Introduction
Age-related macular degeneration (AMD) is the
leading cause of irreversible visual impairment in elderly
populations worldwide (1). It may
be classed as wet or dry, with wet AMD accounting for ~90% of the
severe vision loss associated with AMD, despite only affecting ~10%
of individuals with the disease.
Wet AMD is associated with choroidal
neovascularization (CNV) (1),
which is characterized by the proliferation of blood vessels that
originates in the choroid and grow through the Bruch's membrane and
into the sub-retinal space. These blood vessels eventually bleed
and/or leak fluid, leading to central vision loss (2). The proliferation, migration and
capillary-like structure formation of choroidal endothelial cells
(CECs) has critical involvement in CNV development (3). The mechanisms underlying CNV
development beneath the neurosensory retina in AMD are complex and
have not been fully elucidated.
In wet AMD, the retinal pigment epithelium (RPE) may
separate from the Bruch's membrane and become dysfunctional
(4,5). Physiologically, RPE cells maintain
the structural integrity of the retina and choriocapillaris by
secreting numerous growth factors and nutrients essential to the
survival of the photoreceptors. The alteration in RPE growth factor
secretion contributes to CNV development (4,6–8). In
neovascularized tissues obtained from patients with AMD, the RPE
secretes vascular endothelial growth factor (VEGF) at an increased
rate in comparison to tissues with no neovascularization (9). Furthermore, the RPE may be induced to
secrete inflammatory chemokines including C-C motif chemokine 2
(CCL2, also termed monocyte chemotactic protein 1) and C-X-C motif
chemokine ligand 8 (CXCL8, also termedinterleukin-8) by numerous
stimuli, which have been demonstrated to promote neovascularization
in vitro (10,11).
Interleukin-17 (IL-17) is primarily produced by
cluster of differentiation CD4+ T cells and is a major
pro-inflammatory cytokine associated with the pathogenesis of
various autoimmune and inflammatory diseases (12,13).
IL-17 is detected at high levels in the serum of patients with AMD
(14). In a laser-induced
choroidal neovascularization rat model, IL-17A mRNA expression is
increased in the retina (15). A
previous study demonstrated that IL-17 induces RPE to secrete CCL2
and CXCL8 (16), exhibiting direct
angiogenic activity via activation of the phosphoinositide
3-kinase-Ras-related C3 botulinum toxin substrate 1 (Rac1) and Ras
homolog gene family member A (RhoA) pathways in CECs in
vitro (17). However, it is
still unclear if IL-17 affects CECs through RPE activation.
In the present study, the supernatant of the RPE was
stimulated by IL-17 to obtain an IL-17-conditioned medium
(IL-17-CM). Its effects on CEC proliferation, migration and tube
formation were investigated in a human CEC model.
Materials and methods
Cell culture
Informed consent was obtained from the relatives of
all donors. The study was conducted in accordance with The
Declaration of Helsinki and was approved by the institutional
review board of The First Affiliated Hospital of Chongqing Medical
University (Chongqing, China). Human ocular posterior segments free
of any known ocular disease were obtained from Chongqing Eye Bank
(Chongqing, China). The retina was torn from RPE with forceps, and
the RPE was rubbed off of the choroid with a cotton swab. Choroids
were torn from sclera, and endothelial cells were isolated from 2
choroids of 1 donor by treatment with 0.2% type II collagenase in
minimum essential medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C and 5% CO2 until the tissue
was visibly digested. The cell suspension was filtered through
sterile 70 and 40-mm filters (BD Biosciences, Franklin Lakes, NJ,
USA). The filtered suspension was centrifuged at 300 × g and
suspended in medium containing anti-CD31 magnetic beads
(MiltenyiBiotec, GmbH, Bergisch Gladbach, Germany) according to the
manufacturer's protocol. CECs were isolated from human choroid and
cultured in endothelial growth medium (EGM-2 MV; Lonza Group Ltd.,
Basel, Switzerland) supplemented with 5% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
atmosphere with 5% CO2, as previously described
(17). CECs between passages three
and five were used for experiments. ARPE-19 cells were obtained
from the American Type Culture Collection and maintained in
Dulbecco's modified Eagle's medium/F12 (DMEM/F12; 1:1; Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin in a humidified incubator at 37°C in 5%
CO2.
