Introduction
Breast cancer is ranked the first cause of
cancer-related deaths in women worldwide (1). As in most other countries, breast
cancer is now the most common cancer in Chinese women (2,3).
Estrogen receptor (ER)-positive breast cancer accounts for 70–80%
of all breast cancers, for which endocrine therapy is the most
effective treatment (4,5). Fulvestrant is a selective estrogen
receptor downregulator, which has been approved by FDA for the
treatment of advanced ER-positive breast cancer (6,7).
However, fulvestrant resistance is unavoidable during the treatment
period.
Bisphosphonates (BPs) have been widely and
successfully used for the treatment of bone metastases in breast
cancer patients (8). Zoledronic
acid (ZOL) is a third-generation BP, which has the most potent
inhibitory effect on osteoclast-mediated bone resorption among
currently available BPs (9,10).
In addition to its potent anti-osteoclast effects, preclinical
studies have reported that ZOL induces apoptosis in breast cancer
cells (11,12). It has also been demonstrated that
ZOL inhibits cancer cell invasion (13,14)
and angiogenesis (15,16). However, the effects of ZOL on
endocrine resistance of breast cancer have not been extensively
investigated.
Previous studies have shown that hypoxia may lead to
endocrine resistance in ER-positive breast cancer patients
(17). Hypoxia inducible factor
(HIF)-1α expression is significantly increased in residual tumors
following endocrine therapy (18).
The MCF-7 breast cancer cell line stably expressing HIF-1α has been
reported to be insensitive to fulvestrant in vivo (18). ZOL inhibits HIF-1α expression in
the neoadjuvant endocrine therapy set (18). In the present study, the effect of
ZOL on fulvestrant response and the underlying mechanisms were
investigated in a mouse model and in vitro.
Materials and methods
Chemicals and antibodies
Fulvestrant and ZOL were kindly provided by
AstraZeneca PLC (Cambridge UK) and Novartis Pharma AG (Basel
Switzerland), respectively. Cobalt chloride (CoCl2) and
epidermal growth factor (EGF) were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Mouse monoclonal antibody against
HIF-1α was purchased from BD Biosciences (cat no. 610958; 1:1,000;
Franklin Lakes, NJ, USA). Rabbit polyclonal antibodies against
phosphoinositide 3-kinase (PI3K; cat no. 4255; 1:1,000), AKT
serine/threonine kinase 1 (AKT; cat no. 9272; 1:1,000),
phosphorylated (p-) AKT (cat no. 4060; 1:1,000), extracellular
signal-regulated kinase (ERK) 1/2 (cat no. 4695; 1:2,000), p-ERK1/2
(cat no. 4376; 1:2,000) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies against β-actin
(cat no. 60008-1-Ig; 1:2,000) and GAPDH (cat no. 60004-1-Ig;
1:2,000) were from ProteinTech Group, Inc. (Chicago, IL, USA).
Generation of HIF-1α stably expressing
cells
Generation of the HIF-1α stably expressing cell
lines has been reported previously (18). Full-length cDNA of HIF-1α was
amplified by polymerase chain reaction (PCR). The lentiviral
expression and control vectors were packed into HEK 293T cells to
generate the corresponding lentiviruses. Transfections were
performed using olyethylenimine. MCF-7 cells infected with HIF-1α
or vector control lentiviruses (designated MCF-7/HIF-1α or
MCF-7/vector, respectively) were selected and maintained in the
same medium containing 2 µg/ml puromycin (Sigma-Aldrich; Merck
KGaA). Non-infected cells were completely eradicated by puromycin
selection for 72 h. The surviving lentivirus-infected cells were
confirmed to successfully express HIF-1α by western blot
analysis.
Cell culture and treatments
All cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640
(Shanghai Basalmedia Technologies Co., Ltd., Shanghai, China)
supplemented with 10% fetal bovine serum (Biological Industries,
Cromwell, CT, USA) and 5% penicillin/streptomycin at 37°C under 5%
CO2. Cells were treated with drugs at the indicated
final concentrations. To establish hypoxic conditions, cells were
treated with 200 µmol cobalt chloride (CoCl2) for 24 h,
with or without the addition of 100 µmol ZOL for 18 h after 6 h of
CoCl2 treatment.
