Introduction
Post-traumatic stress disorder (PTSD) is an abnormal
mental reaction to severe stress factors, such as major disasters,
war, terrorist attacks, traffic accidents and abuse, and has a high
incidence overall (1,2). Clinical and community-based studies
of PTSD among the elderly have identified elevated rates of
anxiety, mood variation and substance use disorders which typically
develop after PTSD (3–5). Such cognitive or behavioral changes
are frequently ignored or underestimated during initial
hospitalization, when severe psychological reactions or obvious
physical injuries are presented (6). Recent studies have demonstrated that
PTSD is dependent on environmental factors, which stimulate changes
in the expression of biological susceptibility genes and associated
proteins; however, but the specific mechanism remains to be
elucidated (7,8). Current PTSD treatment includes
antidepressant medication and psychotherapy, but a significant
number of patients remain refractory to the treatment mostly due to
substance use disorders, general medical illnesses and suicides
(9–11).
Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme
in prostaglandin E2 (PGE2) synthesis. The expression of COX-2, an
important mediator of cell injury in inflammation, is induced by
cytokines and inhibited by glucocorticoids (12). It was reported that the expression
of COX-2 could also be induced by neuronal excitation and increased
intracellular calcium. COX-2 also serves an important role in the
pathophysiology of neuronal death in ischemia and a variety of
neurodegenerative diseases (13,14).
The expression of the immediate early gene COX-2 is induced by
vulnerable CA1 neurons. Several studies demonstrated that treatment
with a COX-2selective inhibitor can decrease the damage following
temporary focal ischemia (15,16).
Therefore, inhibition of COX-2 expression might reduce the
oxidative stress-induced damage to nerve cells.
Although it has been known for decades that
cyclooxygenase inhibitors ameliorate brain injury (12), whether inhibition of COX-2 can have
a positive effect on the symptoms of PTSD remain to be elucidated.
In the present study, changes in COX-2 expression in rats with PTSD
treated with COX-2 inhibitor were detected. Celecoxib, a first
selective COX-2 inhibitor, is widely used in clinical practice and
research (17–19). The apoptosis of rat hippocampi and
levels of inflammatory factors, such as tumor necrosis factor α
(TNF-α), interleukin (IL)-6, prostaglandin E2 (PGE2) and nitric
oxide (NO), were used to evaluate the effect of COX-2 inhibition on
PTSD. The objective of the present study was to reveal the
mechanism of COX-2 in the pathogenesis of PTSD, and the therapeutic
potential of COX-2 inhibition was investigated.
Materials and methods
Experimental animals
A total of 60 healthy, male Wistar rats (2–3 months,
150–200 g) were obtained from experimental animal center (Tongji
Medical College, Huazhong University of Science and Technology) and
acclimatized in the laboratory for 2 weeks prior to the
experimental manipulation. Rats were reared in a cage kept at
25±3°C in a normal atmosphere and a 12 h light/dark cycle. Rats had
free access to dry pellets and water with intermittent feeding of
green fodder. Rats were randomly divided into three groups:
Control, (n=20), PTSD (n=20) and intervention (PTSD+COX-2 inhibitor
treatment, n=20). A PTSD model was established by single prolonged
stress (SPS) as previously described by Liberzon et al
(20,21). SPS was induced in three stages:
Rats were restrained for 24 h, forced to swim for 20 min and
administered ether anesthesia. All protocols involving animals were
approved by the Ethics Committee for Experimental Animals (Wuhan
No. 1 Hospital, Wuhan, China).
Rats in the control and PTSD groups were
administered 0.9% normal saline and in the intervention group rats
were treated with 25 mg/(kgd) COX-2 inhibitor celecoxib (Pfizer,
Inc., New York, NY, USA) through a nasogastric gavage. Samples were
collected after treatment for 2 weeks.
A pre-experiment was performed to select the optimum
celecoxib concentration for treatment. A total of 9 rats from the
PTSD group were divided into three groups and received celecoxib in
dose of 15, 25 or 35 mg/(kgd) respectively via intraperitoneal
route for one week, as previously described (22). COX-2 mRNA expression levels in all
groups were detected by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR).
Behavioral detection
A total of four incandescent lamps (60 W) were
placed in each of the four corners and served as an indoor light
source for a behavioral laboratory, at a constant illumination of
150–300 Lux. In order to ensure clear observation of rat activity,
the lights did not directly irradiate the detection equipment. The
outdoor light was blocked by shading curtains. The open-field (OF),
elevated plus maze (EPM) and Morris water maze (MWM) tests used in
the present study are widely used procedures for examining the
behavioral effects of anxiety (23,24).
Horizontal movement distance and residence time are important
indices of the open field test, while the open arm entry times and
open arm residence times are indices for the EPM test. The results
of the Morris water maze test were evaluated based on the escape
latency. The open field test was performed as previously described
by Choleris et al (25),
the EPMtest was performed as previously described by Walf and Frye
(26) and the Morris water maze
test was performed as previously described by Morris (27). The primary method for data
collection was a video-tracking system, which automatically
detected and recorded the horizontal movement distance and
residence time.
Immunohistochemical staining
Glass slides were pre-heated in an oven at 65°C for
1 h. Paraffin-embedded sections were dewaxed, rehydrated and
treated with 3% hydrogen peroxide for quenching of endogenous
peroxidase activity at room temperature. Sections were incubated
with a rabbit anti-rat polyclonal COX-2 antibody (1:1,000; cat. no.