ARPE-19 monolayer cultures were seeded at
2×105 cells/cm2 onto Transwell filters
(diameter, 12 mm; pore size, 0.4 µm) which had been coated with 160
ml Matrigel (dilution, 1:30; BD Biosciences, Franklin Lakes, NJ) in
DMEM/F12 (Invitrogen; Thermo Fisher Scientific, Inc.) and air-dried
overnight, as previously described (18). DMEM/F12 (Invitrogen; Thermo Fisher
Scientific, Inc.) medium supplemented with 0.5% FBS (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to culture the ARPE-19
cells. The medium was replaced every 4 days and cells were
maintained for 8 weeks.
The ARPE-19 monolayer was subsequently cultured with
or without 20 ng/ml IL-17 (R&D Systems, Inc., Minneapolis, MN,
USA) in EGM-2 MV medium (Lonza Group Ltd.) for 48 h, and
conditioned medium with 20 ng/ml IL-17 (IL-17-CM) or without
IL-17(CM) was obtained from the lower chamber of the ARPE-19
monolayer. To avoid the stimulation of CECs by redundant IL-17 in
IL-17-CM and to reasonably match the control conditions, both CM
and IL-17-CM were supplemented with 0.2 ug/ml neutralizing
antibodies (cat. no. AF-317-NA; R&D Systems, Inc.) against
IL-17for 10 min, at room temperature, prior to use.
Cell proliferation assay
CECs were seeded at 2×103 cells per well
in 96-well plates (Corning Incorporated, Corning, NY, USA) and
starved in FBS free EGM-2 MV medium (Lonza Group Ltd) for 12 h.
Cells were subsequently treated with CM or IL-17-CM for 24, 48 or
72 h. Cell proliferation was measured according to the Cell
Proliferation Reagent Water Soluble Tetrazolium 1 (WST-1) kit
manufacturer's protocol (C0035; Beyotime Institute of
Biotechnology, Shanghai, China). Cells were incubated in WST-1
solution for 2 h at 37°C and the absorbance at a wavelength of 450
nm was measured using a spectrophotometer (SpectraMax; Molecular
Devices, LLC, Sunnyvale, CA, USA). All measurements were taken in
quadruplicate.
Cell migration assay
CECs were cultured to confluence in 6-well plates
containing EGM-2 MV (Lonza Group Ltd) supplemented with 5% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) and subsequently
starved in FBS free EGM-2 MV for 12 h. The monolayer was injured
with a sterile pipette tip and washed with PBS to remove debris.
The injured monolayer was treated with CM, IL-17-CM or IL-17-CM
with 0.1 µg/ml neutralizing antibody against CXCL8 (R&D
Systems, Inc., cat. no. AF-208-NA) and/or 0.2 µg/ml neutralizing
antibody against CCL2 (R&D Systems, Inc. cat. no. AF-279-NA),
for 10 min at room temperature. Images were taken at 0, 4, 8 and 12
h with a fluorescence microscope (DM6000; Leica, Wentzler,
Germany). Wound closure rate was measured using Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA) and
measurements were taken in triplicate.
Tube formation assay
Ice-cold growth factor reduced basement membrane
matrixgel (Matrigel; BD Biosciences) was added at 300 µl per well
to pre-cooled 24-well plates at 4°C and allowed to polymerize at
37°C for 30 min. CECs (1×105) were plated onto the gel
surface and incubated at 37°C for 16 h with 200 µl CM, IL-17-CM or
IL-17-CM with anti-CXCL8 and/or anti-CCL2 antibodies. Cell
rearrangement and tube formation was visualized using
phase-contrast microscopy. Images of five random fields at ×5
magnification per well were taken and endothelial tube length was
quantified using Image Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
The ARPE-19 monolayer was maintained in DMEM/F12
(Invitrogen; Thermo Fisher Scientific, Inc.) medium supplemented
with 0.5% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) for 8
weeks to allow polarization. The cells were then treated with or
without IL-17 (50 ng/ml) for 24 h. The supernatant of the lower
chamber was centrifuged at 300 × g, 37°C for 5 min to remove
particulates and stored at −70°C until further use. Total protein
for each sample was determined using a protein assay kit (Bio-Rad
Laboratories, Inc. Hercules, CA, USA). VEGF levels were measured
using a human ELISA development kit (R&D Systems, Inc. cat. no.
SVE00) according to the manufacturer's protocol. Each experiment
was repeated four times.
