Introduction
Cervical cancer is the second most prominent type of
malignant tumor affecting women and the second leading cause of
cancer mortality affecting females in China (1). Primary treatments for cervical cancer
include surgery (including pelvic lymphadenectomy and radical
hysterectomy), radiotherapy and chemotherapy. Radical hysterectomy
and radiotherapy are considered to be curative for localized
disease; whereas, for the treatment of advanced stage cancer,
concurrent radiochemotherapy remains a cornerstone intervention.
However, increasing systemic toxicity caused by radiochemotherapy,
lack of tumor control, recurrence and metastasis significantly
reduce the survival rate of advanced cervical cancer (2). Chemotherapy, despite continuing to be
the most common auxiliary treatment and palliative care option for
recurrent and metastatic cancer, results in unfavorable prognoses
and frequently causes an increase in systemic toxicity and
chemoresistance. Thus, there is a requirement for the development
of novel antitumor reagents for the treatment of recurrent and
metastatic cervical cancers. Currently, despite investigations into
the development of alternative adjuvant treatment methods,
including molecular targeted therapy and immunotherapy, the
majority of research remains at the exploratory stage, and only
anti-vascular endothelial growth factor has been used for the
clinical treatment of cervical cancer (3). Traditional Chinese medicine has long
been used to treat tumors and its anti-tumor role has received
increased research attention (4).
Curcuma zedoaria, also known as Rhizoma
zedoariae, belongs to the Zingiberaceae family and has been
used widely to treat cervical lesions since the early 1940s
(5). Furthermore, zedoary turmeric
oil has previously been used in clinical practice for the treatment
of cervical inflammation and human papilloma virus infection in
China (6). Elemene, a component of
Rhizoma zedoariae oil, may take the form of α, β, δ and γ-elemene.
β-elemene is the main active component of Rhizoma zedoariae
oil and has been revealed to demonstrate antitumor effects in
various cancers, including glioma, laryngeal cancer, leukemia and
ovarian cancer (7–9). Despite certain studies discussing the
therapeutic mechanisms of β-elemene with regards to cancer
(10,11), the underlying molecular mechanisms
of β-elemene have not yet been determined. In addition, the role of
β-elemene in cervical cancer cells has not been widely studied.
Wnt/β-catenin signaling participates in the processes of
embryogenesis and is additionally active in adult organisms. The
upregulation of a number of Wnt/β-catenin signaling pathway members
is closely associated with certain cancer types. Overexpression of
β-catenin has previously been detected in various cancers, such as
intestinal cancer and ovarian carcinomas (12). Furthermore, Wnt/β-catenin signaling
is an essential pathway for the modulation of the proliferation,
differentiation and motility of cells (13). The present study aimed to
investigate whether β-elemene was able to inhibit cell
proliferation, promote cellular apoptosis and decrease the invasive
properties of cervical cancer cells, and to determine whether these
effects occur as a result of the functioning of the Wnt/β-catenin
signaling pathway.
Materials and methods
Chemicals and reagents
SiHa cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). β-elemene was obtained from
Dalian Huali JinGang Pharmaceutical Co., Ltd. (Dalian, China) and
dissolved in PBS in order to generate a 5 mg/ml stock solution for
experimental use. In addition, MTT was purchased from Beijing
Huaxia Ocean Science and Technology Co., Ltd. (Beijing, China).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), PBS and trypsin/EDTA solution were purchased (Gibco; Thermo
Fisher Scientific Inc., Waltham, MA, USA). Matrigel was purchased
from BD Biosciences (Franklin Lakes, NJ, USA). A bicinchoninic acid
(BCA) protein assay kit, in addition to cell cycle and apoptosis
analysis kits, was purchased from Nanjing KeyGen Biotech. Co., Ltd.
(Nanjing, China). Primary antibodies against Cyclin-dependent
kinase inhibitor 2B (P15) (cat. no. AB33457), Cyclin D1 (cat. no.
AB12597), P53 (cat. no. AB41876), apoptosis regulator Bcl-2 (Bcl-2)
(cat. no. AB40639), apoptosis regulator BAX (Bax) (cat. no.
AB40636), β-catenin (cat. no. AB40439), Myc proto-oncogene protein
(c-Myc) (cat. no. AB40766) and GAPDH (cat. no. AB21612), and the
secondary horseradish peroxidase (HRP)-conjugated goat
anti-rabbit-IgG (cat. no. ABL3012-2) and HRP-conjugated goat
anti-mouse-IgG antibodies (cat. no. ABL3031-2), were purchased from
Bioscience Technology, Inc. (www.abscitech.com/; College Park, MD, USA). The
primary antibodies against transcription factor 7 (TCF7; cat. no.
14464-1-AP), 72 kDa type IV collagenase (MMP-2; cat. no.
10373-2-AP) and matrix metalloproteinase-9 (MMP-9; cat. no.
10375-2-AP) were purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). Both the primary and secondary antibodies were diluted to
1:1,000.
Cell cultures
SiHa cells were cultured in DMEM containing 10% FBS
and placed in an incubator with a saturated, humidified atmosphere
with 5% CO2 at 37°C. Logarithmically growing cells were
used in All Subsequent Experiments.
Cell proliferation assay
The MTT assay was used in order to evaluate the
proliferation of SiHa cells. SiHa cells were seeded into 96-well
microtiter plates at 5×103 cells/well and treated with
increasing concentrations of β-elemene (0–50 µg/ml) for 24, 48 and
72 h. Following this, 20 µl MTT solution was added to each well and
incubation continued at 37°C for further 4 h. Dimethyl sulfoxide
(150 µl) was added to each well and incubation was continued at
room temperature for 20 min. The optical density value of each well
was detected at a wavelength of 490 nm. Each assay was performed in
triplicate.
