Introduction
Asthma symptoms frequently include airway
obstruction, airway hyperreactivity (AHR), and airway inflammation.
Allergic asthma is a chronic inflammatory dysfunction exacerbated
by eosinophilia and T-helper type 2 (Th2) cell activation.
Traditional asthma therapies, including corticosteroids and
antihistamines, have limitations and side effects (1,2).
Persistent inflammation does not reverse chronic airway remodeling
and can lead to tissue damage. Neurogenic locus notch homolog
protein (Notch) signaling is a well-conserved pathway involved in
the development of immune cells, and dendritic cells (DCs) and T
cells express Notch receptors and ligands. Notch signaling is
involved in T-cell activation and proliferation, as reported by
Rutz et al (3), who
demonstrated that delta-like ligand (delta)-1 and jagged-1
inhibited T-cell activation, whereas delta-4 promoted T-cell
proliferation. In mice and humans, differentiation of forkhead box
protein P3 (FoxP3)-positive regulatory T cells (Tregs) from naive
CD4+ T cells followed transforming growth factor-β1
signal activation of the Notch pathway (4,5).
Mesenchymal stem cells (MSCs) are self-renewing and multipotential
(6,7), and due to their unique immunological
characteristics, including immunoregulatory activity and low
immunogenicity, MSCs are attractive tools for regenerative medicine
(8,9). MSCs may regulate T-cell
proliferation, inhibit B-cell proliferation (10,11)
and affect the regulation of immune responses by DCs (12) by inducing Treg formation from naive
CD4+ T cells (13,14).
MSCs are able to expand anti-specific Tregs to prevent autoimmunity
(15,16) and have been demonstrated to
directly or indirectly induce Tregs in the regulation of DCs.
MSC-induced Tregs have an important immunosuppressive effect on
allergic asthma (17). The
involvement of the Notch pathway in the MSC modulation of the
adaptive immune response in asthma is not completely understood.
The objective of the present study was to investigate the
importance of placenta-derived MSCs (hPMSCs) in the modulation of
the Notch signaling pathway in asthma and to increase understanding
of the crosstalk between Notch signaling and MSC modulation of
adaptive immune responses. MSCs can regulate the immune system
through cytokine production and the Notch pathway, leading to
reduced inflammation. Thus, MSC-based therapies may be effective in
the treatment of asthma.
Materials and methods
Animal model
A total of 60 Wistar male rats (185±12.5 g) were
obtained at 6–8 weeks of age from the Shandong Lukang Record
Pharmaceutical Co., Ltd. (Jining, China) and were maintained in a
specific pathogen-free facility with a 12 h light/dark cycle at the
Medicine and Pharmacy Research Center of Binzhou Medical University
(Yantai, China). Rats were housed at 18–26°C and 40–70% relative
humidity with free access to food and water. All animal
experimentation and procedures were approved by the Institutional
Animal Care and Use Committee of Binzhou Medical University.
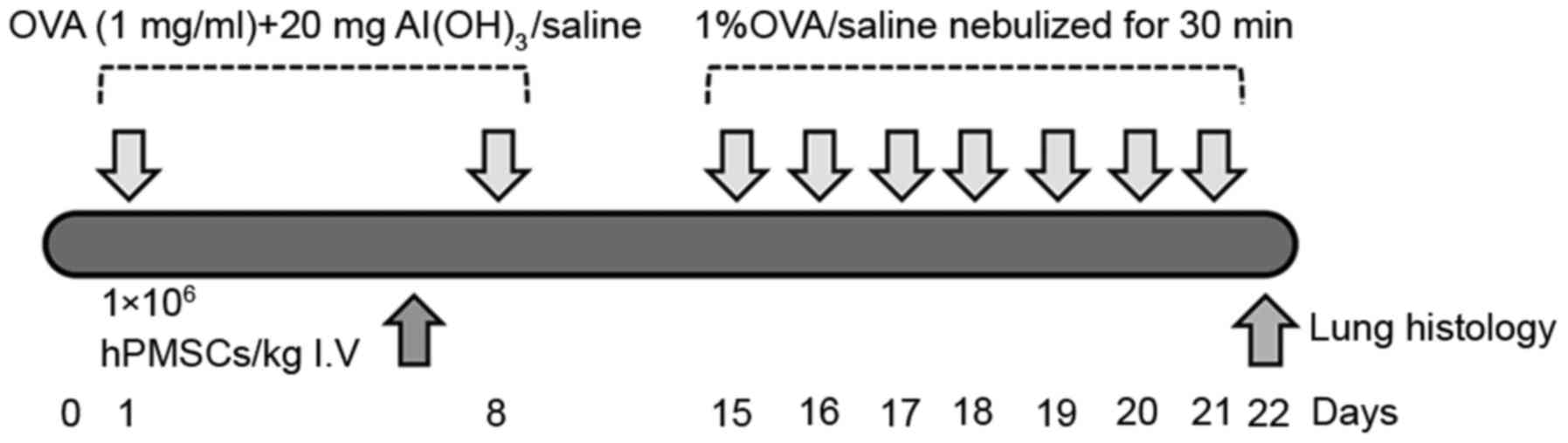
The asthma rat model was established as previously
described (18). Rats were
sensitized by intraperitoneal injection of sterile ovalbumin (OVA;
1 mg in 1 ml saline; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
with 200 mg aluminum hydroxide as an adjuvant, on days 1 and 8.
Following sensitization, the rats were nebulized daily with 1% OVA
(w/v) solution for 30 min in a 30×24×50-cm chamber between days 15
and 21. A control group received intraperitoneal injections of
saline on days 1 and 8, and was exposed to aerosolized saline on
the provocation days. Rats in the OVA + hPMSC group received
1×106 hPMSCs/kg bodyweight suspended in 1 ml sterile
Dulbecco's PBS via tail vein injections, prior to the final OVA
challenge. Rats were sacrificed via cervical dislocation 24 h
subsequent to treatment. The protocol is illustrated in Fig. 1.
Isolation and culture of hPMSCs
hPMSCs were derived from full-term placentas
subsequent obtaining informed consent from the donors, with the
approval of the Institutional Ethics Committee. The cells were
cultured in L-Dulbecco's modified Eagle's medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), with 10% fetal bovine
serum (Hyclone; GE Healthcare Life Sciences) and 100 U/ml each of
penicillin and streptomycin. Cells were cultured at 37°C in a
humidified atmosphere with 5% CO2. The immunophenotype
of hPMSCs [cluster of differentiation (CD73, CD90, CD105, CD45 and
human leukocyte antigen-antigen D related) were determined by flow
cytometry at the third culture passage. Neurogenic, osteogenic, and
adipose differentiation were promoted to identify pluripotent
hPMSCs.
