Introduction
As a highly evolved biological response,
immunoregulation not only coordinates inflammation and innate
immunity, however may additionally modulate adaptive immunity and
establish self-tolerance. Continuous access to nutrients is a
primary requirement for cell proliferation, and controlling
nutrient supply is an ancient survival strategy that may
additionally regulate immune response. Indoleaine 2,3-dioxygenase
(IDO) is a tryptophan enzyme composed of two-helical α-domains with
a heme group located between them (1–2). A
total of two forms of the IDO gene (IDO1 and IDO2) have been
identified, however, the majority of studies have investigated the
function of IDO1, and the physiological role of IDO2 remains
unclear.
The immune regulating properties of IDO were first
described in the prevention of T-cell-mediated allogenetic fetus
rejection in mice, verifying that IDO synthesized in placental
cells protects the mammalian fetus from maternal T lymphocyte
attack (3). The degradation of
tryptophan and accumulation of tryptophan-derived catabolites by
IDO may lead to the suppression of T-cell proliferation at mid-G1
phase, the inhibition of activated T effector cells, and the
induction of T, B, and natural killer cell apoptosis (1,3). IDO
has previously been demonstrated to be expressed in various
tissues, including human lung, placenta, and small intestine and is
upregulated during inflammation. Physiologically, IDO is pivotal in
regulating the immune response to antigenic challenges at mucosal
surfaces in the digestive tract and lungs (4). Furthermore, the induction of IDO and
the subsequent deprivation of tryptophan in the microenvironment,
exerts an antiproliferative effect on T cells and infectious
pathogens (3). IDO is recognized
to be an authentic regulator of immunity in a variety of
pathophysiological settings, including infections, transplantation
and cancer (1,5). The present review aimed to evaluate
recent progress and evidence regarding how IDO activity impacts the
immune responses to inflammatory and immunological signals.
Properties of the IDO enzyme
IDO, a 407-amino acid heme-containing cytoplasmic
protein, is coded by the INDO gene located on chromosome 8p12 in
humans. The enzyme is responsible for the primary mechanism of
extra-hepatic tryptophan metabolism and is expressed in
professional APCs, epithelial cells, vascular endothelium and tumor
cells (1,2,5). The
IDO gene is regulated by upstream interferon (IFN)-γ responsive
elements that bind to activated signal transducer and activator of
transcription 1 (STAT1), interferon regulatory factor-1 and nuclear
factor-κB (NF-κB) (6). IDO
activity is strongly associated with an environment enriched with
redox active compounds, which are required to generate the active
Fe2+ form for tryptophan metabolism. IDO enzymatically
degrades tryptophan and other indoleamine compounds via oxidative
cleavage of the pyrrole ring, resulting in an accumulation of
downstream breakdown products of kynurenine, in addition to other
defined metabolic products, which have been previously reported to
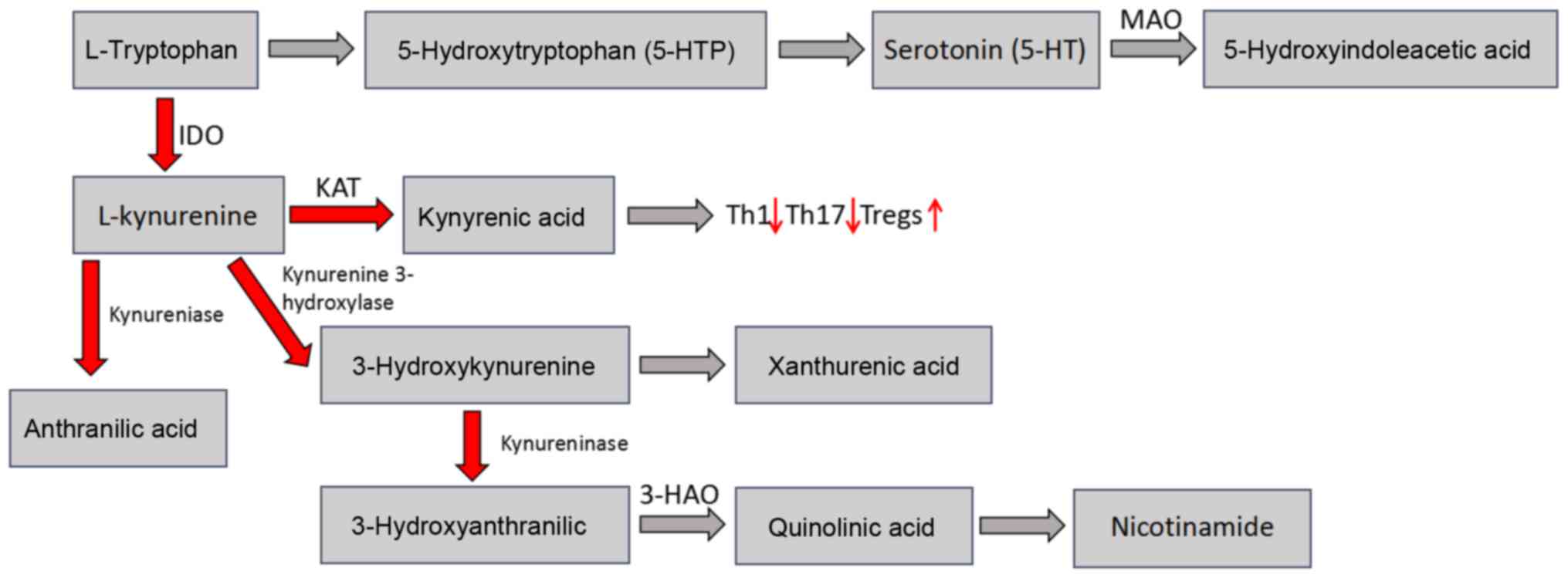
exhibit biological activity in the immune system (Fig. 1) (7). Currently, the ratio of
kynurenine/tryptophan is regarded as a method to determine the
enzymatic activity of IDO. Furthermore, adding strong reductants
(methylenum coeruleum, vitamin C) into the IDO culture system
results in the maintenance of the superoxide anion, an accessory
factor of IDO, at a high concentration, thus enhancing its
activity. The enzymatic activity of IDO is mitigated by natural
immunomodulators including nitric oxide, which are able to combine
with the heme tetrapyrrole group (8).