Flow cytometry analysis of the cell
cycle and apoptosis
SiHa cells (1×106) were exposed to
different concentrations of β-elemene (0, 20, 30 and 40 µg/ml) for
48 h and harvested. The cell cycle was investigated using a Cell
Cycle Detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Briefly, SiHa cells were washed with PBS and then fixed
with 70% ethanol at 4°C for 30 min. Following this, the cells were
suspended in 300 µl PBS and incubated with propidium iodide (PI; 20
mg/ml) and RNase (1 mg/ml) for 30 min. Cellular DNA was stained
with propidium iodide (Nanjing KeyGen Biotech Co., Ltd.). Cell
cycle distributions were determined by flow cytometry using a BD
FACSCalibur system (BD Biosciences) and data was analyzed using the
ModFit software version 4.1 (Verity Software House, Inc., Topsham,
ME, USA). An Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen
Biotech Co., Ltd.) was used to investigate cellular apoptosis. SiHa
cells were washed with PBS and resuspended in 500 µl binding
buffer. Annexin V-fluorescein isothiocyanate (5 µl) and PI (5 µl)
were added to the samples, according to the manufacturer's
protocol. Finally, the processed cells were subjected to flow
cytometry and data were analyzed using the Cell Quest software
version 5.1 (BD Biosciences). Each experiment was performed in
triplicate.
Transwell assay
In preparation for the motility assay, SiHa cells
were resuspended at a density of 1×105 cells/ml in
serum-free DMEM. The cell suspension (200 µl) was added to
different concentrations of β-elemene (0, 20, 30 and 40 µg/ml) and
placed in an upper Transwell chamber (BD Biosciences).
Simultaneously, 600 µl conditioned medium containing 20% FBS, in
addition to the different aforementioned concentrations of
β-elemene, was added to the bottom Transwell chamber. Following a
further 24 h incubation period at 37°C, the SiHa cells that had
migrated to the bottom chamber were fixed in methanol for 30 min at
room temperature, stained with crystal violet for 30 min at room
temperature, and counted using a light microscope under a 10-fold
mirror vision. In order to perform the invasion assay, 40 µl
Matrigel (0.5 mg/ml; Beckman Coulter, Inc., Brea, CA, USA) was
spread onto the upper Transwell chamber and incubated for 4 h at
37°C. The plating of the lower chamber was performed in accordance
with the aforementioned protocol. Following this, the cells were
incubated for 72 h at 37°C in the Transwell device and then fixed,
stained and counted according to the aforementioned protocol. Each
assay was performed in triplicate.
Wound healing assay
SiHa cells at a density of 1×105
cells/well in medium containing 10% FBS were plated into a 6-well
plate and incubated for 24 h (37°C, 5% CO2) until the
cell monolayer covered the plate. A sterile 200 µl plastic pipette
tip was used to scratch vertically on the cell layer in order to
make a wound. Following this, the cells were incubated for 48 h
(37°C, 5% CO2) with different concentrations of
