Introduction
Borna disease virus (BDV) is an enveloped,
non-segmented negative single-stranded RNA virus, which is highly
neurotropic and is able to infect a range of warm-blooded animals,
from birds to primates (1–3). Epidemiological investigations have
demonstrated that neuropsychiatric disorders, including
schizophrenia and mood disorders, are associated with BDV infection
(4–11). Notably, BDV RNA and antigens have
been detected in brain tissues from patients with psychiatric
diseases and brain tumors (12–17),
in addition to in human lymphocytes (18).
The genome of BDV, at a length of 8.9 kb, consists
of six open reading frames which respectively encode six proteins
(19). Among these proteins,
phosphoprotein (BDV p24) and nucleoprotein (BDV p40) are frequently
detected together in human serological studies (20–23);
therefore, they were selected as the primary target antigens in the
present study.
There are four principal methods of detecting BDV
infection: i) Isolating virus from blood samples or tissue
homogenate (5,8,14);
ii) detecting BDV-RNA with reverse transcription-polymerase chain
reaction (RT-PCR) analysis (24);
iii) serological testing, including indirect immunological
fluorescence assays (25), western
blotting (26,27) and ELISA (7); and iv) detecting the primary antigens
(p24 or/and p40) via immunohistochemical staining in situ
(28). The first three methods
require fresh specimens. Although serum or plasma samples are the
easiest diagnostic sources, in clinical practice, pathological
tissue from patients undergoing brain surgery has may be fixed and
embedded into paraffin blocks for long-term preservation in
pathology laboratories, which also are favorable materials for use
in screening for BDV infection in the population via
immunohistochemistry. Previous studies have used antibodies donated
from other collaborating laboratories (29,30).
However, there are no commercially available anti-BDV protein
antibodies.
Monoclonal and polyclonal anti-p24 and anti-p40
antibodies have been prepared previously by animal immunization
technology and hybridoma technology (31). Therefore, the primary purpose of
the present study was to examine the application of the constructed
antibodies against recombinant proteins for detecting p24 and p40
in paraffin sections of BDV-infected rat/mouse brain tissue by
immunohistochemical staining (including appropriate dilutions,
antigen retrieval and evaluation of the results). The present study
may provide a foundation for detecting BDV infection in brain
tissue paraffin sections, particularly from brain tumors, by
immunohistochemistry (12,14).
Materials and methods
BDV strain
Human BDV strain Hu-H1 was isolated from the blood
cells of a severely depressed patient with bipolar disorder in
Germany, and co-cultured with a permanent human oligodendrocyte
(OL) cell line (5). BDV-Hu-H1
(kindly supplied by Professor Hanns Ludwig, Free University of
Berlin, Berlin, Germany) virus solution was obtained by repeated
freezing and thawing of BDV-infected OL cells and stored at
−80°C.
Viral infection
Infection of animals
Sprague-Dawley (SD) rats born within 24 h were
intracranially inoculated in their right cerebral hemispheres with
either: i) 30 µl Hu-H1 virus solution [104 focus forming
units/ml; n=20; 11 males, weight (305±12 g); 9 females, weight
(217±15 g)], as previously described (32); or ii) PBS [30 µl; negative control;
n=20; males, weight (313±14 g); 12 females, weight (222±12 g)]. In
addition, 20 SD rats without injection [10 males, weight (310±12
g); 10 females, weight (224±10 g)], and 20 uninjected C57 mice [11
males, weight (23±1 g); 9 females, weight (20±1 g)] as the normal
control. Rats and mice were group-housed under a 12-h light/dark
cycle (on between 7:00 a.m. and 7:00 p.m.) with unrestricted access
to food and water at a constant room temperature of 22–24°C and 50%
humidity. All animals that we used in this experiment were provided
by the Experimental Animal Center at Chongqing Medical University
(Chongqing, China). All the procedures performed in the present
study were approved by the Ethics Committee of Chongqing Medical
University (Chongqing, China).
Preparation of samples
All rats and mice were sacrificed on postnatal day
56. Each right brain hemisphere was collected and stored at −80°C.
The total protein was extracted from the right brain tissue from
the −80°C refrigerator (n=20) using the Total Protein Extraction
kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). The protein
concentrations were determined with an Enhanced Bicinchoninic Acid
Protein Assay kit (Beyotime Biotechnology Co., Ltd., Zhejiang,
China). Protein samples (15 µg per lane) were subjected to SDS-PAGE
on a 12% gel (100 V for 2 h) following heat denaturing at 95°C for
5 min. Proteins were electrophoretically transferred to
polyvinylidene fluoride (PVDF) membranes (100 V for 75 min) and
were blocked in 5% non-fat dried milk for 1 h at room temperature.
