18), thereby making
this molecule an attractive target for therapeutic intervention. To
this end, the present study seeks to improve the clinical
feasibility of therapies that target IGFBP-2 using a relatively
safe viral vector: Adeno-associated virus 2.
Materials and methods
Ethics statement
The study was approved by the institutional review
board (CWO) of Medical School of Nanjing University (Nanjing,
China). All experimental procedures were conducted in conformity
with institutional guidelines for the care and use of laboratory
animals.
Cell culture and infection with
recombinant AAV2
The adenoviral packaging 293T/17 cell line
(ATCC#CRL-11268), as well as the MCF-7 (ATCC#HTB-22), SKBR-3
(ATCC#HTB-30), MDA-MB-231 (ATCC#HTB-26) and MDA-MB-468 (ATCC #132)
human breast cancer cell lines originated from the American Type
Culture Collection (ATCC, Manassas, VA, USA). MCF-10A cells were
purchased from Shanghai Baili Biotechnology Ltd (Baili, Shanghai,
China). MDA-MB-468 cells were maintained in Leibovitz's L-15 medium
(GIBCO) and the remaining cell lines were maintained in
high-glucose Dulbecco's Modified Eagle Medium (DMEM, GIBCO)
supplemented with 110 mg/l sodium pyruvate, 2 mg/l pyridoxine
hydrochloride, 2 g/l sodium bicarbonate, 10% FBS, penicillin (100
U/ml) and streptomycin (100 mg/ml). All cell cultures were
maintained at 37°C in 5% CO2. MDA-MB-468 cells were
grown to approximately 80% confluence before infection with
adenovirus. Specifically, culture medium was aspirated from the
plates, and infections were conducted using
rAAV2-ZsGreen-shRNA-scramble or rAAV2-ZsGreen-shRNA-hIGFBP-2 (our
laboratory cooperated with Shenzhen Biowit Technologies Company) in
serum-free L-15 medium at an optimized concentration of
1.5×1011 viral genomes/ml (vg/ml). Mock infections were
also performed using only serum-free L-15 medium. Plates were
incubated at 37°C for 12 h with intermittent agitation. At the end
of the incubation, residual medium was aspirated from the plates
and replaced with fresh L-15 medium supplemented with 10% serum.
Infected cells were then stimulated with paclitaxel or IGFBP-2 on
the fourth day after infection, and total RNA or protein were
collected at this time. Fluorescent micro-graphs were also obtained
at this time point using a Nikon TE 2000 microscope (magnification
×100) to confirm infection efficiency.
Preparation of AAV2-ZsGreen virus
carrying short hairpinRNA targeting human IGFBP-2
The pAAV-ZsGreen-shRNA plasmid was supplied by
Biowit Technologies (Shenzhen, China). The 68 bp shRNA template
sequences were designed and synthesized as follows: hIGFBP2-F:
5′-GATCCGGAGCAGGTTGCAGACAATTTCAAGAGAATTGTCTGCAACCTGCTCCTTTTTTAGATCTA-3′;
hIGFBP2-R:
5′-AGCTTAGATCTAAAAAAGGAGCAGGTTGCAGACAATTCTCTTGAAATTGTCTGCAACCTGCTCCG-3′;
hScramble-F:
5′-GATCCGCTCGCCTGTCTACTAACTAATTCAAGAGAATTGTCTGCAACCTGCTCCTTTTTTAGATCTA-3′;
hScramble-R:
5′-AGCTTAGATCTAAAAAAGGAGCAGGTTGCAGACAATTCTCTTGAATTAGTTAGTAGACAGGCGAGCG-3′.
BamHI and HindIII restriction sites were used for cloning. An
equimolar mixture of the sense and anti-sense shRNA templates were
denatured by boiling and were annealed at the speed of 5°C/h to
20°C in a thermocycler to form double-stranded DNA. The purified
products were then directly inserted between BamHI and HindIII
restriction sites downstream of the hU6 promoter in the
pAAV-ZsGreen-shRNA vector. The final recombinant plasmids were
named, ‘pAAV-ZsGreen-shRNA-hIGFBP2’ and
‘pAAV-ZsGreen-shRNA-hScramble,’ and were verified by restriction
enzyme digestion and sequencing at Shanghai SANGON Biological
Engineering Technology and Service Co, Ltd. Adenoviral packaging
293T/17 cells were transfected with pAAV-ZsGreen-shRNA-hIGFBP2 or
pAAV-ZsGreen-shRNA-hScramble together with pAAV-RC and
pAAV-Helpervia modified calcium phosphate co-precipitation when
cell monolayers were 50–60% confluent. The cells were harvested
after 72 h of transfection, and re-suspended in cell lysis buffer
(10 mM Tris-HCl, pH 8.5, 150 mM NaCl). Next, the cells were frozen
and thawed three times in liquid nitrogen and at 37°C,
respectively. The lysate was then centrifuged at 4,000 × g for 10
min, and the supernatant was collected. Next, solid NaCl and
PEG-8000 were added to the supernatant to final concentrations of 1
M and 10% (w/v), respectively, and the mixture was agitated to
dissolve these components during a 1 h incubation on ice. After a
15 min centrifugation at 9,000 × g, the pellet was mixed with 1.38
g/ml of CsCl and centrifuged at 500,000 × g for 24 h at 4°C to
isolate the rAAV2-ZsGreen-shRNA viral particles. Determination of
viral titer was performed via qPCR amplification for purified rAAV2
and was calculated at 5×1012 vg/ml.
MTT assay
MDA-MB-231 and MCF-7 cells were cultured in 96-well
plates and were serum-starved for 24 h when they reached 70%
confluency. Next, culture medium with 5% FBS was added to the
cells, supplemented with either 50 µM paclitaxel alone, or both 50
µM paclitaxel and 100 ng/ml IGFBP-2. To avoid attenuation the
effect of IGFBP-2, cells were treated with IGFBP-2 a second time 24
h after the initial exposure. Cell viability was then determined
both 48 and 72 h following drug treatment using
the3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
(MTT) assay (Sigma). Briefly, the cells were incubated with a 0.5%
MTT solution (diluted in culture medium) for 4 h. Then 100 µl of a
0.04 M dimethylsulfoxide solution were added to each well, followed
by incubation at 37°C for an additional 4 h. The absorbance of the
reaction product was measured at 570 nm and is proportional to cell
viability. The data were expressed as the mean ± S.D. and were used
to generate growth curves. Each treatment group was assayed in
triplicate.
