Introduction
Bone regeneration is a biochemical process, which is
required in response to the progression of cartilage wear and tear,
damage and deformation caused by various types of joint disease
(1). Previous studies have
investigated the abilities of various proteins and cells to
decrease inflammation associated with bone marrow, bone
regeneration, vessel walls, adipose tissue, muscle, periosteum,
tendons, peripheral circulation, umbilical cord blood, skin and
dental tissues (2–4). In recent years, research has focused
on the molecular mechanisms underlying bone regeneration and
fracture repair (5,6). In addition, extracellular signaling
molecules, including platelet-derived growth factor, fibroblast
growth factor (FGF) and vascular endothelial growth factor (VEGF)
have been reported to promote fracture healing and bone
regeneration (7). Furthermore,
bone-healing processes, including vascularization, inflammatory
inhibition and mesenchymal cell entry to the fracture site, have
been reported to be mediated by extracellular signaling molecules
(8). These findings indicate that
targeted bone production and regeneration may contribute to the
reduction of convalescence following treatment of fractures
(1,9). The present study investigated the
role of the extracellular signaling molecule hepatocyte growth
factor (HGF) in inflammation, osteoblast and osteoclast balance,
bone production and bone regeneration in a mouse fracture model. In
addition, bone morphogenetic protein (BMP)-2 production and
alterations in the nuclear factor (NF)-κB signaling pathway were
detected in the mouse model.
HGF is produced by mesenchymal cells during organ
injury and serves a crucial role in the process of bone
regeneration (10). HGF is an
active biological factor that exerts multifunctional effects on
osteogenesis, and also serves a critical role in kidney
development, acute injury and regeneration; HGF is activated by
proteolytic cleavage at the site of injury resulting in formation
of the biologically active HGF protein (11). A previous study reported that
levels of HGF in the serum were correlated with quality of life in
patients undergoing hemodialysis (12). In addition, biologically active HGF
can suppress fibrosis; the molecular basis for HGF-mediated
regression of renal fibrosis was elaborated in a previous report
(13,14). Therefore, it was hypothesized that
HGF may be regarded as a local acute phase protein that is
beneficial for bone regeneration in various bone diseases. The
results of the present study suggested that HGF promoted bone
regeneration via regulation of BMP-2 expression in osteocytes in
vitro and in vivo.
BMPs are members of the transforming growth factor-β
superfamily, which regulate numerous cellular activities in bone
regeneration (15). A previous
study on BMP-2-releasing gelatin/β-tricalcium phosphate sponges
revealed that BMP-2 may be considered a potential protein for the
induction of bone regeneration in segmental bone defects (16). In addition, it has been reported
that BMP-2 serves an important role in various forms of arthritis
and disease activity. Grcevic et al (17) indicated that peripheral blood
expression profiles of BMPs may be used as markers for arthritis,
disease activity, therapeutic responsiveness and prognosis. Lories
and Luyten also suggested that BMPs are beneficial for the repair
of joint destruction and tissue responses that determine the
outcome of chronic arthritis (18). Furthermore, BMP-2 is a member of
the BMP family, which has been reported to possess high potential
for bone formation, inflammatory inhibition in joints and synovial
repair (19,20). In the present study, the in
vivo effects and molecular mechanisms of BMP-2 were
investigated in a mouse fracture model. Inflammation was also
analyzed.
The present study aimed to investigate the
association between bone tissue regeneration and inflammation in a
mouse model of fracture. The results indicated that HGF treatment
markedly promoted bone tissue regeneration and inhibited the
expression of inflammatory factors. Notably, the present study
demonstrated that HGF improves bone tissue regeneration via the
BMP-2-mediated nuclear factor (NF)-κB signaling pathway, thus
suggesting that HGF may be considered a promising agent for the
treatment of patients with fractures.
Materials and methods
Ethics statement
The present study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals at Chengde Medical College Affiliated Hospital
(Chengde, China). The study was approved by the ethics committee of
Chengde Medical College Affiliated Hospital. All surgeries and
euthanasia were performed under intravenous sodium pentobarbital
anesthesia (37 mg/kg).
Animal study
A total of 20 male C57BL/6J mice (6–8 weeks, 28–35
g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). All animals were housed in a
temperature-controlled facility at 23±1°C with a relative humidity
of 50±5%, a 12 h light/dark cycle and unlimited access to food and
water. Mice were subjected to an artificial fracture in the right
paw as described previously (21).
The mice were divided into two groups and were maintained under a
normal schedule with free access to standard diet and water. On day
2 after model establishment, mice received treatment with HGF (10
mg/kg; Sigma Aldrich; Merck KGaA, Darmstadt, Germany) or the same
volume of PBS (control) by intravenous injection. The body weights
of the experimental mice were measured prior to each injection.
Each mouse received 15 daily treatments. All mice were sacrificed
on day 30 for histological analysis. Osteoblasts and osteoclasts
were obtained from experimental mice and then isolated from one
another as described previously (22,23)
on day 30.
Cell culture
Osteocytes were obtained from mice with fractures
prior to treatment as described previously (22,23)
and then cultured in minimum essential media (MEM, Sigma-Aldrich;
Merck KGaA) and supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA). Osteocytes were then treated with HGF
(2 mg/ml) and cultured in a humidified incubator containing 5%
CO2 for 24 h at 37°C.