Rac1 and RhoA pull-down assay
Rac1 and RhoA activity was analyzed using Rac1 and
RhoA pull-down activation assay kits (Cytoskeleton, Inc., Denver,
CO, USA), according to the manufacturer's protocols. The CECs were
cultured in conditions identical to those previously described in
the cell migration assay. The cells were washed with ice-cold PBS
and lysed in ice-cold lysis buffer with the addition of a 1:100
final dilution of a protease inhibitor cocktail (Cytoskeleton,
Inc., Denver, CO, USA). Lysed cells were harvested with a cell
scraper and centrifuged at 10,000 × g at 4°C for 1 min. The
supernatant was aliquoted, snap frozen in liquid nitrogen and
stored at −70°C until further use. An equal volume of supernatant
was incubated for 1 h at 4°C in the presence of glutathione
S-transferase fused p21 activated kinase 1-p21 binding domain beads
for GTP-Rac1 adsorption, or Rhotekin-Rho binding domain bound to
glutathione-sepharose beads for GTP-Rho adsorption. The supernatant
was subsequently washed three times with the wash buffer. Bound
proteins were eluted in sample buffer and analyzed by western blot
analysis using antibodies against Rac1 and RhoA. All materials used
including those for the western blot analysis, were included in the
pull-down assay kits.
Statistical analysis
Data are presented as the mean ± standard deviation,
using SPSS software v11.0 (SPSS, Chicago, IL, USA) the cell
proliferation assay and ELISA results between groups were
statistically compared using the Student's t-test. Tube formation
assay and migration assays results between CM and IL-17-CM were
analyzed using the Student's t-test. Tube formation, migration and
the Rac1 and RhoA pull-down assays were analyzed using one-way
analysis of variance, followed by a Bonferroni correction test as a
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
IL-17-CM enhances migration and tube
formation, however not proliferation of CECs
CM and IL-17-CM induced the proliferation of CECs,
however there was no significant difference in the relative