Animal model
Xenograft tumors in mice were generated with MCF-7
cells, as previously reported (18). Briefly, a 0.72 mg 90-day-release
17β-estradiol pellet (Innovative Research of America, Sarasota, FL,
USA) was implanted subcutaneously one week prior to injection.
MCF-7/vector and MCF-7/HIF-1α cells (1×107) were
resuspended in PBS, mixed with Matrigel (1:1; BD Biosciences) and
injected subcutaneously into the right flank of each mouse in a
final volume of 200 µl. Treatment began when tumors reached an
average size of 150–200 mm3. The animals were randomly
allocated to four groups: Control (PBS; 0.1 ml administered
subcutaneously once per week), fulvestrant (5 mg/kg administered
subcutaneously once per week), ZOL (120 µg/kg administered
subcutaneously twice per week) or fulvestrant plus ZOL). Tumor
xenografts were measured with calipers twice a week, and tumor
volume was determined using the formula: V=length2×width2. Tumors were harvested
following 4 weeks of treatment. Half of each tumor was flash-frozen
in liquid nitrogen, and the other half was fixed in 10% formalin
for 24 h prior to paraffin-embedding.
All of the animal experiments were approved by the
Ethical Committee of Fudan University Shanghai Cancer Hospital
(Shanghai, China).
Animal
[18F]-fluoromisonidazole (FMISO) static positron
emission tomography-computer tomography (PET/CT) scan
[18F]-FMISO PET/CT scans were acquired
before the mice were euthanized. [18F]-FMISO is the most
widely used nitroimidazole derivative in clinical PET/CT. Because
[18F]-FMISO has affinity only for hypoxic cells with
functionally active nitroreductase enzymes, [18F]-FMISO
accumulates in activated hypoxic cells but not in necrotic
cells.
All mice were injected intravenously with 500 µci of
[18F]-FMISO. At 4 h following injection, static emission
scans were obtained. The data acquisition time was 5 min per table
position. [18F]-FMISO PET/CT images at 4 h were noted as
SUV4 hT. In addition, six 0.5×0.5× 0.5 cm small spheres
(background) were located at the triceps brachii muscles, the
scapula muscles, and the latissimus dorsi muscles both in the
homonymy and in the opposite side. The mean value of the six
background volume of interest (VOI) peaks was noted as SUV4 hB. The
tumor-to-background ratio (TBR) was calculated as follows:
TBR4h=SUV4hTSUV4hB.
Immunohistochemical (IHC)
staining
The paraffin-embedded mouse tumor tissue sections (5
µm) were dehydrated and subjected to peroxidase blocking with 5%
goat serum for 1 h at room temperature. HIF-1α primary antibody
(1:100) was added and incubated at 4°C overnight. Immunoreactivity
was detected by using the EnVision+System (DAKO; Agilent
Technologies, Inc., Santa Clara, CA, USA) with diaminobenzidine
chromogen, according to the manufacturer's protocol. The stained
slides were observed with microscopy, and images were acquired with
Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose, CA, USA).
HIF-1α levels were assessed within the entire tumor section with a
semi-quantitative scale that combined proportional expression (0,
no expression; 1, <10%; 2, 10–50%; 3, 50–80%; or 4, >80% of
cells with positive nuclear staining) and staining intensity (0,
none; 1, weak; 2, intermediate; or 3, strong) to obtain a total IHC
score ranging from 0 to 7.
Cell proliferation and cell clonogenic
assays
Cell proliferation assays and cell clonogenic assays
were performed as previously described (18). For cell proliferation assays, cells
were seeded in 96-well plates (3,000 cells/well) in triplicate and
cultured overnight. Then cells were treated with PBS, ZOL,
fuvestrant, or ZOL plus fuvestrant for 48 h, followed by Cell
Counting Kit-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's instructions. For
clonogenic assays, cells were seeded in 6-well plates (300
cells/well) in triplicate and cultured overnight. Then the cells
were treated with PBS, ZOL, fuvestrant, or ZOL plus fuvestrant for
14 days. Representative results of three independent experiments
with similar trends are presented.