Lung histology
Rats were anaesthetized with an intraperitoneal
injection of 4% chloral hydrate (300–350 mg/kg) and sacrificed via
cervical dislocation; lungs were harvested, perfused with PBS, and
fixed in 10% neutral buffered formalin overnight. Lung tissue was
embedded for light microscopy (×200) for the evaluation of
inflammatory cell infiltration. Bronchoalveolar lavage fluid (BALF)
was collected by cannulation of the trachea and flushing of the
left lung bronchus three times with normal saline. The supernatant
was discarded following centrifugation at 500 × g for 10 min at
25°C. The total cell, eosinophil, lymphocyte, macrophage and
neutrophil counts were obtained following Wright's staining for
~1–2 min at room temperature. All the obtained cells were counted
by a pathological image analysis system (Image-Pro Plus 6.0; Media
Cybernetics, Inc., Rockville, MD, USA).
ELISA analysis
Interleukin (IL)-4 (cat. no. R4000), interferon
(IFN)-γ (cat. no. DY585) and immunoglobulin (Ig)E (cat. no. DY6900)
levels were assayed using commercially available ELISA kits
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from lung samples and MNCs
using TRIzol reagent (Thermo Fisher Scientific, Inc.), dissolved in
diethyl pyrocarbonate in double distilled water and stored at
−80°C. cDNA was synthesized using a Revert Aid First-Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) and subsequently
amplified. qPCR was performed using the TransStart Tip Green qPCR
SuperMix (TransGen Biotech Co., Ltd., Beijing, China) on the
Real-Time PCR Detection system (iQ5; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The PCR conditions were DNA denaturation at
94°C for 30 sec, followed by 40 cycles at 94°C for 5 sec, 60°C for
30 sec and 72°C for 1 min. All experiments were performed according
to the manufacturer's protocol. The gene-specific primers were as
follows: Notch-1 forward, AACTCACCAGAGCAGCCTACA; Notch-1 reverse,
CCACATTCCAGCACACTCAA; Notch-2 forward, TGGAGGCAGGAGGAAAGAC; Notch-2
reverse, GATGGGCAAAGGTCAGTAGG; Notch-3 forward,
CGGCTTGATTTCCCATACC; Notch-3 reverse, ACACCCAGGACGAAGATGAC;
jagged-1 forward, AGTAAACGGGATGGGAACAG; jagged-1 reverse,
CAGCAGAGGAACCAGGAAAT; delta-4 forward, GCCCAGACTCCATCCTTACA;
delta-4 reverse, GCTCCTGCTAAATGCCAGAC; and GAPDH forward,
ACAGCAACAGGGTGGTGGAC; GAPDH reverse, TTTGAGGGTGCAGCGAACTT. PCR
results were quantified using the 2−ΔΔCq method
(19).
Western blot analysis
Lung tissue was homogenized and lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) and the supernatant was collected
following centrifugation at 12,000 × g for 5 min at 4°C. Protein
was quantified using a bicinchoninic acid (BCA) assay with the
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol; 50 µg aliquots of protein
were loaded and electrophoresed on a 10% SDS-PAGE gel, and
transferred onto polyvinylidene fluoride membranes. Following
blocking for 2 h in 5% non-fat milk at room temperature, membranes
were incubated with primary antibodies overnight at 4°C, rinsed,
and incubated for 1 h with the corresponding secondary antibodies
at room temperature for 1 h. Blots were visualized with
diaminobenzidine and read with a Clinx GenoSens1600 electrophoresis
gel imaging analyzer (Clinx Science Instruments Co., Ltd.,
Shanghai, China). The bands were semi-quantified using ImageJ 1.51
software (National Institutes of Health, Bethesda, MD, USA).
Primary antibodies included anti-Notch-1 (1:500; cat. no. 3447;
Cell Signaling Technology, Inc., Danvers, MA, USA), Notch-2 (1:50;
5732; Cell Signaling Technology, Inc.), Notch-3 (1:500; 2889; Cell
Signaling Technology, Inc.), jagged-1 (1:50; ab7771; Abcam,
Cambridge, UK) and delta-4 (1:500; ab183523; Abcam). Horseradish
peroxidase-conjugated secondary antibodies were obtained from Wuhan
Sanying Biotechnology (1:500; SA00001-15; Wuhan, China).