absorbance of the supernatant between the two groups at 24, 48 and
72 h (Fig. 1A). IL-17-CM
significantly increased tube formation in CECs. The data was
normalized to a CM value of 1, with the relative tube length of
CECs stimulated by IL-17-CM at 1.25±0.08 (P=0.001; Fig. 1B). The migration distance of the
CECs increased with duration in both the IL-17-CM and the CM
treated groups. The migration distance of CECs stimulated with
IL-17-CM was significantly increased at 12 h in comparison with CM
(Fig. 1C; CM, 185±26 µm; IL-17-CM,
285±30 µm; P=0.012).
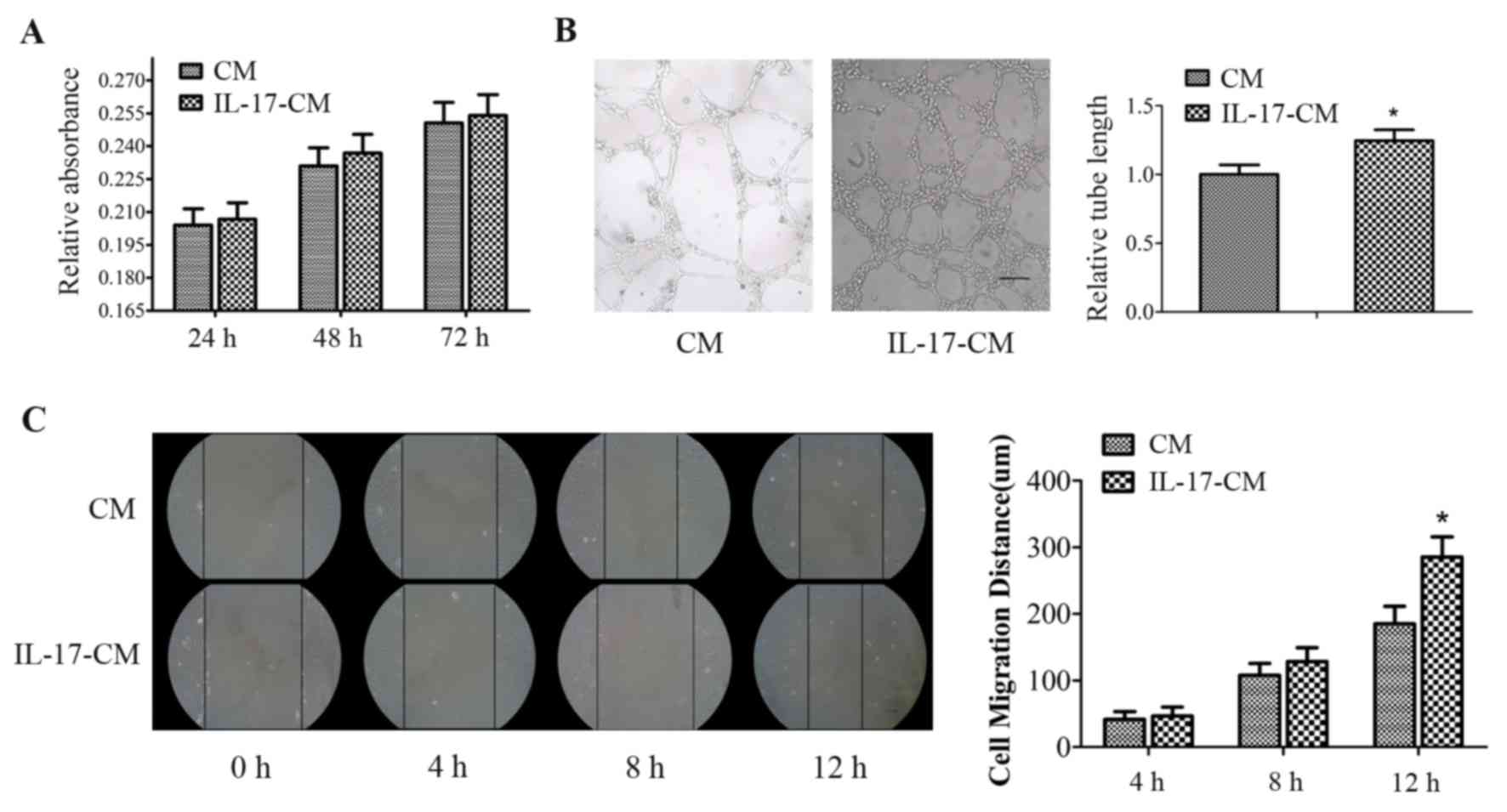 | Figure 1.Effect of IL-17-CM on CEC
proliferation, migration and tube formation. (A) Cell proliferation
was measured with a water soluble tetrazolium 1 kit at 24, 48 and
72 h. (B) Cells were seeded onto Matrigel-coated plates and exposed
to CM or IL-17-CM for 16 h. Endothelial tube formation was
visualized by microscopy and the tube length was analyzed. Scale
bar, 100 µm. Relative absorbance and tube length data was
normalized to a CM value of 1. (C) CEC monolayers were scratched
and incubated with CM or IL-17-CM. Wound healing distance was
recorded by phase contrast microscopy at 0, 4, 8 and 12 h following
the scratch injury. Scale bar, 100 µm. Data are presented as the
mean ± standard deviation of four replicate experiments in A, five
in B and three in C. *P<0.05 vs. the CM. IL-17, interleukin-17;
CM, conditioned medium; CEC, choroidal endothelial cell. |
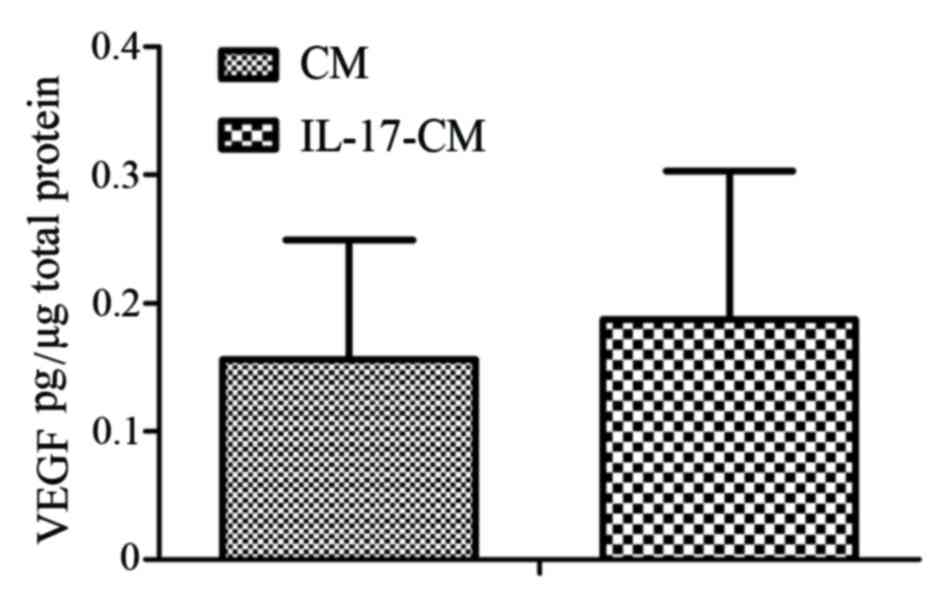
IL-17 fails to upregulate VEGF
secretion by the ARPE-19 monolayer
To investigate whether migration and tube formation
of CECs was induced by VEGF secreted by the RPE, the expression of
VEGF was detected in the supernatant of the ARPE-19 monolayer with
or without IL-17 stimulation. The expression levels of VEGF in the
supernatant of ARPE-19 with or without IL-17 stimulation were not
significantly different (Fig. 2;
IL-17-CM, 0.19±0.12 pg/µg total protein; CM, 0.16±0.09 pg/µg total
protein).