Trypan blue cell counting
After infection with 1.5×1011 vg/ml
rAAV2-ZsGreen-shRNA-scramble or AAV2-ZsGreen-shRNA-hIGFBP-2 for
four days, MDA-MB-468 cells were switched to growth medium
containing 5% serum and were treated with paclitaxel or vehicle
control for 24 h. For each experiment, all treatments were
performed in triplicate, and each experiment was repeated at least
three times. Both attached and floating cells were collected by
centrifugation and were stained with 0.04% Trypan blue. The number
of viable and non-viable cells was quantified with a hemocytometer,
and dead cells were divided by all cell counts (18).
Cell invasion assays
After infection with 1.5×1011 vg/ml
rAAV2-ZsGreen-shRNA-scramble or rAAV2-ZsGreen-shRNA-hIGFBP-2 for 4
days, or stimulation with 100 ng/ml IGFBP-2 for 24 h alone,
MDA-MB-468 cells were trypsinized and re-suspended in serum-free
culture medium. The upper chambers of Transwell inserts (8.0 µm
membrane pores, Costar, USA) were subsequently coated with 2.5
mg/ml Matrigel (LOT: 356234, BD Biosciences, USA) and incubated at
37°C for 30 min. A total of 105 cells in 200 µl of
medium were then added to the upper chambers of the inserts and
were allowed to migrate toward the bottom chambers, which contained
medium with 20% FBS as a chemoattractant. After 24 h, the cells on
the apical surfaces of the membranes were removed using a cotton
swab, and cells on the underside were fixed in 3.7%
paraformaldehyde (PFA) and stained with crystal violet.
Quantification of migrated cells was performed by dissolving
crystal violet with 10% acetic acid, and the optical density of
each sample was read with a micro-plate reader at 595 nm. Phase
contrast micro-graphs were also obtained at a magnification of
×100. Experiments were performed with technical duplicates and were
repeated at least three times with consistent results.
Western blotting and
immunohistochemistry
When MDA-MB-231, MDA-MB-468, MCF-7 and SKBR-3 cells
reached approximately 60–70% confluence in their conventional
growth media, they were then switched to either serum-free medium
containing 100 ng/ml IGFBP-2, or serum-free medium alone. Protein
was collected after 24 h. Protein from MDA-MB-468 cells infected
with AAV2 was harvested four h after infection. Western blotting
was performed according to established protocols (19), and the following antibodies were
used: IGFBP-2 (1:1,000, Abcam4243), ER-a (1:1,000, sc-73479),
integrin-α5β (1:1,000, sc-10729), p-ErbB2 (1:1,000, CST#2241),
ErbB2 (1:1,000, CST#2242) and GAPDH (1:1,000, BD Biosciences).
Immunohistochemical staining was performed on 5 µm formalin-fixed,
paraffin-embedded tumor tissue sections. Tissue sections were
deparaffinized in xylenes and hydrated in a graded sequence of
ethanol solutions. For antigen retrieval, sections were then heated
in a microwave in citrate buffer (pH 6.0). After cooling,
nonspecific binding was blocked with diluted serum (5% bovine serum
albumin) followed by incubation with antibodies against CK-7
(1:500, Abcam 183344), IGFBP-2 (1:300, Abcam 109284), MMP-2 (1:500,
Abcam 1818) and Ki67 (1:1,000, Abcam 92742) at room temperature in
a humidified chamber for 2 h. Negative control sections incubated
without primary antibodies were also used. After incubation with
primary antibodies, sections were washed with phosphate-buffered
saline (PBS) and subsequently treated using the corresponding
biotinylated secondary antibody from an Ultravision ONE kit (Thermo
Fisher Scientific, USA) according to the manufacturer's protocol.
Peroxidase activity was visualized using 3,3′-diaminobenzidine, and
sections were counterstained with hematoxylin.
Detection of IGFBP-2 gene
expression
MDA-MB-231, MDA-MB-468, MCF-7 and SKBR-3 cells were
stimulated with 100 ng/ml IGFBP-2 for 24 h in serum-free medium,
and total cell RNA was extracted using TRIzol reagent (Invitrogen)
according to the manufacturer's instructions. Total viral RNA from
infected MDA-MB-468 and MCF-10A cells was also extracted. RNA
yields were measured using a NanoDrop D-2000 (Thermo Fisher
Scientific, USA) instrument. First-strand cDNA was then synthesized
from 1 µg total RNA using oligo-dT rimers and the SuperScript II
Reverse Transcriptase kit (Invitrogen), according to the
manufacturer's instructions. Quantitative real-time PCR was
performed in 20 µl reaction mixtures containing 1 µl of the cDNA
preparation, 10 µl 2X SYBR Green Premix Ex Taq (Takara, DRR041S),
and 1 µM primer pairs on an ABI Step One PCR Instrument. The primer
sequencesused for IGFBP-2 amplification were R:
5-GTCTACTGCATCCGCTGGGT-3; F: 5-GCAAGGGTGGCAAGCATC-3, and the primer
sequences used for GAPDH amplification were R:
5-GGAAGATGGTGATGGGATT-3; F: 5-AACGGATTTGGTCGTATTG-3. The thermal
profile for the real-time PCR reaction consisted of 5 min at 95°C
followed by 40 cycles of 30 sec at 95°C and 1 min at 60°C. Each
sample was assayed in triplicate. Threshold values were determined
for each sample/primer pair, and the average and standard error of
gene expression were calculated. The specificity of the PCR
products was then verified by melt curve analysis, and products
from each reaction were electrophoresed on a 1.8% agarose gel.
GAPDH was used as an internal standard for mRNA expression.
rAAV2-ZsGreen-shRNA-hIGFBP-2 in breast
cancer hypodermic model
The effect of rAAV2-ZsGreen-shRNA-hIGFBP-2 on breast
tumor growth was determined using the athymic mouse model of breast
cancer by hypodermic injection. Female BALB/c nude mice used in
this study (4–5 weeks old and weighing 20.0±2.0 g) were obtained
from the Changzhou Vince Experimental Animal Co. Ltd (Qualification
certificate numbers: 201505694 and 201508683) and were housed in a
defined pathogen-free environment. All procedures were approved by
the National Animal Care and Use Committee in China and the
Laboratory Animal Center of the Academy of Medical Sciences of
Nanjing University. Briefly, MDA-MB-468 cells were trypsinized and
washed with serum-free L-15 medium three times to remove residual
enzyme. Next, mice were anesthetized via inhalation of isoflurane,
and 107 MDA-MB-468 cells in 100 µl of a mixture
consisting of 50 µl complete medium and 50 µl Matrigel (BD, 356234)
were injected subcutaneously into the left flank. After the tumor
volume reached approximately 753 mm (around the 15th day
post-injection), AAV2-ZsGreen-shRNA-hIGFBP-2 or scramble viral
particles (1.0×108 vg/ml in PBS) were administered by
direct injection into the xenograft (4 sites/lump, 25 µl/site)
using a syringe with a 30-gauge needle every five days for 30 days.
