Introduction
Spinal cord injury (SCI) is a clinically-common form
of accidental damage, due to the development of the social economy
and increasing use of motor vehicles. Cases of SCI caused by work
and traffic accidents are increasing (1). It has been reported that there are
>300,000 new cases of SCI worldwide every year, predominantly in
young adults (2). However, no
accurate statistics are available in China at present. Data
published in 2013 indicated that the number of patients with SCI
patients totaled >1,000,000, with an average annual increase of
10,000 (3). The treatment of SCI
is difficult and is associated with high rates of disability
(3). SCI represents a
psychological and economic burden for patients, their families and
society. The treatment of SCI has been a focus of research
worldwide (4). Existing clinical
treatment methods for SCI include surgical decompression, topical
drugs, acupuncture, local cryoablation, connective tissue and
scarring elimination, and physical rehabilitation. Although these
treatments may be effective, a number of long-term neurological
sequelae may occur (4,5).
SCI encompasses primary and sequential damage. The
primary damage is instant and irreversible, and may directly lead
to cell death at the site of injury. Effective intervention may be
challenging to deliver in the clinic (6). Sequential injury occurs within
several h or days, primarily as chronic reactive damage to the
spinal cord (7). In addition, the
injury may progress in a sustained manner (8). Sequential SCI causes more severe
damage to the spinal cord compared with primary injury (9). The sequential injury mechanisms of
the spinal cord primarily include inflammatory reactions,
neurogenic shock, cellular apoptosis, excitatory poisoning,
mitochondrial dysfunction, free radicals and reperfusion injury
(10).
Toll-like receptors (TLRs) are a type of pattern
recognition receptor, which were originally detected in
Drosophila embryos (11).
Subsequently, homologous receptors were identified in mammals.
These receptors are collectively known as the TLR family. It has
been determined that the TLR family includes at least 12 members
(12). TLRs are extensively
distributed on the surface of macrophages, monocytes, dendritic
cells, natural killer cells and lymphocytes (12). The TLR4 signal pathway has been
extensively studied, due to its role in mediating inflammatory