Statistical analysis
Statistical calculations were performed using SPSS
18.0 (SSPS, Inc., Chicago, IL, USA). Results are presented as the
mean ± standard deviation. When applicable, one-way analysis of
variance and Holm-Sidak tests were used to determine significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
hPMSCs engraftment results in a
significant reduction in inflammatory cells in BALF and lung
tissue
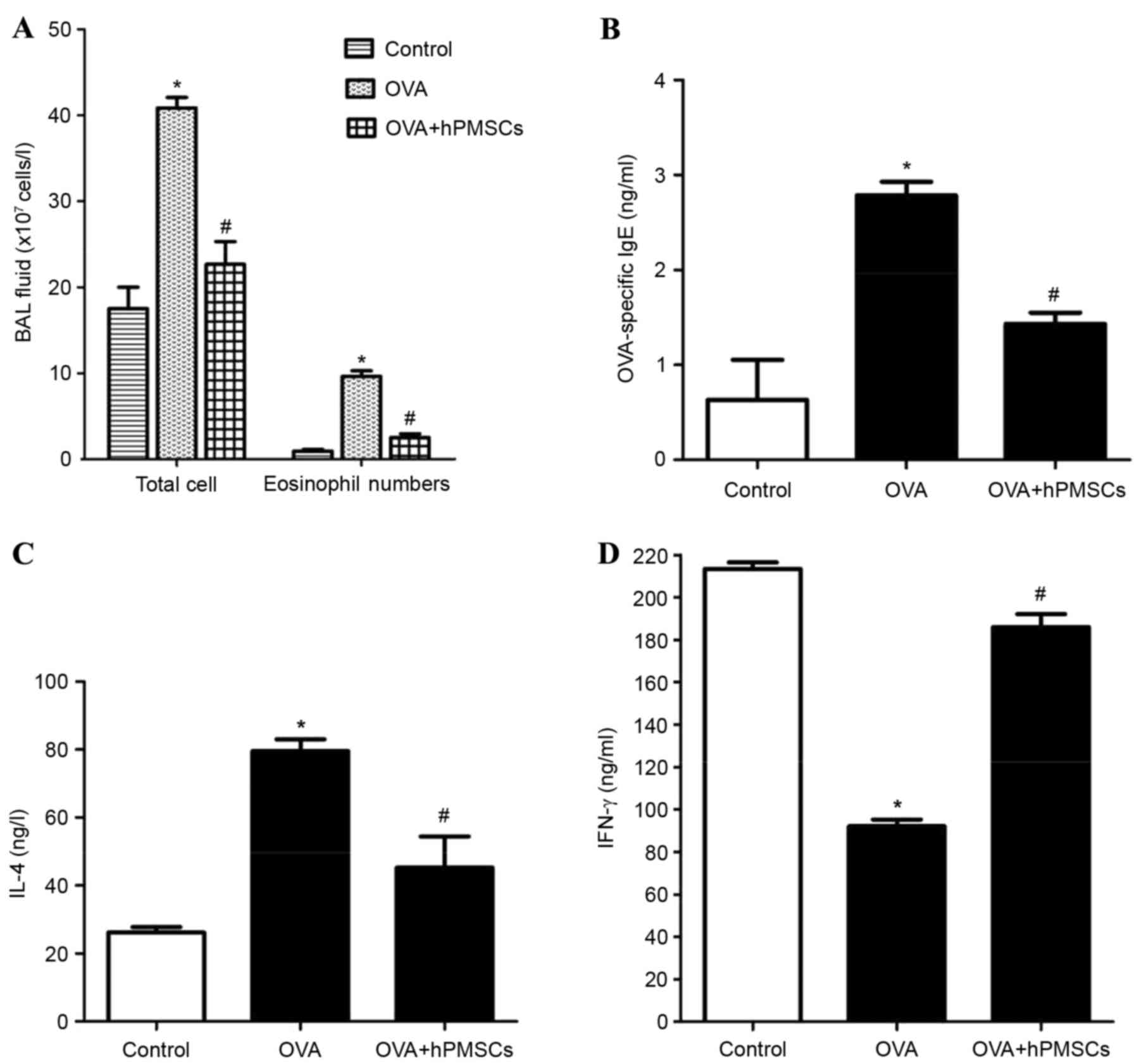
The cells in the BALF collected from controls, OVA-
and hPMSC-treated rats were counted on a hemocytometer. The total
number of cells and the number of eosinophils were both
significantly greater in the OVA group compared with the OVA +
hPMSCs and control groups; these counts were also greater in the
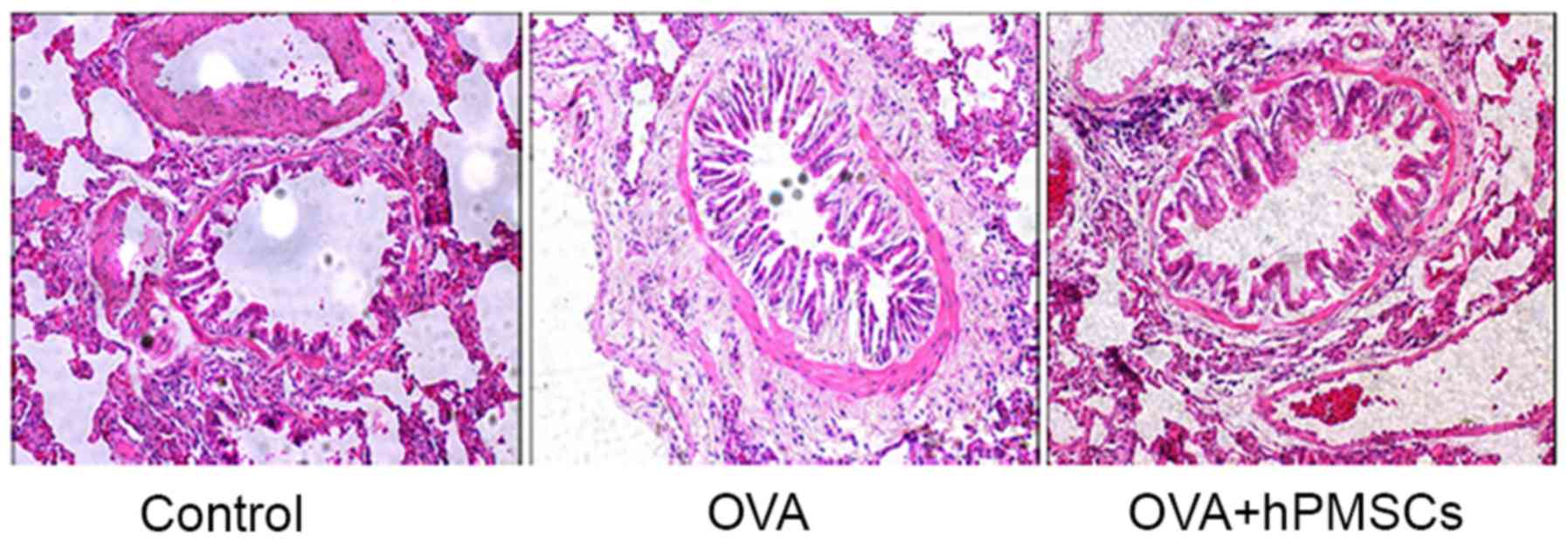
OVA + hPMSCs group in the OVA group (Fig. 2). Histological evaluation revealed
inflammatory alterations in the bronchial tissue of rats in the OVA
group, including inflammatory cell infiltration of the trachea, and
surrounding perivascular tissue and subepithelial smooth muscle,
along with luminal narrowing. The asthma model was thus
successfully established. The inflammatory alterations were
significantly reduced in the OVA + hPMSCs group compared with the
OVA group. Inflammatory cell infiltration and goblet cell
hyperplasia were reduced, mucus secretion was downregulated, and
airway smooth muscle proliferation was attenuated. Only slight
luminal narrowing was apparent, and only occasional alveolar
inflammatory cells were observed (Fig.