A control group of mice received intratumoral injections of PBS in
parallel with the treatment groups. Tumor dimensions were measured
with vernier calipers every five days beginning at the 15th day
post-injection by euthanatizing three mice and excising their
tumors. Tumor volumes were calculated using the following formula:
volume (V) = L × W2 × π/6, where L is the length and W is the width
of the tumor. For histological analysis, subcutaneous tumors were
excised and fixed in 4% paraformaldehyde for 12 h and embedded in
paraffin. Additionally, frozen tumor tissue was embedded in optimal
cutting temperature (OCT) compound, and sectioned into 6 µm
sections with a freezing microtome. The infection efficiency of
in vivo viral gene transfer was assessed in cryosectioned
tumor tissue using fluorescence microscopy (wave length 488
nm).
Statistical analysis
Results are expressed as means ± standard deviation
(SD). Differences between the groups were determined with the
Student's t-test. All analyses were performed with SPSS software
(IBM, USA), version 10.0. Differences among groups were considered
to be significantly different at P<0.05.
Results
IGFBP-2 promoted survival and impacted
chemosensitivity of breast cancer cells
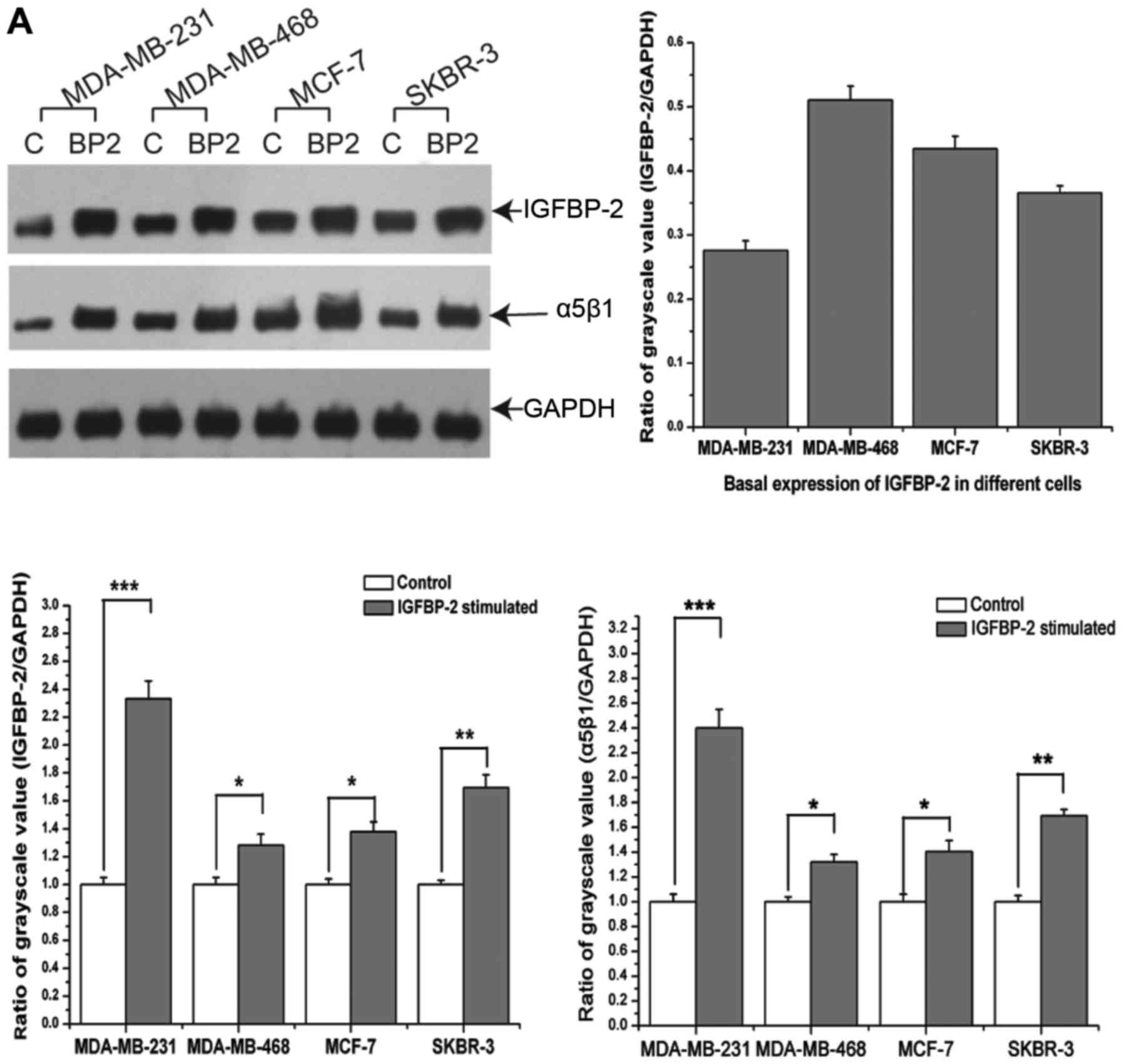
Exogenous addition of IGFBP-2 increased the
protein-level expression of IGFBP-2 and integrin-α5β1 in
MDA-MB-231, MDA-MB-468, MCF-7 and SKBR-3 cells, which represent
different subtypes of breast cancer (Fig. 1A). Among these cell lines, the
triple-negative breast cancer cell line, MDA-MB-468, exhibited the
most robust levels of basal IGFBP-2 expression (Fig. 1A). In addition, exogenous IGFBP-2
(100 ng/ml) enhanced the viability of paclitaxel-treated MDA-MB-231
and MCF-7 cells (Fig. 1B and C),
although no further increases in survival were observed when the
dose of IGFBP-2 was increased to 500 ng/ml. Moreover, IGFBP-2 was
down-regulated on the fourth day after infection with
rAAV2-ZsGreen-shRNA-hIGFBP-2. By the fifth day after infection,
loss of IGFBP-2 significantly increased the number of dead
MDA-MB-468 cells compared to control cells infected with
rAAV2-ZsGreen-shRNA-scramble. With respect to chemosensitivity,
MDA-MB-468 cells infected with rAAV2-ZsGreen-shRNA-hIGFBP-2
exhibited a greater paclitaxel sensitivity compared to cells
infected with rAAV2-ZsGreen-shRNA-scramble. Indeed, the number of
dead cells infected with rAAV2-ZSGreen-shRNA-hIGFBP-2 increased to
56% of the total cell population, a 1.9-fold increase over the
scramble control (Fig. 1D).