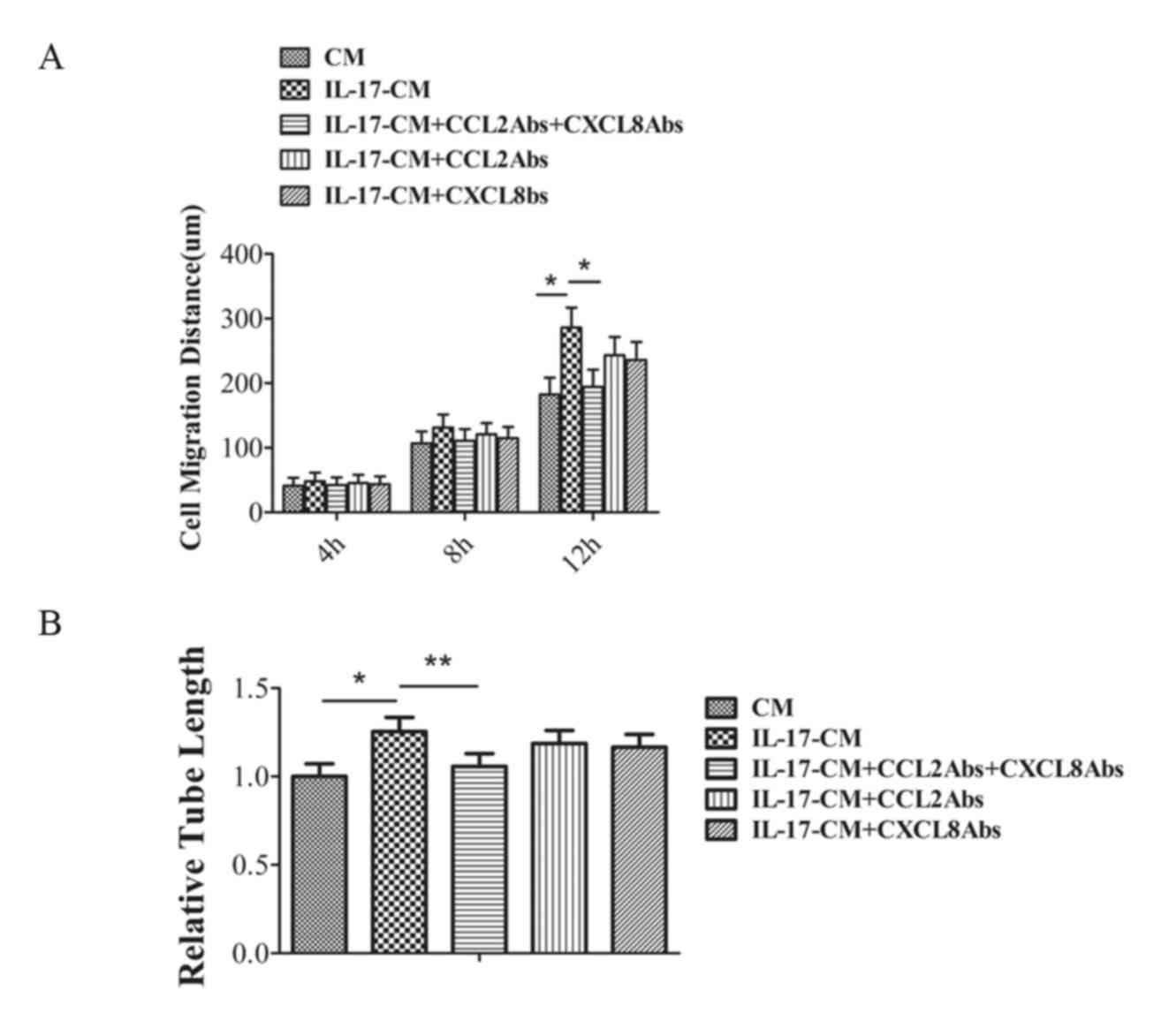
IL-17-CM promotes the migration and
tube formation of CECs via CXCL8 and CCL2
In the author's previous study, the levels of CXCL8
and CCL2 increased markedly in the supernatant from RPE cells
treated with IL-17 (16). To
investigate the potential role of CXCL8 and CCL2 in the migration
of CECs in IL-17-CM, CXCL8 and/or CCL2 expression was neutralized
in IL-17-CM prior to migration and tube formation assays. The
migration distance and tube formation ability of CECs in CM,
IL-17-CM, IL-17-CM with anti-CXCL8 and anti-CCL2, IL-17-CM with
anti-CCL2 only or IL-17-CM withanti-CXCL8 only was investigated
(Fig. 3). The data was normalized
to a CM value of 1.CEC migration was significantly inhibited by
IL-17-CM with the combination ofanti-CXCL8 and anti-CCL2 (P=0.024),
however the inhibitory effect was not present in treatment with
either antibody alone (Fig. 3A).