BA0738, Boster Biological Technology, Pleasanton, CA, USA)
overnight at 4°C. The spatial localization of COX-2 was visualized
by incubation with mouse IgG horseradish peroxidase
(HRP)-conjugated secondary antibody (1:10,000; cat. no. PV-9000;
ZSGB-Bio; OriGene Technologies, Inc., Beijing, China) for 1 h at
room temperature. A 3,3′-diaminobenzidine tetrahydrochloride
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) chromogenic reagent
was added dropwise until the nucleus had stained brown. After
wards, the sections were rinsed with PBS, counterstained with
hematoxylin for 3 min at room temperature, dehydrated with graded
ethanol (75, 85, 95% and anhydrous ethanol) and xylene and mounted
with Entellan (cat. no. 1.07960.0500; Merck KGaA). Sections were
observed under a light microscope, six visual fields were selected
and the mean number of positive cells was counted.
RNA extraction and RT-qPCR
Total RNA from rat hippocampi was extracted using
TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian, China) and
detected with an ultraviolet spectrophotometer and agarose-gel
electrophoresis. For each sample, 1 µg RNA was reverse-transcribed
to obtain first-strand cDNA using the PrimeScript® RT
Reagent kit with a gDNA Eraser (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. Expression levels of
COX-2were analyzed using RT-qPCR. The primers were designed and
synthesized by TsingKe Biological Company (Wuhan, China). The
primer specific to COX-2 was: Forward, 5′-TCGCTGTGCCTGATGATTG-3′
and reverse, 5′-TCGCTTATGATCTGTCTTG-3′; and a primer specific to
the internal control β-actin was: Forward,
5′-TGACGTGGACATCCGCAAAG-3′ and reverse, 5′-CTGGAAGGTGGACAGCGAGG-3′.
Each reaction mixture (20 µl total volume) contained 10 µl of 2×
SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.), 0.4 µmol/l
each forward and reverse primer and 0.2±0.02 µg cDNA template. The
thermocycling conditions were: 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec, 58°C for 20 sec and 72°C for 20 sec. The
relative transcription levels of genes were calculated using the
2−∆∆Cq method (28).
The quantitation cycle (Cq) was determined for each reaction, and
the Cq values for each gene of interest were normalized to the
endogenous control gene β-actin. The quantification of target and
reference genes was evaluated using standard curves and the ratio
between the target and reference gene represented the relative
expression levels of target gene. For each group three technical
replicates of each measurement were obtained.
Western blot analysis
Western blot analysis was performed to determine the
expression of COX-2. Rat hippocampi were homogenized and the total
protein was extracted. The protein concentration was determined
using a Bicinchoninic Acid kit (Bio-Swamp, Wuhan, China). Equal
amounts of protein (30 µg) were separated by 10% SDS-PAGE and then
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked for 2 h at room
temperature with 5% skimmed milk in Tris-buffered saline (20 mmol/l
Tris, 500 mmol/l NaCl and 0.05% Tween 20). Subsequently, the
membrane was incubated with anti-COX-2 antibody (1:100; cat. no.
ab52237; Abcam, Cambridge, UK) overnight at 4°C. An anti-β-actin
antibody (1:10,000, cat. no. ab227387, Abcam) was selected as an
internal reference. The membranes were washed with Tris-buffered
saline and incubated with a rabbit anti-goat secondary antibody
(1:10,000, cat. no. E030130, EarthOx, LLC, Millbrae, CA, USA) for 2
h at room temperature. Immunoreactivity was visualized by
colorimetric reaction using enhanced chemiluminescence substrate
buffer (EMD Millipore, Billerica, MA, USA). Membranes were scanned
with a Gel DocEZ imager (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and bands were quantified using Quantity One software version
5.0 (Bio-Rad Laboratories, Inc.).
Terminal deoxynucleotidyl transferase
mediated dUTP nick end labeling (TUNEL) staining
Paraffin-embedded sections of rat hippocampi were
obtained. TUNEL assay was performed using a TUNEL kit (Boster
Biological Technology, Pleasanton, CA, USA) according to the
manufacturer's protocol. The sections were sealed with natural gum.
Apoptotic cells were observed under a light microscope
(magnification, ×400) and counted in 6 fields of vision. The
apoptosis index was expressed as the number of apoptotic cells
within 1 mm2, and the apoptosis of rat hippocampal
neurons was defined as the number of apoptotic neurons/total number
of neurons ×100%.
ELISA
The levels of IL-6, TNFα and PGE2 in rat hippocampi
were evaluated by an ELISA assay. The supernatant of 10% rat
hippocampal homogenate was extracted. IL-6 (cat. no. RA20607),
TNF-α (cat. no. RA20035) and PGE2ELISA kits (cat. no. RA20013) were
obtained from Bio-Swamp (Wuhan, China) and the assay was carried
out according to the manufacturer's protocol.
NO detection
The NO content in rat hippocampi was detected using
the Griess test (29). The Nitric
Oxide Assay kit (cat. no. S0021, Beyotime Institute of
Biotechnology, Shanghai, China) was used according to the
manufacturer's protocol. The hippocampal tissue was treated with
S3090 lysate (Beyotime Institute of Biotechnology) prior to
detection.
Statistics analysis
All data are expressed as the mean ± standard
deviation. Statistical differences were analyzed by one-way using
SPSS (version 18.0, SPSS, Inc., Chicago, IL, USA). Duncan's test
was used as a post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioral changes in rats
In the pre-experiment, in the 25 mg/(kgd) celecoxib
treatment group, the COX-2 mRNA expression level was lower than in
rats administered 15 and 35 mg/(kgd) celecoxib (Fig. 1). Therefore, 25 mg/(kgd) celecoxib
treatment was selected for the following experiments.