β-elemene (0, 20, 30 and 40 µg/ml) under serum-free conditions. The
gap distance was measured at 0 and 48 h using a light microscope
under a 4-fold mirror vision. Each assay was conducted in
triplicate.
Preparation of proteins and western
blot analysis
Following treatment of SiHa cells with β-elemene (40
µg/ml) for 48 h, radioimmunoprecipitation assay buffer (cat. no.
WLA014a; Wanlei Life Science, Shenyang, China) containing
phenylmethylsulfonyl fluoride was added in order to extract the
total protein. A BCA assay kit (Nanjing KeyGen Biotech. Co., Ltd.)
was used to measure the protein concentration. Equal amounts of
protein (60 µg) were separated by SDS-PAGE on a 10% gel and
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked using 5% skimmed milk at room temperature for 2 h and
incubated with appropriate primary antibodies against P15, Cyclin
D1, p53, Bcl-2, Bax, β-catenin, c-Myc, TCF7, MMP-2, MMP-9 and GAPDH
at 4°C overnight. Following this, the membranes were incubated with
the secondary antibodies (anti-rabbit or anti-mouse) for 1 h at
37°C. Finally, the immune reactive proteins were detected using an
enhanced chemiluminescence kit (cat. no. WLA003a; Wanlei Life
Science). Protein bands were quantified using Quantity One software
(version 4.6.3; BioRad Laboratories, Inc.) and normalized against
GAPDH. Each experiment was performed in triplicate.
Statistical analysis
SPSS software (version 17.1; SPSS, Inc., Chicago,
IL, USA) was used to analyze the statistical data, and the data are
presented as the mean ± standard deviation. The Student's t-test
was used to evaluate the differences between the control group and
the experimental group, and one-way analysis of variance followed
by Tukey's test was used to evaluate the differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
β-elemene inhibits the proliferation
and survival of SiHa cells
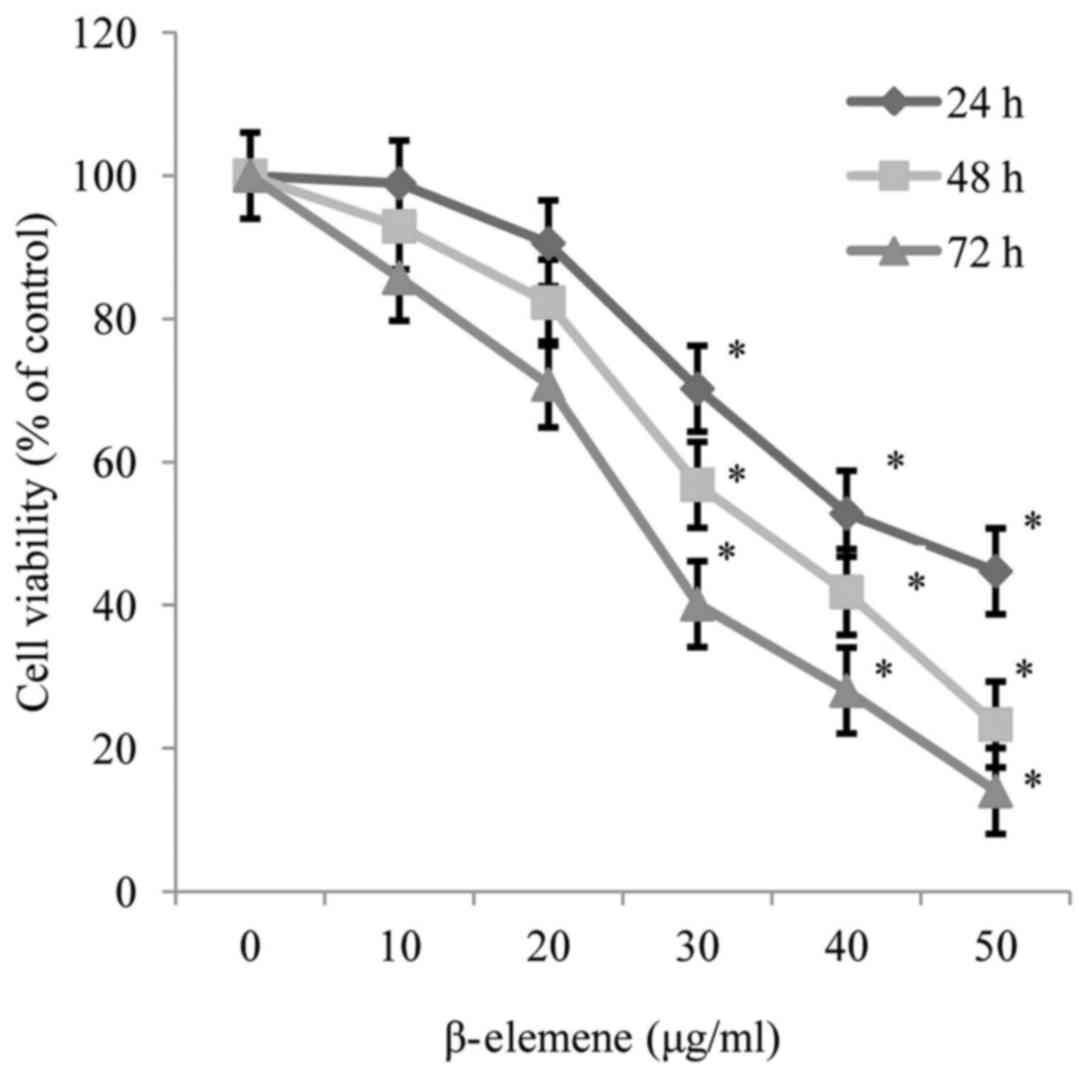
The results of the MTT assay revealed that β-elemene
reduced the viability of SiHa cells in a dose and time-dependent
manner, and the half-maximal inhibitory concentration values of
β-elemene for SiHa cells were 42.17, 29.20 and 21.29 µg/ml at 24,
48 and 72 h, respectively (P<0.05; Fig. 1).
β-elemene induces cell-cycle arrest at
the G1 phase in SiHa cells
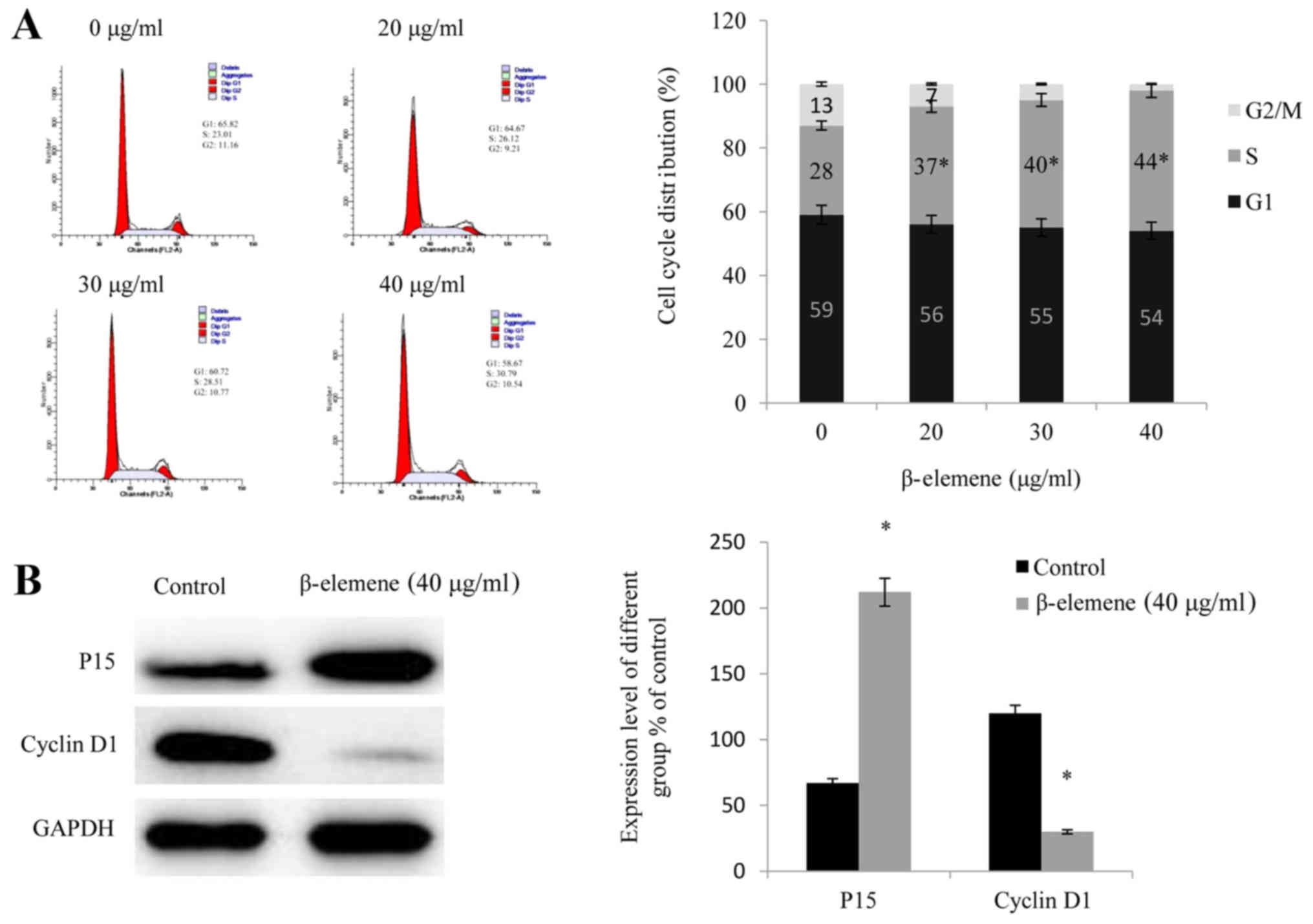
Cell cycle analysis demonstrated that following
treatment with increasing doses of β-elemene (20, 30 and 40 µg/ml),
the number of SiHa cells arrested at the G1 phase of the cell cycle
significantly increased compared with 0 µg/ml (P<0.05; Fig. 2A), which suggested that the
administration of β-elemene arrested SiHa cells at the G1 phase by
reducing their ability to divide and inhibiting their
proliferation. Furthermore, cell cycle-associated proteins were
investigated, and it was revealed that the administration of
β-elemene upregulated the expression of P15 and downregulated the
expression of Cyclin D1 (P<0.05; Fig. 2B).