Western blotting
Cells were washed twice with cold PBS and
centrifuged at 500 × g for 3 min. The cell pellet was suspended in
80 µl lysis buffer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The suspension was incubated on ice for 40 min and
centrifuged for 10 min at 16, 000 × g. Protein concentration was
determined with a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China). Cell lysates (50 µg)
were resolved by 10% SDS-PAGE, and electrophoretically transferred
to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA),
Following blocking with 5% BSA for 1 h at room temperature,
membranes were hybridized overnight at 4°C with primary antibodies
specific for the detection of each protein and GAPDH (used as a
loading control). Horseradish peroxidase-conjugated secondary
antibodies (cat nos. 715-035-150 and 415-035-166; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) were used
at 1:5,000 dilution in TBS-Tween 20 solution for 1 h at room
temperature. Protein-antibody complexes were detected by
chemiluminescence with the Super Signal West Dura Extended Duration
Substrate (EMD Millipore), and images were captured with an
ImageQuant LAS 4000 biomolecular imager (GE Healthcare Life
Sciences, Little Chalfont, UK). The experiments were repeated at
least three times.
Statistical analysis
Each experiment was repeated independently three
times. Statistical significance of differences between two groups
were analyzed with Student's t-test, and among multiple groups with
one-way analysis of variance followed by the Student-Newman-Keuls
test for post-hoc analysis. All analyses were performed with SPSS
18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
ZOL exerts antitumor activity on
HIF-1α-overexpressing breast cancer cells and synergizes with
fulvestrant in vitro
Previous studies have reported that HIF-1α is
overexpressed following neoadjuvant endocrine therapy and breast
cancer cells overexpressing HIF-1α are resistant to fulvestrant,
which suggests that HIF-1α may confer endocrine resistance
(18). To identify the effect of
ZOL in fulvestrant treatment, HIF-1α-overexpressing MCF-7 breast
cancer cells were used (termed MCF-7/HIF-1α; Fig. 1A). It was observed that HIF-1α
expression was decreased following ZOL treatment, and this decrease
was more evident in MCF-7/HIF-1α cells under hypoxic conditions
(Fig. 1B). MCF-7/HIF-1α cells were
then treated with fulvestrant alone, ZOL alone, or fulvestrant plus
ZOL, and their effects on cell growth was determined in
vitro. Either treatment alone did not show an inhibitory effect
on the growth of MCF-7/HIF-1α cells, suggesting that HIF-1α
overexpression renders these cells resistant to both treatments
(Fig. 1C and D). The combination
treatment of fulvestrant and ZOL, however, exerted a synergistic
effect on MCF-7/HIF-1α cells to strongly inhibit cell proliferation
and growth (P<0.001; Fig.
1D).
 | Figure 1.ZOL exerts antitumor activity in
HIF-1α-overexpressing breast cancer cells and synergizes with
fulvestrant in vitro. (A) Western blot analysis of the
control MCF-7/vector and MCF-7/HIF-1α cells, demonstrating that
HIF-1α overexpression was successfully established. (B)
MCF-7/HIF-1α and MCF-7/vector cells were pre-treated with 200 µmol
CoCl2 for 6 h followed by treated with CoCl2
and 100 µmol ZOL for 18 h and western blot analysis was used to
detect HIF-1α expression. (C) Growth of MCF-7/HIF-1α and
MCF-7/vector cells treated with 100 µmol ZOL and/or 0.1 nmol/l
fulvestrant for two weeks, as determined by colony formation assay.
Control is untreated cells. (D) Viability of MCF-7/HIF-1α and
MCF-7/vector cells treated with 100 µmol ZOL and/or 0.1 nmol/l
fulvestrant for 0, 12, 24, 48 h, as determined by Cell Counting
Kit-8 assay. *P<0.05 and ***P<0.001 vs. untreated control.
ZOL, zoledronic acid; HIF, hypoxia-inducible factor; FUL,
fulvestrant; OD, optical density. |
Combination treatment with fulvestrant
and ZOL reduces the growth of HIF-1α-overexpressing breast cancer
cells in vivo
To further investigate the effect of ZOL and
fulvestrant combination treatment on breast cancer cell growth, an
ER-positive breast cancer mouse model was established by using
MCF-7-derived xenograft tumors. The ZOL dose (120 µg/kg) used in
the present study is equivalent to the intravenous clinical dose of
4 mg every 3 to 4 weeks. HIF-1α-overexpressing tumors grew faster
and larger compared with control MCF-7 tumors (P<0.001; Fig. 2A). The [18F]-FMISO
uptake (TBR4 h) was significantly higher in HIF-1α-overexpressing
xenograft tumors compared with control tumors (P<0.001; Fig. 2B). Treatment with ZOL alone exerted
no significant effect on the growth of HIF-1α-overexpressing
tumors, although it significantly decreased the size of
MCF-7/vector tumors (P<0.001; Fig.