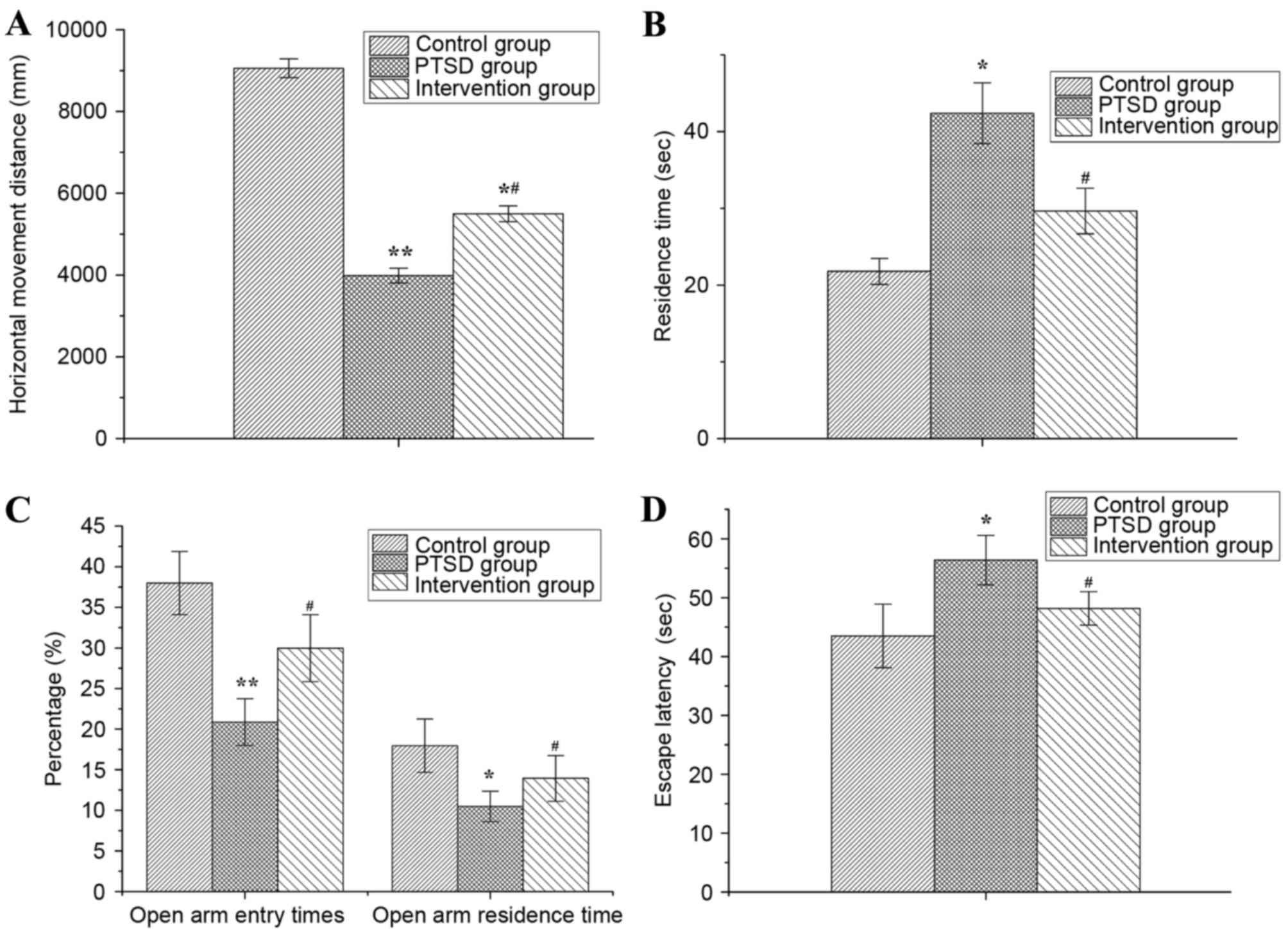
As presented in Fig.
2, behavioral changes were observed in rats with PTSD and in
rats from the intervention group, compared with the control group.
The alertness, anxiety and environmental adaptability of rats were
evaluated by the OF test. The results of the OF test demonstrated
that the horizontal movement distance of rats within 5 min in the
PTSD and intervention groups were significantly decreased compared
with the control group (P<0.01 or P<0.05) and the horizontal
movement distance in intervention group was greater than in the
PTSD group (P<0.05) (Fig. 2A).
The residence time of rats in the PTSD group was increased
significantly compared with the control group (P<0.05), but in
the intervention group the residence time was decreased
significantly compared with the PTSD group (P<0.05; Fig. 2B).
The anxiety levels of rats were evaluated by EPM and
compared with the control group. The percentage of open arm entry
times and the percentage of open arm residence time of rats in the
PTSD group were decreased significantly (P<0.01 or P<0.05).
Compared with the PTSD group, the percentage of open arm entry
times and open arm residence time of rats were increased in the
intervention group (P<0.05; Fig.
2C).
The escape latency of rats was detected by MWM and
the results are presented in Fig.
2D. Compared with the control group, the escape latency was
increased in the PTSD group (P<0.05) and decreased compared with
the PTSD group (P<0.05).