IDO expression has been revealed to be markedly
different between various cells depending on the specific cell
type, the maturational state and the activation status of the
cells. The catabolic pathway regulated by IDO activity results from
activity of two different genes, termed IDO1 and IDO2. Mice and
humans possess the two associated genes tightly in a syntenic
region of chromosome 8 (9). The
two genes exhibit sequence homology, however, they respond
differently to various signals in distinct cell types, and their
patterns of gene regulation and expression are not identical.
Currently, IDO1 is the more comprehensively studied of the two
genes, however IDO2 is gradually being recognized. It has
previously been demonstrated that IDO expression and enzymatic
activity is mediated by suppressor of cytokine signaling 3 (SOCS3),
NF-κB, DNAX-activation protein 12 and interferon regulatory
factor-8 pathways. In addition, IDO protein levels and activity are
modulated posttranslationally via ubiquitination or protein
nitration via inducible nitric oxide synthase (iNOS).
Administration of iNOS blockers following transplantation has been
demonstrated to improve allograft function and attenuate graft
damage (10).
IDO pathways and immune regulation
Uncontrollable immune responses may be fatal,
therefore the immune system is delicately balanced between immunity
and tolerance. IDO, as a major inhibitor of the immune response,
appears to be pivotal in imposing restrictions on potentially
exaggerated inflammatory reactions to danger signals. IDO
contributes to immune regulation via catalyzing tryptophan along
the kynurenine pathway. IDO modifies the immune response via three
pathways: i) by depleting tryptophan in the local microenvironment,
IDO results in metabolic stress sensed by general control
nondepressible-2 (GCN2) kinase and mammalian target of Rapamycin
(mTOR), which eventually promotes anergy in responding T cells and
directs the conversion of regulatory T cells (Treg) (11); ii) by producing tryptophan
catabolism that binds to a natural ligand for the aryl hydrocarbon
receptor (AhR), IDO similarly results in immunosuppressive effects
on the immunogenicity of dendritic cell (DCs) reduction and the
Treg conversion (12); iii) by
inducing Treg function, IDO as a signaling protein shapes the
immunological microenviroment in vivo. The following section
identifies mechanisms by which IDO pathways are implicated in
modulating immune regulation.
IDO depletes tryptophan and produces
bioactive downstream metabolites
Effector pathways: GCN2 activation and
mTOR suppression
IDO activity decreases the local concentration of
tryptophan in the IDO-expressing cell and in the microenvironment
surrounding nearby cells. Tryptophan depletion by IDO may act as a
potential regulatory signal via two signaling pathways: activation
of the molecular stress-response pathway, including GCN2 kinase,
which directly binds to uncharged tryptophan tRNAs, and suppression
of the mTOR kinase pathway, which is known to regulate immune
reactions (13). DCs expressing
IDO may induce the immunosuppressive activity of Tregs, however
this effect is observed to be abrogated by genetic disruption of
GCN2. Depletion of tryptophan has been demonstrated to mediate the
activation of the GCN2 pathway for downregulating the CD3 ζ-chain
in CD8+ T cells and blocking Th17 cell differentiation
(14). GCN2 blunts protein
translation by phosphorylating its downstream target, initiating
eukaryotic translation initiation factor 2 α (eIF-2α), resulting in
blockade readout of the majority of RNA transcripts, except for the
LIP RNA transcript. IDO supports interleukin (IL)-6 production
through GCN2 activation and was revealed to affect myeloid-derived
suppressor cell function and tumor progression (15). A previous study in nephritis mouse
models, conducted by Chaudhary et al (16), suggested that kidney injury of mice
were improved by amino acid metabolism and protected from the
autophagic response. The IFN-γ-mediated induction of IDO activity
with subsequent activation of GCN2 is implied in the metabolic
signaling process. These results outline the IDO-GCN2 pathway, as a
critical negative feedback mechanism in glomerular stromal cells,
via inducing autophagy that limits inflammatory renal pathological
alterations. Therefore, the IDO-GCN2 pathway mediates amino-acid
levels and may contribute to a generalized mechanism of regulating
the immune response.
Amino acid withdrawal additionally has an effect on
the nutrient-sensing mTOR pathway. IDO-mediated catabolism of
tryptophan inhibits mTOR and T cell receptor protein kinase C-sita
(PKC-θ), which are regulatory targets of the master amino
acid-sensing galactokinase 1 acting upstream of mTOR (17). Furthermore, in the tumor
microenvironment, blockade of mTOR by IDO was reported to trigger
autophagy in anergized T cells, whereas the opposite effect was
observed in tryptophan restoration, which relieved mTOR blockade
(18). The observation of blockade
of mTOR by IDO suggests it may act as a pivotal regulator, however
direct effects of IDO on the mTOR pathway have not yet been fully
elucidated, and further research is required to verify the specific
mechanism.