2C). However, the combination treatment of fulvestrant and ZOL
significantly reduced the tumor volumes of both the control
MCF-7/vector and the HIF-1α-overexpressing MCF-7/HIF-1α xenograft
tumors, compared with either single drug treatment (P<0.001;
Fig. 2D). Of note, the drug
treatments did not exert any side effects on animal body weight of
either the MCF-7/vector and MCF-7/HIF-1α xenograft-bearing mice
(Fig. 2E).
 | Figure 2.Effect of combination treatment of
fulvestrant and ZOL on the growth of HIF-1α-overexpressing breast
cancer cells in vivo. Mice bearing MCF-7/vector or
MCF-7/HIF-1α xenograft tumors were treated with vehicle (control),
fulvestrant (5 mg/kg, once per week), ZOL (120 µg/kg, twice a
week), or fulvestrant plus ZOL. (A) MCF-7/HIF-1α cells grew faster
and larger xenograft tumors compared with MCF-7/vector cells. (B)
Analysis of xenografts tumors by
[18F]-fluoromisonidazole static positron emission
tomography-computer tomography. (C) The drug-sensitive MCF-7/vector
xenograft tumor volumes were significantly reduced following ZOL
treatment, while the growth of drug-resistant MCF-7/HIF-1α tumors
were not affected by ZOL treatment. (D) Combination of fulvestrant
and ZOL significantly reduced the growth of MCF-7/vector and
MCF-7/HIF-1α xenograft tumors. (E) Body weight of the animals in
the experimental groups. **P<0.01 and ***P<0.001 vs.
untreated control. ZOL, zoledronic acid; HIF, hypoxia-inducible
factor; FUL, fulvestrant; TBR, tumor-to-background ratio. |
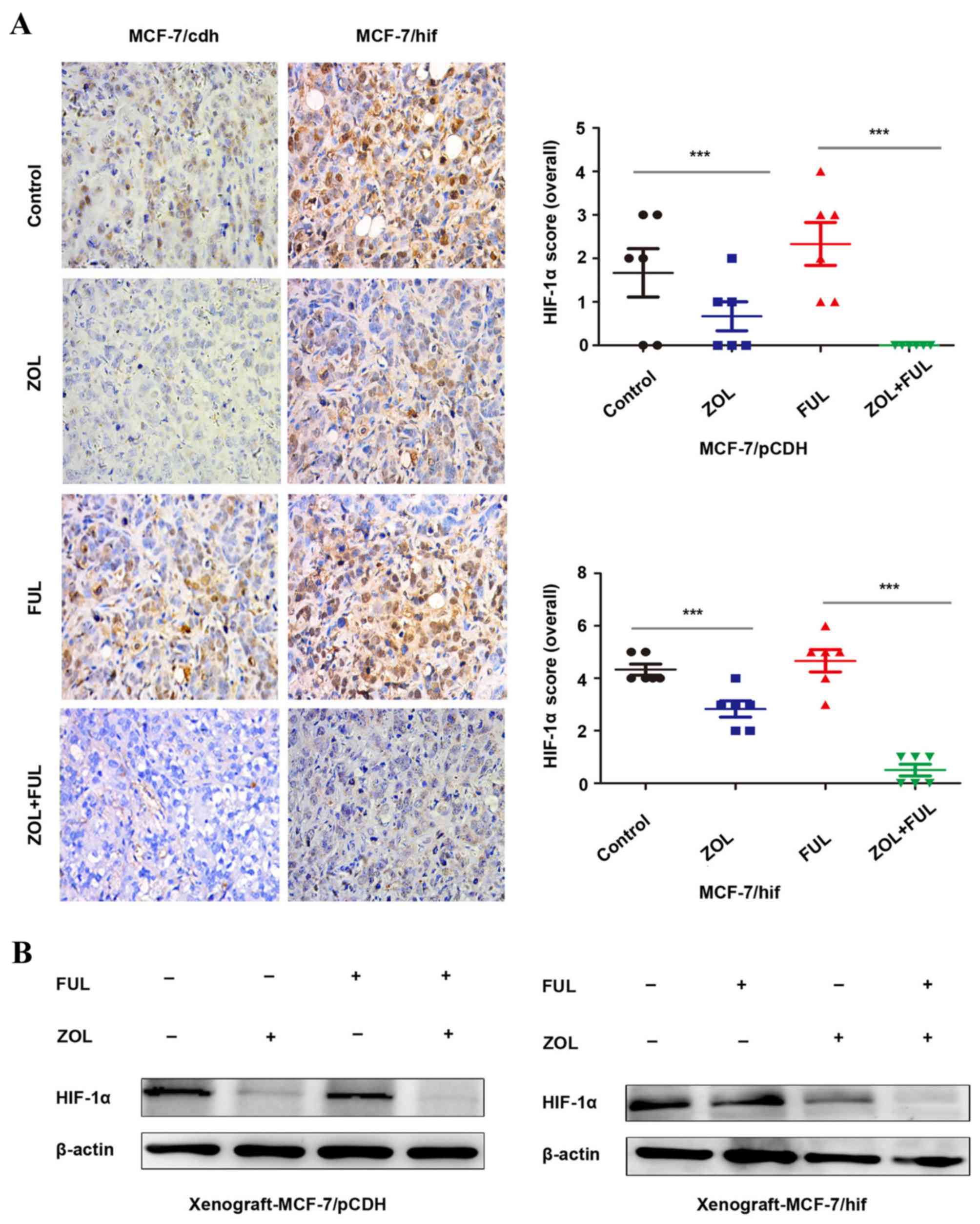
ZOL inhibits HIF-1α expression in
vitro and in vivo
A previous study has reported that ZOL inhibited
HIF-1α expression in vitro and in vivo (18). In the present study, HIF-1α
expression was demonstrated to be significantly decreased following
ZOL treatment in vitro (Fig.
1B). In vivo, compared to untreated mice, IHC staining
revealed that HIF-1α expression was downregulated following ZOL
treatment in both the MCF-7/vector and MCF-7/HIF-1α xenograft
tumors (Fig. 3A). No change was
observed in either xenograft model following fulvestrant treatment
(Fig. 3A). Combination of ZOL with