The membranes were incubated overnight at 4°C with mouse anti-BDV
nucleoprotein (p24) monoclonal antibody (1:5,000; kindly provided
by Professor Hanns Ludwig) (31,32)
and used anti-GAPDH antibody (1:5,000; ab9485, Abcam, Cambridge,
UK) served as the loading control. The membranes were washed with
TBS-Tween 20 (TBST), and further incubated with goat anti-mouse
immunoglobulin (Ig)G-HRP (1:10,000; bs-0296G-HRP, Beijing Bioss
Co., Ltd., Beijing, China) at room temperature for 2 h. Subsequent
to washing the membranes, the protein bands were visualized using
enhanced chemiluminescence detection reagents (Nanjing KeyGen
Biotech Co., Ltd.). The signal was detected using the Bio-Rad
ChemiDoc XRS system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Preparation of antibodies
Preparation of antigens
Full-length cDNAs of BDV p24 and p40 were generated
using the RT-PCR (33). The PCR
conditions were: An initial denaturation at 94°C for 5 min; 30
cycles of 94°CC for denaturation (40 sec), annealing at 60°C (40
sec) and elongation at 72°C (30 sec); and extension at 72°C for 5
min. Total RNAs isolated from BDV-Hu-H1-infected OL cells were used
as templates. The primers used for RT-PCR were:
5′-TAACATATGACCATGCCACCCAAGAGACGC-3′ (forward) and
5′-CGCGGATCCAAGTTTAGACCAGTCACAC-3′ (reverse) for p40; and
5′-CCGCATATGGCAACGCGACCATCGAGT-3′ (forward) and
5′-CCGCTCGAGTGGTATGATGTCCCATTCATCC-3′ (reverse) for p24. The cDNAs
of p24 and p40 were ligated into the vectors (Novagen; Merck KGaA,
Darmstadt Germany) pET-41a by NdeI and XhoI sites and
pET-14b by NdeI and BamHI sites, respectively. These
vectors were transformed into Escherichia coli BL21 (DE3;
the Key Laboratory of Neurobiology, Chongqing, China). The
authenticity of the expression constructs was verified by
sequencing. The recombinant histidine-tagged p24 and p40 proteins
were used following purification using HisTrap HP (GE Healthcare,
Chicago, IL, USA) column chromatography. The column was
equilibrated with equilibration buffer and the bacterial lysate was
filtered to obtain the supernatant, which was purified via a
purification column. Unbound hybrid proteins were removed to obtain
recombinant proteins by using wash buffer containing 20 mmol/l
Tris-HCl (pH 8.0), 50 mmol/l imidazole and 500 mmol/l NaCl.
Polyclonal antibody preparation
Two adult male New Zealand white rabbits with the
weight of 3 kg obtained from the Experimental Animal Center of
Chongqing University were used for the experiment. Under specific
pathogen-free conditions at a constant room temperature of 22–24°C
and 50% humidity with a 12-h light/dark cycle (lights on: 8:00
a.m., off: 8:00 p.m.) and provided ad libitum access to food
and water. The purified histidine-tagged p24 protein (500 µg
diluted with 1 ml of physiological saline), was emulsified with an
equal volume of complete Freund's adjuvant (Sigma-Aldrich, Merck
KGaA) and injected intradermally into a rabbit over 10 to 12 sites.
Following pre-immunization blood drawing, the rabbit received
booster injections twice, at monthly intervals, with the same
amount of protein mixed with incomplete Freund's adjuvant.
Subsequent to the third injection, the serums were collected and
purified by antigen immunoaffinity chromatography. The antibody
against recombinant p40 was similarly produced. The activated
agarose gel and antigen were coupled overnight to prepare an
antigen-immobilized column. The closed column was pre-washed with
PBS to equilibrate the column. Following, the serums were added to
a well-balanced column and incubated overnight at 4°C and washed
with the eluate to obtain purified antibody. The eluent was
measured at 280 nm.
Monoclonal antibody preparation
A total of 5 BALB/c mice [female, 6–8 weeks old,
weight (21±1 g)] were obtained from the Experimental Animal Center
of Chongqing Medical University. Under specific pathogen-free
conditions at a constant room temperature of 22–24°C and 50%
humidity with a 12 h light/dark cycle (lights on: 8:00 a.m., off:
8:00 p.m.) and provided ad libitum access to food and water.
The mice were first immunized intraperitoneally with
histidine-tagged p24 protein (50 µg diluted with 100 µl saline,
emulsified in Freund's complete adjuvant). Immunization was
repeated three times at intervals of 2 weeks. Hybridoma was
prepared by fusing SP2/0 myeloma cells (Nanjing GenScript Co.,
Ltd., Nanjing, China) and spleen cells of immunized BALB/c mice.