Effector pathways: tryptophan
catabolites as ligands for AhR
In addition to GCN2 kinase, it has previously been
demonstrated that the breakdown products of tryptophan catabolism
are important crucial mediators in the IDO pathway. It was revealed
that high IDO expression and tryptophan metabolites
(3-hydroxykynurenine and 3-hydroxyanthranilic acid) in transgenic
DCs, irreversibly inhibit allogeneic T-cell proliferation.
Conversely, increased kynurenine/tryptophan ratios in plasma
IDO+ DC may promote the production of Tregs and induce
the effector T cells in the state of anergy or apoptosis.
Kynurenine catabolites exert a cytotoxic function on
CD3+ cells, however the molecular mechanism remains to
be fully elucidated, and potentially involves in inhibition of T
cell costimulatory signaling via 3′-phosphoinositide-dependent
kinase 1 (an essential mediator of CD28-induced NF-κB activation)
pathway and activation of signaling via AhR (19).
AhR is a transcription factor that is activated by
dioxin-like kynurenine-related ligands. AhR has previously been
implicated in the inflammatory and immune regulations that IDO is
involved in. The immunological effects of AhR activation on T cell
subsets appear to be immunosuppressive, including arrest of T cell
activation, induction of differentiation of Foxp3+ Treg
cells, alterations in the functional immunogenicity of DCs and
suppression of anti-tumor immune responses (20). AhR signaling in DCs is additionally
required to induce the expression of functional IDO, indicating
occurrence of crosstalk between the two pathways. Kynurenine
regulates an effector signaling pathway from IDO in activating AhR,
however additionally regulates tryptophan catabolizing enzyme,
tryptophan 2,3-dioxygenase, which has the capacity to inhibit
anti-tumor immune responses and promote tumor cell survival
(21). Metabolites of tryptophan
are directly toxic to CD8+T cells and CD4+Th1
cells, however not to Th2 cells, therefore enhanced IDO activity
appears to redirect T helper cell polarization toward a Th2
phenotype. IDO activity may be partially counteracted by two
negative feedback loops, including kynurenine increasing IL-6
expression through AhR and eIF-2α, leading to incremental
B-lymphocyte induced maturation protein 1 levels, which impede the
INDO promoter region (22). This
allows for fine tuning of IDO activity to maintain a balance
between immune activation and suppression, as necessary.
Therapeutically, administration of kynurenine compounds may protect
transplanted tissues from an inflammatory reaction response and
promote immune tolerance.
Effector pathways: a signaling protein
in NF-κB pathway
It was indicated that IDO may function as a
signaling protein responsible for the self-amplification and
maintenance of a stably regulatory phenotype in plasmacytoid
dendritic cells (pDCs) rather than a catalyst. For pDCs treated
with transforming TGF-β in mice, this signaling function occurs via
recruitment and activation of Src homology region 2
domain-containing phosphatase proteins bonding to immunoreceptor
tyrosine-based inhibitory motifs in the Fyn-dependent
phosphorylation of IDO molecule. IDO then initiates a circuit of
downstream signaling effectors, including the noncanonical NF-κB
pathway, that result in sustained tumor growth factor (TGF)-β
production, induction of type I interferons and the regulatory pDC
phenotype, ultimately inducing long-lasting IDO expression and
autocrine TGF-β secretion in a positive feedback loop (23). Furthermore, noncanonical NF-κB
signaling downregulates proinflammatory cytokine production in DCs,
and selective activation of the noncanonical NF-κB pathway gives
rise to noninflammatory DCs that suppress T cell activation and
promote the development of T cells with regulatory properties
(24). Accordingly, noncanonical
NF-κB signaling in DCs is required for IDO induction and immune
regulation.
Regulatory effect of IDO on T cells
and Tregs
IDO pathways have important effects on T cells in
response to antigenic stimulation (Fig. 2). T cells activated by DCs
expressing IDO recognize the antigen and enter into cell cycle,
however IDO-induced activation of GCN2 blocks subsequent cell cycle
progression, leading to the inhibition of Th17 differentiation and
the increase of T cell apoptosis. The IDO activity fails to
regulate T cells in the case of GCN2 dependent amino acid stress
response pathways defected (13,15).
Additionally, the IDO-mediated redox in DCs may affect T cell
sensitivity. In a model of pulmonary aspergillosis in mice, a
superoxide-dependent step in tryptophan metabolism along the
kynurenine pathway is inhibited, leading to unrestrained T-cell
reactivity, dominant production of IL-17, and defective regulatory
T-cell activity (25). However,
whether T cells are regulated by IDO activity solely in DCs that
present antigens directly to T cells, or whether T cells activated
by DCs not expressing IDO would be affected by local IDO activity
in bystander DCs, remains to be clarified.