3). hPMSCs were thus demonstrated to alleviate the development
of asthma, reducing OVA-induced bronchial airway inflammation, cell
infiltration and mucin production.
Effect of hPMSC transplantation on
cytokine levels in rats with asthma
Serum cytokines were measured by ELISA. A high IgE
level indicates a recent allergic attack. The IgE concentrations
were significantly higher in the OVA group compared with the
control and OVA + hPMSCs groups. The injection of hPMSCs was
associated with a significant decrease in IgE in rats challenged
with OVA (Fig. 2B). The IL-4 level
was higher in the OVA group compared with the control group,
although the IFN-γ level was lower. In the OVA + hPMSCs group, IL-4
was decreased and IFN-γ was increased compared with rats in the OVA
group (Fig. 2C and D; P<0.05).
The results indicated that hPMSCs inhibited Th2 cytokine secretion
and IgE production in rats with OVA-induced asthma.
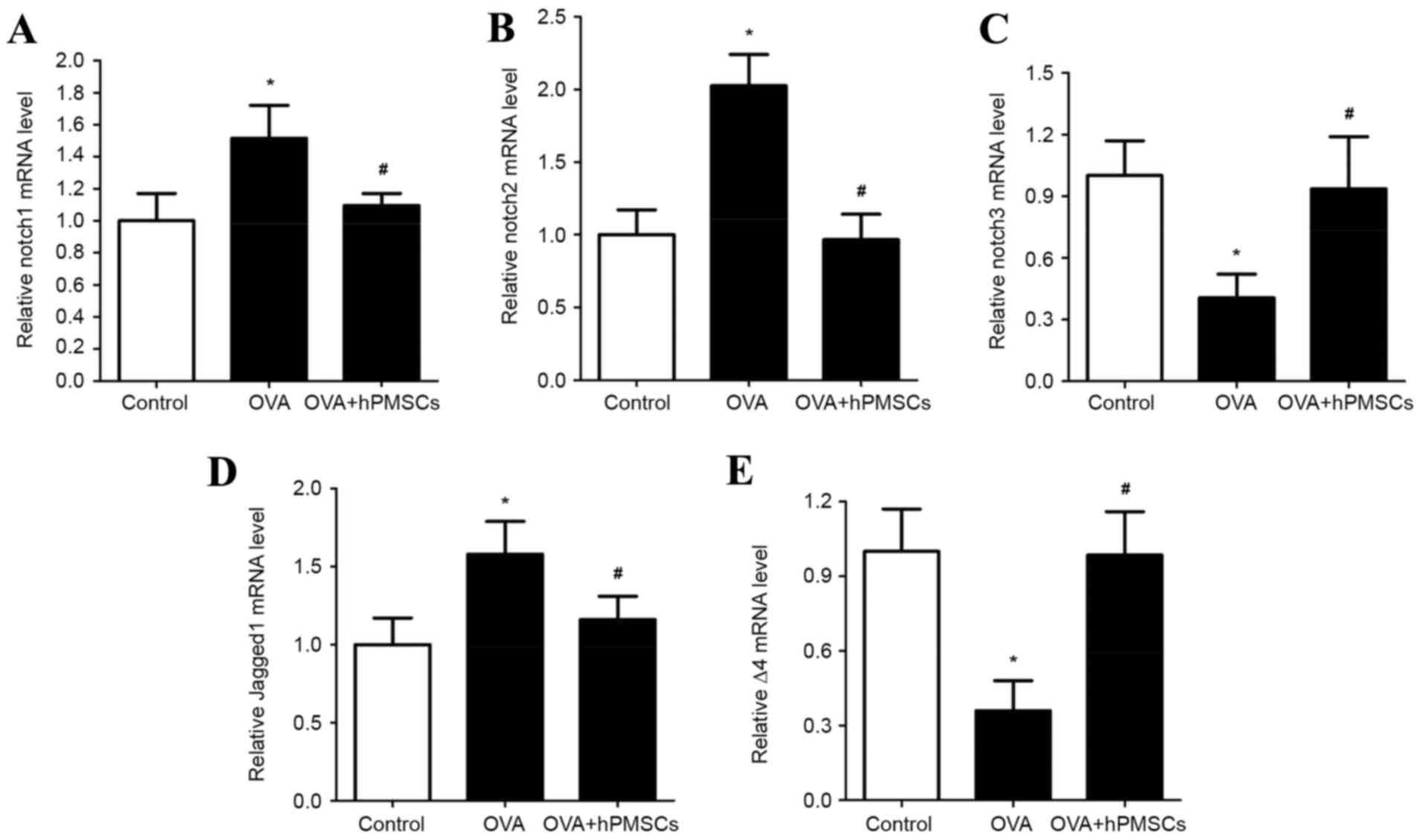
hPMSC administration alters Notch
signaling in lung tissue
The involvement of Notch signaling in asthma and the
effects of hPMSCs on Notch signaling were investigated via
assessment of Notch-1, Notch-2, Notch-3, delta-4 and jagged-1
expression in lung tissue (Fig.