Immunized mouse spleen cells and SP2/0 myeloma cells were
hybridized with 50% PEG 4000 (Sigma-Aldrich Merck KGaA) at a 5:1
ratio and cultured in RPMI-1640 culture medium (Hyclone; GE
Healthcare Life Sciences, Logan UT, USA). The recombinant BDV
nucleus protein was used as the antigen, and the positive cell
clone strains were measured by indirect ELISA (Cosmo ELISA Plate,
1105–096; Cosmo Biosciences, Inc., San Diego, CA, USA). The optical
density (OD) of each well was measured at 450 nm. The criteria were
as follows: OD was greater than or equal to 2.1X the negative
control. Candidates with positive ELISA clones were subjected to
limited dilution and further clones were screened. The hybridoma
cell strains with stable secretory monoclonal antibody were
determined by the limited dilution method for three-times sub
clones with the preliminary screening of the suspected positive
cell clones: The selected positive cell clones (1 cell/well) were
seeded into a 96-well plate in HT medium (Hyclone; GE Healthcare
Life Sciences) and were cultured for 4 weeks. The supernatant was
then collected and detected via indirect ELISA, which was used
until the positive rate was 100%. For the production of large
amounts of monoclonal antibodies, mouse ascites of monoclonal
antibodies were collected and purified by protein G affinity
chromatography. Firstly, a purification column containing
Sepharose-protein G was washed with binding buffer. Secondly, mouse
ascites were diluted 4 times with binding buffer, and centrifuged
at 11,900 × g for 30 min at 4°C. Thirdly, the supernatant was added
into the washed purification column. The bound monoclonal
antibodies were eluted with elution buffer and loaded in a
collection tube containing 0.1 ml potassium phosphate buffer.
Finally, the eluent was measured at A280 nm. The antibody against
the recombinant p40 was similarly produced.
Titer and specificity of antibodies
Titer detection by ELISA
The recombinant proteins were diluted to a
concentration of 100 ng/100 µl with 0.05 mol/l
Na2CO3-NaHCO3 coating buffer (pH
9.6) and were added to 96-well plates (100 µl proteins/well).
Following overnight incubation at 4°C, plates were washed with
PBS-Tween 20 (PBST) three times. Wells were blocked with 5% bovine
serum albumin (Hyclone; GE Healthcare Life Sciences) solution for 1
h at 37°C and washed with PBST three times. The pre-immunization
serum was diluted 1:1,000 and the purified antibodies were diluted
1:1,000, and serially diluted two-fold from 1:1,000 to 1:512,000.
The plates were incubated with 100 µl diluted antibodies for 1 h at
37°C. Wells were washed and further incubated with goat anti-rabbit
immunoglobulin G antibody (Beijing Bioss Co., Ltd.) diluted 1:2,500
(100 µl/well), conjugated with horseradish peroxidase (HRP).
Following washing of the plates, 3,3,5,5,-tetramethylbenzidine was
added to each well. The chromogenic reaction was stopped by 50
µl/well 2 mol/l H2SO4 following a 10-min
incubation at 37°C. The optical density of each well was measured
at 450 nm. All samples were run in triplicate. PBS was used as a
blank control and the pre-immunization serum was used as a negative
control.
Specificity identification by western
blotting
Proteins were extracted from normal OL cells and
BDV-infected OL cells using the KeyGen Whole Cell Lysis Assay
(Nanjing KeyGen Biotech Co., Ltd.). Protein concentrations were
quantified according to the Bradford procedure (Bradford protein
quantitation assay; Nanjing KeyGen Biotech Co., Ltd.) to maintain
the same loads. Proteins (normal OL cells lysates, 25 µg;
BDV-infected OL cell lysates, 25 µg; recombinant p24 and p40 BDV
proteins, 1 µg) were separated by 12% SDS-PAGE (100 V for 2 h)
following heat denaturation at 95°C for 5 min, and transferred onto
PVDF membranes in Tris-glycine buffer containing 20% methanol (100
V for 75 min). The membranes were stripped according to marker
bands and were blocked in 5% non-fat milk at room temperature for 1
h. The primary antibodies (1:1,500) were diluted with blocking
buffer and incubated with membranes overnight at 4°C. The membranes
were washed with TBST, and further incubated with HRP-conjugated
secondary antibodies (1:2,000; bs-0295G-HRP and bs-0296G-HRP,
Beijing Bioss Co., Ltd.) at room temperature for 2 h. Following
washing of the membranes, the protein bands were visualized using
enhanced chemiluminescence detection reagents (Nanjing KeyGen
Biotech Co., Ltd.). The signal was detected using the Bio-Rad
ChemiDoc XRS system (Bio-Rad Laboratories, Inc.). Additionally, the
isolated recombinant BDV proteins by 12% SDS-PAGE were stained with
Coomassie brilliant blue at room temperature for 3 h and then
scanned by Epson Graphic Arts Scanner. The OD of each band was
semi-quantified using Quantity One software (version 4.4.0; Bio-Rad
Laboratories, Inc.).