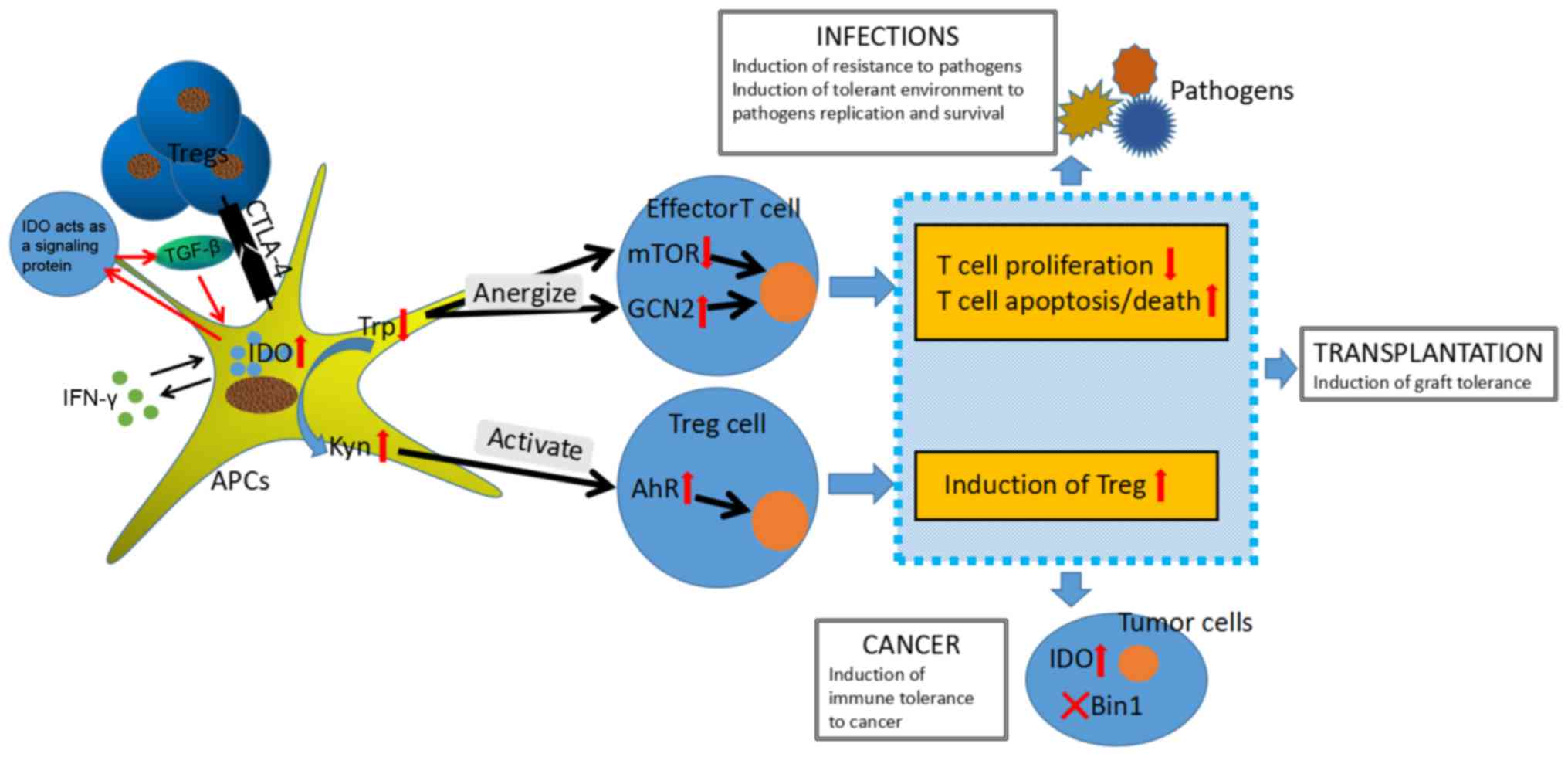 | Figure 2.IDO pathway control of T cell and
Treg responses. IDO is expressed on professional APCs and tumor
cells, and is critical in immune regulation of cancers,
transplantation and infections by catalyzing oxidative catabolism
of the essential amino acid tryptophan, along the kynurenine
pathway. IDO modifies immune response in three pathways: By
depleting tryptophan in the local microenvironment, IDO results in
metabolic stress sensed by GCN2 kinase and mTOR, which eventually
promotes anergy in responding T cells and directs the conversion of
Foxp3+ T cell; by producing tryptophan catabolism that
binds to a natural ligand for AhR, IDO similarly achieves
immunosuppressive effects on the immunogenicity of dendritic cell
reduction and the Foxp3+ Tregs conversion; by inducing
regulatory T cell function, IDO as a signaling protein shapes the
immunological microenviroment in vivo. IDO, indoleamine 2,
3-dioxygenase; mTOR, mammalian target of Rapamycin; GCN2, general
control nondepressible-2; APC, antigen presenting cell; AhR, aryl
hydrocarbon receptor; CTLA4, cytotoxic T-lymphocyte antigen 4;
Foxp3+, Forkhead Box P3; Treg, T regulatory cells;
TGF-β, tumor growth factor-β; IFN-γ, interferon-β; Trp, tryptophan;
Kyn, kynurenine; BIN-1, bridging integrator 1. |
The majority of immune responses are naturally
blocked by increasing
CD4+CD25+Foxp3+ Tregs. The IDO
pathway is conducive to regulation of Treg lineage commitment and
function. In vitro, tryptophan depletion (sensed by GCN2)
acts synergistically with kynurenine metabolites to redirect
CD4+ T cells to differentiate into Foxp3+
Treg cells. In vivo, inhibition or knockout of IDO genes
prevents the antigen-specific Tregs in response to pathogen
challenge and decreases the ratio of T regulatory/T effector cells
(26). The B7 receptors on DCs
expressing IDO bind to cytotoxic T-lymphocyte antigen 4 (CTLA4) on
Tregs, resulting in their proliferation and antigen-specific anergy
(5,11). Under conditions of IDO ablation,
resting Tregs convert uniformly into a phenotype resembling
proinflammatory Th17 cells. The reprogrammed Treg cells following
IDO-blocking have been characterized as analogous to that of
‘polyfunctional’ T-helper cells co-expressing IL-17, IL-2 and tumor
necrosis factor-α (27).