COX-2 levels in rat hippocampi
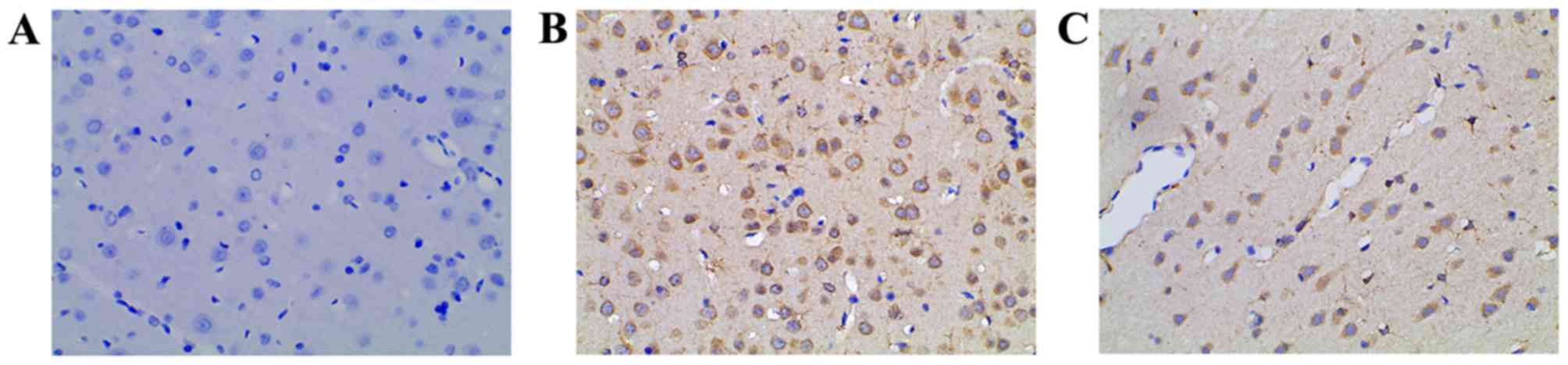
The COX-2 levels in rat hippocampi were evaluated by
immunohistochemical staining. In the control group, low or no COX-2
expression was observed (Fig. 3A).
Compared with the control, the level of COX-2 expression in the
PTSD group was increased significantly (Fig. 3B). In the intervention group, the
COX-2 level was lower than that of the PTSD group (Fig. 3C).
Transcription level of COX-2 mRNA in
rat hippocampi
The mRNA level of COX-2 in rat hippocampi was
evaluated by RT-qPCR (Fig. 4).
Compared with the control group, COX-2 mRNA levels were increased
significantly in the PTSD group (P<0.01) and intervention group
(P<0.05). Compared with the PTSD group, COX-2 levels in the
intervention group were decreased (P<0.05).
Protein level of COX-2 in rat
hippocampi
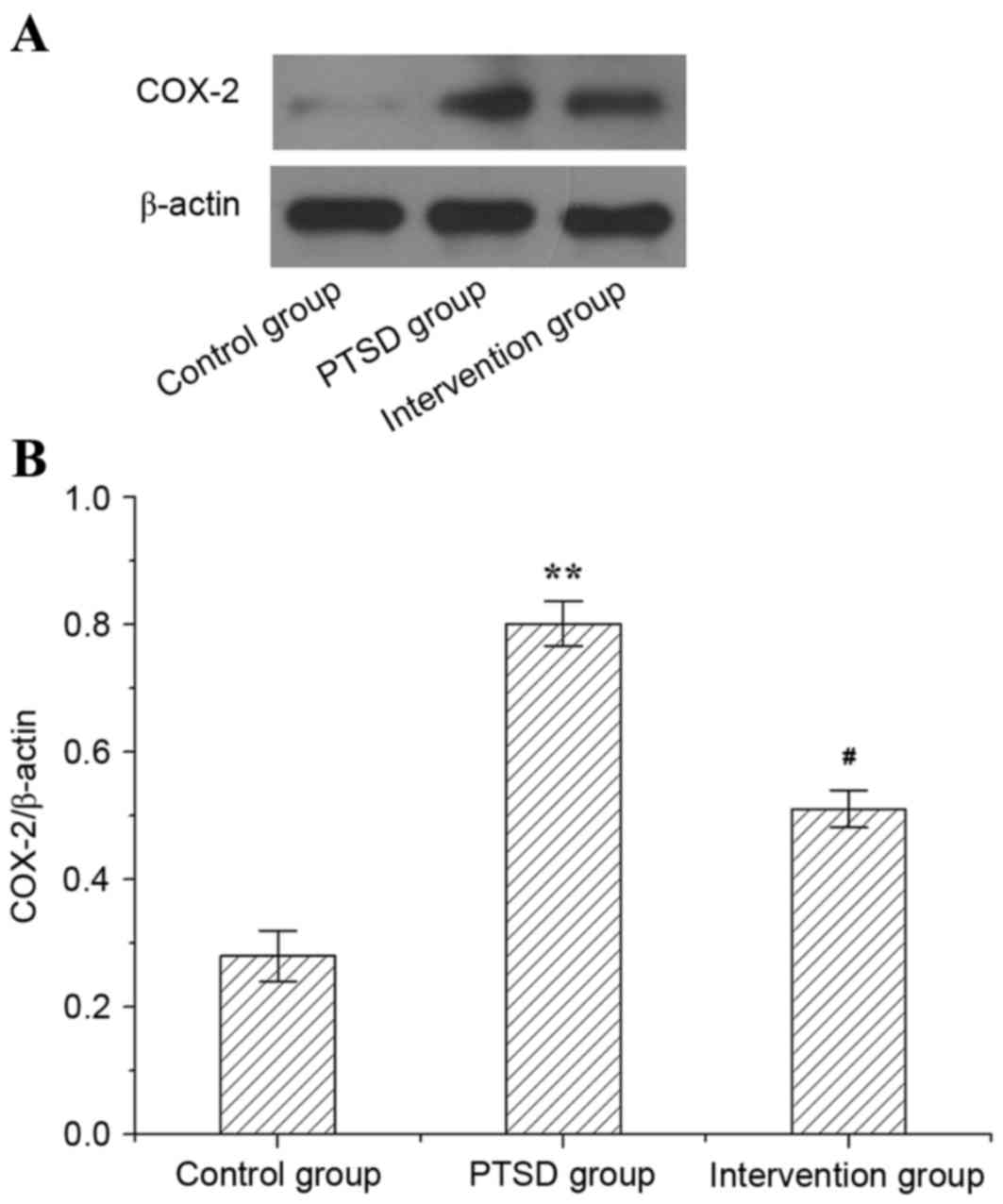
The protein level of COX-2 in rat hippocampi is
presented in Fig. 5. In the PTSD
group, the protein expression level of COX-2 was higher than in the
control group (P<0.01). However, compared with the PTSD group,
the expression level of protein COX-2 was lower in the intervention
group (P<0.05).