4). The gene expression of Notch-1, Notch-2 and jagged-1 was
significantly higher in the OVA group compared with the control
group (P<0.05); however, it was significantly lower in the OVA +
hPMSCs group compared with the OVA group (Fig. 4A, B and D; P<0.05). Notch-3 and
delta-4 expression exhibited the opposite trend, with significantly
decreased expression in the OVA group compared with the control and
OVA + hPMSCs groups (Fig. 4C and
E; P<0.05). hPMSC transplantation thus increased Notch-3 and
delta-4 expression in the lung tissues of rats treated with OVA +
hPMSCs. No significant differences were observed in the expression
of Notch receptors and ligands in the control and OVA + hPMSCs
groups (Fig. 4C and E). In order
to investigate the effects of hPMSCs on Notch signaling responses,
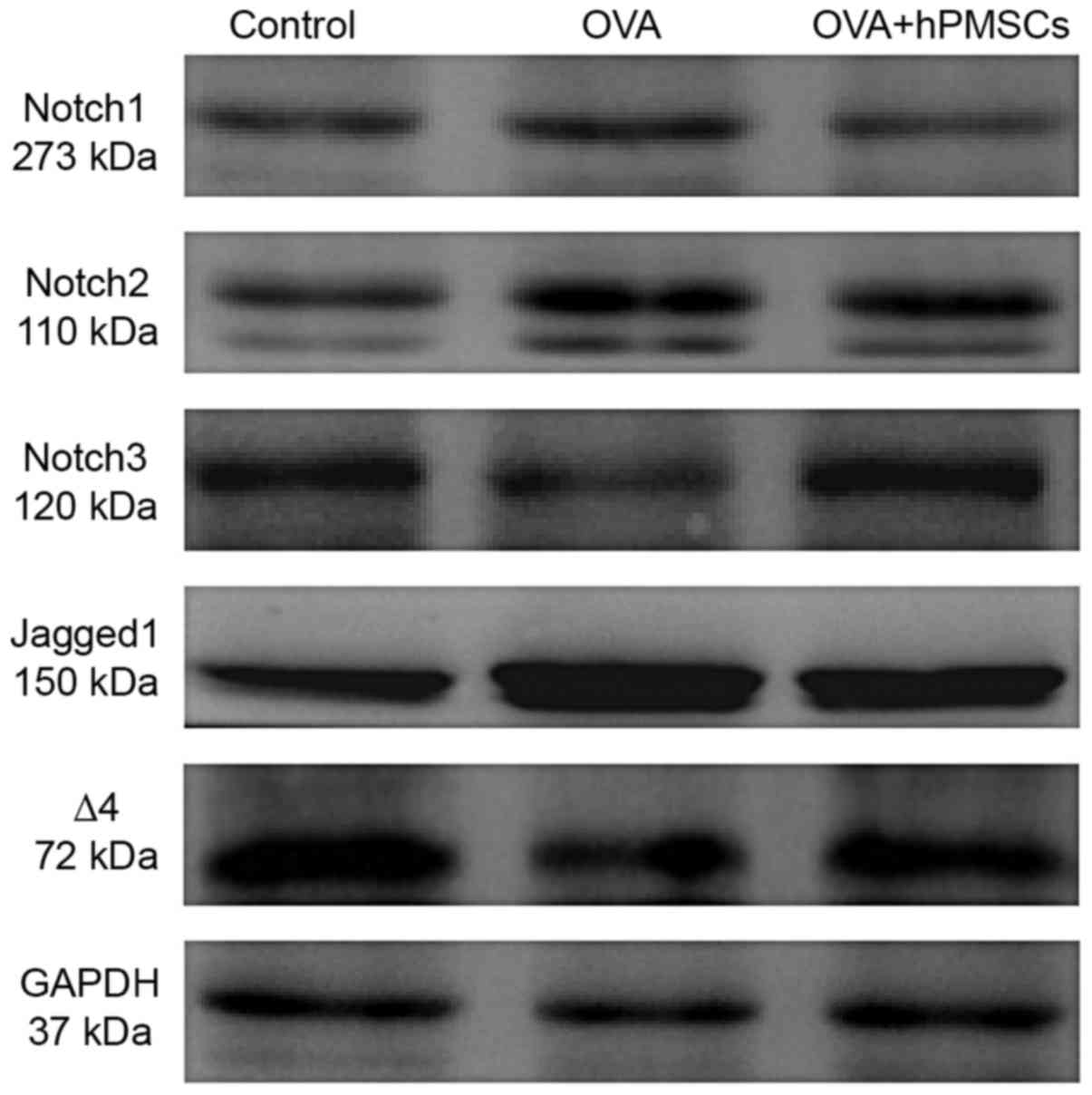
protein expression in lung tissue was assayed by western blotting.
Consistent with the qPCR results, Notch-1, Notch-2 and jagged-1
protein expression increased in the OVA group compared with the
control group (P<0.05); Notch-3 and delta-4 expression was
reduced. The results demonstrated that the administration of hPMSCs
decreased the expression of Notch-1, Notch-2, and Jagged-1 and
increased Notch-3 and Delta-4 protein expression compared with the
expression in the OVA group (Fig.
5). Collectively, the results demonstrated that the Notch
pathway had an important role in the lung pathogenesis of asthma
and that hPMSCs exerted various effects on Notch receptors and
ligands.
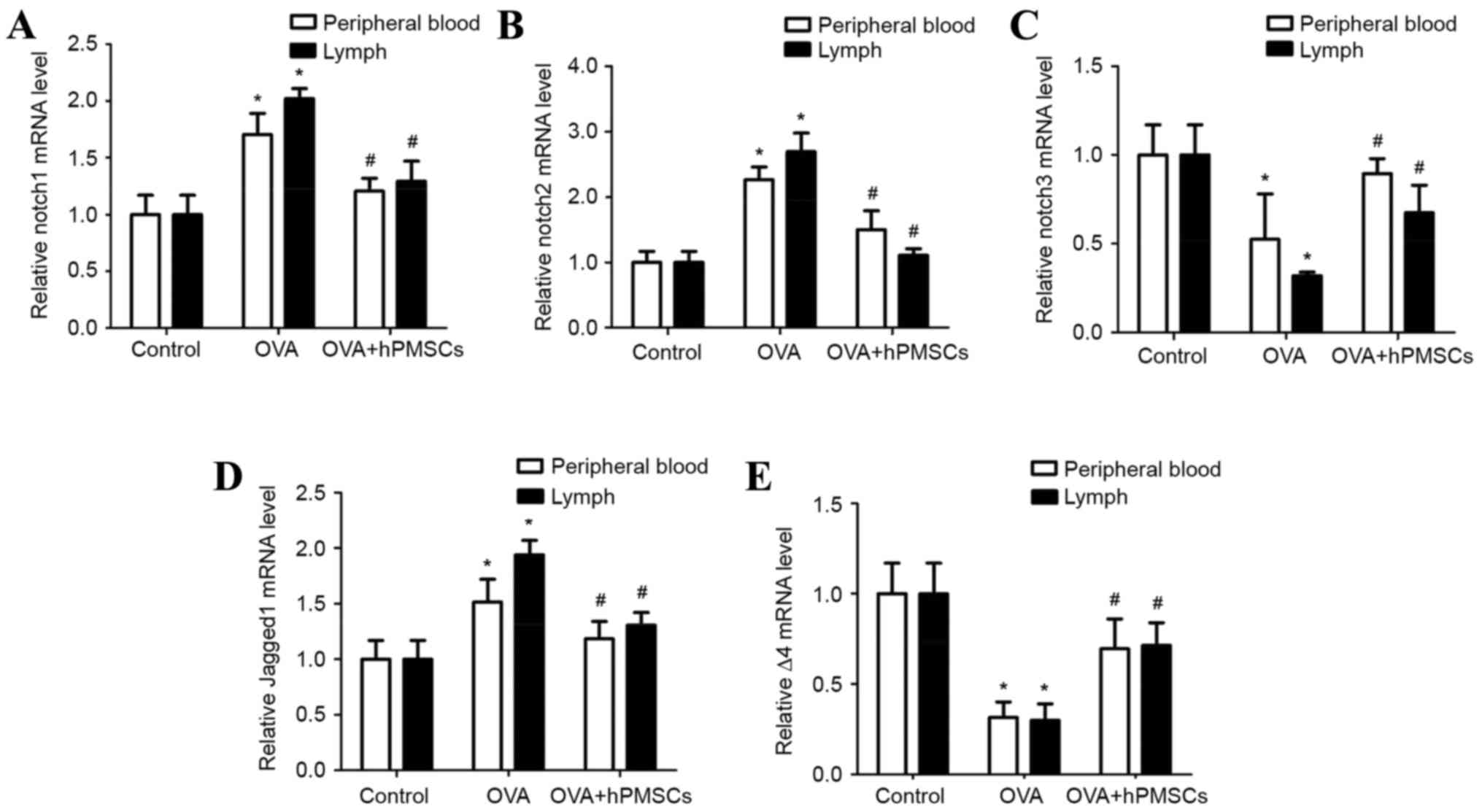
Effect of hPMSCs on Notch signal
expression in peripheral blood and lymph
In order to examine the effects of hPMSCs on Notch
signaling, Notch-1, Notch-2, Notch-3, delta-4 and jagged-1 gene
expression was assayed in peripheral blood and lymph (Fig. 6). Notch-1, Notch-2 and jagged-1
expression was significantly increased in the OVA group compared
with the control group (P<0.05); hPMSC administration
significantly increased Notch-1, Notch-2 and jagged-1 expression in
the OVA + hPMSCs group (P<0.05; Fig. 6A, B and D). A similar trend was
observed in lymph from the OVA group, with significantly increased
Notch-1, Notch-2 and jagged-1 expression compared with the control
and OVA + hPMSCs group (P<0.05). Notch-1, Notch-2 and jagged-1
gene expression was significantly reduced in the OVA + hPMSCs group
compared with the OVA group. The results demonstrated that Notch-3
and delta-4 expression in blood and lymph was decreased in the OVA
group compared with the control group (P<0.05; Fig. 6C and E), and was increased by hPMSC
transplantation (P<0.05).
In the OVA group, Notch-1, Notch-2 and jagged-1 gene
expression was increased in the lymph compared with the blood
(P<0.05). Meanwhile, Notch-3 and delta-4 expression was
decreased (P<0.05; Fig. 6). The
results confirmed a greater imbalance in Notch signaling in the
lymph compared with the blood, which is consistent with a severe
imbalance in T cells, and Notch receptor and ligand expression in
the present asthma model.
Discussion
The present study demonstrated that hPMSCs modulated
a regulatory environment in a rat model of asthma via the Notch
signaling pathway. Previous studies demonstrated that MSCs have
important therapeutic potential in a number of clinical disorders,
including asthma (7).
Placental-MSCs are similar to bone marrow-derived MSCs in
morphology and function, although they exert stronger immune