MTT assay
Osteoblasts and osteoclasts were separately cultured
in Dulbecco's Modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) and supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) for 24 h at 37°C.
Osteoblasts and osteoclasts were then incubated with HGF (2 mg/ml)
in a 96-well plate for 24 h at 37°C. A total of 20 µl MTT (5 mg/ml)
in PBS solution was added to each well and cells were then
incubated for a further 4 h at 37°C. Medium was then removed and
100 µl dimethyl sulfoxide was added to dissolve the formazan
crystals. The viability of the cells was then determined using an
ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a
wavelength of 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from osteocytes using the
RNA Easy Mini Extract kit (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's protocol. RNA was reversed transcribed using a
PrimeScript RT Master Mix kit (Takara Bio, Inc., Otsu, Japan).
Subsequently, cDNA (10 µg) was diluted 1/10 with distilled water
and 10 µl was used for amplification. Specific primer sets for C-C
motif chemokine ligand 2 (Ccl)2, Ccl5 and intercellular adhesion
molecule 1 (Icam1) were conserved in the laboratory (Table I). RT-qPCR was performed using a
qPCR system (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with SYBR Green Master Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. A
total of 45 amplification cycles were performed, including 94°C for
30 sec, denaturation at 96°C for 5 sec, primer annealing at 64°C
for 5 sec, touchdown to 56°C for 15 sec and extension at 72°C for
10 sec. Relative mRNA expression levels were then determined using
the 2−ΔΔCq method (24). The final results were presented as
the fold of β-actin.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Forward primer | Reverse primer |
|---|
| Ccl2 |
5′-AAGAAGCTGTAGTATTTGTCACCAAGCTCA-3′ |
5′-CATCAGGTACGATCCAGGCT-3′ |
| Ccl5 |
5′-GCAGCTGCATCCCTCACCGT-3′ |
5′-GCAGCAGGGAGTGGTGTCCG-3′ |
| Icam1 |
5′-GCTGTGCTTTGAGAACTGTG-3′ |
5′-GTGAGGTCCTTGCCTACTTG-3′ |
| β-actin |
5′-GTGGGCGCCCAGGCACCA-3′ |
5′-CTCCTTAATGTCACGCACGATTT-3′ |
Osteocyte migration assay
Osteocytes were obtained from mice with fractures,
and osteoblasts and osteoclasts were isolated from mice that had
undergone fracture prior to treatment as described in a previous
study (25). Osteoblasts and
osteoclasts were suspended at a density of 1×106 in 100
µl serum-free MEM for 12 h. The cells were then seeded into the
upper chambers of a BD BioCoat Matrigel Invasion Chamber (BD
Biosciences, Franklin Lakes, NJ, USA) and then incubated with HGF
(2 mg/ml) for 12 h at 37°C. The migration assay was conducted
according to the manufacturer's protocol.
Determination of NF-κB activity
Osteoblasts (1×106 cells) and osteoclasts
(1×106 cells) were separately cultured in 6-well plates
and incubated with either HGF (2 mg/ml) or PBS for 48 h. The
efficacy of HGF on NF-κB activity was analyzed by determination of
NF-κB-luciferase activity in cells. Briefly, cells were transfected
with a pNF-κB-luciferase vector (Promega Corporation, Madison, WI,
USA) for 48 h at 37°C using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to
manufacturer's instructions. Luciferase activity in the cells was
determined 72 h post-transfection at 37°C by using the
Dual-Luciferase Reporter Assay System (Promega Corporation)
following the manufacturer's protocol. Relative luciferase activity
was determined by analyzing the firefly luciferase activity and
normalizing it with the Renilla luciferase activity.
Cells differentiation
Osteoblasts and osteoclasts were obtained from mice
with fractures prior to treatment and then separately cultured with
MEM medium supplemented with 10% FBS. Cells were incubated with HGF
(2 mg/ml) or the same volume of PBS (2 mg/ml) and then cultured for
72 h at 37°C. Analysis of tartrateresistant acid phosphatase
activity was used to determine osteoblast and osteoclast
differentiation.
Western blotting
Osteocytes were obtained from mice with fractures
and were homogenized in lysis buffer containing protease inhibitor
to perform protein extraction (Sigma-Aldrich; Merck KGaA), after
which the cells were centrifuged at 6,000 × g at 4°C for 10 min.
The supernatant was used for protein analysis. Protein
concentration was determined using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Protein samples (10 µg) were separated
using 12.5% SDS-PAGE, as described in a previous study (23), and then transferred to
polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica,
MA, USA). For western blotting, following blocking with 5% skimmed
milk for 1 h at 37°C, primary antibodies: VEGF (cat. no. ab32152;
1:1,000; Abcam, Cambridge, UK), BMP-2 receptor [(R); cat. no.
ab38560; 1:1,000; Abcam)], macrophage colony-stimulating factor
(M-CSF; cat. no. ab9693; 1:1,000; Abcam), receptor activator of
NF-κB ligand (RANKL; cat. no. ab216484; 1:1,000; Abcam) and β-actin
(cat. no. ab8226; 1:1,000; Abcam) were then incubated with PVDF
membranes for 12 h at 4°C. Subsequently, membranes were incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
mAb (cat. no. PV-6001; 1:5,000; OriGene Technologies, Inc.,
Beijing, China) for 24 h at 4°C. Following this, protein bands were
visualized using Western Bright Enhanced Chemiluminescent HRP
Substrate (Advansta, Inc., Menlo Park, CA, USA).