Therefore, IDO is important in the differentiation of
Foxp3+Tregs to Th17-like effector cells. IDO stimulates
Treg cell bystander suppressor activity and simultaneously inhibits
the IL-6 secretion, which is required for the conversion of Tregs
into Th17-like effector cells. Notably, human monocyte-derived DCs
upregulate IDO expression induced by proinflammatory cytokines,
expanding the population of allogenetic autologous Tregs. These
Tregs suppress autologous and allogeneic proliferation of T-cells
and repress the generation of antigen-specific CTL (28).
Therefore, IDO pathways and IDO-mediated tryptophan
degradation, regulate the balance between effector T cells and
Tregs in favor of Tregs. This transition may control excess
inflammation and prevent immune-mediated pathology. However,
whether IDO represents a predominant mechanism or operates in a
synergistic manner in combination with other tolerogenic effects,
requires further investigation.
Role of IDO in immune suppression
The role of IDO in cancer
IDO is widely overexpressed in tumor cells and acts
at multifarious levels to establish a more hospitable environment
for tumor progression. It has previously been demonstrated that
increased expression of IDO is predominantly associated with poor
prognosis in a variety of cancer types, including ovarian,
endometrial, colorectal and cervical cancers (29–30).
Studies using murine models have indicated that IDO expression is
not always present in tumor cells, however may be located in
tumor-draining lymph nodes or in surrounding stroma near the tumor
margins (31). IDO activation in
either tumor cells or nodal regulatory DCs appears to be adequate
to facilitate tumor immune escape. There are two potential sites
for the immunosuppressive action of IDO in tumor-bearing hosts:
Firstly, IDO expressed by the tumor cells enables creation of a
localized immunosuppressive milieu in the tumor, either by
inhibiting effector function and proliferation of T cells in the
tumor, or by utilizing toxic metabolites of tryptophan to directly
kill infiltrating T cells. Alternatively, host DCs expressing IDO
are able to identify tumor-derived antigens and transfer them to
tumor-draining lymph nodes where they would present the antigen to
naive T cells for induction of T-cell deletion, a failure of clonal
expansion, or perhaps even the biasing of various cells toward a
regulatory phenotype. DCs respond to low tryptophan by increasing
expression of the inhibitory receptors Ig-like transcript 3 (ILT3),
ILT4 and TGF-β1, which renders them immunosuppressive APCs
(32). Due to the potent
immunological functions of IDO, upregulation of IDO by host APCs or
tumor cells may be critical for the induction of the tumor-tolerant
environment.
Mouse genetic research has demonstrated that IDO
overexpression may be regulated by inactivation of bridging
integrator (Bin)1 (a tumor suppressor gene), which appears to
prevent cancer development to a significant extent by limiting
immune escape (6). However, Bin1
expression in human tumors is extinguished by aberrant RNA splicing
patterns and altered gene methylation patterns. Furthermore, it has
been indicated that inactivated Bin1 generates cancer
cell-intrinsic benefits for cell proliferation and survival. In an
in vitro study, transformation of Bin1-null and
Bin1-expressing primary mouse embryo keratinocytes with oncogenes,
produced cell lines which then were grafted into syngeneic animals.
The Bin1-null cells appeared to form larger tumors, whereas only
indolent nodules were seen in the Bin1-expressing cell group.
Overall, overexpression of IDO accompanied by Bin1 inactivation or
loss promotes tumorigenesis by enabling immune escape (33).
The role of IDO in
transplantation
Successful and sustained allograft tolerance is
based on effective control of the potential immune reaction.
Current treatments employ a general immunosuppressant, which
results in patient susceptibility to pathogenic infection and
potentially severe adverse effects. Mice with IDO knockout
experience acute rejection injury of transplanted major
histocompatibility complex mismatched grafts, whereas long-term
survival occurs in wild-type mice with high-level tryptophan
catabolism (34). Further
experiments of liver transplantation have demonstrated that IDO
expression of Kupffer cells demonstrates a time-dependent increase
in the tolerance group, and the number of IDO-positive cells are
closely associated with the severity of acute reaction (35). A further study demonstrated that
IDO+ DC transfusion prolongs the survival of recipients
in small bowel transplantation models, and more efficient results
may be obtained with 3-hydroxyanthranilic acid treatment (36). IDO induction of DC is dependent on
transcription factor Foxp3. Surface CTLA-4 expressed by Treg cells
binds B7 molecules on DCs to induce IDO expression and promote a DC
regulatory phenotype, and this phenomenon from CTLA-4 to B7 is
likely modulated by induced Foxp3+ Treg cells. In
addition, IDO may activate Treg cells via the aforementioned AhR
and GCN2 pathways. Therefore, there is latent for a mutually
reinforcing loop, which maintains a lasting transplant tolerance
microenvironment. Additionally, in a study of rat lung allografts,
functionally inhibiting cytotoxic CD8+ T cells were
demonstrated to be critical in the mechanism of immune modulation
of IDO, which reduces infiltrating CD8+ T cells and
impairs cytotoxic function on perforin and granzyme A/B secretion
(37).
The role of IDO in infectious
diseases
In infectious disease states, IDO exerts pleiotropic
effects, acting as a suppressor of intracellular pathogen
replication and as an immune regulator. IFN-β and IFN-γ induced
tryptophan degradation leads to enhanced IDO activity against
pathogens including cytomegalovirus, Herpes simplex virus type 2,
Chlamydia psittaci strains and Leishmania donovani
(5). A clinical experiment
demonstrated that IDO expression increases in chronically infected
hepatitis C patients and acute hepatitis B patients, however not in
those from recovered patients or patients with hepatic flare
(38,39), suggesting that IDO may be an
indicator of subsequent immune responses operative during the early
phase of infection. Conversely, pathogens are capable of
highjacking the immunosuppressive effects of IDO and using them to
facilitate their own life cycle. Leishmania parasites
circumvent immune clearance via promoting the induction of IDO
among host DCs. The immunocompromised response on IDO induction
enables a triumphant localization of Leishmania (40). IDO may be crucial in forming
pathogen-induced lung inflammation in the influenza infection, and
predisposes the lung to secondary bacterial infection. Inhibition
of IDO activity contributes to the activation of the heterosubtypic
memory T cell response for cross-protective immunity against the
influenza virus (41).