Immunohistochemical analysis
Paraffin section preparation
All rats and mice were sacrificed on postnatal day
56. All left-brain hemispheres were fixed with 4% paraformaldehyde
at room temperature for 24 h and embedded in paraffin. Tissue
section was sliced along the sagittal plane into 5-µm sections, for
the purpose of immunohistochemical staining.
All tissue sections from BDV-infected rats (n=20),
PBS-injected rats (n=20), normal SD rats (n=20) and normal C57 mice
(n=20) were tested with four antibodies against recombinant
proteins respectively: Poly- and monoclonal anti-p24 antibodies
(pAbp24 and mAbp24), and poly- and monoclonal anti-p40 antibodies
(pAbp40 and mAbp40). All primary antibodies (pAbp24, mAbp24, pAbp40
and mAbp40) were diluted 1:50, 1:100, 1:200, 1:400, 1:600, 1:800,
1:1,000, 1:1,200, 1:1,400, 1:1,600, 1:1,800, 1:2,000 and
1:5,000.
Immunohistochemical staining
The technique of detection of BDV in the brain by
immunohistology was pioneered by Gosztonyi et al (34). Immunohistochemical staining was
performed according to the EnVision two-step protocol
(EnVision™; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Varying concentrations of alcohol used for
rehydration were as follows: Two washes with absolute ethanol 3
min, 95% ethanol 3 min, 85% ethanol 3 min and 80% ethanol 3 min.
Heat-mediated antigen retrieval was performed within 3 min using
retrieval buffer sodium citrate (1 mM; pH 6.0) with a pressure
cooker. Then the slices were washed with PBS for 3 min, three times
and blocked with 3% hydrogen peroxide at room temperature for 10
min. The primary antibodies were incubated overnight at 4°C and the
blank control was treated with PBS instead of primary antibody,
overnight at 4°C. Following incubation with HRP-conjugated
secondary antibody (K406511-2, EnVision™; Dako; Agilent
Technologies, Inc.) at room temperature for 30 min, the sections
were stained with diaminobenzidine for a few sec and counterstained
with hematoxylin for 3 min at room temperature.
Evaluation criteria
The present study focused on the evaluation of the
intensity of positive staining, positive proportion of neurons and
neuroglial cells and background staining in three brain regions:
Cerebral cortex, hippocampus and cerebellum. p24 is has been
demonstrated to exhibit strong positive staining in the cytoplasm
and weak staining in the nucleus; p40 has been demonstrated to
exhibit strong positive staining in the nucleus and weak staining
in the cytoplasm (35). Positive
staining was graded according to the intensity and proportion. For
intensity: 0, no positive cells; 1, light yellow; 2, brown-yellow;
and 3, tan. For proportion: 0, no positive cells; 1, 1–25%; 2,
26–50%; 3, 51–75%; and 4, 76–100% (36).