Small interfering (si)RNA
transfections
Osteocytes (1×106 cells) were cultured to
80% confluence and were transfected with siRNA sequences (100 pM;
Invitrogen; Thermo Fisher Scientific, Inc.) that targeted BMP-2:
si-BMP-2 sense, 5′-GUGCUAUCUCGAUGCUGUATT-3′ and antisense,
5′-AAUACAGCAUCGAGAUAGCAC-3′; or scramble siRNA sense,
5′-GUGCUAUCUCGAUGCUGUA-3′ and antisense,
5′-UACAGCAUUCGAGAUAGCAC-3′. Transfection was performed using
Lipofectamine® RNAi MAX (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
were cultured for 48 h at 37°C following transfection for further
analysis.
Flow cytometry
On day 30, serum was obtained from experimental mice
via centrifugation at 6,000 × g for 15 min at 4°C. Serum levels of
lymphocytes, plasmacytes, neutrophils and monocytes in mice with
fracture were analyzed by flow cytometry. All procedures were
performed as described in a previous study (26). In addition, the ratios of apoptotic
cells were determined using a Coulter EPICS XL Flow Cytometer
(Beckman Coulter, Inc., Brea, CA, USA), and results were analyzed
using Expo32-ADC v. 1.2B software (Beckman Coulter, Inc.).
ELISA
On day 30, to detect serum protein expression levels
of inflammatory factors, mouse tumor necrosis factor (TNF)-α (cat.
no. MTA00B; Bio-Rad Laboratories, Inc.), monocyte chemotactic
protein (MCP)-1 (cat. no. CPCA00; Bio-Rad Laboratories, Inc.),
interleukin (IL)-1 (cat. no. MLB00C; Bio-Rad Laboratories, Inc.)
and IL-6 (cat. no. M6000B; Bio-Rad Laboratories, Inc.) ELISA kits
were used. The experiments were conducted according to the
manufacturer's protocols. Finally, absorbance of the samples was
measured at 450 nm using an ELISA plate reader.
Histological analysis
To determine the therapeutic effects of HGF on a
mouse model of fracture, the mice were sacrificed under
pentobarbital anesthesia on day 30 and the bone tissues located in
the fracture were separated and fixed in 10% formalin for 30 min at
37°C. The tissues were subsequently decalcified and embedded in
paraffin for 2 h at 37°C. Paraffin-embedded bone tissues sections
(4 µg) from experimental mice were stained with hematoxylin and
eosin for 2 h at 37°C. Images were obtained using an inverted light
microscope (Olympus Corporation, Tokyo, Japan).
Vascular density measurements and
evaluation of bone resorption activity
Vascular density measurements and bone resorption
activity were analyzed in mice following treatment with HGF,
according to methods described in previous studies (27,28).
Statistical analysis
All data are presented as the mean ± standard
deviation of triplicate experiments. All data were analyzed using
SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA). Unpaired data
were analyzed using Student's t-test P<0.05 was considered to
indicate a statistically significant difference.
Results
In vitro effects of HGF treatment on
migration and differentiation of osteocytes from a mouse model of
fracture
The present study analyzed the effects of HGF on
migration and differentiation of osteoblasts and osteoclasts from
osteocytes obtained from mice with fracture. As shown in Fig. 1A, HGF promoted cell viability of
osteoblasts and inhibited the cell viability of osteoclasts. A
migration assay indicated that HGF treatment slightly enhanced
migration of osteoblasts; however, no effects were detected on
osteoclast migration (Fig. 1B). In
addition, the results demonstrated that HGF promoted
differentiation of osteoblasts and inhibited differentiation of
osteoclasts at the fracture location (Fig. 1C). Furthermore, the balance of
osteoblasts and osteoclasts (Ob/Oc) was improved in the fracture
location (Fig. 1D). Taken
together, these results suggested that HGF treatment may regulate
migration and differentiation of osteoblasts and osteoclasts in a
mouse model of fracture.
Effects of HGF treatment on
inflammatory factors and biochemical analysis in a mouse model of
fracture
A previous study indicated that aggravation of
inflammatory responses can inhibit bone regeneration (29). Therefore, the present study
detected inflammatory factors and biochemical indicators in a mouse
model of fracture following treatment with HGF. As shown in
Fig. 2A-D, the expression levels
of TNF-α, MCP-1, IL-1 and IL-6 were downregulated in serum samples
from HGF-treated experimental mice. Biochemical analysis indicated
that the percentage of lymphocytes, plasmacytes, neutrophils and
monocytes were also decreased in mice treated with HGF compared
with PBS (Fig. 2E-H). These
results suggested that HGF may be beneficial for the treatment of
fractures in a mouse model via the downregulation of inflammatory
responses.
Effects of HGF treatment on the
expression levels of extracellular signaling molecules in a mouse
model of fracture
After analyzing the alterations in inflammatory
responses in a mouse model of fracture, the present study
investigated the expression levels of extracellular signaling
molecules. As presented in Fig. 3,
the expression levels of VEGF, BMP-2R, RANKL and M-CSF were
upregulated in the fracture location of HGF-treated mice.