In particular, the human immune deficiency virus
(HIV) may induce IDO synthesis to escape the direct killing
mechanism of CD8+ cytotoxic lymphocyte recognition. The
induction of IDO in APCs via the N-terminal domain of HIV-1
transactivator regulatory protein (Tat) is engendered through an
intracellular signaling cascade reaction including Janus activated
kinases (Jak) I, phosphatidyl inositol 3-kinase (PI3K) or CTLA-4-B7
interaction, which consequently results in a breakdown of
tryptophan to kynurenine and a suppression of T-cell proliferation.
IFN-γ signaling resulting in IDO expression may be blocked by JAKs
and PI3K inhibitors, however this does not occur with Tat-induced
IDO expression, suggesting further investigation is necessary in
order to elucidate the novel mechanism underlying IDO induction
from Tat proteins in HIV infection (42). In HIV patients, it has been
observed that elevated IDO enzymatic activity in APCs is negatively
associated with Th22:Treg and Th17:Treg ratios in the
anti-retroviral therapy-naive group, suggesting that imbalance of
the ratio of Th22/Th17 to Tregs may contribute to widespread immune
dysfunction in HIV-1 infection (43).
Strategies to target IDO
Currently, a broad range of candidate compounds have
been developed as IDO-inhibitors for clinical application. Of the
IDO inhibitors, 1-methyl-DL-tryptophan (1-MT) is the most
extensively studied. There are two available stereoisomers of 1-MT;
D and L isomers. L-1MT is advantageous in suppressing the enzymatic
activity of IDO (tryptophan degradation into kynurenine) in cell
lines. The D-1MT stereoisomer induces T-cell proliferation in
allogeneic mixed lymphocyte reactions. IDO-mediated products of
tryptophan repress the immunoregulatory kinases mTOR and PKC-θ,
which may be relieved by D-1MT. D-1MT acts as a potential
tryptophan mimetic in mTOR regulation by restoration of mTOR
pathway. Conversely, the immunostimulatory effect of L-1MT is
restricted by activation of the AhR pathway in response to
production of N-methyl-kynurenine (44). Overall, D-1MT exhibits a broader
range of benefits and is therefore clinically applied to a greater
extent, compared with L-1MT.
Additionally, IDO blockade enhances the
effectiveness of chemotherapy. Mice administered with IDO-inhibitor
plus disparate chemotherapeutic agents, including cyclophosphamide,
doxorubicin, or cisplatin, congruously demonstrate smaller tumors
compared with those treated with hemotherapeutic agents alone
(45). However, it remains unclear
whether this effect may occur as a result of various roles of IDO
in restoring Treg-mediated suppression following chemotherapy.
Regarding in-situ modification of Tregs, therapeutic anti-tumor
vaccinations have improved immune responses in mice with B16
melanoma tumors (46), inducing
extensive conversion of Tregs into polyfunctional ‘reprogrammed’
IL-17 expressing Th17 cells. Other immune modulators, including
anti-CTLA-4 monoclonal antibodies, may ultimately be associated
with IDO inhibitors in therapeutic application.
Conclusions
In conclusion, IDO is a physiological host mechanism
for immunological tolerance in various settings. IDO functions at
the level of metabolic regulation and effects the activation or
inhibition of immunity and cellular metabolism via controlling
pathways including GCN2, mTOR and AhR. The pivotal role of IDO in
immune inhibition is dependent on the depletion of cellular
tryptophan levels and the generation of kynurenines that result in
T effector cell anergy and induce the proliferation of Tregs.
Therefore, regulation of IDO biosynthesis or activity in the immune
system exhibits immunological implication in various biological
processes, including cancer, transplantation and infection. The
targeting of IDO is currently applied in clinics as a therapeutic
strategy, however, further investigations are required in order to
fully elucidate the mechanisms of the various pathways affected by
IDO activation.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IDO
|
Indoleamine 2, 3-dioxygenase
|
|
APCs
|
antigen presenting cells
|
|
STAT1
|
activated signal transducer and
activator of transcription 1
|
|
NF-κB
|
nuclear factor-kappa B
|
|
iNOS
|
inducible nitric oxide synthase
|
|
mTOR
|
mammalian target of Rapamycin
|
|
GCN2
|
general control nondepressible-2
|
|
AhR
|
aryl hydrocarbon receptor
|
|
DCs
|
dendritic cells
|
|
eIF-2α
|
eukaryotic translation initiation
factor 2 α
|
|
PKC-θ
|
protein kinase C-sita
|
|
ILT3
|
Ig-like transcript 3
|
|
Tat
|
N-terminal domain of human immune
deficiency virus-1 transactivator regulatory protein
|
|
JAKs
|
janus activated kinases
|
|
PI3K
|
phosphatidyl inositol 3-kinase
|
|
1-MT
|
1-methyl-DL-tryptophan
|
|
BIN-1
|
bridging integrator 1
|
References
|
1
|
Munn DH: Indoleamine 2,3-dioxygenase,
Tregs and cancer. Curr Med Chem. 18:2240–2246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerriets VA and Rathmell JC: Metabolic
pathways in T cell fate and function. Trends Immunol. 33:168–73.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munn DH, Zhou M, Attwood JT, Bondarev I,
Conway SJ, Marshall B, Brown C and Mellor AL: Prevention of
allogeneic fetal rejection by tryptophan catabolism. Science.