Statistical analysis
A total of 10 representative areas were selected
under a high magnification field (×400) for each slide. The mean
intensity score was multiplied by the mean proportion score to
produce the total score under all the dilution levels of the four
primary antibodies, for BDV-infected and uninfected (negative
control and normal control) groups, respectively. The total scores
between the two groups were tested using the non-parametric
Mann-Whitney test in SPSS (version 21.0; IBM Corp., Armonk, NY,
USA). Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Viral infection
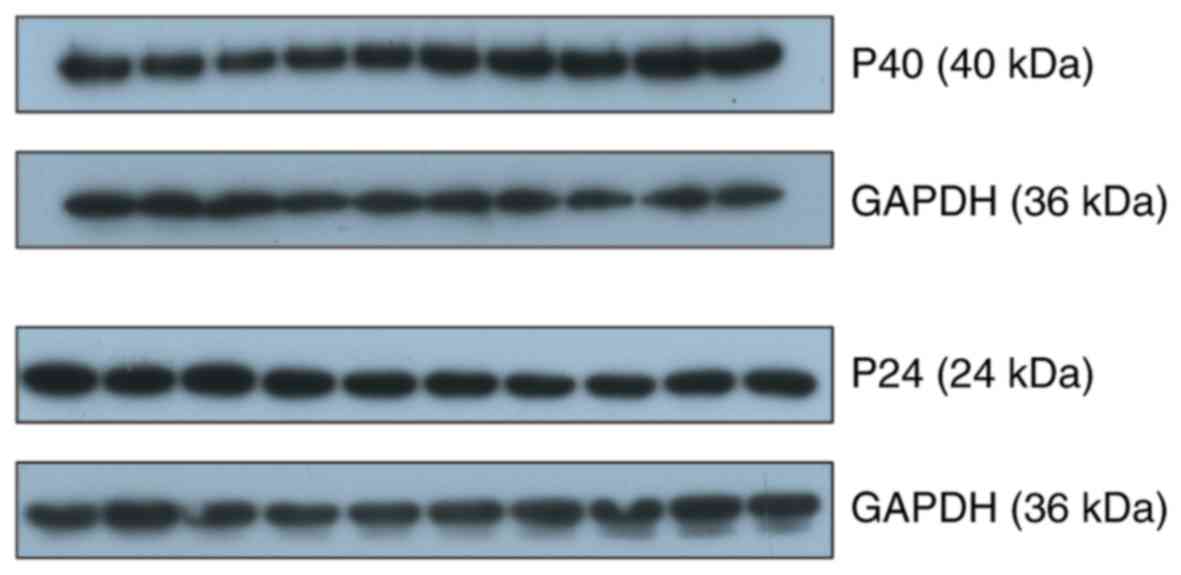
The infection of 20 BDV-infected rats were confirmed
by western blotting with monoclonal anti-p24 antibody (kindly
provided by Professor Hanns Ludwig) (Fig. 1).
Purification of recombinant
proteins
The purity of the recombinant p24 and p40 were
analyzed by SDS-PAGE on a 12% gel (Fig. 2). The recombinant protein was
primarily expressed in soluble form and purified by affinity
chromatography. The band of His-p24 was ~24 kDa with a purity of
85%, and the band of His-p40 appeared at ~40 kDa with a purity of
90%. Results were confirmed by western blotting with monoclonal
antibodies against p24 and p40, respectively (donated by Professor
Hanns Ludwig), and the reaction bands were observed at 24 and 40
kDa. The results of western blotting demonstrated that the purified
recombinant p24 or p40 protein was specifically bound to the
monoclonal antibody. It was, therefore, immunologically confirmed
that the expressed recombinant proteins had the correct antigenic
activity and were able to be used to detect the antibody
constructed.
Titer of antibodies
Positive dilutions were determined when the
absorbance ratio of antibodies to the negative control was >2:1
(Fig. 3). The data demonstrated
that the titer of the polyclonal anti-p24 antibody was 1:128,000,
as well as the monoclonal antibody. The titer of polyclonal and
monoclonal anti-p40 antibody was 1:256,000 and 1:128,000,
respectively.
 | Figure 3.Titer of each antibody, detected by
ELISA analysis. The serial dilution of each antibody was from
1:1,000 to 1:512,000. 3,3′,5,5′-tetramethylbenzidine was used as
the chromogenic reagent. A450nm was measured. Absorbance
ratios of results with antibodies to results with pre-immunization
serum >2.1 were considered to be positive titers. *, minimal
positive titer. A450nm, absorbance at 450 nm; p40, Borna
disease virus nucleoprotein; p24, Borna disease virus
phosphoprotein. |
Western blot analysis
Western blot analysis demonstrated that proteins
from BDV-infected OL cells and recombinant proteins were detected
positively by pAbp24, mAbp24, pAbp40 and mAbp40 (Fig. 4). All normal OL cells exhibited no
signal. Pre-immunization sera were used as the control, instead of
primary antibodies, and there was no signal for either the
recombinant or native proteins (data not shown). The results showed
that the four antibodies had a good specificity for BDV p24 and
p40, respectively.
 | Figure 4.Specificity of antibodies, detected
by western blotting. Secondary antibodies were conjugated with
horseradish peroxidase. The protein bands were visualized by
enhanced chemiluminescence. Lane 1, lysate of BDV-infected OL
cells; lane 2, lysate of normal OL cells; lane 3, recombinant
proteins. pAbp24, polyclonal anti-p24 antibody; mAbp24, monoclonal
anti-p24 antibody; pAbp40, polyclonal anti-p40 antibody; mAbp40,
monoclonal anti-p40 antibody. p40, Borna disease virus
nucleoprotein; p24, Borna disease virus phosphoprotein. |
Immunohistochemical analysis
The results of the four primary antibodies (pAbp24,
mAbp24, pAbp40 and mAbp40) were positive in the BDV-infected group
(n=20) and were negative in the BDV-uninfected group (n=60)
(Figs. 5 and 6). For polyclonal anti-p24 and anti-p40
antibodies, the dilutions of 1:100 to 1:400 obtained strong
positive staining with slight background coloring, and were
therefore considered to be a suitable dilution. For monoclonal
anti-p24 and anti-p40 antibodies, the dilutions of 1:200 to 1:400
obtained strong positive staining without background coloring, and
were therefore considered to be a suitable dilution. The total mean
scores for the four antibodies were 8 (mean) and 0 (mean) in
BDV-infected and uninfected group, respectively. P-values (P=0.000)
were generated by the nonparametric Mann-Whitney test between the
BDV-infected and uninfected group and the mean ± standard deviation
of the BDV-infected group was 7.38±3.59, and 0.07±0.28 for the
uninfected group.