HGF-induced expression of extracellular signaling molecules may
contribute to bone regeneration and bone healing in a mouse model
of fracture. Taken together, these results suggested that HGF
treatment increased the expression levels of extracellular
signaling molecules in osteocytes in a mouse model of fracture.
Analysis of the mechanism underlying
HGF-mediated NF-κB signaling in a mouse model of fracture
A previous study reported that NF-κB serves an
essential role in bone regeneration via the regulation of numerous
genes involved in cellular activity (30). Therefore, the present study
analyzed the association between HGF and the NF-κB signaling
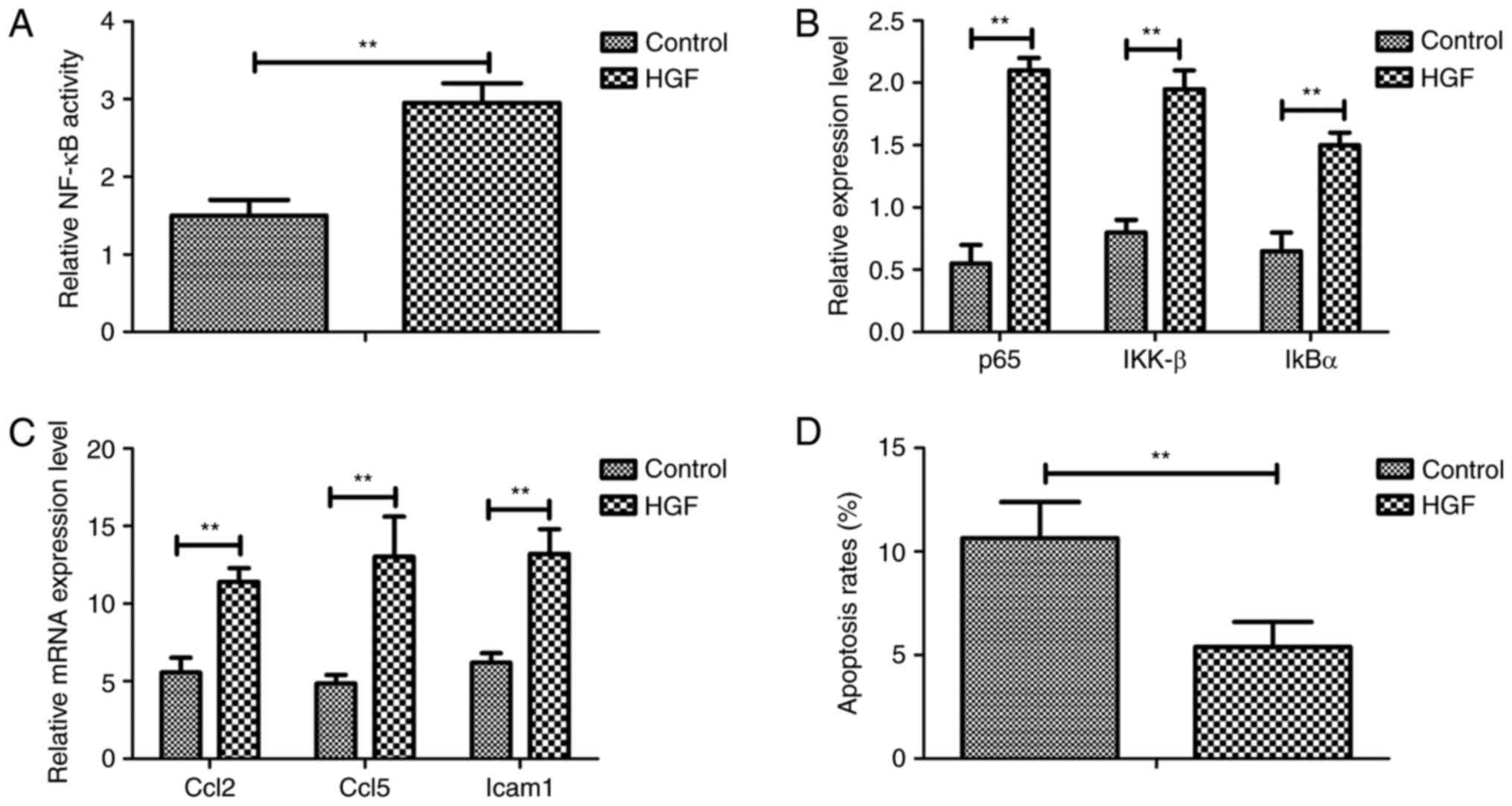
pathway in a mouse model of fracture. As shown in Fig. 4A, treatment with HGF promoted NF-κB
activity in osteocytes. In addition, p65, IKK-β and IκBα expression
levels were upregulated in osteocytes from experimental mice
treated with HGF (Fig. 4B). HGF
treatment also increased the expression of the following
anti-inflammatory genes, Ccl2, Ccl5 and Icam1, which are regulated
by the NF-κB signaling pathway, as determined by RT-qPCR (Fig. 4C). Furthermore apoptosis of
osteocytes was decreased in experimental mice treated with HGF
(Fig. 4D). Taken together, these
results indicated that HGF may regulate the physiological function
of osteocytes via regulation of the NF-κB signaling pathway.