fulvestrant had a synergistic effect in significantly further
decreasing HIF-1α expression in both the MCF-7/vector and
MCF-7/HIF-1α xenograft tumors (Fig.
3A). Western blotting analysis of the xenograft tumor tissues
from the four experimental groups confirmed the IHC results for
HIF-1α protein expression (Fig.
3B).
ZOL inhibits HIF-1α expression by
blocking the ERK pathway
The present study demonstrated that HIF-1α
expression was inhibited by ZOL treatment in vitro (Fig. 4A) and in vivo (Fig. 3). However, the mechanisms remain
unclear. PI3K/AKT and ERK1/2 signaling pathways are the main
pathways involved in HIF-1α activation (19). Therefore, the protein expression
levels of key proteins associated with the PI3K/AKT and ERK1/2
pathways were examined by western blotting in two ER-positive
breast cancer cell lines, MCF-7 and T47D, treated with increasing
concentrations of ZOL. The results demonstrated that ZOL treatment
significantly reduced p-ERK1/2 levels in both MCF-7 and T47D cells,
compared with untreated control, but had no obvious effects on
p-AKT and PI3K levels (Fig. 4B).
When treated with EGF, a ligand binding to EGF receptor and
activating the ERK pathway, the inhibition of HIF-1α by ZOL was
significantly reversed (Fig. 4C).
These results indicate that ZOL inhibited HIF-1α expression, at
least in part, through the ERK1/2 signaling pathway (Fig. 5).
Discussion
ER-positive breast cancer accounts for 70–80% of all
breast cancers, for which endocrine therapy is the standard
treatment. However, 30–40% of patients relapse following endocrine
therapy, which indicates drug resistance (4). In previous studies from our group,
[18F]- FMISO PET/CT, a useful tool to detect hypoxia,
was demonstrated to predict primary endocrine therapy resistance in
breast cancer (17). HIF-1α is an
effective factor that adapts to hypoxia, and is associated with
tumor initiation, progression and resistance to radiotherapy and
chemotherapy (20–22). Mitochondrial metabolism
dysregulation and tumor growth factor-β/SMAD signaling promote
breast cancer metastasis (23,24),
which may be associated with hypoxia to promote tumor progression
and drug resistance.