β-elemene induces apoptosis in SiHa
cells
The results of the flow cytometry analysis revealed
that treatment with increasing doses of β-elemene (20, 30 and 40
µg/ml), significantly increased the apoptotic rate of SiHa cells in
a dose-dependent manner (P<0.05; Fig. 3A). Furthermore, the levels of
apoptosis-associated proteins were investigated, and it was
demonstrated that the administration of β-elemene significantly
upregulated the expression levels of p53 and Bax, and significantly
downregulated the expression of Bcl-2 (P<0.05; Fig. 3B).
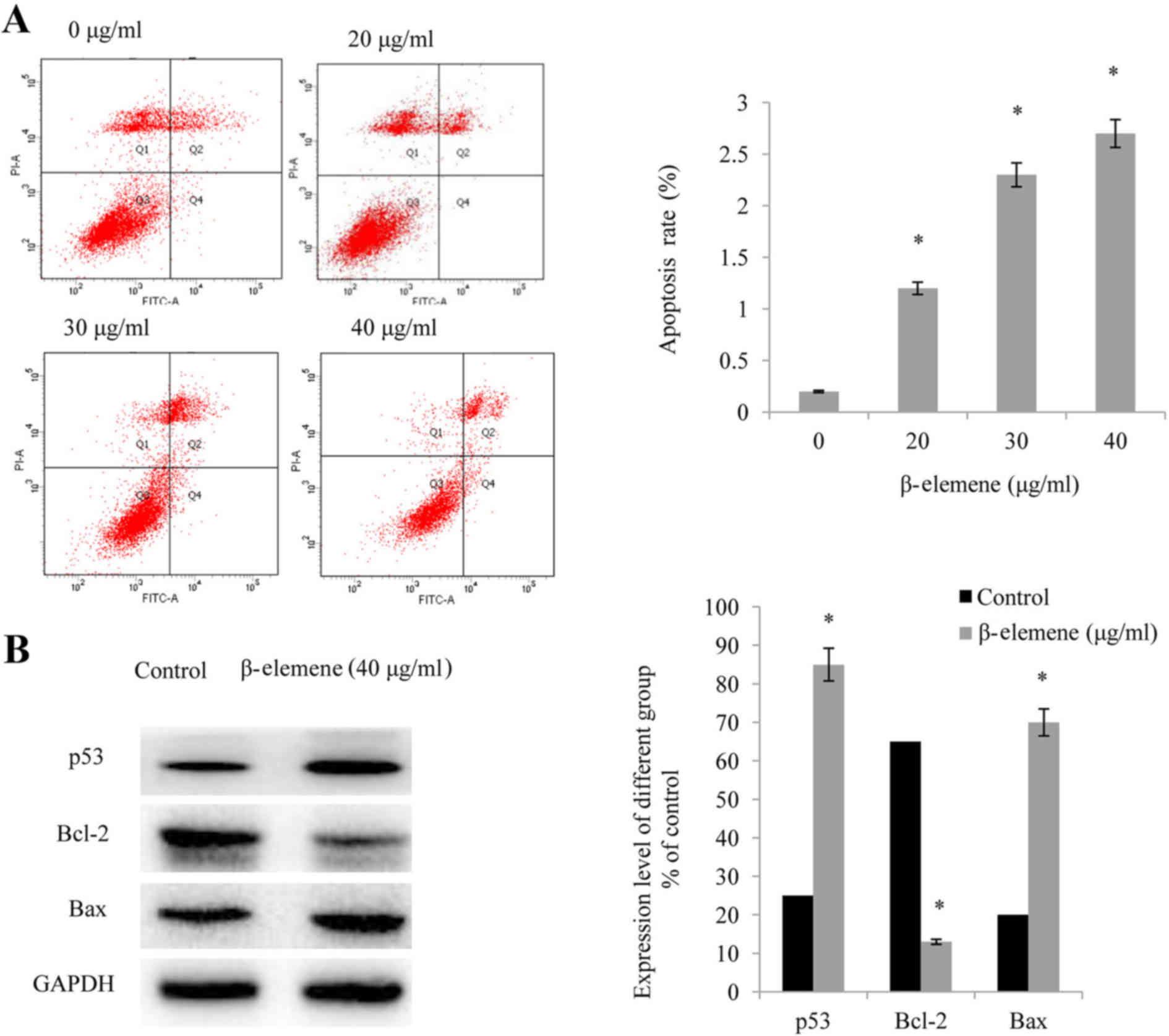 | Figure 3.β-elemene induces apoptosis in SiHa
cells. (A) Following treatment of SiHa cells with increasing doses
of β-elemene (0, 20, 30, and 40 µg/ml) for 48 h, flow cytometry was
performed in order to detect cellular apoptosis; *P<0.05 vs. 0
µg/ml. (B) Western blot analysis of p53, Bax, and Bcl-2 expression
levels in SiHa cells. Each experiment was performed in triplicate.