Apoptosis of rat hippocampi
The apoptosis of rat hippocampi was evaluated by
TUNEL staining and the results are presented in Fig. 6. In the PTSD group and the
intervention group, the TUNEL staining was positive. Compared with
the control group, the apoptosis in the PTSD group increased
significantly (Fig. 6B). However,
apoptosis in the intervention group was decreased significantly
compared with the PTSD group (Fig.
6C). The apoptotic rate in the PTSD group and the intervention
group were higher than in the control group (P<0.01; Fig. 6D). Compared with the PTSD group,
the apoptotic rate in the intervention group decreased
significantly (P<0.01; Fig.
6D).
Levels of TNF-α, IL-6, PGE2and NO in
rat hippocampi
Levels of TNF-α, IL-6, PGE2 and NO in rat hippocampi
are presented in Fig. 7. In the
PTSD group, the levels of TNF-α, IL-6, PGE2 and NO were higher than
in the control group (P<0.01). The levels of TNF-α and IL-6 in
the intervention group were higher than in the control group
(P<0.05, P<0.01), but lower than in the PTSD group
(P<0.01; Fig. 7A). Compared
with the PTSD group, the levels of PGE2 and NO in the intervention
group were increased significantly (P<0.01; Fig. 7B).
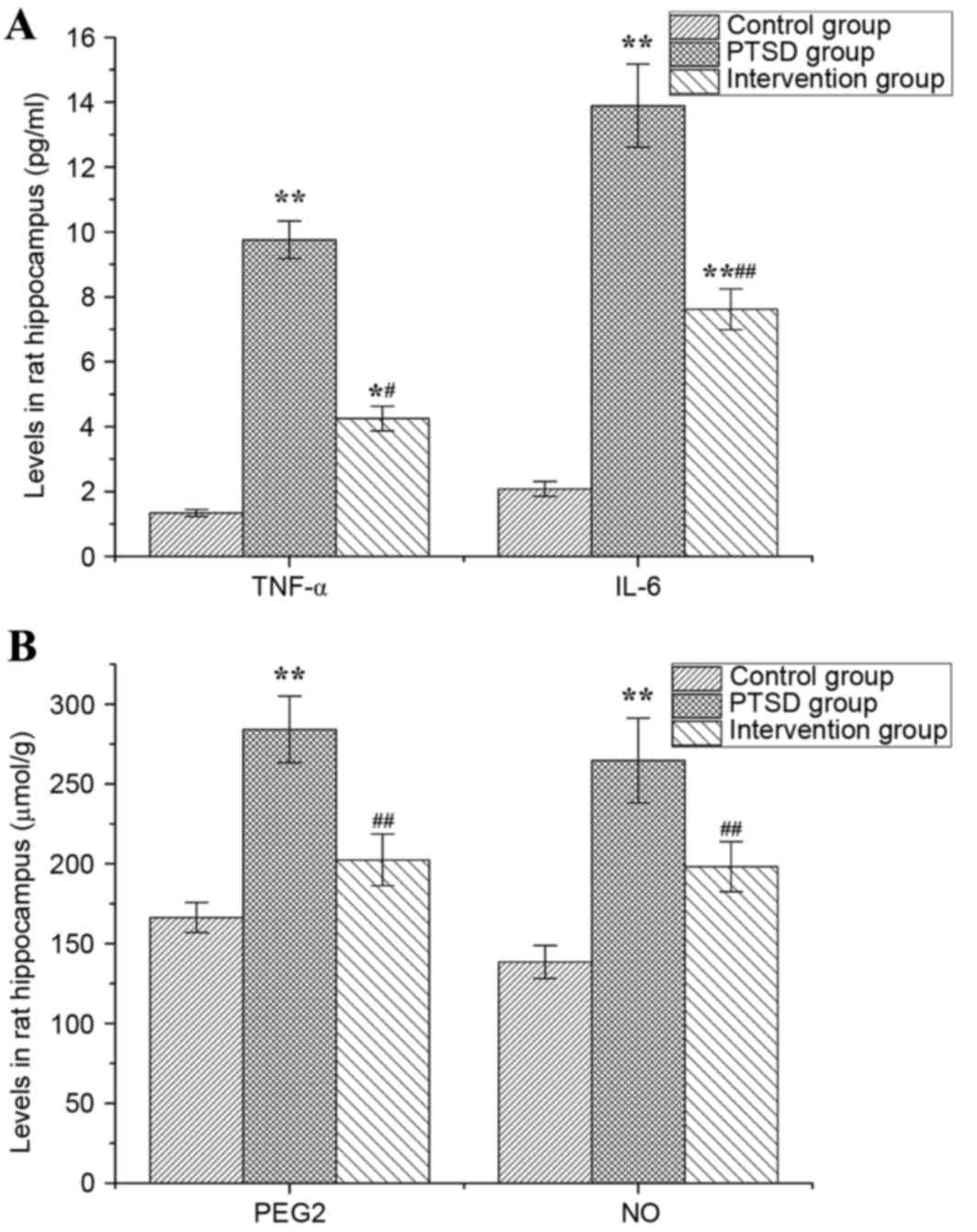 | Figure 7.Levels of TNF-α, IL-6, PGE2 and NO in
rat hippocampi. The levels of (A) TNF-α and IL-6, and (B) PGE2 and
NO. Data are expressed as the mean ± standard deviation (n=6).