281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciorba MA, Bettonville EE, McDonald KG,
Metz R, Prendergast GC, Newberry RD and Stenson WF: Induction of
IDO-1 by immunostimulatory DNA limits severity of experimental
colitis. J Immunol. 184:3907–3916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mbongue JC, Nicholas DA, Torrez TW, Kim
NS, Firek AF and Langridge WH: The Role of indoleamine 2,
3-dioxygenase in immune suppression and autoimmunity. Vaccines
(Basel). 3:703–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prendergast GC, Smith C, Thomas S,
Mandik-Nayak L, Laury-Kleintop L, Metz R and Muller AJ: Indoleamine
2,3-dioxygenase pathways of pathogenic inflammation and immune
escape in cancer. Cancer Immunol Immunother. 63:721–735. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trabanelli S, Ocadlikova D, Evangelisti C,
Parisi S and Curti A: Induction or regulatory T Cells by dendritic
cells through indoleamine 2,3-dioxygenase: A potent mechanism of
acquired peripheral tolerance. Curr Med Chem. 18:2234–2239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang L, Baban B, Johnson BA and Mellor
AL: Dendritic cells, indoleamine 2,3 dioxygenase and acquired
immune privilege. Int Rev Immunol. 29:133–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ball HJ, Sanchez-Perez A, Weiser S, Austin
CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS and
Hunt NH: Characterization of an indoleamine 2,3-dioxygenase-like
protein found in humans and mice. Gene. 396:203–213. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poormasjedi-Meibod MS, Jalili RB,
Hosseini-Tabatabaei A, Hartwell R and Ghahary A: Immuno-Regulatory
Function of Indoleamine 2,3 Dioxygenase through Modulation of
Innate Immune Responses. PLoS One. 8:e710442013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Munn DH and Mellor AL: Indoleamine 2,3
dioxygenase and metabolic control of immune responses. Trends
Immunol. 34:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nguyen NT, Kimura A, Nakahama T, Chinen I,
Masuda K, Nohara K, Fujii-Kuriyama Y and Kishimoto T: Aryl
hydrocarbon receptor negatively regulates dendritic cell
immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad
Sci USA. 107:pp. 19961–19966. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sundrud MS, Koralov SB, Feuerer M, Calado
DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek
R, Waldner H, et al: Halofuginone inhibits TH17 cell
differentiation by activating the amino acid starvation response.
Science. 324:1334–1338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baban B, Chandler PR, Johnson BA, Huang L,
Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, et
al: Physiologic control of IDO competence in splenic dendritic
cells. J Immunol. 187:2329–2335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith C, Chang MY, Parker KH, Beury DW, Du
Hadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP,
Laury-Kleintop LD, et al: IDO Is a nodal pathogenic driver of lung
cancer and metastasis development. Cancer Discov. 2:722–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaudhary K, Shinde R, Liu H,
Gnana-Prakasam JP, Veeranan-Karmegam R, Huang L, Ravishankar B,
Bradley J, Kvirkvelia N, McMenamin M, et al: Amino acid metabolism
inhibits antibody-driven kidney injury by inducing autophagy. J
Immunol. 194:5713–5724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chuang HC, Lan JL, Chen DY, Yang CY, Chen
YM, Li JP, Huang CY, Liu PE, Wang X and Tan TH: The kinase GLK
controls autoimmunity and NF-κB signaling by activating the kinase
PKC-θ in T cells. Nat Immunol. 12:1113–1138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Metz R, Rust S, Duhadaway JB, Mautino MR,
Munn DH, Vahanian NN, Link CJ and Prendergast GC: IDO inhibits a
tryptophan sufficiency signal that stimulates mTOR: A novel IDO
effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology.
1:1460–1468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaher SS, Germain C, Fu H, Larkin DF and
George AJ: 3-Hydroxykynurenine suppresses CD4+ T-cell
proliferation, induces T-regulatory-cell development, and prolongs
corneal allograft survival. Investig. Invest Ophthalmol Vis Sci.
52:2640–2648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao K, Zhou Q, Chen W, Jia W, Zheng J,
Kang J, Wang K and Duan T: Possible role of the ‘IDO-AhR axis’ in
maternal-foetal tolerance. Cell Biol Int. 37:105–108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pilotte L, Larrieu P, Stroobant V, Colau
D, Dolusic E, Frédérick R, De Plaen E, Uyttenhove C, Wouters J,
Masereel B and Van den Eynde BJ: Reversal of tumoral immune
resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl
Acad Sci USA. 109:pp. 2497–2502. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DiNatale BC, Murray IA, Schroeder JC,
Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ and Perdew GH:
Kynurenic acid is a potent endogenous aryl hydrocarbon receptor
ligand that synergistically induces interleukin-6 in the presence
of inflammatory signaling. Toxicol Sci. 115:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pallotta MT, Orabona C, Volpi C, Vacca C,
Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M,
Bicciato S, et al: Indoleamine 2,3-dioxygenase is a signaling
protein in long-term tolerance by dendritic cells. Nat Immunol.
12:870–878. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tas SW, Vervoordeldonk MJ, Hajji N,
Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML,
Tak PP and de Jong EC: Noncanonical NF-kappaB signaling in
dendritic cells is required for indoleamine 2,3-dioxygenase (IDO)
induction and immune regulation. Blood. 110:1540–1549. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Romani L, Fallarino F, De Luca A,
Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti
MC, Grohmann U, et al: Defective tryptophan catabolism underlies
inflammation in mouse chronic granulomatous disease. Nature.