Similarly, the addition of bothanti-CXCL8 and anti-CCL2 in IL-17-CM
significantly reduced tube length compared with IL-17-CM (IL-17-CM,
1.25±0.08; IL-17-CM + anti-CCL2 + anti-CXCL8, 1.06±0.07; P=0.04),
however addition of either antibody alone had no significant effect
on tube length (Fig. 3B). The
values were normalized to a CM value of 1. This data suggested that
the combination of CXCL8 and CCL2 expression in IL-17-CM was
essential to induce the migration and tube formation of CECs.
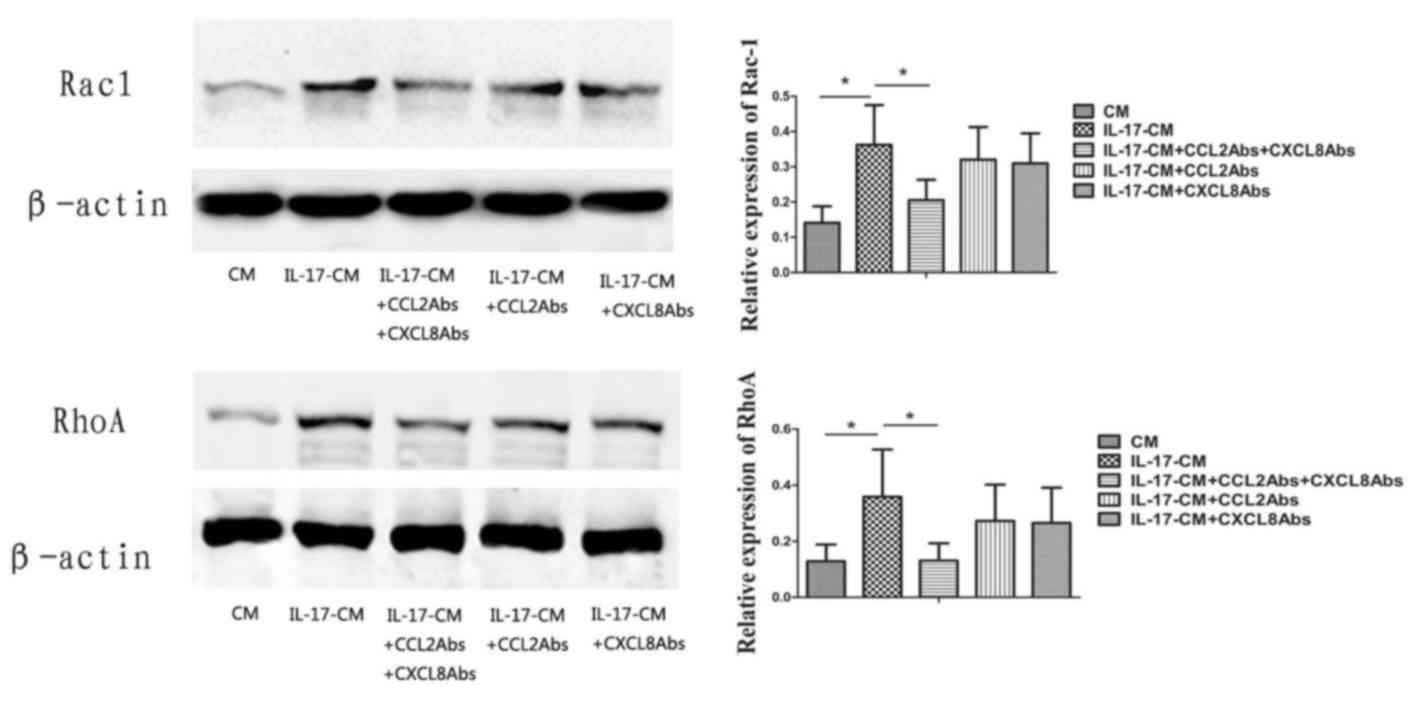
IL-17-CM upregulates Rac1 and RhoA
activity in CECs via CXCL8 and CCL2
The expression levels of Rac1 and RhoA in CECs was
significantly increased in IL-17-CM treated groups in comparison
with the CM group (Fig. 4; Rac1,
P=0.008; RhoA, P=0.042). Neutralizing antibodies to both CXCL8 and
CCL2 significantly inhibited Rac1 and RhoA activity in the IL-17-CM
group following 10 min of treatment (Rac1, P=0.037; RhoA, P=0.038).