Discussion
There are no commercially available antibodies for
BDV p24 and p40 on the market. In the present study, recombinant
full-length BDV p24 and p40 were used as antigens to immunize
rabbits and mice. Antibodies usually only recognize specific
epitopes with conformational structures. Since in western blotting
the antigen is denatured into a linear structure, this may lead to
different results compared with methods using the conformational
antigen and antibody (37,38). The issue of conformational epitopes
on BDV-p24/p40 has been addressed. The conformational epitopes of
the donated monoclonal antibodies used in the present study and
their characterization have been examined previously (31,39).
However, the conformational epitopes of the antibodies against
recombinant proteins produced in the present study remain to be
elucidated, and further analysis is required. However, the high
specificity and sensitivity of the four antibodies produced in the
present study were confirmed by western blotting, ELISA analysis
and immunohistochemistry on paraffin sections.
Analysis of BDV protein expression (particularly
p24/p40) in human brain tissue may determine BDV infection in
humans; however, this method is unsuitable for epidemiological
screening due to the low viral titer in patients and the necessity
for time-consuming surgery. Serum or plasma samples are the easiest
diagnostic sources and infection may be determined by easily
accessible blood samples. As BDV is a highly neurotropic virus,
brain tissue represents an ideal sample source to detect BDV
infection. Researchers have observed BDV RNA and antigens in the
brains of patients with neuropsychiatric disorders (14,40).
Clinical human brain tissue samples are frequently stored in
paraffin blocks; therefore, it may be convenient to detect BDV
infection in these samples by immunohistochemistry, and to study
the correlation between BDV infection and morbidity (40).
Compared with the polyclonal antibodies in the
present study, the sensitivity of the monoclonal antibodies was
consistent and the specificity was increased; additionally, the
nonspecific staining of the background was decreased with the
monoclonal antibodies. The results of the present study
demonstrated that the polyclonal and monoclonal anti-p24 and
anti-p40 antibodies against recombinant proteins were highly
specific and sensitive for BDV p24 and p40 detection, and may be
efficiently used to detect BDV infection in paraffin sections of
human brain tissue in future studies.
Acknowledgements
The authors of the present study would like to thank
Professor Liv Bode and Professor Hanns Ludwig of the Robert Koch
Institute and Institute of Virology at the Free University of
Berlin (Berlin, Germany) for providing the human oligodendroglioma
cell line and the Borna disease virus strain Hu-H1 used in the
present study. The present study was supported by the National
Basic Research Program of China (973 Program) (grant no.
2009CB918300) and the Natural Science Foundation of Chongqing City
of China (grant no. cstc2013jcyjA10003).
References
|
1
|
Ludwig H and Bode L: Borna disease virus:
New aspects on infection, disease, diagnosis and epidemiology. Rev
Sci Tech. 19:259–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzalez-Dunia D, Sauder C and de la Torre
JC: Borna disease virus and the brain. Brain Res Bull. 44:647–664.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuhn JH, Dürrwald R, Báo Y, Briese T,
Carbone K, Clawson AN, deRisi JL, Garten W, Jahrling PB,
Kolodziejek J, et al: Taxonomic reorganization of the family
Bornaviridae. Arch Virol. 160:621–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waltrip RW II, Buchanan RW, Summerfelt A,
Breier A, Carpenter WT Jr, Bryant NL, Rubin SA and Carbone KM:
Borna disease virus and schizophrenia. Psychiatry Res. 56:33–44.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bode L, Dürrwald R, Rantam FA, Ferszt R
and Ludwig H: First isolates of infectious human Borna disease
virus from patients with mood disorders. Mol Psychiatry. 1:200–212.
1996.PubMed/NCBI
|
|
6
|
Zhang L, Xu MM, Zeng L, Liu S, Liu X, Wang
X, Li D, Huang RZ, Zhao LB, Zhan QL, et al: Evidence for Borna
disease virus infection in neuropsychiatric patients in three
western China provinces. Eur J Clin Microbiol Infect Dis.