Maximal responses to IGFBP-2 knockdown were obtained when viral
titers of 1.5×1011 vg/ml were used.
rAAV2-ZsGreen-shRNAs did not infect
normal human mammary epithelial cells MCF-10A
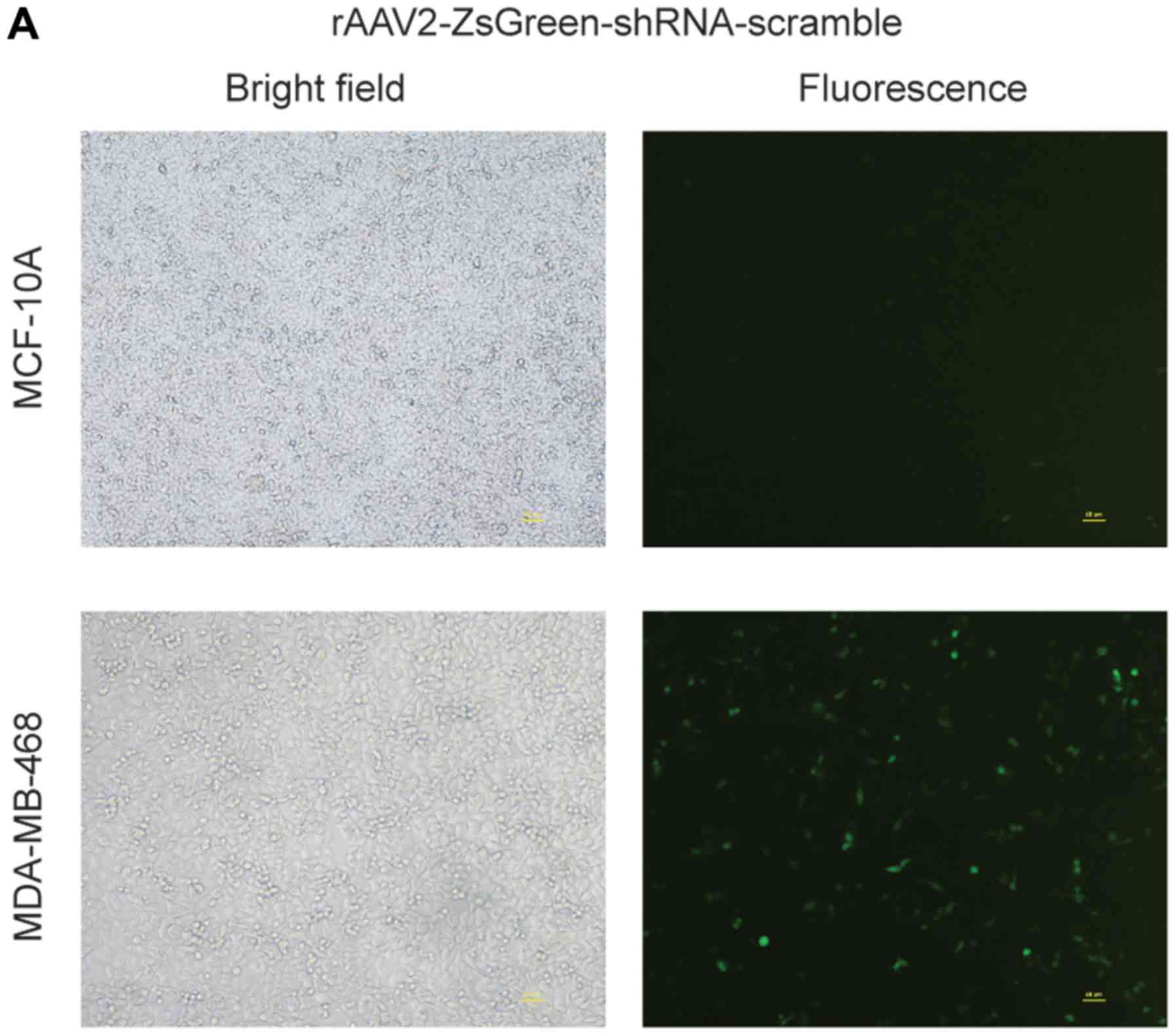
MDA-MB-468 and MCF-10A cells were infected with
rAAV2-ZsGreen-shRNA-scramble or rAAV2-ZsGreen-shRNA-hIGFBP-2 at the
same viral titers. On the fourth day post-infection, MDA-MB-468
cells exhibited robust green fluorescence, while MCF-10A cells were
only weakly fluorescent (Fig. 2A).
In addition, MDA-MB-468 cells infected with rAAV2-ZsGreen-shRNA
exhibited reduced proliferation, which eventually culminated in
apoptosis. These responses were not observed in MCF-10A cells (data
not shown). Next, IGFBP-2 expression in MDA-MB-468 but not MCF-10A
cells was demonstrated to be down-regulated at both the mRNA and
protein levels following infection with
rAAV2-ZsGreen-shRNA-hIGFBP-2 (Fig. 2B
and C). These results suggest that AAV2 can specifically
targeting breast cancer cells, but not normal mammary epithelial
cells.