*P<0.05, **P<0.01 vs. the control group;
#P<0.05, ##P<0.01 vs. the PTSD group.
TNF-α, tumor necrosis factor-α; IL, interleukin; PGE2,
prostaglandin E2; NO, nitric oxide; PTSD, post-traumatic stress
disorder. |
Discussion
Although the morbidity of PTSD has been reported
multiple times, the pathogenesis of the disease remains to be
elucidated. PTSD can significantly reduce the quality life of
patients, causing psychiatric and physical comorbidity (30). Research over the past two decades
has focused on identifying the neurocircuitry mediating the core
clinical symptoms of PTSD, in attempt to develop optimal, effective
interventions for PTSD (31). The
hypothalamic-pituitary-adrenal (HPA) axis, sympathetic and
parasympathetic nervous system and 5-hydroxytryptamine (5-HT) are
the main regulators of the stress response, emotion and arousal of
an organism (32). Intense
external stimulation might induce over-excitement of HPA, which can
lead to an increased cytokine secretion (33). It has been reported that
transmembrane proteins can transport TNF-α and IL-6 into the
central nervous system through active transport, causing a series
of inflammatory injuries to the central nervous system (34,35).
Increased stress-associated protein expression is a key pathogenic
symptom of PTSD. In the present study, the levels of TNF-α and IL-6
in rats with PTSD were increased significantly, indicating that
TNF-α and IL-6 may serve an important role in the pathogenesis of
PTSD. The increase in TNF-α and IL-6 expression in rats with PTSD
was prevented by the inhibition of COX-2, which suggested that a
feedback loop might exist between COX-2 and inflammation. Stress
can stimulate the immune system to release pro-inflammatory
factors. IL-6 in plasma of patients with depression was positively
correlated with the severity of their clinical symptoms (36). Therefore, the authors of the
present study hypothesized that excessive inflammatory cytokine
expression can lead to secondary intracranial injury and resulting
pathological changes associated with PTSD.
Studies have demonstrated that COX-2 expression is
abnormal in patients with depression (36,37).
In the present study, rats with PTSD were treated with COX-2
inhibitor celecoxib which alleviated their anxiety and cognitive
function. In rats with PTSD, the level of COX-2 was higher than in
the control group, while it was decreased significantly following
treatment with celecoxib. The above observations suggest that the
enhanced expression of COX-2 is involved in the pathogenesis of
PTSD and COX-2 is a potential target for PTSD treatment. Celecoxib
serves an important role in the treatment of PTSD through the
inhibition of the expression of COX-2. The results of the present
study are consistent with previous studies, in which inhibition of
COX-2 production lead to a reduction in a variety of stress-induced
behavioral pathologies in mice (38). The protein level of COX-2 is
closely associated with inflammation in vivo (39,40).
The present study confirmed that the increase in the abundance of
pro-inflammatory factors is associated with an increased COX-2
expression and causes up-regulation of PGE2, NO and other products
of oxidative stress. The mechanism of cell apoptosis induced by
oxidative stress has been confirmed (41,42).
PEG2 is one of the causative factors of apoptosis. Takadera et
al (43) demonstrated that
apoptosis was observed following treatment of mouse neurons with
PGE2 for 48 h and that PGE2 induced apoptosis in a dose-dependent
manner via activation of cysteine protein kinase. Li et al
(44) reported that COX-2/PGE2
signaling serves a pivotal role in the accumulation and function of
myeloid-derived suppressor cells following traumatic stress and
that COX-2 blockade inhibits accumulation and function of
myeloid-derived suppressor cells and restores T cell response
following traumatic stress. In the present study, the level of PEG2
in hippocampi of rats with PTSD was increased and following
intervention with celecoxib, the PEG2 level was down-regulated.
These results of the present study indicated that celecoxib
intervention can inhibit COX-2 production and induce a protective
effect on rat hippocampi.
NO, as a highly reactive free radical, can induce
free radical chain reaction with ONOO−, which can
directly inhibit mitochondrial respiratory chain and cause damage
to DNA. Previous studies have shown that activation of NF-κB and
up-regulation of the inducible nitric oxide synthase can lead to
the synthesis of NO causing tissue injury (45). In the present study, NO expression
in hippocampi of rats with PTSD was increased significantly
compared with the control group but in the intervention group NO
levels were lower than in the PTSD group, indicating that COX-2
inhibition may protect the nervous system by reducing the
expression of NO and its downstream pathway.
PTSD as a stress disorder disease and COX-2 may
serve an important role in its pathogenesis. In the present study,
the levels of TNF-α, IL-6 and COX-2 in the hippocampi of rats with
PTSD were increased significantly compared with healthy rats, which
is consistent with the PTSD pathology detected by imaging. Levels
of proinflammatory cytokines were increased by stress stimuli.
Up-regulation of COX-2 was involved in the oxidative stress and its
downstream products such as NO and PGE2 were elevated causing
neuronal injury and apoptosis. Oxidative stress responses are a
common pathway for multiple intracellular signal transduction
pathways and drug therapy has been shown to significantly reduce
the symptoms of PTSD (46).
Therefore, exploring the pathogenesis of PTSD-related signal
transduction pathways can contribute to the development of new
drugs and clinical treatments.