In a previous study from our group, we reported that
high HIF-1α expression predicted resistance to endocrine therapy
(18). ZOL, a standard drug for
patients with bone metastasis and osteoporosis, increased the
sensitivity to antiestrogen treatment through HIF-1α inhibition in
ER-positive breast cancer (18).
In the present study, we demonstrated that combination treatment of
fulvestrant and ZOL significantly inhibited the growth of
HIF-1α-overexpressing MCF-7 cells in vitro and in a
xenograft model, while single fulvestrant treatment did not inhibit
the growth of HIF-1α-overexpressing MCF-7 cells. These results
indicated that HIF-α may reduce the sensitivity of breast cancer
cells to fulvestrant, but ZOL treatment restored the sensitivity to
fulvestrant in vitro and in vivo.
ZOL is a nitrogen-containing bisphosphonate, which
attaches to the mineralized bone matrix, inhibits bone resorption
and prevents the occurrence of skeletal-related events (9). Increasing evidence has indicated that
ZOL exerts antitumor activity in vitro and in vivo
(25–27). Various in vivo studies have
investigated the therapeutic value of ZOL alone or in combination
with conventional chemotherapy and mechanistic target of rapamycin
(mTOR) inhibitors on the growth of tumors (28–30).
In the present study, the mechanism by which ZOL restored
sensitivity to fulvestrant was examined. ZOL significantly
inhibited ERK1/2 phosphorylation in breast cancer cells, while the
PI3K/AKT signaling pathway was not affected. Addition of EGF, an
ERK activator, reversed the inhibition of ERK1/2 activation and
HIF-1α expression following ZOL treatment. These results
demonstrated that ZOL inhibited HIF-1α expression by blocking the
ERK pathway.
In conclusion, the present study suggests that
inhibition of ERK/HIF-1α by ZOL may increase the sensitivity of
ER-positive breast cancer cells to fulvestrant. The combination of
ZOL and fulvestrant may serve as a new therapeutic scheme for
patients with recurrent ER-positive breast cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. NSFC81301246).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia X, Liu G, Cheng J, Shen Z and Shao Z:
CYR61 contributes to poor response to letrozole in ER positive
breast carcinoma. Curr Cancer Drug Targets. 2016.
|
|
5
|
Masood S: Estrogen and progesterone
receptors in cytology: A comprehensive review. Diagn Cytopathol.
8:475–491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bross PF, Baird A, Chen G, Jee JM,
Lostritto RT, Morse DE, Rosario LA, Williams GM, Yang P, Rahman A,
et al: Fulvestrant in postmenopausal women with advanced breast
cancer. Clin Cancer Res. 9:4309–4317. 2003.PubMed/NCBI
|
|
7
|
Ciruelos E, Pascual T, Arroyo Vozmediano
ML, Blanco M, Manso L, Parrilla L, Muñoz C, Vega E, Calderón MJ,
Sancho B and Cortes-Funes H: The therapeutic role of fulvestrant in
the management of patients with hormone receptor-positive breast
cancer. Breast. 23:201–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polascik TJ and Mouraviev V: Zoledronic
acid in the management of metastatic bone disease. Ther Clin Risk
Manag. 4:261–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russell RG: Bisphosphonates: Mode of
action and pharmacology. Pediatrics. 119 Suppl 2:S150–S162. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fromigue O, Lagneaux L and Body JJ:
Bisphosphonates induce breast cancer cell death in vitro. J Bone
Miner Res. 15:2211–2221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jagdev SP, Coleman RE, Shipman CM, Rostami
HA and Croucher PI: The bisphosphonate, zoledronic acid, induces
apoptosis of breast cancer cells: Evidence for synergy with
paclitaxel. Br J Cancer. 84:1126–1134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Virtanen SS, Väänänen HK, Härkönen PL and
Lakkakorpi PT: Alendronate inhibits invasion of PC-3 prostate
cancer cells by affecting the mevalonate pathway. Cancer Res.