rAAV2-ZsGreen-shRNA inhibited
proliferation of MDA-MB-468 cells in vivo
We next asked if direct injection of
rAAV2-ZsGreen-shRNA into tumor xenografts could inhibit their
growth in vivo. To this end, we injected a mixture of
MDA-MB-468 cells and Matrigel into the mammary fat pads of nude
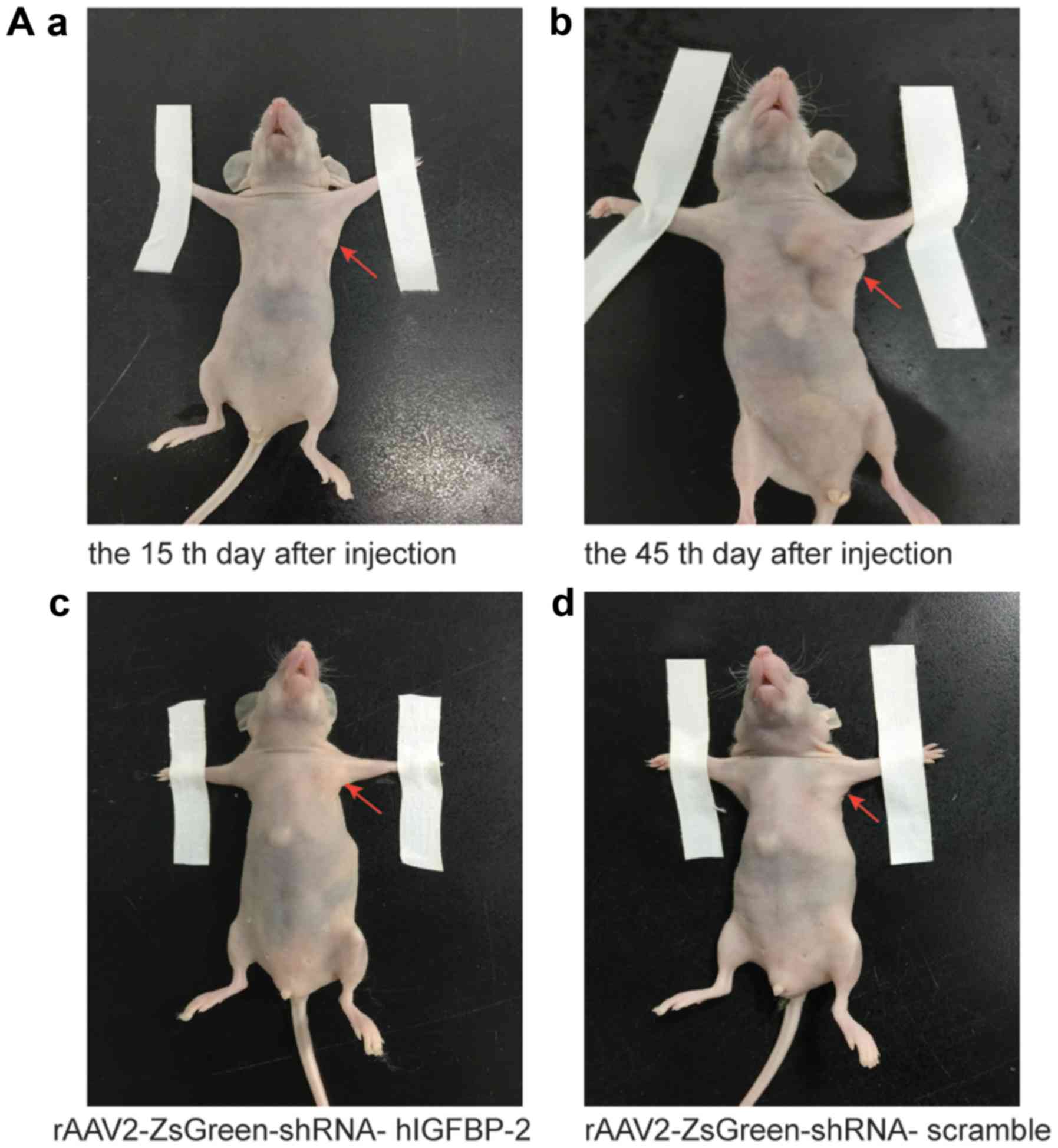
mice. Fifteen days or 45 days after injection, the mice were
euthanized and the tumors were excised (Fig. 3A). Hematoxylin and eosin (H&E)
staining was then performed to verify the morphology of the tumor
cells. We also used frozen tissue sections to confirm that the
viruses had successfully targeted the tumor cells (Fig. 3B). With respect to tumor volume,
tumors injected with rAAV2-ZsGreen-shRNA-scramble and
rAAV2-ZsGreen-shRNA-hIGFBP-2 both exhibited significantly reduced
primary tumor volume compared to control tumors injected with PBS
(Fig. 3C), but
rAAV2-ZsGreen-shRNA-hIGFBP-2 had more marked effect. In addition,
immunohistochemical staining revealed that injection of
rAAV2-ZsGreen-shRNA-scramble and rAAV2-ZsGreen-shRNA-hIGFBP-2 both
decreased tumor Ki-67 expression (Fig.
3D). A general post mortem examination revealed no obvious
organ-specific toxicity in any of the virus-treated animals.
rAAV2-ZsGreen-shRNA-IGFBP-2 suppressed
the invasive potential of MDA-MB-468 cells
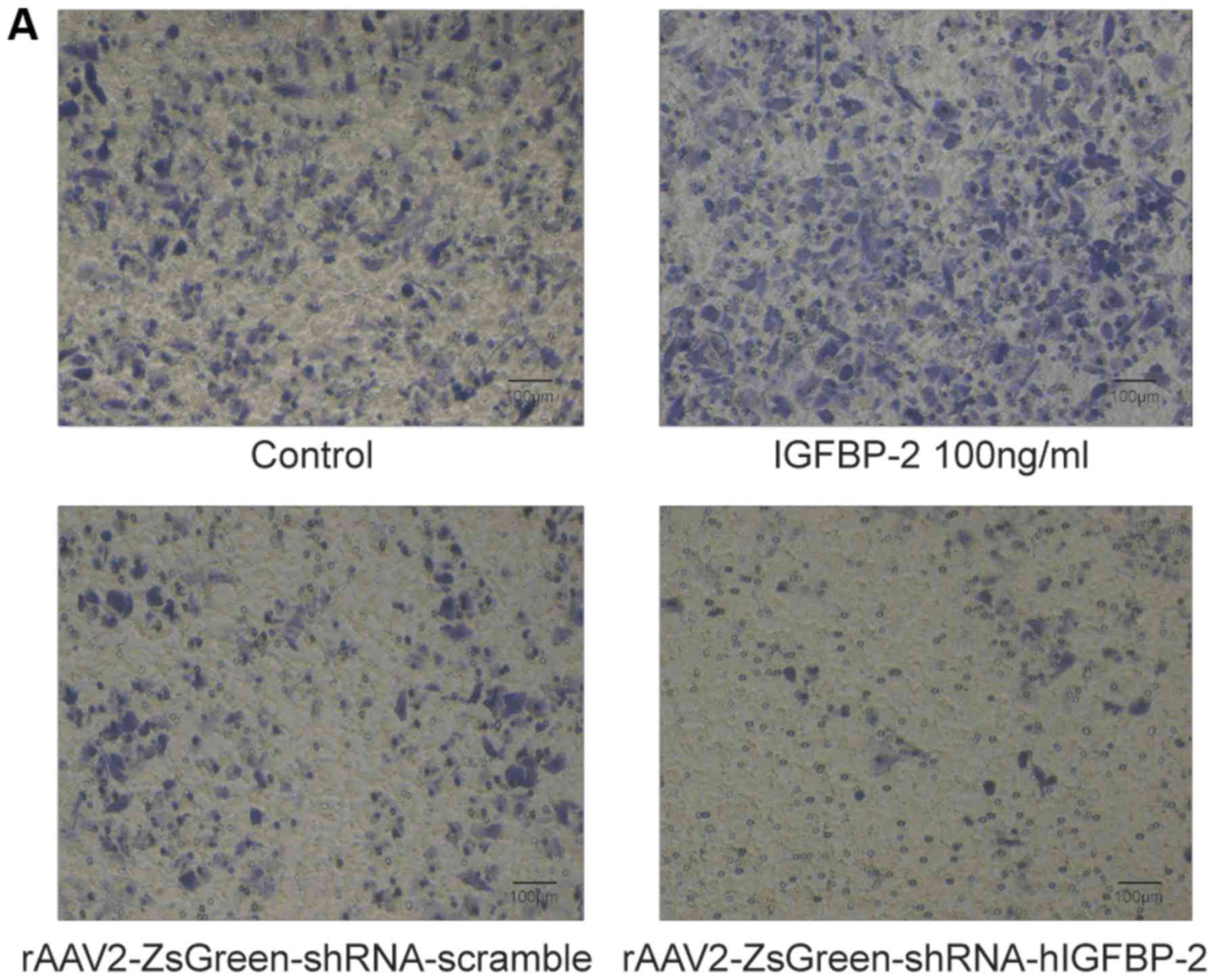
While IGFBP-2-stimulated MDA-MB-468 cells showed
markedly enhanced invasion potential, rAAV2-ZsGreen-shRNA-hIGFBP-2
infected MDA-MB-468 cells showed impaired invasion potential
compared to control cells infected with
rAAV2-ZsGreen-shRNA-scramble (Fig.
4A). These results were quantified by dissolving crystal violet
with a micro-plate reader (Fig.
4B). Moreover, mouse tumors injected with
rAAV2-ZsGreen-shRNA-hIGFBP-2 exhibited markedly reduced MMP-2
expression compared to tumors injected with either PBS or
rAAV2-ZsGreen-shRNA-scramble (Fig.
4C).
Discussion
Breast cancer is the most common cancer type in
women and is associated with a high mortality rate due to the
propensity of this disease to metastasize. Personalized treatment
is often recommended based on histological sub-types characterized
by the expression of estrogen (ER), progesterone (PR) and Her-2
receptors. Importantly, patients with triple-negative
(ER−/PR−/HER2−) breast cancer are
often faced with the worst prognosis. Previous work has shown that
IGFBP-2 can stimulate the proliferation of a number of different
cell types, including prostate cancer cells (20), glioma (21), and chondrocytes (22). Our previous work has also found
that IGFBP-2 is secreted into the breast cancer micro environment
by adipocytes, where by it promotes cancer metastasis. In this
study, we attempted to clarify further the effects of IGFBP-2 in
breast cancer cells and establish its role as a putative
therapeutic target. This hypothesisis further supported by the
observation that exogenous IGFBP-2 could promote survival of
MDA-MB-231 and MCF-7 breast cancer cells in the presence of
paclitaxel, while loss of IGFBP-2 enhanced the chemosensitivity of
MDA-MB-468 cells. We had tried doxorubicin to do the same tests in
Fig. 1 but there was not
significant difference between different treatments.