Analysis of HGF treatment on the
expression levels of BMP-2 and revascularization-associated factors
in a mouse model of fracture
The present study investigated the expression levels
of BMP-2 and revascularization-associated factors in osteocytes
from a mouse model of fracture. As shown in Fig. 5A, HGF upregulated BMP-2 expression
in osteocytes from a mouse model of fracture. In addition, the
expression levels of epidermal growth factor and FGF were increased
in osteocytes from a mouse model of fracture (Fig. 5B). Inhibition of BMP-2 expression
using siRNA (Si-BMP-2) decreased BMP-2 protein expression and
inhibited NF-κB activity in osteocytes in vitro (Fig. 5C and D). Furthermore, the results
indicated that Si-BMP-2 suppressed the expression levels of p65,
IKK-β and IκBα in osteocytes in vitro (Fig. 5E). Taken together, these results
suggested that HGF may enhance the NF-κB pathway via regulation of
BMP-2 expression.
In vivo effects of HGF treatment on
pathological alterations in a mouse model of fracture
The in vivo effects of HGF on a mouse model
of fracture were also analyzed in the present study. As shown in
Fig. 6A, HGF treatment increased
body weight compared with in the PBS group. HGF also increased the
bone resorption activity of mice (Fig.
6B). In addition, the vascular density of the HGF group was
significantly increased compared with in the PBS group (Fig. 6C). The extent of bone regeneration
and healing was also improved in HGF-treated fractured mice
(Fig. 6D). These data suggested
that HGF treatment may markedly improve bone resorption,
neovascularization and bone regeneration, as determined by
histological staining.
Discussion
Bone regeneration serves an essential role in
fracture recovery, and is associated with bone resorption and
bone-bonding ability (31,32). Previous studies have demonstrated
that HGF is associated with cell regeneration and regulation of
material-related bone formation (33,34).
Although previous studies have indicated that HGF is constitutively
produced by donor-derived bone marrow cells and can promote
regeneration of pancreatic β-cells (35,36),
the effects of HGF on bone regeneration have not been reported in
previous studies. The present study investigated the effects, and
potential molecular mechanisms, of HGF on bone regeneration in
osteoblasts and osteoclasts in vitro and in fractured mice
in vivo. The present study also studied the effects of HGF
on inflammation in serum and apoptosis of osteocytes. The results
indicated that HGF enhances the NF-κB pathway through the
regulation of BMP-2 expression, which may markedly improve bone
resorption, neovascularization and bone regeneration in a mouse
model of fracture.
Theoretically, inflammatory cytokines are crucial
regulatory factors in fracture progression that are mediated by
various intracellular kinase signaling pathways, and regulate the
local inflammatory response following acute tibial plateau fracture
(37,38). Although a previous study reported
inflammatory/necrosis biomarkers in fractured femurs treated with
proximal femoral nail antirotation (39), the molecular mechanism has not been
clearly elaborated in previous reports. In addition, IL-6
production modulated by HGF has been investigated in bone
marrow-derived macrophages; the results revealed the role of HGF in
inflammatory response-mediated diseases (40). Furthermore, overexpression of HGF
in polycythemia vera attenuates the growth of clonal erythroblasts
independently of V617F activating mutation of janus kinase 2
(41). Therefore, blocking
inflammatory factors may interrupt the inflammatory process, thus
breaking the vicious cycle of inflammation and promoting bone
regeneration (42,43). In the present study, HGF exerted
beneficial effects on the treatment of a mouse model of fracture by
downregulation of inflammatory responses. In addition, HGF
upregulated BMP-2 expression in a mouse model of fracture.
BMPs are members of the transforming growth factor-β
superfamily, which regulate cellular metabolism, signaling pathways
and numerous cell functions, including cell proliferation,
migration, apoptosis, differentiation and adhesion (44,45).
In recent years, reports have suggested that BMP-2 may be used to
reconstruct segmental mandibular defects and effectively repair
ischemic damage by inducing angiogenesis and osteogenesis, and by
decreasing osteoclast bone resorption activity (46,47).
In the present study, the association between HGF and BMP-2 was
analyzed in a mouse model of fracture. Previous studies have
reported that HGF contributes to fracture repair by upregulating
the expression of BMPRs (48,49).
It has also been suggested that BMP-2 promotes bone formation and
osteoblastic differentiation by endochondral ossification (50,51).
In addition, downregulation of BMPR expression is exhibited in
mesenchymal cells from the joints of patients with rheumatoid
arthritis (52). Furthermore,
BMP-2 been clinically applied for spinal fusion procedures and it
has been reported effectively regulate joint inflammation and
damage (53,54). The results of the present study
confirmed that HGF is an efficient drug for promotion of bone
regeneration via BMP-2-induced NF-κB signaling.
A previous study indicated that the NF-κB signaling
pathway has an essential role in bone regeneration and
neovascularization via regulating the expression of VEGF, BMP-2,
RANKL and M-CSF expression in fracture progression (55). Notably, HGF has been reported to
preferentially stimulate NF-κB signaling, in order to protect renal
proximal tubular epithelial cells against inflammation (43). In addition, BMP-2 exerts regulatory
effects on apoptosis of chondrocytes, which serves a crucial role
in the survival of chondrocytes (56). Furthermore, the NF-κB signaling
pathway may stimulate BMP-2 gene expression in growth plate
chondrocytes in vivo and in a chondrocyte cell line in
vitro (57). In the present
study, HGF was revealed to stimulate BMP-2-mediated NF-κB
signaling, which may be responsible for the downregulation of the
expression of TNF-α, IL-6 and IL-1 inflammatory factors. Outcomes
have indicated that HGF-mediated NF-κB signaling may be attributed
to BMP-2 upregulation, which may promote bone regeneration.