A significant inhibition of Rac1 and RhoA activity was not present
when CXCL8 or CCL2 alone were neutralized with the respective
antibodies. Collectively, these results suggested that IL-17-CM
induced the upregulation of Rac1 and RhoA activity in CECs, which
may be in part mediated by CXCL8 and CCL2.
Discussion
The present study demonstrated that a potent
angiogenic effect was induced in human CECs treated with IL-17-CM
from RPE cells, via the promotion of migration and capillary tube
formation, however not proliferation. The IL-17-CM-induced
angiogenic response was largely mediated by CCL2 and CXCL8. VEGF
expression levels were not affected by the IL-17-CM.
To physiologically mimic RPE cells in vivo,
ARPE-19 monolayer was cultured for 8 weeks on Transwell filter,
which may mimic differentiation to some degree. No significant
difference was observed between CM and IL-17-CM treated groups in
the proliferation assay, suggesting that IL-17 failed to induce
mitogenic activity in RPE. However, it was demonstrated that
IL-17-CM significantly increased the migration and capillary tube
formation of CECs in comparison with CM from unstimulated RPE. A
wound healing assay was performed to detect cell migration
(19). Results suggested that the
wound closure was a result of both cell migration and
proliferation. Proliferation cannot be completely excluded from the
involvement of wound closure in the present study, as there was no
significant difference in proliferation between the CM and
IL-17-CM-treated groups. These results suggest that cell migration
may be a more important contributor to wound healing. Therefore,
other angiogenic factors in IL-17-CM were further explored and the
expression levels of VEGF were detected. A previous study
demonstrated that IL-17 may induce angiogenesis through the
upregulation of VEGF expression (20). The results of the present study
indicated that RPE in vitro was capable of secreting VEGF,
however IL-17 had no significant effect on VEGF expression. In the
aforementioned previous study, a different cell type was used to
investigate VEGF expression, which may explain the contradicting
results. In the present study, the increase in migration and
capillary tube formation ability in CECs induced by IL-17-CM was
independent of VEGF. Therefore, the angiogenic effect of IL-17-CM
may be mediated by factors other than VEGF.
The author's previous study suggested that CCL2 and
CXCL8 expressionis significantly increased in RPE stimulated by
IL-17 (16). CCL2 and CXCL8 are
angiogenic, promoting the migration and capillary tube formation of
endothelial cells (21). In the
present study, no anti-angiogenic effect was observed through the
addition of anti-CCL2 or anti-CXCL8 antibodies in IL-17-CM.
However, the migration and capillary tube formation ability of CECs
was significantly decreased in the presence of anti-CCL2 and
anti-CXCL8 antibodies combined. Therefore, CCL2 and CXCL8 may be
key pro-angiogenic factors in IL-17-CM, however CCL2 or CXCL8 alone
do not induce a distinct pro-angiogenic effect.
The migration of CECs towards the RPE and
neurosensory retina is a key feature f CNV development. Filopodia,
lamellipodia and stress fibers may promote endothelial cell
migration via actin rearrangement, through the activation of
biochemical pathways that are primarily regulated by Rho GTPases,
including Rac1 and RhoA (22).
Rac1 is required for lamellipodium extension, and RhoA is
associated with stress fiber formation (23,24).
In the present study, neutralizing CCL2 and CXCL8 expression
decreased Rac1 and RhoA activity in IL-17-CM, suggesting that IL-17
increased CCL2 and CXCL8 expression in RPE to promote CEC
migration, which may be mediated by Rac1 and RhoA.
In conclusion, IL-17-CM was demonstrated to induce
potent angiogenic activity. The suppression of CEC migration and
capillary formation in IL-17-CM by anti-CCL2 and CXCL8 antibodies
indicated that IL-17 may indirectly mediate the development of new
choroidal vessels via the RPE in an in vitro wet AMD model.
Results suggest that IL-17 may be a novel therapeutic target for
the treatment of CNV in AMD.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81200704 and
81200677), The National Basic Research Program of China (program
973, grant no. 2011CB510200) and The National Key Clinical
Specialties Construction Program of China.