In conclusion, COX-2 can induce inflammation and
cell apoptosis in rats and promote the development of PTSD. COX-2
inhibition can decrease the levels of TNF-α, IL-6, PEG2 and NO in
the hippocampi of rats with PTSD and reduce the apoptosis of cells
in this tissue. COX-2 inhibition reduces the prevalence of
oxidative stress products and apoptosis, and may in the future
serve an important role in the clinical research and treatment of
PTSD.
Acknowledgements
This study was supported by the grant from Nature
Science Foundation of Hubei Province (grant no. 2015CFB694).
References
|
1
|
Jones KD, Young T and Leppma M: Mild
traumatic brain injury and posttraumatic stress disorder in
returning Iraq and Afghanistan War Veterans: Implications for
assessment and diagnosis. J Counsel Dev. 88:372–376. 2010.
View Article : Google Scholar
|
|
2
|
Olatunji BO, Cisler JM and Tolin DF:
Quality of life in the anxiety disorders: A meta-analytic review.
Clin Psychol Rev. 27:572–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pietrzak RH, Goldstein RB, Southwick SM
and Grant BF: Psychiatric comorbidity of full and partial
posttraumatic stress disorder among older adults in the United
States: Results from wave 2 of the National Epidemiologic Survey on
Alcohol and Related Conditions. Am J Geriatr Psychiatry.
20:380–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen H, Kaplan Z, Koresh O, Matar MA,
Geva AB and Zohar J: Early post-stressor intervention with
propranolol is ineffective in preventing posttraumatic stress
responses in an animal model for PTSD. Eur Neuro Psychopharmacol.
21:230–240. 2011. View Article : Google Scholar
|
|
5
|
Rauch SA, Morales KH, Zubritsky C, Knott K
and Oslin D: Posttraumatic stress, depression, and health among
adults in primary care. Am J Geriatr Psychiatry. 14:316–324. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knight JA and Taft CT: Assessing
neuropsychological concomitants of trauma and PTSD. Assessing
Psychological Trauma and PTSD. Wilson JP and Keane TM: 2nd. The
Guilford Press; New York, NY: pp. 344–388. 2004
|
|
7
|
Neylan TC, Schadt EE and Yehuda R:
Biomarkers for combat-related PTSD: Focus on molecular networks
from high-dimensional data. Eur J Psychotraumatol. 5:239382014.
View Article : Google Scholar
|
|
8
|
Moeller DR, Duffy JM, Goolsby AM and
Gallimore JT: Use of a removable mandibular neuroprosthesis for the
reduction of posttraumatic stress disorder (PTSD) and mild
traumatic brain injury/PTSD/associated nightmares, headaches, and
sleep disturbances. J Spec Oper Med. 14:64–73. 2014.PubMed/NCBI
|
|
9
|
Mills KL, Teesson M, Ross J and Peters L:
Trauma, PTSD, and substance use disorders: Findings from the
Australian national survey of mental health and well-being. Am J
Psychiatry. 163:652–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen BE, Marmar CR, Neylan TC, Schiller
NB, Ali S and Whooley MA: Posttraumatic stress disorder and
health-related quality of life in patients with coronary heart
disease: Findings from the heart and soul study. Arch Gen
Psychiatry. 66:1214–1220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang HK and Bullman TA: Risk of suicide
among US veterans after returning from the Iraq or Afghanistan war
zones. JAMA. 300:652–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakayama M, Uchimura K, Zhu RL, Nagayama
T, Rose ME, Stetler RA, Isakson PC, Chen J and Graham SH:
Cyclooxygenase-2 inhibition prevents delayed death of CA1
hippocampal neurons following global ischemia. Proc Nati Acad Sci
USA. 95:pp. 10954–10959. 1998; View Article : Google Scholar
|
|
13
|
Rothman SM and Olney JW: Glutamate and the
pathophysiology of hypoxic-ischemic brain damage. Ann Neurol.
19:105–111. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi DW: Glutamate neurotoxicity and
diseases of the nervous system. Neuron. 1:623–634. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh DP and Chopra K: Flavocoxid, dual
inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits
neuroprotection in rat model of ischaemic stroke. Pharmacol Biochem
Behav. 120:33–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yagami T, Koma H and Yamamoto Y:
Pathophysiological roles of cyclooxygenases and prostaglandins in
the central nervous system. Mol Neurobiol. 53:4754–4771. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grösch S, Tegeder I, Niederberger E,
Bräutigam L and Geisslinger G: COX-2 independent induction of cell
cycle arrest and apoptosis in colon cancer cells by the selective
COX-2 inhibitor celecoxib. FASEB J. 15:2742–2744. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altorki NK, Keresztes RS, Port JL, Libby
DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah
K, Pasmantier MW and Dannenberg AJ: Celecoxib, a selective
cyclo-oxygenase-2 inhibitor, enhances the response to preoperative
paclitaxel/carboplatin in early stage lung cancer. J Clin Oncol.
21:2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karim A, Tolbert DS, Hunt TL, Hubbard RC,
Harper KM and Geis GS: Celecoxib, a specific COX-2 inhibitor, has
no significant effect on methotrexate pharmacokinetics in patients
with rheumatoid arthritis. J Rheumatol. 26:2539–2543.
1999.PubMed/NCBI
|
|
20
|
Liberzon I, Krstov M and Young EA:
Stress-restress: Effects on ACTH and fast feedback.