Values presented represent the mean ± standard deviation of three
independent experiments. *P<0.05 vs. control. p53, cellular
tumor antigen p53; Bax, apoptosis regulator BAX; Bcl-2, apoptosis
regulator Bcl-2; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
β-elemene inhibits the invasion and
migration of SiHa cells
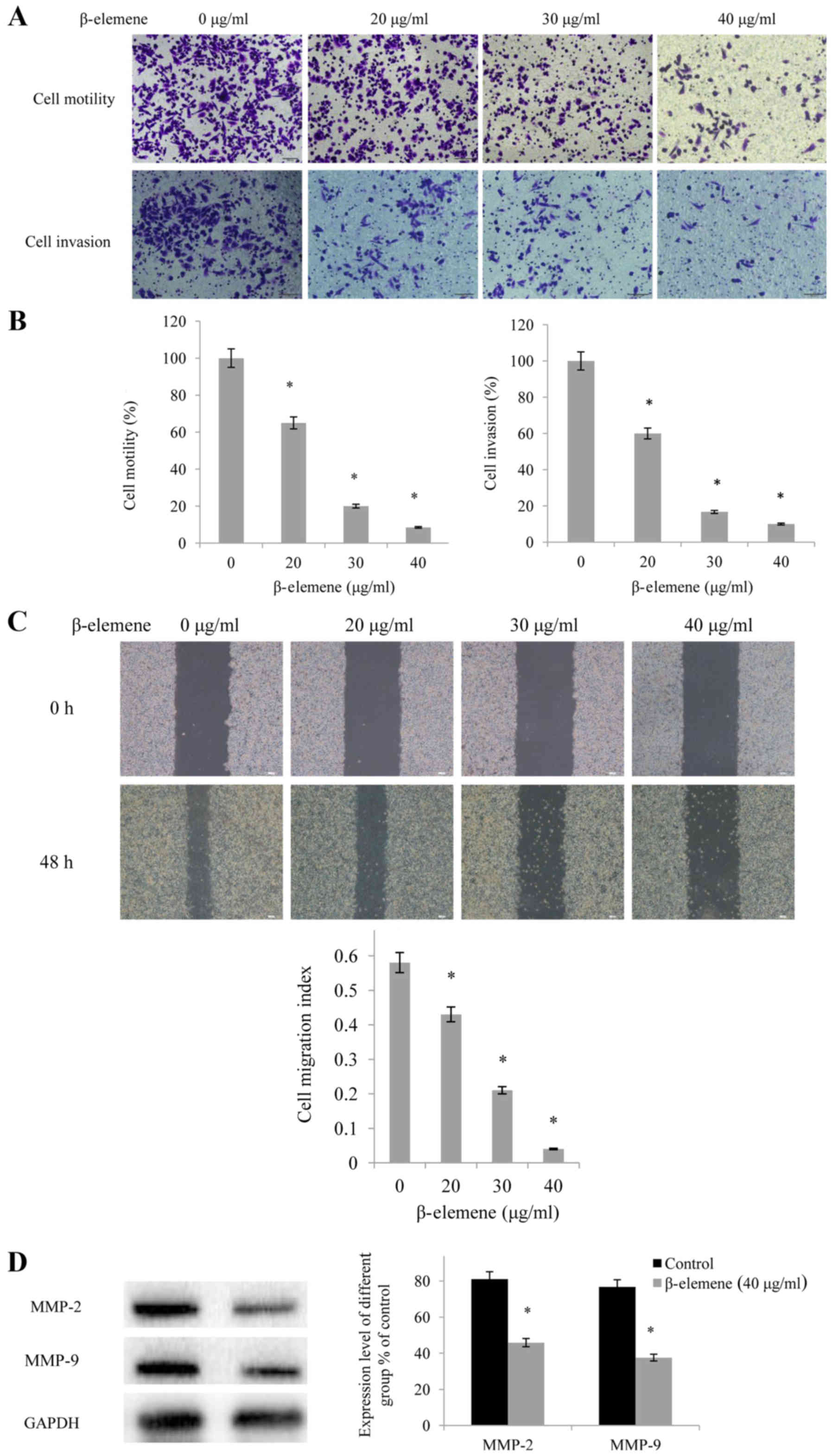
The results of the Transwell assays demonstrated
that β-elemene administration inhibited the motility and invasion
of SiHa cells in a dose-dependent manner (Fig. 4A), the quantification results of
which are presented in Fig. 4B
(P<0.05). Furthermore, when SiHa cells were treated with
β-elemene (40 µg/ml) for 72 h, the invasive ability of the cells
declined by 90%. In addition, wound healing migration assays
revealed that β-elemene administration inhibited the migration of
SiHa cells in a dose-dependent manner, thus suggesting that
β-elemene may significantly inhibit the migratory ability of SiHa
cells (Fig. 4C). Furthermore, the
expression levels of invasion-associated proteins were
investigated, and it was revealed that β-elemene significantly
downregulated the expression levels of MMP-2 and MMP-9 (P<0.05;
Fig. 4D).