IGFBP-2 possesses Arg-Gly-Asp integrin-binding
motifs and is among the many proteins that interact with
integrin-α5β1. This interaction has been reported to be involved in
modulating the effects of IGFBP-2 on glioma cell migration and
invasion (23). In our study,
exogenous IGFBP-2 could promote the protein-level expression of
integrin-α5β1 in four breast cancer cell lines. We also found that
exogenous IGFBP-2 up-regulated the expression of endogenous IGFBP-2
in our panel of breast cancer cell lines. Similarly, in a study by
Sehgal et al, IGFBP-2 was shown to up-regulate β-catenin
expression in breast cancer cells, which then further enhanced
IGFPB-2 expression via a positive-feedback mechanism (24). Therefore, suppressing the effects
of IGFBP-2 may help to impede breast cancer progression.
Among the cell lines tested, the highly metastatic,
triple-negative breast cancer cell line, MDA-MB-231, exhibited the
most significant increase in IGFBP-2 expression. Moreover,
exogenous IGFBP-2 promoted the survival of MDA-MB-231 cells, and
enhanced the expression of integrin-α5β1 in this cell type.
Therefore, although MDA-MB-231 cells do not exhibit high basal
expression levels of IGFBP-2, the progression of tumors derived
from this cell line may also be impeded by rAAV2-shRNA-IGFBP-2
therapy. Breast tumors in human patients reside in a complex micro
environment, which could significantly modulate cancer cell protein
expression. Therefore, in vitro studies using human cell
lines may not fully recapitulate all features of clinical breast
tumors. Nevertheless, given that MDA-MB-468 cells exhibit abundant
levels of basal IGFBP-2 expression, this cell type represents a
favorable breast cancer model in which to test the effects of
rAAV2-shRNA-hIGFBP-2 treatment in vitro and in vivo.
In our in vitro study, infection of MDA-MB-468 cells with
rAAV2-ZsGreen-shRNA-hIGFBP-2 led to IGFBP-2 down-regulation,
increased cell death and enhanced chemosensitivity to paclitaxel
compared to infection with rAAV2-ZsGreen-shRNA-scramble. These
results supported the notion that rAAV2-ZsGreen-shRNA-hIGFBP-2 may
have therapeutic effects on triple-negative breast cancer cells.
Moreover, the inability of AAV2 to infect normal mammary epithelial
(MCF-10A) cells renders the potential clinical applications of this
therapy even more exciting. Actually AAV2 transfection efficiency
was strong in a certain number of breast cancer lines which are not
confined to TNBC. We can see evidence based on southern blot
analysis that MCF-7 cells with ER(+) can also be transfected with
AAV2 in reference (17). In
contrast, neither the 4.7 kb replicative form DNA monomer nor the
Rep protein expression could be detected in nHMECs. To our
knowledge, AAV2 transfection efficiency was strong in human breast
cancer cell line MCF-7, MDA-MB-468, MDA-MB-231 and MDA-MB-435. We
hope this virus which can not reproduce in normal human epithelial
cells may attribute to future clinical therapy of human breast
cancer. We speculate that this virus could not replicate in MCF-10A
cells because AAV2-encoded Rep proteins may be inhibited by certain
factors present in normal mammary epithelial cells.