In conclusion, the results of the present study
suggest that further investigation is required to determine the
overall role of HGF in entire joint cytokine homeostasis,
neovascularization and bone regeneration. The present findings
indicated that HGF is beneficial for bone regeneration via
increased expression of BMP-2, which leads to neovascularization
and bone regeneration through regulation of the NF-κB signaling
pathway. These preclinical data provided information that may be
useful for the future treatment of patients with fractures.
References
|
1
|
Furuya H, Tabata Y and Kaneko K: Bone
regeneration for murine femur fracture by gelatin hydrogels
incorporating basic fibroblast growth factor with different release
profiles. Tissue Eng Part A. 20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Wang Y, Gou W, Lu Q, Peng J and Lu
S: Role of mesenchymal stem cells in bone regeneration and fracture
repair: A review. Int Orthop. 37:2491–2498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorimbert V, König D, Marro J, Ruggiero F
and Jaźwińska A: Bone morphogenetic protein signaling promotes
morphogenesis of blood vessels, wound epidermis, and actinotrichia
during fin regeneration in zebrafish. FASEB J. 29:4299–4312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez RA, Kim JH, Buitrago JO, Wall IB and
Kim HW: Novel therapeutic core-shell hydrogel scaffolds with
sequential delivery of cobalt and bone morphogenetic protein-2 for
synergistic bone regeneration. Acta Biomater. 23:295–308. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rozen N, Lewinson D, Bick T, Meretyk S and
Soudry M: Role of bone regeneration and turnover modulators in
control of fracture. Crit Rev Eukaryot Gene Expr. 17:197–213. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutchison C, Pilote M and Roy S: The
axolotl limb: A model for bone development, regeneration and
fracture healing. Bone. 40:45–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hankenson KD, Gagne K and Shaughnessy M:
Extracellular signaling molecules to promote fracture healing and
bone regeneration. Adv Drug Deliv Rev. 94:3–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki A, Uemura T and Nakamura H: Control
of bone remodeling by nervous system. Neural involvement in
fracture healing and bone regeneration. Clin Calcium. 20:1820–1827.
2010.(In Japanese).
|
|
9
|
Tewari D, Khan MP, Sagar N, China SP,
Singh AK, Kheruka SC, Barai S, Tewari MC, Nagar GK, Vishwakarma AL,
et al: Ovariectomized rats with established osteopenia have
diminished mesenchymal stem cells in the bone marrow and impaired
homing, osteoinduction and bone regeneration at the fracture site.
Stem Cell Rev. 11:309–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramezani A, Nägga K, Hansson O, Lönn J,
Sjöwall J, Katoozian F, Mansouri S and Nayeri F: Hepatocyte growth
factor in cerebrospinal fluid differentiates community-acquired or
nosocomial septic meningitis from other causes of pleocytosis.
Fluids Barriers CNS. 12:222015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faletto DL, Kaplan DR, Halverson DO, Rosen
EM and Vande Woude GF: Signal transduction in c-met mediated
motogenesis. EXS. 65:107–130. 1993.PubMed/NCBI
|
|
12
|
Baum E, Pawlaczyk K, Mackowiak B,
Maćkowiak B, Sosinska P, Matecka M, Kolodziejczak B, Musielak M and
Breborowicz A: Levels of hepatocyte growth factor in serum
correlate with quality of life in hemodialysis patients. Int J Clin
Exp Pathol. 8:13477–13482. 2015.PubMed/NCBI
|
|
13
|
Mizuno S and Nakamura T: Molecular basis
for HGF-mediated regression of renal fibrosis. Nihon Rinsho. 64
Suppl 2:S312–S321. 2006.
|
|
14
|
Zhang SH, Wen KM, Wu W, Li WY and Zhao JN:
Efficacy of HGF carried by ultrasound microbubble-cationic
nano-liposomes complex for treating hepatic fibrosis in a bile duct
ligation rat model, and its relationship with the
diffusion-weighted MRI parameters. Clin Res Hepatol Gastroenterol.
37:602–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh K, Massel DH, Mayo BC, Bohl DD, Long
WW and Modi KD: Bone morphogenetic proteins in lumbar arthrodesis:
What is all the debate about? Commentary on an article by Daniel C.
Beachler, PhD, MHS et al: ‘Bone morphogenetic protein use and
cancer risk among patients undergoing lumbar arthrodesis: A
case-cohort study using the SEER-medicare database’. J Bone Joint
Surg Am. 98:e572016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto M, Hokugo A, Takahashi Y, Nakano
T, Hiraoka M and Tabata Y: Combination of BMP-2-releasing
gelatin/β-TCP sponges with autologous bone marrow for bone
regeneration of X-ray-irradiated rabbit ulnar defects.