β-elemene suppresses the Wnt/β-catenin
signaling pathway
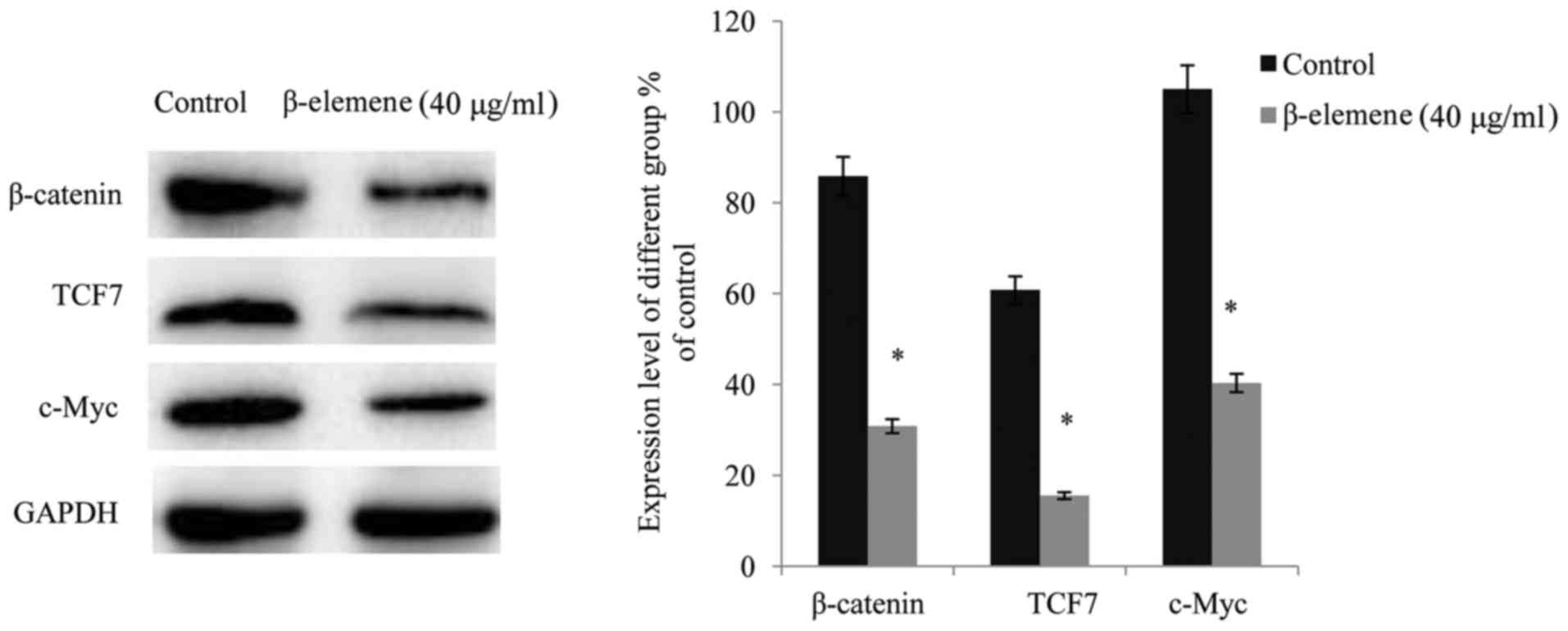
Following exposure of SiHa cells to β-elemene (40
µg/ml) for 48 h, western blot analysis was performed in order to
detect alterations in the expression levels of β-catenin, its
target molecule TCF7, and their target protein, c-Myc. The results
demonstrated that administration of β-elemene significantly
suppressed the expression of β-catenin and its downstream target
molecule TCF7 in SiHa cells, thus resulting in a reduced expression
level of their target, c-Myc (P<0.05; Fig. 5).
Discussion
Curcuma zedoaria, the dried rhizome of
Zingiberaceae plants, has previously been revealed to exhibit
antitumor, antithrombotic and antibacterial effects, and has been
used to treat many types of cancer in traditional Chinese medical
practices (14). β-elemene, an
extractable monomer from Curcuma zedoaria oil, has a wide
antineoplastic spectrum, without toxic effects, and has been
approved as an auxiliary treatment in China (8). Previous studies have revealed that
the anticancer effects of β-elemene are predominantly due to the
inhibition of tumor cell proliferation at a certain stage in the
cell cycle, in addition to increased levels of apoptosis (7–9).
However, the exact signaling pathways responsible for such
therapeutic effects have not yet been determined. In the present
study, the antineoplastic effects of β-elemene, in addition to the
possible involvement of the Wnt/β-catenin signaling pathway in
cervical cancer cells, was investigated.
In the present study, the results of the MTT assay
demonstrated that the administration of β-elemene resulted in
significantly decreased viability of SiHa cells, in a dose and
time-dependent manner. Furthermore, previous studies have revealed
that treatment with β-elemene leads to inhibition of the
proliferation of prostate cancer cells (15) and glioblastoma cells (16). In order to verify the ability of
β-elemene to inhibit the cell cycle, flow cytometry was performed
in order to determine the phases of the cell cycle, and the results
demonstrated that treatment with β-elemene resulted in G1 phase
cell cycle arrest in SiHa cells. Previous studies have demonstrated
that β-elemene may inhibit cell proliferation via G0/G1 cell cycle
arrest in human glioblastoma cells (17), and that β-elemene may arrest the
cell cycle at the G2/M phase in ovarian carcinoma cells (18). The results of the present study
differed from those of Zhu et al (18) with regards to the phase at which
the cell cycle was arrested. Therefore, the results of the present
study and those of previous studies suggest that the cell cycle
stage at which β-elemene is able to cease cell proliferation varies
across different cancer types.
The present study additionally demonstrated that
treatment with β-elemene increased and decreased the expression
levels of P15 and Cyclin D1, respectively. The P15 protein is able
to induce cell cycle arrest at the G1/S phase via inhibition of the
downstream Cyclin-dependent kinase 4/6, which further suppresses
the activity of Cyclin D1 (19,20).
Therefore, it may be concluded that β-elemene inhibits the cell
proliferation of SiHa cells via promotion of G1 phase cell cycle
arrest. Furthermore, flow cytometry analysis revealed that the
apoptotic rates of SiHa cells were significantly increased in
groups treated with β-elemene in a dose-dependent manner. In
addition, it was demonstrated that treatment with β-elemene
markedly upregulated the expression levels of p53 and Bax, and
markedly downregulated the expression level of Bcl-2. Furthermore,
Bax and Bcl-2, two key regulators of mitochondria-mediated
apoptosis, are integral members of the Bcl-2 protein family
(21). The Bax/Bcl-2 ratio
controls cell apoptosis: A higher Bax/Bcl-2 ratio results in
caspase 3 activation and a subsequent increase in the rate of
apoptosis (22). p53, a notable
tumor suppressor protein, is able to promote cellular apoptosis via
transcriptional activation of multiple genes, including those
encoding the Bcl-2 and caspase protein families. Furthermore,
upregulation of p53 may further elevate the Bax/Bcl-2 ratio, thus
inducing cellular apoptosis (23).
Therefore, it was concluded that β-elemene induced apoptosis via
upregulation of p53 expression and elevation of the Bax/Bcl-2 ratio
in SiHa cells.
Transwell and wound-healing migration assays
revealed that β-elemene inhibited the motility, invasion and
migration of SiHa cells in a dose-dependent manner. In addition,
the present study revealed that β-elemene was able to significantly
downregulate the expression levels of MMP-2 and MMP-9. MMPs are
extracellular matrix-degrading enzymes that are involved in the
initiation of cell invasion and migration. MMP2 and MMP9, two
important members of the MMP family, are able to degrade type V,
Vll, and X collagen, in addition to gelatin and elastic fibers in
the basement membrane of the extracellular matrix, which may
destroy the integrity of the basement membrane and further promote
tumor cell invasion and migration through the basement membrane
structure (24,25). Therefore, it was concluded that
β-elemene may inhibit cell invasion and migration via
downregulation of MMP-2 and MMP-9 expression in SiHa cells.