33:621–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bode L and Ludwig H: Borna disease virus
infection, a human mental-health risk. Clin Microbiol Rev.
16:534–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikuta K, Ibrahim MS, Kobayashi T and
Tomonaga K: Borna disease virus and infection in humans. Front
Biosci. 7:d470–d495. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Zhang L, Lei Y, Liu X, Zhou X, Liu
Y, Wang M, Yang L, Zhang L, Fan S and Xie P: Meta-analysis of
infectious agents and depression. Sci Rep. 4:45302014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaliunaite V, Steibliene V, Bode L,
Podlipskyte A, Bunevicius R and Ludwig H: Primary psychosis and
Borna disease virus infection in Lithuania: A case control study.
BMC Psychiatry. 16:3692016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Donfrancesco R, Gregori P, Vulcano A,
Candelori E, Ronchetti R, Miano S, Pagani J, Villa MP and Patti AM:
Borna disease virus infection in children with psychiatric
disorders. APMIS Suppl. 1–82. 2008.
|
|
12
|
Nakaya T, Tada M, Takahashi H, Fujiwar S,
Sakuma S, Sawamur Y, Abe H and Ikuta K: Expression of borna disease
virus messages in clinical samples from patients with brain
malignant tumors. Proc Japan Academy Ser B Physl Biol Sci. 72:pp.
157–162. 1996; View Article : Google Scholar
|
|
13
|
Salvatore M, Morzunov S, Schwemmle M and
Lipkin WI: Borna disease virus in brains of North American and
European people with schizophrenia and bipolar disorder. Bornavirus
Study Group. Lancet. 349:1813–1814. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura Y, Takahashi H, Shoya Y, Nakaya
T, Watanabe M, Tomonaga K, Iwahashi K, Ameno K, Momiyama N,
Taniyama H, et al: Isolation of Borna disease virus from human
brain tissue. J Virol. 74:4601–4611. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haga S, Yoshimura M, Motoi Y, Arima K,
Aizawa T, Ikuta K, Tashiro M and Ikeda K: Detection of Borna
disease virus genome in normal human brain tissue. Brain Res.
770:307–309. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi H, Nakaya T, Nakamura Y, Asahi
S, Onishi Y, Ikebuchi K, Takahashi TA, Katoh T, Sekiguchi S,
Takazawa M, et al: Higher prevalence of Borna disease virus
infection in blood donors living near thoroughbred horse farms. J
Med Virol. 52:330–335. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ludwig H, Bode L and Gosztonyi G: Borna
disease: A persistent virus infection of the central nervous
system. Prog Med Virol. 35:107–151. 1988.PubMed/NCBI
|
|
18
|
Bode L, Zimmermann W, Ferszt R, Steinbach
F and Ludwig H: Borna disease virus genome transcribed and
expressed in psychiatric patients. Nat Med. 1:232–236. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lipkin W and Briese T: Bornaviridae.
Fields Virology. 2. 5th. Knipe DHP, Griffin D, Lamb R, Martin M,
Roizman B and Straus S: Lippincott, Williams & Wilkins;
Philadelphia: pp. 1829–1851. 2007
|
|
20
|
Bode L, Reckwald P, Severus WE, Stoyloff
R, Ferszt R, Dietrich DE and Ludwig H: Borna disease virus-specific
circulating immune complexes, antigenemia and free antibodies-the
key marker triplet determining infection and prevailing in severe
mood disorders. Mol Psychiatry. 6:481–491. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mazaheri-Tehrani E, Maghsoudi N, Shams J,
Soori H, Atashi H, Motamedi F, Bode L and Ludwig H: Borna disease
virus (BDV) infection in psychiatric patients and healthy controls
in Iran. Virol J. 11:1612014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flower RL, Kamhieh S, McLean L, Bode L,
Ludwig H and Ward CM: Human Borna disease virus infection in
Australia: Serological markers of infection in multi-transfused
patients. Apmis Suppl. 1–93. 2008.
|
|
23
|
Liu X, Bode L, Zhang L, Wang X, Liu S,
Zhang L, Huang R, Wang M, Yang L, Chen S, et al: Health care
professionals at risk of infection with Borna disease
virus-evidence from a large hospital in China (Chongqing). Virol J.
12:392015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miranda HC, Nunes SO, Calvo ES, Suzart S,
Itano EN and Watanabe MA: Detection of Borna disease virus p24 RNA
in peripheral blood cells from Brazilian mood and psychotic
disorder patients. J Affect Disord. 90:43–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rott R, Herzog S, Fleischer B, Winokur A,
Amsterdam J, Dyson W and Koprowski H: Detection of serum antibodies
to Borna disease virus in patients with psychiatric disorders.