reactions (13). Previous studies
have demonstrated that microglial cells express TLR1-TLR9,
primarily TLR4; it was identified through culturing of neuronal
cells, microglial cells, oligodendrocytes and astrocytes taken from
the prosencephalon of neonatal mice that TLR4 is highly-expressed
on the surface of microglial cells (14,15).
Scirpus yagara is classified as a small grass
in the family Cyperaceae; it is the dry lotus of S.
fluviatilis (Torr.) A. Gray (S. yagara Ohwi) (14). S. yagara is bitter in taste
and is believed to exhibit pharmacological functions, following the
principles of traditional Chinese medicine, including alleviating
stagnated blood, promoting the circulation of qi, and relieving
dyspepsia and pain (15). S.
yagara may be applied to the treatment of abscesses,
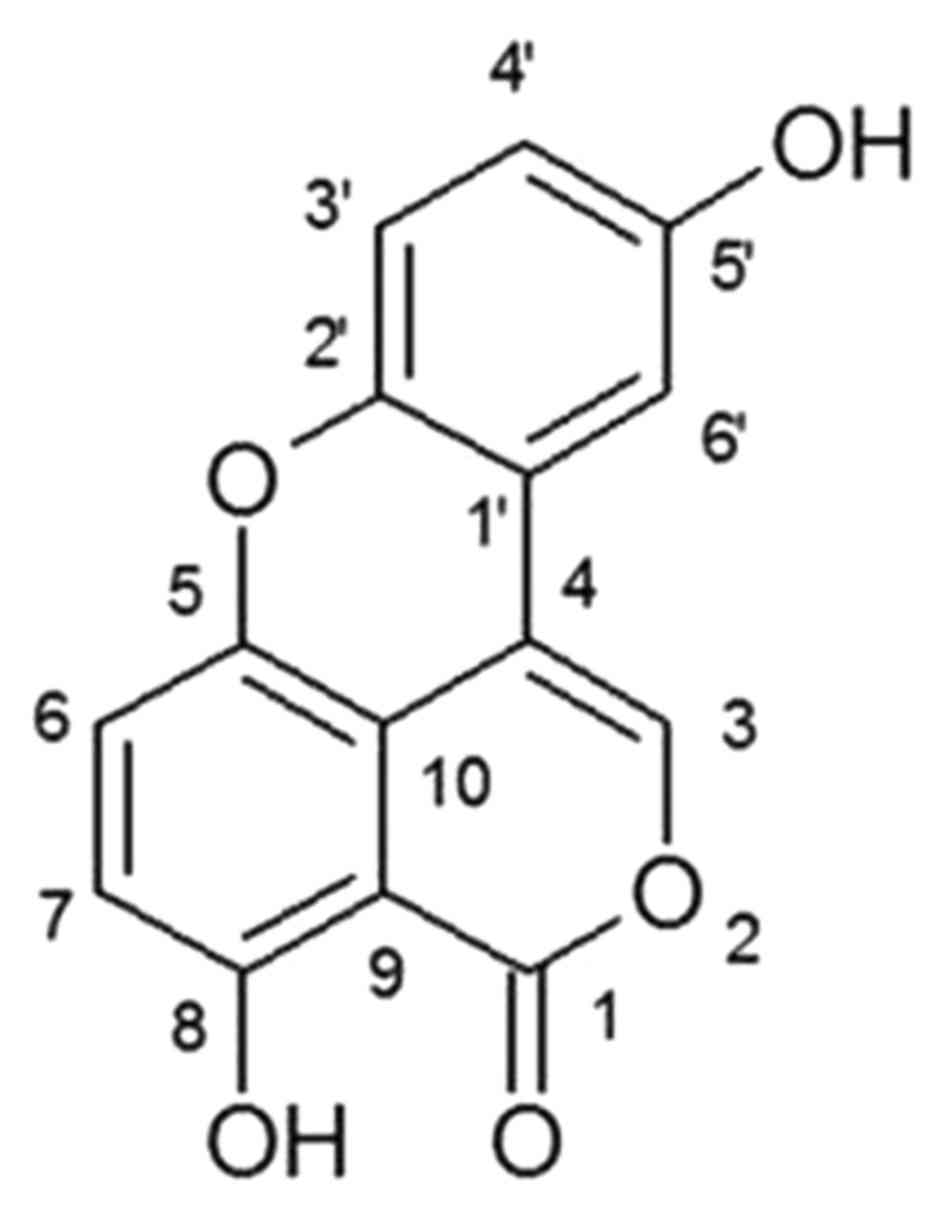
congestion, amenorrhea and pain associated with dyspepsia (16). Sparstolonin B (Fig. 1) is a novel oxygen-mixed anthracene
compound detected in a traditional Chinese medicine, Sparganium
stoloniferum (15), and is
additionally present in S. yagara. A previous study
demonstrated that sparstolonin B is a low-toxicity TLR2 and
TLR4-selective antagonist, which is able to inhibit inflammation
induced by lipopolysaccharide in in vivo mouse models
(16). Therefore, sparstolonin B
has been hypothesized to be a potential novel treatment for
inflammation and associated diseases (17). In the present study, the potential
protective effect of sparstolonin B against inflammation and
apoptosis in an SCI model was examined, and the molecular mechanism
underlying the effects of sparstolonin B in SCI was investigated by
studying alterations in TLR4 signaling pathways.
Materials and methods
Ethics and animals
Male Sprague-Dawley rats (n=30; weight, 200–230 g;
age, 6 weeks) were purchased from Experiment Center of Tianjin
Medical University (Tianjin, China) and were housed under standard
environmental conditions (humidity, 45–55%; temperature, 22–23°C,
7-h light/19-h dark cycle) and maintained on a normal rodent diet
and tap water ad libitum. The present study was approved by
the institutional Animal Care and Use Committee of Tianjin People's
Hospital (Tianjin, China) and was performed according to the
principles of the National Institutes of Health Guide for the Care
and Use of Laboratory Animals (National Institutes of Health,
Bethesda, MD, USA).