The Wnt/β-catenin signaling pathway consists of a
series of proteins encoded by oncogenes and anti-oncogenes, and is
notably implicated in embryonic development, intracellular
transport and cellular apoptosis. In addition, abnormal activation
of the Wnt/β-catenin signaling pathway is associated with
tumorigenesis, invasion and metastasis of various types of cancer
(12,26). During the activation of
Wnt/β-catenin signaling, β-catenin interacts with DNA via the
TCF/lymphoid enhancer factor DNA binding protein and subsequently
activates the expression of downstream target genes, encoding
Cyclin D1 and c-Myc, which promote cell proliferation (27). In the present study, treatment with
β-elemene downregulated the levels of β-catenin and its downstream
target molecule TCF7, leading to downregulation of their target
molecules, including Cyclin D1 and c-Myc; thus, this indicated that
β-elemene inhibits SiHa cell proliferation via inhibition of the
Wnt/β-catenin pathway. Yao et al (10) demonstrated that β-elemene
administration inhibited proliferation via the p38
mitogen-activated protein kinase-dependent pathway in glioblastoma
cells. In addition, Liang et al (11) demonstrated that β-elemene inhibits
cell viability via downregulation of the phosphatidylinositol
3-kinase/RAC-α serine/threonine-protein
kinase/serine/threonine-protein kinase mTOR signaling pathway in
human osteosarcoma cells. There are two pathways that control
cellular apoptosis: The mitochondria-dependent pathway and the
mitochondria-independent pathway. Upregulation of Bax in the
mitochondria-dependent pathway may promote cytochrome release from
the mitochondrial intermembrane space, which subsequently activates
caspase 3 and consequently renders the cells susceptible to
apoptosis. The mitochondria-independent pathway induces apoptosis
via activation of a caspase cascade. Procaspase 8 triggers the
downstream caspase effector and is responsible for the cleavage of
caspase 8, which activates caspase 3 and promotes cellular
apoptosis (28). Cyclin D1 and
survivin are able to induce the inhibition of caspase 3 expression.
Wnt/β-catenin signaling activates Bax, Cyclin D1 and surviving
(29). The present study revealed
that β-elemene increases the expression of Bax, and suppresses the
expression of Cyclin D1; therefore, suggesting that β-elemene may
induce cellular apoptosis via inhibition of the Wnt/β-catenin
signaling pathway.
p53 is a notable tumor suppressor gene, and the p53
protein is involved in cell cycle regulation, DNA repair and the
induction of apoptosis. Previous studies have revealed that the
intersection of the p53 and Wnt/β-catenin pathways is TCF4, a
member of the Wnt/β-catenin signaling pathway that may be
downregulated by p53 (30) and
Dickkopf-1, and transcriptionally upregulated by p53, in order to
further inhibit Wnt/β-catenin activity (31). Further studies have revealed that
p53 acts upstream of Wnt/β-catenin in order to suppress the latter
pathway (32). In the present
study, it was demonstrated that β-elemene upregulates p53
expression levels, thus suggesting that β-elemene promotes SiHa
cellular apoptosis via upregulation of p53 and subsequent
inhibition of the Wnt/β-catenin signaling pathway. Li et al
(33) demonstrated that β-elemene
induces apoptosis via Akt and extracellular-signal-regulated kinase
signaling in order to deliver apoptotic signals to lung cancer
cells. MMPs, highly conserved zinc ion-dependent proteolytic
enzymes, are able to degrade the epithelial basement membrane or
extracellular matrix and thus promote the invasion and metastasis
of tumor cells. As targets of the Wnt/β-catenin signaling pathway,
MMP-2, MMP-7 and MMP-9 are involved in the process of inflammation
regulation, tumorigenesis and tumor progression (34), in which MMP-7 activates both MMP-2
and MMP-9 in order to degrade collagen (35). Increased expression levels of
β-catenin result in the upregulation of the expression levels of
MMPs, which then act as contributors to tumor invasion and
metastasis (34). The results of
the present study suggest that β-elemene reduces the expression
levels of β-catenin and its downstream target molecules, MMP-2 and
MMP-9, further suggesting that β-elemene may inhibit cell invasion
and migration via inhibition of the Wnt/β-catenin signaling pathway
in SiHa cells. Similarly, Zhang et al (36) demonstrated that β-elemene inhibited
cell invasion and migration via downregulation of nuclear
transcription factor expression mediated by mothers against
decapentaplegic homolog 3 in MCF-7 cells.
In conclusion, the present study revealed that
β-elemene inhibits the proliferation, invasion and migration of
cervical cancer cells in vitro, and induces cellular
apoptosis. Furthermore, it was demonstrated that β-elemene may
exert its therapeutic effects via attenuation of the Wnt/β-catenin
signaling pathway. However, this remains to be investigated in a
clinical setting. The results of the present study suggest that
β-elemene may be a potential novel therapeutic agent for the
treatment of cervical cancer.
References
|
1
|
Chen W, Zheng R, Zhang S, Zeng H, Xia C,
Zuo T, Yang Z, Zou X and He J: Cancer incidence and mortality in
China, 2013. Cancer Lett. 401:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Leeuwen CM, Oei AL, Chin KWTK, Crezee
J, Bel A, Westermann AM, Buist MR, Franken NAP, Stalpers LJA and
Kok HP: A short time interval between radiotherapy and hyperthermia
reduces in-field recurrence and mortality in women with advanced
cervical cancer. Radiat Oncol. 12:752017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Tao L, Ni T, Gu H, Jin F, Dai X,
Feng J, Ding Y, Xiao W, Guo S, et al: Anticancer efficacy of the
ethyl acetate extract from the traditional Chinese medicine herb
Celastrus orbiculatus against human gastric cancer. J
Ethnopharmacol. 205:147–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar D, Basu S, Parija L, Rout D, Manna
S, Dandapat J and Debata PR: Curcumin and Ellagic acid
synergistically induce ROS generation, DNA damage, p53 accumulation
and apoptosis in HeLa cervical carcinoma cells. Biomed Pharmacoth.