inhibitory effects on T cells (20,21).
A number of studies have used transplanted human
MSCs to treat different diseases, and have used them in a variety
of animal models, including mice (22), rats (23) and rabbits (24). The findings from these previous
studies and those of the present study demonstrate that human MSCs
are able to survive and function following transplantation. Yun
et al (22) and Chan et
al (25) used the method of
tail vein injection to transplant MSCs into animals. MSCs may be
distributed to the brain and lung through the blood circulation,
and the effect of treatment is the same as that when transplanted
directly into the location of the lesion. The majority of studies
have demonstrated that the primary function of MSCs is an
immunosuppressive function, which regulates the immune balance.
The results of the present study demonstrated that
hPMSCs decreased the number of lung inflammatory cells and the IgE
serum concentration in OVA-sensitized rats. A significant reduction
in the number of eosinophils in the BALF and decreased serum IL-4
added to the evidence that hPMSCs alleviated the asthmatic symptoms
and suppressed airway inflammation. The Notch signaling pathway is
evolutionarily conserved, and interactions between Notch receptors
and ligands regulate cellular differentiation, proliferation and
apoptosis. The Notch pathway is involved in a number of diseases,
including asthma (26). The Notch
pathway has been associated with T-cell development,
differentiation and activation, and serves a key role in the
induction of Th2 differentiation (27,28).
Notch expression was observed in the lung tissue and
lung T cells in a BALB/c mouse model of asthma, in which Th2
polarization and Th1/Th2 disturbances in asthma pathogenesis were
associated with the Notch pathway (29–31).
Guo et al (32)
demonstrated Notch-1 expression in lungs and lung T cells of BALB/C
mice. The introduction of an activated allele of Notch-1 into
CD4+ T cells has been demonstrated to activate IL-4
expression and Th2 cell responses (33). In the present study, significant
increases were observed in Notch-1 and Notch-2 expression in the
lung tissue, blood and lymph of rats with OVA-induced asthma, and
serum IL-4 mRNA was increased. Following hPMSC administration,
Notch-1 and Notch-2 expression decreased and IFN-γ increased.
Previous studies have demonstrated that Notch-1 and Notch-2
interact with jagged-1/2 to induce Th2-cell differentiation
following treatment with Th2-cell stimuli (34–36).
Notch-1 and Notch-2 were analyzed in a mouse asthma model, and the
effect of Notch-1 was stronger compared with that of Notch-2
(37).
In the present study, a difference was not observed
between Notch-1 and Notch-2 expression. The results confirmed a
role for Notch signaling in asthma pathogenesis, and that hPMSCs
may inhibit Th2 polarization by blocking Notch signaling, which may
attenuate the excessive production of Th2 cytokines. Previous
reports have suggested that CD4+ T-cell subsets,
including Th17 cells and Treg cells, may serve key roles in asthma
(38–41). Notch-3 signaling and cell-to-cell
contact facilitates Treg-cell expansion. MSCs were demonstrated to
decrease cell infiltration and lung pathology via regulation of the
Treg-cell population in a previous model of allergic asthma
(17). In the present study,
Notch-3 expression was observed in the lung tissue, blood and lymph
in all three study groups.