451:211–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matteoli G, Mazzini E, Iliev ID, Mileti E,
Fallarino F, Puccetti P, Chieppa M and Rescigno M: Gut CD103+
dendritic cells express indoleamine 2,3-dioxygenase which
influences T regulatory/T effector cell balance and oral tolerance
induction. Gut. 59:595–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma MD, Hou DY, Liu Y, Koni PA, Metz R,
Chandler P, Mellor AL, He Y and Munn DH: Indoleamine
2,3-dioxygenase controls conversion of Foxp3? Tregs to TH17-like
cells in tumor-draining lymph nodes. Blood. 113:6102–6111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chung DJ, Rossi M, Romano E, Ghith J, Yuan
J, Munn DH and Young JW: Indoleamine 2,3-dioxygenase-expressing
mature human monocyte-derived dendritic cells expand potent
autologous regulatory T cells. Blood. 114:555–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thaker AI, Rao MS, Bishnupuri KS, Kerr TA,
Foster L, Marinshaw JM, Newberry RD, Stenson WF and Ciorba MA: IDO1
metabolites activate beta-catenin signaling to promote cancer cell
proliferation and colon tumorigenesis in mice. Gastroenterology.
145:416–425.e1-4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferns DM, Kema IP, Buist MR, Nijman HW,
Kenter GG and Jordanova ES: Indoleamine-2,3-dioxygenase (IDO)
metabolic activity is detrimental for cervical cancer patient
survival. Oncoimmunology. 4:e9814572015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johnson TS, Munn DH and Maria BL:
Modulation of tumor tolerance in primary central nervous system
malignancies. Clin Dev Immunol. 2012:9372532012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munn DH and Mellor AL: IDO and tolerance
to tumors. Trends Mol Med. 10:15–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palucka K, Banchereau J and Mellman I:
Designing vaccines based on biology of human dendritic cell
subsets. Immunity. 33:464–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brandacher G, Cakar F, Winkler C,
Schneeberger S, Obrist P, Bösmüller C, Werner-Felmayer G, Werner
ER, Bonatti H, Margreiter R and Fuchs D: Non-invasive monitoring of
kidney allograft rejection through IDO metabolism evaluation.
Kidney Int. 71:60–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun X, Gong ZJ, Wang ZW, Li T, Zhang JY,
Sun HC, Liu S, Huang L, Huang C and Peng ZH: IDO-Competent-DCs
induced by IFN-γ attenuate acute rejection in rat liver
transplantation. J Clin Immunol. 32:837–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie FT, Cao JS, Zhao J, Yu Y, Qi F and Dai
XC: IDO expressing dendritic cells suppress allograft rejection of
small bowel transplantation in mice by expansion of Foxp3+
regulatory T cells. Transpl Immunol. 33:69–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu WL, Lin YH, Xiao H, Xing S, Chen H,
Chi PD and Zhang G: Epstein-Barr virus infection induces
indoleamine 2, 3-dioxygenase expression in human monocyte-derived
macrophages through p38/mitogen-activated protein kinase and NF-κB
pathways: Impairment in T cell functions. J Virol. 88:6660–6671.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schulz S, Landi A, Garg R, Wilson JA and
van Drunen Littel-van den Hurk S: Indolamine 2,3-dioxygenase
expression by monocytes and dendritic cell populations in hepatitis
C patients. Clin Exp Immunol. 180:484–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshio S, Sugiyama M, Shoji H, Mano Y,
Mita E, Okamoto T, Matsuura Y, Okuno A, Takikawa O, Mizokami M and
Kanto T: Indoleamine-2,3-dioxygenase as an effector and an
indicator of protective immune responses in patients with acute
hepatitis B. Hepatology. 63:83–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Donovan MJ, Tripathi V, Favila MA, Geraci
NS, Lange MC, Ballhorn W and McDowell MA: Indoleamine
2,3-dioxygenase (IDO) induced by Leishmania infection of human
dendritic cells. Parasite Immunol. 34:464–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sage LK, Fox JM, Mellor AL, Tompkins SM
and Tripp RA: Indoleamine 2,3-dioxygenase (IDO) activity during the
primary immune response to influenza infection modifies the memory
T Cell response to influenza challenge. Viral Immunol. 27:112–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Planès R and Bahraoui E: HIV-1 Tat protein
induces the production of IDO in human monocyte derived-dendritic
cells through a direct mechanism: Effect on T cells proliferation.
PLoS One. 8:e745512013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Page EE, Greathead L, Metcalf R, Clark SA,
Hart M, Fuchs D, Pantelidis P, Gotch F, Pozniak A, Nelson M, et al:
Loss of Th22 cells is associated with increased immune activation
and IDO-1 activity in HIV-1 infection. J Acquir Immune Defic Syndr.
67:227–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moon YW, Hajjar J, Hwu P and Naing A:
Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J
Immunother Cancer. 3:512015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Andersen MH: The specific targeting of
immune regulation: T-cell responses against Indoleamine
2,3-dioxygenase. Cancer Immunol Immunother. 6:1289–1297. 2012.
View Article : Google Scholar
|
|
46
|
Sharma MD, Hou DY, Baban B, Koni PA, He Y,
Chandler PR, Blazar BR, Mellor AL and Munn DH: Reprogrammed
foxp3(+) regulatory T cells provide essential help to support
cross-presentation and CD8(+) T cell priming in naive mice.
Immunity. 33:942–954. 2010. View Article : Google Scholar : PubMed/NCBI
|