Science. 228:755–756. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu ZF, Amsterdam JD, Kao M, Shankar V,
Koprowski H and Dietzschold B: Detection of Borna disease
virus-reactive antibodies from patients with affective disorders by
western immunoblot technique. J Affect Disord. 27:61–68. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sauder C, Müller A, Cubitt B, Mayer J,
Steinmetz J, Trabert W, Ziegler B, Wanke K, Mueller-Lantzsch N, de
la Torre JC and Grässer FA: Detection of Borna disease virus (BDV)
antibodies and BDV RNA in psychiatric patients: Evidence for high
sequence conservation of human blood-derived BDV RNA. J Virol.
70:7713–7724. 1996.PubMed/NCBI
|
|
28
|
Dietzel J, Kuhrt H, Stahl T, Kacza J,
Seeger J, Weber M, Uhlig A, Reichenbach A, Grosche A and Pannicke
T: Morphometric analysis of the retina from horses infected with
the Borna disease virus. Vet Pathol. 44:57–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horimoto T, Takahashi H, Sakaguchi M,
Horikoshi K, Iritani S, Kazamatsuri H, Ikeda K and Tashiro M: A
reverse-type sandwich enzyme-linked immunosorbent assay for
detecting antibodies to Borna disease virus. J Clin Microbiol.
35:1661–1666. 1997.PubMed/NCBI
|
|
30
|
Nakamura Y, Watanabe M, Kamitani W,
Taniyama H, Nakaya T, Nishimura Y, Tsujimoto H, Machida S and Ikuta
K: High prevalence of Borna disease virus in domestic cats with
neurological disorders in Japan. Vet Microbiol. 70:153–169. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludwig H, Furuya K, Bode L, Klein N,
Dürrwald R and Lee DS: Biology and neurobiology of Borna disease
viruses (BDV), defined by antibodies, neutralizability and their
pathogenic potential. Arch Virol Suppl. 7:111–133. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei Y, Li D, Deng J, Shao WH, Fan SH, Wang
X, Huang H, Chen SG, Zhang HZ, Zhang L, et al: Metabolomic
profiling of three brain regions from a postnatal infected Borna
disease virus Hu-H1 rat model. Metabolomics. 10:484–495. 2014.
View Article : Google Scholar
|
|
33
|
Chen CH, Chiu YL, Wei FC, Koong FJ, Liu
HC, Shaw CK, Hwu HG and Hsiao KJ: High seroprevalence of Borna
virus infection in schizophrenic patients, family members and
mental health workers in Taiwan. Mol Psychiatry. 4:33–38. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gosztonyi G and Ludwig H: Borna
disease-neuropathology and pathogenesis. Curr Top Microbiol
Immunol. 190:39–73. 1995.PubMed/NCBI
|
|
35
|
Kamitani W, Ono E, Yoshino S, Kobayashi T,
Taharaguchi S, Lee BJ, Yamashita M, Kobayashi T, Okamoto M,
Taniyama H, et al: Glial expression of Borna disease virus
phosphoprotein induces behavioral and neurological abnormalities in
transgenic mice. Proc Natl Acad Sci USA. 100:pp. 8969–8974. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iizasa T, Chang H, Suzuki M, Otsuji M,
Yokoi S, Chiyo M, Motohashi S, Yasufuku K, Sekine Y, Iyoda A, et
al: Overexpression of collagen XVIII is associated with poor
outcome and elevated levels of circulating serum endostatin in
non-small cell lung cancer. Clin Cancer Res. 10:5361–5366. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Billich C, Sauder C, Frank R, Herzog S,
Bechter K, Takahashi K, Peters H, Staeheli P and Schwemmle M:
High-avidity human serum antibodies recognizing linear epitopes of
Borna disease virus proteins. Biol Psychiatry. 51:979–987. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsunaga H, Tanaka S, Sasao F, Nishino Y,
Takeda M, Tomonaga K, Ikuta K and Amino N: Detection by radioligand
assay of antibodies against Borna disease virus in patients with
various psychiatric disorders. Clin Diagn Lab Immunol. 12:671–676.
2005.PubMed/NCBI
|
|
39
|
Bode L: Human bornavirus infection-towards
a valid diagnostic system. APMIS Suppl. 1–39. 2008.
|
|
40
|
De La Torre JC, Gonzalez-Dunia D, Cubitt
B, Mallory M, Mueller-Lantzsch N, Grässer FA, Hansen LA and Masliah
E: Detection of borna disease virus antigen and RNA in human
autopsy brain samples from neuropsychiatric patients. Virology.
223:272–282. 1996. View Article : Google Scholar : PubMed/NCBI
|