81:31–37. 2016. View Article : Google Scholar
|
|
6
|
Xu QY: The clinical value of local
application of compound zedoary turmeric oil suppository for
cervical human papilloma virus infection. Med Inform. 2:337–338.
2015.(In Chinese).
|
|
7
|
Zhang X, Zhang Y and Li Y: β-elemene
decreases cell invasion by upregulating E-cadherin expression in
MCF-7 human breast cancer cells. Oncol Rep. 30:745–750. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao F, Qiu J and Zhang H: Potential role
of β-elemene on histone H1 in the H22 ascites hepatoma cell line.
Mol Med Rep. 6:185–190. 2012.PubMed/NCBI
|
|
9
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang D, Yang M, Guo B, Yang L, Cao J and
Zhang X: HIF-1α induced by β-elemene protects human osteosarcoma
cells from undergoing apoptosis. J Cancer Res Clin Oncol.
138:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
13
|
Chen C, Zhao M, Tian A, Zhang X, Yao Z and
Ma X: Aberrant activation of Wnt/β-catenin signaling drives
proliferation of bone sarcoma cells. Oncotarget. 6:17570–17583.
2015.PubMed/NCBI
|
|
14
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae. J Ethnopharmacol. 143:406–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li QQ, Wang G, Huang F, Banda M and Reed
E: Antineoplastic effect of beta-elemene on prostate cancer cells
and other types of solid tumor cells. J Pharm Pharmacol.
62:1018–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao YS, Zhu TZ, Chen YW, Yao YQ, Wu CM,
Wei ZQ, Wang W and Xu YH: β-elemene inhibits Hsp90/Raf-1 molecular
complex inducing apoptosis of glioblastoma cells. J Neurooncol.
107:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li CL, Chang L, Guo L, Zhao D, Liu HB,
Wang QS, Zhang P, Du WZ, Liu X, Zhang HT, et al: β-elemene induces
caspase-dependent apoptosis in human glioma cells in vitro through
the upregulation of Bax and Fas/FasL and downregulation of Bcl-2.
Asian Pac J Cancer Prev. 15:10407–10412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y
and Xu Y: β-elemene inhibits proliferation of human glioblastoma
cells and causes cell-cycle G0/G1 arrest via mutually compensatory
activation of MKK3 and MKK6. Int J Oncol. 38:419–426.
2011.PubMed/NCBI
|
|
19
|
Jares P, Colomera D and Campo E: Genetic
and molecular pathogenesis of mantle cell lymphoma: Perspectives
for new targeted therapeutics. Nat Rev Cancer. 7:750–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Q, Dong B, Nan F, Guan D and Zhang Y:
5-Aminolevulinic acid photodynamic therapy in human cervical cancer
via the activation of microRNA-143 and suppression of the Bcl-2/Bax
signaling pathway. Mol Med Rep. 14:544–550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan Y, Liang H, Li T, Li M, Li R, Qin X
and Li S: The MMP-1, MMP-2, and MMP-9 gene polymorphisms and
susceptibilityto bladder cancer: A meta-analysis. Tumour Biol.
35:3047–3052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao H, Yuan X, Jiang J, Wang P, Sun X,
Wang D and Zheng Q: Antimetastatic effects of licochalcone B on
human bladder carcinoma T24 by inhibition of matrix
metalloproteinases9 and NF-κB activity. Basic Clin Pharmacol
Toxicol. 115:527–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoffmeyer K, Raggioli A, Rudloff S, Anton
R, Hierholzer A, Del Valle I, Hein K, Vogt R and Kemler R:
Wnt/β-catenin signaling regulates telomerase in stem cells and
cancer cells. Science. 336:1549–1554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koff JL, Ramachandiran S and
Bernal-Mizrachi L: A time to kill: Targeting apoptosis in cancer.
Int J Mol Sci. 16:2942–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu K, Ma L and Zhu J: miR-483-5p promotes
growth, invasion and self-renewal of gastric cancer stem cells by
Wnt/β-catenin signaling. Mol Med Rep. 14:3421–3428. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rother K, Johne C, Spiesbach K, Haugwitz
U, Tschöp K, Wasner M, Klein-Hitpass L, Möröy T, Mössner J and
Engeland K: Identification of Tcf-4 as a transcriptional target of
p53 signalling. Oncogene. 23:3376–3384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Shou J and Chen X: Dickkopf-1, an
inhibitor of the Wnt signaling pathway, is induced by p53.
Oncogene. 30:1843–1848. 2000. View Article : Google Scholar
|
|
32
|
Kim NH, Kim HS, Kim NG, Lee I, Choi HS, Li
XY, Kang SE, Cha SY, Ryu JK, Na JM, et al: p53 and microRNA-34 are
suppressors of canonical Wnt signaling. Sci Signal. 4:ra712011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Xu L, Qu X, Zhao M, Yu P, Kang J,
Liu Y and Hu X: Cbl-regulated Akt and ERK signals are involved in
β-elemene-induced cell apoptosis in lung cancer cells. Mol Med Rep.
4:1243–1246. 2011.PubMed/NCBI
|
|
34
|
Wu B, Crampton SP and Hughes CC: Wnt
signaling induces matrix metalloproteinase expression and regulates
T cell transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Imai K, Yokohama Y, Nakanishi I, Ohuchi E,
Fujii Y, Nakai N and Okada Y: Matrix metalloproteinase 7
(matrilysin) from human rectal carcinoma cells. Activation of the
precursor, interaction with other matrix metalloproteinases and
enzymic properties. J Biol Chem. 270:6691–6697. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Li Y, Zhang Y, Song J, Wang Q,
Zheng L and Liu D: Beta-elemene blocks epithelial-mesenchymal
transition in human breastcancer cell line MCF-7 through
Smad3-mediated down-regulation of nuclear transcription factors.
PLoS One. 8:e587192013. View Article : Google Scholar : PubMed/NCBI
|