Psychoneuroendocrinology. 22:443–453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liberzon I, López JF, Flagel SB, Vázquez
DM and Young EA: Differential regulation of hippocampal
glucocorticoid receptors mRNA and fast feedback: Relevance to
post-traumatic stress disorder. J Neuroendocrinol. 11:11–17. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nadeem MN and Maqdoom M: Evaluation of
anticonvulsant effect of celecoxib, a selective cyclooxygenase-2
inhibitor in experimentally induced convulsions in albino rats. Int
J Basic Clin Pharmacol. 5:1466–1470. 2016. View Article : Google Scholar
|
|
23
|
Carola V, D'Olimpio F, Brunamonti E,
Mangia F and Renzi P: Evaluation of the elevated plus-maze and
open-field tests for the assessment of anxiety-related behaviour in
inbred mice. Behav Brain Res. 134:49–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brandeis R, Brandys Y and Yehuda S: The
use of the Morris Water Maze in the study of memory and learning.
Int J Neurosci. 48:29–69. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choleris E, Thomas AW, Kavaliers M and
Prato FS: A detailed ethological analysis of the mouse open field
test: Effects of diazepam, chlordiazepoxide and an extremely low
frequency pulsed magnetic field. Neurosci Biobehav Rev. 25:235–260.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walf AA and Frye CA: The use of the
elevated plus maze as an assay of anxiety-related behavior in
rodents. Nat Protoc. 2:322–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morris R: Development of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han Y, Li X, Zhou S, Meng G, Xiao Y, Zhang
W, Wang Z, Xie L, Liu Z, Lu H and Ji Y: 17ß-estradiol antagonizes
the down-regulation of ERα/NOS-3 signaling in vascular endothelial
dysfunction of female diabetic rats. PLoS One. 7:e504022012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vermetten E, Vythilingam M, Southwick SM,
Charney DS and Bremner JD: Long-term treatment with paroxetine
increases verbal declarative memory and hippocampal volume in
posttraumatic stress disorder. Biol Psychiatry. 54:693–702. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cisler JM, Bush K, James GA, Smitherman S
and Kilts CD: Decoding the traumatic memory among women with PTSD:
Implications for neurocircuitry models of PTSD and real-time fMRI
neurofeedback. PLoS One. 10:e01347172015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Babson KA and Feldner MT: Temporal
relations between sleep problems and both traumatic event exposure
and PTSD: A critical review of the empirical literature. J Anxiety
Disord. 24:1–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boals A and Hathaway LM: The importance of
the DSM-IV E and F criteria in self-report assessments of PTSD. J
Anxiety Disord. 24:161–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bailey JN, Goenjian AK, Noble EP, Walling
DP, Ritchie T and Goenjian HA: PTSD and dopaminergic genes, DRD2
and DAT, in multigenerational families exposed to the Spitak
earthquake. Psychiatry Res. 178:507–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carpenter LL, Gawuga CE, Tyrka AR, Lee JK,
Anderson GM and Price LH: Association between plasma IL-6 response
to acute stress and early-life adversity in healthy adults.
Neuropsychopharmacology. 35:2617–2623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robinson RA: Molecular clue to PTSD. PLoS
Biol. 13:e10022832015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Müller N and Schwarz MJ: The
immune-mediated alteration of serotonin and glutamate: Towards an
integrated view of depression. Mol Psychiatry. 12:988–1000. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gamble-George JC, Baldi R, Halladay L,
Kocharian A, Hartley N, Silva CG, Roberts H, Haymer A, Marnett LJ,
Holmes A and Patel S: Cyclooxygenase-2 inhibition reduces
stress-induced affective pathology. Elife. 5(pii):
e141372016.PubMed/NCBI
|
|
39
|
Gao HM, Liu B, Zhang W and Hong JS: Novel
anti-inflamatory therapy for Parkinson's disease. Trends Pharmacol
Sci. 24:395–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hinz B and Brune K: Cyclooxygenaxe-2-10
years later. J Pharmacol Exp Ther. 300:367–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Said RS, Badr AM, Nada AS and El-Demerdash
E: Sodium selenite treatment restores long-lasting ovarian damage
induced by irradiation in rats: Impact on oxidative stress and
apoptosis. Reprod Toxicol. 43:85–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nury T, Zarrouk A, Vejux A, Doria M,
Riedinger JM, Delage-Mourroux R and Lizard G: Induction of
oxiapoptophagy, a mixed mode of cell death associated with
oxidative stress, apoptosis and autophagy, on
7-ketocholesterol-treated 158n murine oligodendrocytes: Impairment
by α-tocopherol. Biochem Biophys Res Commun. 446:714–719. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takadera T, Yumoto H, Tozuka Y and
Ohyashiki T: Prostaglandin E(2) induces caspase-dependent apoptosis
in rat cortical cells. Neurosci Lett. 317:61–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li RJ, Liu L, Gao W, Song XZ, Bai XL and
Li ZF: Cyclooxygenase-2 blockade inhibits accumulation and function
of myeloid-derived suppressor cells and restores T cell response
after traumatic stress. J Huazhong Univ Sci Technolog Med Sci.
34:234–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morioka N, Inoue A, Hanada T, Kumagai K,
Takeda K, Ikoma K, Hide I, Tamura Y, Shiomi H, Dohi T and Nakata Y:
Nitric oxide synergistically potentiates interleukin-1 beta-induced
increase of cyclooxygenase-2 mRNA levels, resulting in the
facilitation of substance P release from primary afferent neurons:
Involvement of cGMP-independent mechanisms. Neuropharmacology.
43:868–876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Puetz TW, Youngstedt SD and Herring MP:
Effects of pharmacotherapy on combat-related PTSD, anxiety, and
depression: A systematic review and meta-regression analysis. PLoS
One. 10:e01265292015. View Article : Google Scholar : PubMed/NCBI
|