References
|
1
|
Lim LS, Mitchell P, Seddon JM, Holz FG and
Wong TY: Age-related macular degeneration. Lancet. 379:1728–1738.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Lookeren Campagne M, Le Couter J,
Yaspan BL and Ye W: Mechanisms of age-related macular degeneration
and therapeutic opportunities. J Pathol. 232:151–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grossniklaus HE and Green WR: Choroidal
neovascularization. Am J Ophthalmol. 137:496–503. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frank RN: Growth factors in age-related
macular degeneration: Pathogenic and therapeutic implications.
Ophthalmic Res. 29:341–353. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mousa SA, Lorelli W and Campochiaro PA:
Role of hypoxia and extracellular matrix-integrin binding in the
modulation of angiogenic growth factors secretion by retinal
pigmented epithelial cells. J Cell Biochem. 74:135–143. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campochiaro PA: Retinal and choroidal
neovascularization. J Cell Physiol. 184:301–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holz FG, Pauleikhoff D, Klein R and Bird
AC: Pathogenesis of lesions in late age-related macular disease. Am
J Ophthalmol. 137:504–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zarbin MA: Current concepts in the
pathogenesis of age-related macular degeneration. Arch Ophthalmol.
122:598–614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strauss O, Heimann H, Foerster MH,
Agostini H, Hansen LL and Rosenthal R: Activation of L-type Ca2+
channels is necessary for growth factor-dependent stimulation of
VEGF secretion by RPE cells. Invest Ophthalmol Vis Sci.
44:39262003.
|
|
10
|
Elner VM, Burnstine MA, Strieter RM,
Kunkel SL and Elner SG: Cell-associated human retinal pigment
epithelium interleukin-8 and monocyte chemotactic protein-1:
Immunochemical and in-situ hybridization analyses. Exp Eye Res.
65:781–789. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgins GT, Wang JH, Dockery P, Cleary PE
and Redmond HP: Induction of angiogenic cytokine expression in
cultured RPE by ingestion of oxidized photoreceptor outer segments.
Invest Ophthalmol Vis Sci. 44:1775–1782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin JI and Bayry J: A role for IL-17 in
age-related macular degeneration. Nat Rev Immunol. 13:7012013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maddur MS, Miossec P, Kaveri SV and Bayry
J: Th17 cells: Biology, pathogenesis of autoimmune and inflammatory
diseases, and therapeutic strategies. Am J Pathol. 181:8–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li
Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, et al:
Complement component C5a promotes expression of IL-22 and IL-17
from human T cells and its implication in age-related macular
degeneration. J Transl Med. 9:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasegawa E, Sonoda KH, Shichita T, Morita
R, Sekiya T, Kimura A, Oshima Y, Takeda A, Yoshimura T, Yoshida S,
et al: IL-23-independent induction of IL-17 from γδT cells and
innate lymphoid cells promotes experimental intraocular
neovascularization. J Immunol. 190:1778–1787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Kijlstra A, Chen Y and Yang P:
IL-17A stimulates the production of inflammatory mediators via
Erk1/2, p38 MAPK, PI3K/Akt, and NF-κB pathways in ARPE-19 cells.
Mol Vis. 17:3072–3077. 2011.PubMed/NCBI
|
|
17
|
Chen Y, Zhong M, Liang L, Gu F and Peng H:
Interleukin-17 induces angiogenesis in human choroidal endothelial
cells in vitro. Invest Ophthalmol Vis Sci. 55:6968–6975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Yang P, Li F and Kijlstra A: The
effects of Th17 cytokines on the inflammatory mediator production
and barrier function of ARPE-19 cells. PLoS One. 6:e181392011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Honorati MC, Cattini L and Facchini A:
IL-17, IL-1beta and TNF-alpha stimulate VEGF production by
dedifferentiated chondrocytes. Osteoarthritis Cartilage.
12:683–691. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gálvez BG, Genís L, Matías-Román S,
Oblander SA, Tryggvason K, Apte SS and Arroyo AG: Membrane type
1-matrix metalloproteinase is regulated by chemokines
monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in
endothelial cells during angiogenesis. J Biol Chem. 280:1292–1298.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ridley AJ and Hall A: The small
GTP-binding protein rho regulates the assembly of focal adhesions
and actin stress fibers in response to growth factors. Cell.
70:389–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ridley AJ, Paterson HF, Johnston CL,
Diekmann D and Hall A: The small GTP-binding protein rac regulates
growth factor-induced membrane ruffling. Cell. 70:401–410. 1992.
View Article : Google Scholar : PubMed/NCBI
|