62:2708–2714. 2002.PubMed/NCBI
|
|
14
|
Boissier S, Ferreras M, Peyruchaud O,
Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM and
Clézardin P: Bisphosphonates inhibit breast and prostate carcinoma
cell invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|
|
15
|
Fournier P, Boissier S, Filleur S,
Guglielmi J, Cabon F, Colombel M and Clézardin P: Bisphosphonates
inhibit angiogenesis in vitro and testosterone-stimulated vascular
regrowth in the ventral prostate in castrated rats. Cancer Res.
62:6538–6544. 2002.PubMed/NCBI
|
|
16
|
Wood J, Bonjean K, Ruetz S, Bellahcène A,
Devy L, Foidart JM, Castronovo V and Green JR: Novel antiangiogenic
effects of the bisphosphonate compound zoledronic acid. J Pharmacol
Exp Ther. 302:1055–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng J, Lei L, Xu J, Sun Y, Zhang Y, Wang
X, Pan L, Shao Z, Zhang Y and Liu G: 18F-fluoromisonidazole PET/CT:
A potential tool for predicting primary endocrine therapy
resistance in breast cancer. J Nucl Med. 54:333–340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia X, Hong Q, Lei L, Li D, Li J, Mo M,
Wang Y, Shao Z, Shen Z, Cheng J and Liu G: Basal and therapy-driven
hypoxia-inducible factor-1alpha confers resistance to endocrine
therapy in estrogen receptor-positive breast cancer. Oncotarget.
6:8648–8662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aebersold DM, Burri P, Beer KT, Laissue J,
Djonov V, Greiner RH and Semenza GL: Expression of
hypoxia-inducible factor-1alpha: A novel predictive and prognostic
parameter in the radiotherapy of oropharyngeal cancer. Cancer Res.
61:2911–2916. 2001.PubMed/NCBI
|
|
21
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
22
|
Bachtiary B, Schindl M, Pötter R, Dreier
B, Knocke TH, Hainfellner JA, Horvat R and Birner P: Overexpression
of hypoxia-inducible factor 1alpha indicates diminished response to
radiotherapy and unfavorable prognosis in patients receiving
radical radiotherapy for cervical cancer. Clin Cancer Res.
9:2234–2240. 2003.PubMed/NCBI
|
|
23
|
Jiang HL, Sun HF, Gao SP, Li LD, Huang S,
Hu X, Liu S, Wu J, Shao ZM and Jin W: SSBP1 suppresses TGFβ-driven
epithelial-to-mesenchymal transition and metastasis in
triple-negative breast cancer by regulating mitochondrial
retrograde signaling. Cancer Res. 76:952–964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang HL, Sun HF, Gao SP, Li LD, Hu X, Wu
J and Jin W: Loss of RAB1B promotes triple-negative breast cancer
metastasis by activating TGF-β/SMAD signaling. Oncotarget.
6:16352–16365. 2015.PubMed/NCBI
|
|
25
|
Senaratne SG, Pirianov G, Mansi JL, Arnett
TR and Colston KW: Bisphosphonates induce apoptosis in human breast
cancer cell lines. Br J Cancer. 82:1459–1468. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gnant M and Clézardin P: Direct and
indirect anticancer activity of bisphosphonates: A brief review of
published literature. Cancer Treat Rev. 38:407–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clézardin P, Fournier P, Boissier S and
Peyruchaud O: In vitro and in vivo antitumor effects of
bisphosphonates. Curr Med Chem. 10:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ottewell PD, Mönkkönen H, Jones M, Lefley
DV, Coleman RE and Holen I: Antitumor effects of doxorubicin
followed by zoledronic acid in a mouse model of breast cancer. J
Natl Cancer Inst. 100:1167–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heymann D, Ory B, Blanchard F, Heymann MF,
Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F and Redini
F: Enhanced tumor regression and tissue repair when zoledronic acid
is combined with ifosfamide in rat osteosarcoma. Bone. 37:74–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moriceau G, Ory B, Mitrofan L, Riganti C,
Blanchard F, Brion R, Charrier C, Battaglia S, Pilet P, Denis MG,
et al: Zoledronic acid potentiates mTOR inhibition and abolishes
the resistance of osteosarcoma cells to RAD001 (Everolimus):
Pivotal role of the prenylation process. Cancer Res.
70:10329–10339. 2010. View Article : Google Scholar : PubMed/NCBI
|