The clinical application of small interfering RNA
(siRNA)-based therapy is limited by the poor stability, poor
intracellular uptake, and rapid enzymatic degradation of these
molecules. To overcome these limitations, we used adeno-associated
virus type 2 (AAV2) to deliver siRNAs to cancer cells. Importantly,
this method is non-pathogenic, and these viruses do not infect
normal human mammary epithelial cells. Several lines of evidence
suggest that over-expression of IGFBP-2 increases cell growth and
metastatic potential in several tumor types, including ovarian
(25), prostate (26), and bladder cancer (27), as well as glioblastoma (28). Based on the results of our study,
we expect that recombinant AAV2-ZsGreen-shRNA-hIGFBP-2-mediated
degradation of IGFBP-2 mRNA has significant potential in the
treatment of breast cancers characterized by IGFBP-2
over-expression. In addition, Alam et al determined that
infection of nude mice with wild-type AAV2 induced necrosis and
inhibited tumor growth in xenograft models of breast cancer
(29). Using a similar mouse
model, our study also found that intratumoral injection with
1.0×108 vg/ml of rAAV2-ZsGreen-shRNA-scramble or
rAAV2-ZsGreen-shRNA-hIGFBP-2 both reduced tumor volume compared to
injections of PBS alone, as evidenced by reduced Ki-67 expression.
However, the exact mechanism by which AAV2 infection inhibits tumor
growth is not known. According to the literature, it is reasonable
to predict that the structures of certain AAV2 proteins, such as
Rep, may resemble those of eukaryotic cell cycle checkpoint
proteins. Accordingly, AAV2-mediated rescue of defective tumor cell
checkpoint proteins would greatly down-regulate cell proliferation.
Although the specific protein signals downstream of IGFBP-2 have
not been identified, the results of this observational study can
confirm that rAAV2-ZsGreen-shRNA-hIGFBP-2 can inhibit the
proliferation of tumor xenografts derived from MDA-MB-468 cells
more significantly.
Furthermore, the
rAAV2-ZsGreen-shRNA-hIGFBP-2-infected MDA-MB-468 cells exhibited
reduced invasive potential in vitro, whereas exogenous
IGFBP-2 stimulated MDA-MB-468 invasion. Taken together, these
results further suggest that this recombinant virus maybe a
promising method to impede breast cancer cell metastasis. In our
in vivo study, tumor xenografts injected with
rAAV2-ZsGreen-shRNA-hIGFBP-2 exhibited remarkably reduced MMP-2
expression compared to tumors injected with
rAAV2-ZsGreen-shRNA-scramble, suggesting that
rAAV2-ZsGreen-shRNA-hIGFBP-2 maybe a useful tool for preventing
metastasis in breast cancer patients. Importantly, our previous
work has demonstrated that exogenous IGFBP-2 could enhance the
expression of MMP-2 in breast cancer cells (11). Therefore, this result is in accord
with the trans-well assay and indicates that
rAAV2-ZsGreen-shRNA-hIGFBP-2 maybe used to impede tumor metastasis
in breast cancer patients. Taken together, our in vitro and
in vivo results indicate that rAAV2-ZsGreen-shRNA-hIGFBP-2
can inhibit the growth of MDA-MB-468 cells, as well as enhance
chemosensitivity and reduce invasive potential in this cell
type.
In conclusion, we have constructed an AAV-2-mediated
siRNA delivery system designed to target IGFBP-2-regulated pathways
in breast cancer. The effectiveness of this system in both in
vitro and in vivo models has been reported for the first
time in the present study. We show that MCF10A cells are resistant
to rAAV2-shRNA-scramble infection and in vivo injection of
rAAV2-shRNA-hIGFBP-2 inhibits the growth of tumor xenografts
derived from MDA-MB-468cells. We also demonstrate that
rAAV2-shRNA-hIGFBP-2 can reduce the invasive potential of
MDA-MB-468cells in vitro.
Acknowledgements
This article is supported by the Jiangsu Natural
Science Foundation of China (grant no. BK20130591). the authors
would like to thank the company Biowit Technologies (Shenzhen,
China).
Glossary
Abbreviations
Abbreviations:
|
IGFBP-2
|
insulin-like growth factor-binding
protein-2
|
|
AAV2
|
adeno-associated virus type 2
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
shRNA
|
short hairpin ribonucleic acid
|
|
HER2
|
human epidermalgrowth factor
receptor-2
|
|
DMEM
|
Dulbecco's modification of Eagle's
medium
|
|
FBS
|
fetal bovine serum
|
|
MMP-2
|
matrix metalloproteinase 2
|
|
CK-7
|
cytokeratin-7
|
|
MTT
|
Methylthiazolyldiphenyl-tetrazolium
bromide
|
|
GAPDH
|
glyceraldehyde phosphate
dehydrogenase
|
|
OCT
|
optimal cutting temperature
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
El Atiq F, Garrouste F, Remacle-Bonnet M,
Sastre B and Pommier G: Alterations in serum levels of insulin-like
growth factors and insulin-like growth-factor-binding proteins in
patients with colorectal cancer. Int J Cancer. 57:491–497. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karasik A, Menczer J, Pariente C and
Kanety H: Insulin-like growth factor-I (IGF-I) and IGF-binding
protein-2 are increased in cyst fluids of epithelial ovarian
cancer. J Clin Endocrinol Metab. 78:271–276. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baron-Hay S, Boyle F, Ferrier A and Scott
C: Elevated serum insulin-like growth factor binding protein-2 as a
prognostic marker in patients with ovarian cancer. Clin Cancer Res.
10:1796–1806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DY, Kim SJ and Lee YC: Serum
insulin-like growth factor (IGF)-I and IGF-binding proteins in lung
cancer patients. J Kor Med Sci. 14:401–404. 1999. View Article : Google Scholar
|
|
5
|
Cohen P, Peehl DM, Stamey TA, Wilson KF,
Clemmons DR and Rosenfeld RG: Elevated levels of insulin-like
growth factor-binding protein-2 in the serum of prostate cancer
patients. J Clin Endocrinol Metab. 76:1031–1035. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shariat SF, Lamb DJ, Kattan MW, Nguyen C,
Kim J, Beck J, Wheeler TM and Slawin KM: Association of
preoperative plasma levels of insulin-like growth factor I and
insulin-like growth factor binding proteins-2 and −3 with prostate
cancer invasion, progression and metastasis. J Clin Oncol.