Biomaterials. 56:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grcevic D, Jajic Z, Kovacic N, Lukic IK,
Velagic V, Grubisic F, Ivcevic S and Marusic A: Peripheral blood
expression profiles of bone morphogenetic proteins, tumor necrosis
factor-superfamily molecules, and transcription factor Runx2 could
be used as markers of the form of arthritis, disease activity, and
therapeutic responsiveness. J Rheumatol. 37:246–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lories RJ and Luyten FP: Bone
morphogenetic proteins in destructive and remodeling arthritis.
Arthritis Res Ther. 9:2072007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Postigo J, Iglesias M, Álvarez P, Jesús
Augustin J, Buelta L, Merino J and Merino R: Bone morphogenetic
protein and activin membrane-bound inhibitor, a transforming growth
factor β rheostat that controls murine treg cell/Th17 cell
differentiation and the development of autoimmune arthritis by
reducing interleukin-2 signaling. Arthritis Rheumatol.
68:1551–1562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brescia AC, Simonds MM, McCahan SM,
Fawcett PT and Rose CD: The role of transforming growth factor β
signaling in fibroblast-like synoviocytes from patients with
oligoarticular juvenile idiopathic arthritis: Dysregulation of
transforming growth factor β signaling, including overexpression of
bone morphogenetic protein 4, may lead to a chondrocyte phenotype
and may contribute to bony hypertrophy. Arthritis Rheumatol.
66:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holstein JH, Schmalenbach J, Herrmann M,
Ölkü I, Garcia P, Histing T, Herrmann W, Menger MD, Pohlemann T and
Claes L: Excess dietary methionine does not affect fracture healing
in mice. Med Sci Monit. 18:BR469–BR474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan Q, Schoenmaker T, Jansen ID, Bian Z,
de Vries TJ and Everts V: Osteoblasts of calvaria induce higher
numbers of osteoclasts than osteoblasts from long bone. Bone.
86:10–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aino M, Nishida E, Fujieda Y, et al:
Isolation and characterization of the human immature osteoblast
culture system from the alveolar bones of aged donors for bone
regeneration therapy. Expert Opin Biol Ther. 14:1731–1744. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bajnok A, Kaposi A, Kovács L, Vásárhelyi
B, Balog A and Toldi G: Analysis by flow cytometry of calcium
influx kinetics in peripheral lymphocytes of patients with
rheumatoid arthritis. Cytometry A. 83:287–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kinsella S, Murphy K, Breen M, O'Neill S,
McLaughlin P, Coyle J, Bogue C, O'Neill F, Moore N, McGarrigle A,
et al: Comparison of single CT scan assessment of bone mineral
density, vascular calcification and fat mass with standard clinical
measurements in renal transplant subjects: The ABC HeART study. BMC
Nephrol. 16:1882015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morimoto Y, Hoshino H, Sakurai T, Terakawa
S and Nagano A: Quantitative evaluation of bone resorption activity
of osteoclast-like cells by measuring calcium phosphate resorbing
area using incubator-facilitated and video-enhanced microscopy.
Microsc Res Tech. 72:317–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas MV and Puleo DA: Infection,
inflammation, and bone regeneration: A paradoxical relationship. J
Dent Res. 90:1052–1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mallavia B, Recio C, Oguiza A, Ortiz-Muñoz
G, Lazaro I, Lopez-Parra V, Lopez-Franco O, Schindler S, Depping R,
Egido J and Gomez-Guerrero C: Peptide inhibitor of NF-κB
translocation ameliorates experimental atherosclerosis. Am J
Pathol. 182:1910–1921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xinluan W, Yuxiao L, Helena NH, Zhijun Y
and Ling Q: Systemic drug delivery systems for bone tissue
regeneration-a mini review. Curr Pharm Des. 21:1575–1583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gibbs DM, Black CR, Dawson JI and Oreffo
RO: A review of hydrogel use in fracture healing and bone
regeneration. J Tissue Eng Regen Med. 10:187–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tomson PL, Lumley PJ, Alexander MY, Smith
AJ and Cooper PR: Hepatocyte growth factor is sequestered in
dentine matrix and promotes regeneration-associated events in
dental pulp cells. Cytokine. 61:622–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Madonna R, Cevik C, Nasser M and De
Caterina R: Hepatocyte growth factor: Molecular biomarker and
player in cardioprotection and cardiovascular regeneration. Thromb
Haemost. 107:656–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin SZ, Meng XW, Sun X, Han MZ, Liu BR,
Wang XH and Pei FH: Hepatocyte growth factor promotes liver
regeneration induced by transfusion of bone marrow mononuclear
cells in a murine acute liver failure model. J Hepatobiliary
Pancreat Sci. 18:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Izumida Y, Aoki T, Yasuda D, Koizumi T,
Suganuma C, Saito K, Murai N, Shimizu Y, Hayashi K, Odaira M, et
al: Hepatocyte growth factor is constitutively produced by
donor-derived bone marrow cells and promotes regeneration of
pancreatic beta-cells. Biochem Biophys Res Commun. 333:273–282.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harvey EJ: Knee trauma and posttraumatic
osteoarthritis-more science needed: Commentary on an article by
Justin M. Haller, MD et al: ‘Inflammatory cytokine response
following acute tibial plateau fracture’. J Bone Joint Surg Am.