Previous research demonstrated that the percentages
of CD4+ CD25+ Foxp3+ Tregs were
significantly decreased in the serum of OVA group rats compared
with those in the control group and hPMSC-treated rats. The
administration of hPMSCs markedly increased the percentages of
CD4+ CD25+ Foxp3+ Tregs in the
serum and lymph. The Th17/Treg rebalance was induced by hPMSC
administration. The mechanism may be mediated by Treg regulation,
partly involving increased IL-10 levels and Foxp3/RORγt (42). These previous results suggested
that Notch-3 signaling may be involved in the development of asthma
by regulating the generation and the expansion of Treg cells
through hPMSC administration. Notch receptors and ligands are
involved in the regulation of immune cells and tissue
microenvironments (26). In the
present study, jagged-1 and delta-4 mRNA were detected in the
lungs, serum and lymph of the control group, OVA group and
hPMSC-treated group, and Jagged-1 expression was increased in the
rats with OVA-induced asthma. Treatment with hPMSCs decreased the
jagged-1 response to OVA challenge. Delta-4 expression decreased in
the rats with OVA-induced asthma compared with the control group
and the OVA+hPMSCs group. Jagged-1 was required for the expansion
of CD4+ CD25+ FoxP3+ regulatory T
cells following treatment with MSCs (43). Rutz et al (3) demonstrated that delta-1 and jagged-1
served important roles in the inhibition of T-cell activation,
whereas delta-4 expression enhanced T-cell proliferation. These
results provide an insight into the involvement of Notch signaling
in the crosstalk between immune cells and hPMSCs in asthma.
In conclusion, the results of the present study
demonstrated that hPMSCs may reduce airway inflammation via
inhibition of Notch-1, Notch-2 and jagged-1 expression, and
promotion of Notch-3 and delta-4 expression. The effect of hPMSC
administration in alleviating airway hyper-responsiveness and
inflammation may be mediated by Notch regulation, partly involving
reduced IL-4 and IgE levels and increased IFN-γ. hPMSCs may be a
feasible agent for treating asthma in the future. The present
findings add to the understanding of the biological significance of
hPMSCs in mediating the Notch pathway in asthma pathogenesis.
Acknowledgements
The present study was supported by grants from the
Shandong Province Natural Science Foundation of China (grant no.
ZR2011HM081), Yantai Science and Technology Development Funds
(grant no. 2008142-11) and the Taishan Scholar Foundation.
Glossary
Abbreviations
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
hPMSCs
|
human placenta mesenchymal stem
cells
|
|
Treg
|
regulatory T cell
|
|
Th
|
helper T cell
|
|
BM-MSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
AHR
|
airway hyperreactivity
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
NS
|
normal saline
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
Notch
|
neurogenic locus notch homolog
protein
|
|
OVA
|
ovalbumin
|
|
delta
|
delta-like ligand
|
|
IFN-γ
|
interferon-γ
|
|
Ig
|
immunoglobulin
|
|
IL
|
interleukin
|
|
DCs
|
dendritic cells
|
|
FoxP3
|
forkhead box protein P3
|
|
BCA
|
bicinchoninic acid
|
References
|
1
|
Kupczyk M and Wenzel S: U.S. and European
severe asthma cohorts: What can they teach us about severe asthma?
J Intern Med. 272:121–132. 2012.
|
|
2
|
Wenzel S: Severe asthma: From
characteristics to phenotypes to endotypes. Clin Exp Allergy.
42:650–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rutz S, Mordmüller B, Sankano S and
Scheffold A: Notch ligands Delta-like1, Delta-like4 and Jagged1
differentially regulate activation of peripheral T helper cells.
Eur J Immunol. 35:2443–2451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao PE, Petrone AL and Ponath PD:
Differentiation and expansion of T cells with regulatory function
from human peripheral lymphocytes by stimulation in the presence of
TGF-{beta}. J Immunol. 174:1446–1455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Del Papa B, Sportoletti P, Cecchini D,
Rosati E, Balucani C, Baldoni S, Fettucciari K, Marconi P, Martelli
MF, Falzetti F and Di Ianni M: Notch1 modulates mesenchymal stem
cells mediated regulatory T-cell induction. Eur J Immunol.
43:182–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bassi ÊJ, Moraes-Vieira PM, Moreira-Sá CS,
Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva
A and Câmara NO: Immune regulatory properties of allogeneic
adipose-derived mesenchymal stem cells in the treatment of
experimental autoimmune diabetes. Diabetes. 61:2534–2545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mariñas-Pardo L, Mirones I, Amor-Carro O,
Fraga-Iriso R, Lema-Costa B, Cubillo I, Rodríguez Milla MÁ,
García-Castro J and Ramos-Barbón D: Mesenchymal stem cells regulate
airway contractile tissue remodeling in murine experimental asthma.
Allergy. 69:730–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassi EJ, Aita CA and Câmara NO: Immune
regulatory properties of multipotent mesenchymal stromal cells:
Where do we stand? World J Stem Cells. 3:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Machado Cde V, Telles PD and Nascimento
IL: Immunological charac-teristics of mesenchymal stem cells. Rev
Bras Hematol Hemoter. 35:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asari S, Itakura S, Ferreri K, Liu CP,
Kuroda Y, Kandeel F and Mullen Y: Mesenchymal stem cells suppress
B-cell terminal differentiation. Exp Hematol. 37:604–615. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryan JM, Barry FP, Murphy JM and Mahon BP:
Mesenchymal stem cells avoid allogeneic rejection. J Inflamm
(Lond). 2:82005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jonuleit H, Schmitt E, Schuler G, Knop J
and Enk AH: Induction of interleukin 10-producing, nonproliferating
CD4(+) T cells with regulatory properties by repetitive stimulation
with allogeneic immature human dendritic cells. J Exp Med.
192:1213–1222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamazaki S, Iyoda T, Tarbell K, Olson K,
Velinzon K, Inaba K and Steinman RM: Direct expansion of functional
CD25+ CD4+ regulatory T cells by antigen-processing dendritic
cells. J Exp Med. 198:235–247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morelli AE and Thomson AW: Tolerogenic
dendritic cells and the quest for transplant tolerance. Nat Rev
Immunol. 7:610–621. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maldonado RA and von Andrian UH: How
tolerogenic dendritic cells induce regulatory T cells. Adv Immunol.