20:833–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Busund LT, Richardsen E, Busund R, Ukkonen
T, Bjørnsen T, Busch C and Stalsberg H: Significant expression of
IGFBP2 in breast cancer compared with benign lesions. J Clin
Pathol. 58:361–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
So AI, Levitt RJ, Eigl B, Fazli L,
Muramaki M, Leung S, Cheang MC, Nielsen TO, Gleave M and Pollak M:
Insulin-like growth factor binding protein-2 is a novel therapeutic
target associated with breast cancer. Clin Cancer Res.
14:6944–6954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schütt BS, Langkamp M, Rauschnabel U,
Ranke MB and Elmlinger MW: Integrin-mediated action of insulin-like
growth factor binding protein-2 in tumor cells. J Mol Endocrinol.
32:859–868. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pereira JJ, Meyer T, Docherty SE, Reid HH,
Marshall J, Thompson EW, Rossjohn J and Price JT: Bimolecular
interaction of insulin-like growth factor (IGF) binding protein-2
with alphavbeta3 negatively modulates IGF-I-mediated migration and
tumor growth. Cancer Res. 64:977–984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Gao C, Meng K, Qiao H and Wang Y:
Human adipocytes stimulate invasion of breast cancer MCF-7 cells by
secreting IGFBP-2. PLoS One. 10:e01193482015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zaiss AK and Muruve DA: Immunity to
adeno-associated virus vectors in animals and humans: A continued
challenge. Gene Ther. 15:808–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herzog RW, Yang EY, Couto LB, Hagstrom JN,
Elwell D, Fields PA, Burton M, Bellinger DA, Read MS, Brinkhous KM,
et al: Long-term correction of canine hemophilia B by gene transfer
of blood coagulation factor IX mediated by adeno-associated viral
vector. Nat Med. 5:56–63. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Takabe K, Bidlingmaier SM, Ill CR
and Verma IM: Sustained correction of bleeding disorder in
hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 96:pp.
3906–3910. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponnazhagan S, Mahendra G, Kumar S, Shaw
DR, Stockard CR, Grizzle WE and Meleth S: Adeno-associated virus
2-mediated antiangiogenic cancer gene therapy: Long-term efficacy
of a vector encoding angiostatin and endostatin over vectors
encoding a single factor. Cancer Res. 64:1781–1787. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Collins SA, Buhles A, Scallan MF, Harrison
PT, O'Hanlon DM, O'Sullivan GC and Tangney M: AAV2-mediated in vivo
immune gene therapy of solid tumours. Genet Vaccines Ther. 8:82010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alam S, Bowser BS, Conway MJ, Israr M,
Tandon A and Meyers C: Adeno-associated virus type 2 infection
activates caspase dependent and independent apoptosis in multiple
breast cancer lines but not in normal mammary epithelial cells. Mol
Cancer. 10:972011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Foulstone EJ, Zeng L, Perks CM and Holly
JM: Insulin-like growth factor binding protein 2 (IGFBP-2) promotes
growth and survival of breast epithelial cells: Novel regulation of
the estrogen receptor. Endocrinology. 154:1780–1793. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Bian Z, Wei D and Zhang JG:
MiR-29b regulates migration of human breast cancer cells. Mol Cell
Biochem. 352:197–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uzoh CC, Holly JM, Biernacka KM, Persad
RA, Bahl A, Gillatt D and Perks CM: Insulin-like growth
factor-binding protein-2 promotes prostate cancer cell growth via
IGF-dependent or -independent mechanisms and reduces the efficacy
of docetaxel. Br J Cancer. 104:1587–1593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holmes KM, Annala M, Chua CY, Dunlap SM,
Liu Y, Hugen N, Moore LM, Cogdell D, Hu L, Nykter M, et al:
Insulin-like growth factor-binding protein 2-driven glioma
progression is prevented by blocking a clinically significant
integrin, integrin-linked kinase, and NF-κB network. Proc Natl Acad
Sci USA. 109:pp. 3475–3480. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiepe D, Van Der A, Ciarmatori S, Ständker
L, Schütt B, Hoeflich A, Hügel U, Oh J and Tönshoff B: Defined
carboxy-terminal fragments of insulin-like growth factor (IGF)
binding protein-2 exert similar mitogenic activity on cultured rat
growth plate chondrocytes as IGF-I. Endocrinology. 149:4901–4911.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang GK, Hu L, Fuller GN and Zhang W: An
interaction between insulin-like growth factor-binding protein 2
(IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell
mobility. J Biol Chem. 281:14085–14091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sehgal P, Kumar N, Praveen Kumar VR, Patil
S, Bhattacharya A, Vijaya Kumar M, Mukherjee G and Kondaiah P:
Regulation of protumorigenic pathways by insulin like growth factor
binding protein2 and its association along with β-catenin in breast
cancer lymph node metastasis. Mol Cancer. 12:632013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakrabarty S and Kondratick L:
Insulin-like growth factor binding protein-2 stimulates
proliferation and activates multiple cascades of the
mitogen-activated protein kinase pathways in NIH-OVCAR3 human
epithelial ovarian cancer cells. Cancer Biol Ther. 5:189–197. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chatterjee S, Park ES and Soloff MS:
Proliferation of DU145 prostate cancer cells is inhibited by
suppressing insulin-like growth factor binding protein-2. Int J
Urol. 11:876–884. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyake H, Hara I, Yamanaka K, Muramaki M,
Gleave M and Eto H: Introduction of insulin-like growth factor
binding protein-2 gene into human bladder cancer cells enhances
their metastatic potential. Oncol Rep. 13:341–345. 2005.PubMed/NCBI
|
|
28
|
Fukushima T, Tezuka T, Shimomura T, Nakano
S and Kataoka H: Silencing of insulin-like growth factor-binding
protein-2 in human glioblastoma cells reduces both invasiveness and
expression of progression-associated gene CD24. J Biol Chem.
282:18634–18644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alam S, Bowser BS, Israr M, Conway MJ and
Meyers C: Adeno-associated virus type 2 infection of nude mouse
human breast cancer xenograft induces necrotic death and inhibits
tumor growth. Cancer Biol Ther. 15:1013–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|