97:e332015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haller JM, McFadden M, Kubiak EN and
Higgins TF: Inflammatory cytokine response following acute tibial
plateau fracture. J Bone Joint Surg Am. 97:478–483. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marino M, Palmieri G, Peruzzi M, Scuderi F
and Bartoccioni E: A study of inflammatory/necrosis biomarkers in
the fracture of the femur treated with proximal femoral nail
antirotation. Mediators Inflamm. 2015:1898642015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coudriet GM, He J, Trucco M, Mars WM and
Piganelli JD: Hepatocyte growth factor modulates interleukin-6
production in bone marrow derived macrophages: Implications for
inflammatory mediated diseases. PLoS One. 5:e153842010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boissinot M, Cleyrat C, Vilaine M, Jacques
Y, Corre I and Hermouet S: Anti-inflammatory cytokines hepatocyte
growth factor and interleukin-11 are over-expressed in Polycythemia
vera and contribute to the growth of clonal erythroblasts
independently of JAK2V617F. Oncogene. 30:990–1001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Katz MS, Thatch KA and Schwartz MZ:
Hepatocyte growth factor and omega-3-enriched feeds have a
synergistic effect on mucosal mass in an animal model of
inflammatory bowel disease. J Pediatr Surg. 47:194–198. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
da Silva CG, Maccariello ER, Wilson SW,
Putheti P, Daniel S, Damrauer SM, Peterson CR, Siracuse JJ,
Kaczmarek E and Ferran C: Hepatocyte growth factor preferentially
activates the anti-inflammatory arm of NF-κB signaling to induce
A20 and protect renal proximal tubular epithelial cells from
inflammation. J Cell Physiol. 227:1382–1390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sasikumar KP, Elavarasu S and Gadagi JS:
The application of bone morphogenetic proteins to periodontal and
peri-implant tissue regeneration: A literature review. J Pharm
Bioallied Sci. 4 Suppl 2:S427–S430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: A critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hustedt JW and Blizzard DJ: The
controversy surrounding bone morphogenetic proteins in the spine: A
review of current research. Yale J Biol Med. 87:549–561.
2014.PubMed/NCBI
|
|
47
|
Dagostino PR, Whitmore RG, Smith GA,
Maltenfort MG and Ratliff JK: Impact of bone morphogenetic proteins
on frequency of revision surgery, use of autograft bone, and total
hospital charges in surgery for lumbar degenerative disease: Review
of the Nationwide inpatient sample from 2002 to 2008. Spine J.
14:20–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Imai Y, Terai H, Nomura-Furuwatari C,
Mizuno S, Matsumoto K, Nakamura T and Takaoka K: Hepatocyte growth
factor contributes to fracture repair by upregulating the
expression of BMP receptors. J Bone Miner Res. 20:1723–1730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ye L, Lewis-Russell JM, Davies G, Sanders
AJ, Kynaston H and Jiang WG: Hepatocyte growth factor up-regulates
the expression of the bone morphogenetic protein (BMP) receptors,
BMPR-IB and BMPR-II, in human prostate cancer cells. Int J Oncol.
30:521–529. 2007.PubMed/NCBI
|
|
50
|
Okubo Y, Bessho K, Fujimura K, Kusumoto K,
Ogawa Y and Iizuka T: Osteogenesis by recombinant human bone
morphogenetic protein-2 at skeletal sites. Clin Orthop Relat Res.
1–301. 2000.
|
|
51
|
Ozeç Y, Oztürk M, Kýlýç E, Yeler H, Göze F
and Gümüş C: Effect of recombinant human bone morphogenetic
protein-2 on mandibular distraction osteogenesis. J Craniofac Surg.
17:80–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Marinova-Mutafchieva L, Taylor P, Funa K,
Maini RN and Zvaifler NJ: Mesenchymal cells expressing bone
morphogenetic protein receptors are present in the rheumatoid
arthritis joint. Arthritis Rheum. 43:2046–2055. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Issa JP, do Nascimento C, Lamano T,
Iyomasa MM, Sebald W and de Albuquerque RF Jr: Effect of
recombinant human bone morphogenetic protein-2 on bone formation in
the acute distraction osteogenesis of rat mandibles. Clin Oral
Implants Res. 20:1286–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yonezawa H, Harada K, Ikebe T, Shinohara M
and Enomoto S: Effect of recombinant human bone morphogenetic
protein-2 (rhBMP-2) on bone consolidation on distraction
osteogenesis: A preliminary study in rabbit mandibles. J
Craniomaxillofac Surg. 34:270–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang J, Wang K, Shi Z and Zhang M:
Osteoprotegerin mRNA/receptor activator of NF-kappaB ligand mRNA
expressions in bone tissues of glucocorticoid-induced osteonecrosis
of the femoral head. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
22:1161–1164. 2008.(In Chinese). PubMed/NCBI
|
|
56
|
Sugimori K, Matsui K, Motomura H, Tokoro
T, Wang J, Higa S, Kimura T and Kitajima I: BMP-2 prevents
apoptosis of the N1511 chondrocytic cell line through
PI3K/Akt-mediated NF-kappaB activation. J Bone Miner Metab.
23:411–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feng JQ, Xing L, Zhang JH, Zhao M, Horn D,
Chan J, Boyce BF, Harris SE, Mundy GR and Chen D: NF-kappaB
specifically activates BMP-2 gene expression in growth plate
chondrocytes in vivo and in a chondrocyte cell line in vitro. J
Biol Chem. 278:29130–29135. 2003. View Article : Google Scholar : PubMed/NCBI
|