108:111–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kavanagh H and Mahon BP: Allogeneic
mesenchymal stem cells prevent allergic airway inflammation by
inducing murine regulatory T cells. Allergy. 66:523–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang M, Zhao X, Liu Y, Tian Y, Ran X and
Jiang Y: A role for WNT1-inducible signaling protein-1 in airway
remodeling in a rat asthma model. Int Immunopharmacol. 17:350–357.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srour N and Thébaud B: Stem cells in
animal asthma models: A systematic review. Cytotherapy.
16:1629–1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Bai J, Ji X, Li R, Xuan Y and Wang
Y: Comprehensive characterization of four different populations of
human mesenchymal stem cells as regards their immune properties,
proliferation and differentiation. Int J Mol Med. 34:695–704. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yun HM, Kim HS, Park KR, Shin JM, Kang AR,
Il Lee K, Song S, Kim YB, Han SB, Chung HM and Hong JT:
Placenta-derived mesenchymal stem cells improve memory dysfunction
in an Aβ1-42-infused mouse model of Alzheimer's disease. Cell Death
Dis. 4:e9582013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong P, Xie X, Li F, Liu Y and Lu Y:
Placenta mesenchymal stem cell accelerates wound healing by
enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem
Biophys Res Commun. 438:410–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stoff A, Rivera AA, Sanjib Banerjee N,
Moore ST, Michael Numnum T, Espinosa-de-Los-Monteros A, Richter DF,
Siegal GP, Chow LT, Feldman D, et al: Promotion of incisional wound
repair by human mesenchymal stem cell transplantation. Exp
Dermatol. 18:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan CK, Lin TC, Huang YA, Chen YS, Wu CL,
Lo HY, Kuo ML, Wu KH and Huang JL: The modulation of Th2 immune
pathway in the immunosuppressive effect of human umbilical cord
mesenchymal stem cells in a murine asthmatic model. Inflamm Res.
65:795–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radtke F, Wilson A, Mancini SJ and
MacDonald HR: Notch regulation of lymphocyte development and
function. Nat Immunol. 5:247–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishnaswamy S, Verdile G, Groth D,
Kanyenda L and Martins RN: The structure and function of
Alzheimer's gamma secretase enzyme complex. Crit Rev Clin Lab Sci.
46:282–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berezovska O, Jack C, Deng A, Gastineau N,
Rebeck GW and Hyman BT: Notch1 and amyloid precursor protein are
competitive substrates for presenilin1-dependent gamma-secretase
cleavage. J Biol Chem. 276:30018–30023. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui ZL, Gu W, Ding T, Peng XH, Chen X,
Luan CY, Han RC, Xu WG and Guo XJ: Histone modifications of Notch1
promoter affect lung CD4+ T cells differentiation in asthmatic
rats. Int J Immunopathol Pharmacol. 26:371–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Strooper B, Annaert W, Cupers P, Saftig
P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS,
Ray WJ, et al: A presenilin-1-dependent gamma-secretase-like
protease mediates release of Notch intracellular domain. Nature.
398:518–522. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karlström H, Bergman A, Lendahl U, Näslund
J and Lundkvist J: A sensitive and quantitative assay for measuring
cleavage of presenilin substrates. J Biol Chem. 277:6763–6766.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo XJ, Zhou M, Ren LP, Yang M, Huang SG
and Xu WG: Small interfering RNA mediated knockdown of Notch1 in
lung T cells of asthmatic mice affects T cell differentiation. Chin
Med J (Engl). 122:2647–2651. 2009.PubMed/NCBI
|
|
33
|
Fang TC, Yashiro-Ohtani Y, Del Bianco C,
Knoblock DM, Blacklow SC and Pear WS: Notch directly regulates
Gata3 expression during T helper 2 cell differentiation. Immunity.
27:100–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Osborne BA and Minter LM: Notch signalling
during peripheral T-cell activation and differentiation. Nat Rev
Immunol. 7:64–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bailis W, Yashiro-Ohtani Y, Fang TC,
Hatton RD, Weaver CT, Artis D and Pear WS: Notch simultaneously
orchestrates multiple helper T cell programs independently of
cytokine signals. Immunity. 39:148–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okamoto M, Matsuda H, Joetham A, Lucas JJ,
Domenico J, Yasutomo K, Takeda K and Gelfand EW: Jagged1 on
dendritic cells and Notch on CD4+ T cells initiate lung allergic
responsiveness by inducing IL-4 production. J Immunol.
183:2995–3003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zong D, Ouyang R, Li J, Chen Y and Chen P:
Notch signaling in lung diseases: Focus on Notch1 and Notch3. Ther
Adv Respir Dis. 10:468–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi YH, Shi GC, Wan HY, Jiang LH, Ai XY,
Zhu HX, Tang W, Ma JY, Jin XY and Zhang BY: Coexistence of Th1/Th2
and Th17/Treg imbalances in patients with allergic asthma. Chin Med
J (Engl). 124:1951–1956. 2011.PubMed/NCBI
|
|
39
|
Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou
X, Wang J, Fan H, Yan CS, Kuang JL, et al: Adoptive transfer of
induced-Treg cells effectively attenuates murine airway allergic
inflammation. PLoS One. 7:e403142012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kearley J, Robinson DS and Lloyd CM:
CD4+CD25+ regulatory T cells reverse established allergic airway
inflammation and prevent airway remodeling. J Allergy Clin Immunol.
122:617–624.e6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kared H, Adle-Biassette H, Foïs E, Masson
A, Bach JF, Chatenoud L, Schneider E and Zavala F:
Jagged2-expressing hematopoietic progenitors promote regulatory T
cell expansion in the periphery through notch signaling. Immunity.
25:823–834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Li H, Cao Y, Wu F, Ma W, Wang Y and
Sun S: Placenta-derived mesenchymal stem cells improve airway
hyperresponsiveness and inflammation in asthmatic rats by
modulating the Th17/Treg balance. Mol Med Rep. 16:8137–8145. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cahill EF, Tobin LM, Carty F, Mahon BP and
English K: Jagged-1 is required for the expansion of CD4+ CD25+
FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine
mesenchymal stromal cells. Stem Cell Res Ther. 6:192015. View Article : Google Scholar : PubMed/NCBI
|