Rats were anesthetized using xylazine hydrochloride
(5 mg/kg) and ketamine hydrochloride (75 mg/kg). The back of each
rat was cleaned with povidone iodine (10%) and a laminectomy was
performed at T9-T11 to expose the underlying T10 spinal cord,
following a dorsomedial incision to the skin. The spinal cord was
exposed and the paravertebral muscles were dissected bluntly,
exposing the lamina bilaterally. A complete laminectomy was
performed at T9-T11, and the SCI model was induced at the T10
segment using an aneurysm clip (Yasargil FE 760; Aesculap, Inc.,
Corporate Parkway, PA, USA), applied extradurally for 30 sec. The
aneurysm clip was subsequently removed, and the fascia and skin
were sutured separately using silk stitches.
Experimental groups
The rats were randomly divided into three groups:
Group 1, the control group (n=6), comprised normal rats treated
with PBS; group 2, the SCI model group (n=12), comprised SCI model
rats treated with PBS; group 3, the sparstolonin B group (n=12),
comprised SCI model rats treated with 300 mg/kg sparstolonin B
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) every other day for
4 weeks.
Basso, Beattie and Bresnahan (BBB)
score and water content
Following treatment with sparstolonin B, the rats
were assessed for functional recovery using the BBB Locomotor
Rating Scale (18) and carried out
by two independent reviewers. Following sparstolonin B treatment,
rats were sacrificed using decollation under 35 mg/kg of
pentobarbital sodium. The abdomens of the rats were cut open,
spinal level T10 was peeled and spinal cord tissues were collected
and washed with PBS. Spinal cord tissues were weighed as wet
weight, then spinal cord tissue was heated at 68°C for 72 h, and
weighed as dry weight. The water content of spinal cord tissue was
calculated by (wet weight/dry weight)×100.
ELISA
Following treatment with sparstolonin B, rats were
sacrificed by decollation as above and the spinal cord acquired.
Tissue samples (50 mg) were homogenized and dissociated in
radioimmunoprecipitation assay lysis buffer (BestBio Biotechnology,
Shanghai, China) for 30 min on ice. The supernatants were collected
following centrifugation at 10,000 × g for 10 min at 4°C and used
to measure total protein using bicinchoninic acid (BCA) buffer
(BestBio Biotechnology). Protein (10 µg) was used to measure TNF-α
(E-EL-R0019c) and IFN-γ (E-EL-R0009c) levels using ELISA kits
(Elabscience, Wuhan, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Following treatment with sparstolonin B, rats were
sacrificed and the spinal cord acquired as above. Total RNA was
extracted from tissue samples using TRIzol reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA (500 ng) was used
for reverse transcription into cDNA using a First-strand cDNA
Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). qPCR
was performed using iQ SYBR Green Supermix (Bio-Rad Laboratories,
Inc.) on an Eppendorf Realplex2 Mastercycler (BioRad Laboratories,
Inc.). qPCR was performed under the following conditions: 95°C for
10 min, followed by 40 cycles of 95°C for 20 sec, 58°C for 25 sec
and 72°C for 30 sec. The miRNA expression was analyzed using the
2−ΔΔCq method (19).
Western blot analysis
Tissue samples (50 mg) were homogenized and
dissociated in radioimmunoprecipitation assay lysis buffer (BestBio
Biotechnology, Shanghai, China) for 30 min on ice. The supernatants
were collected following centrifugation at 10,000 × g for 10 min at
4°C and used to measure total protein using bicinchoninic acid
(BCA) buffer (BestBio Biotechnology). Proteins (50 µg) were
separated using SDS-PAGE on 8–10% gels and transferred onto
polyvinylidene fluoride membranes. Membranes were blocked with
Tris-buffered saline with Tween-20 (TBST) for 1 h at 37°C and
subsequently immunoblotted with anti-tumor necrosis factor (TNF)-α
(sc-8301; 1:500), interferon (IFN)-γ (sc-393089; 1:500), apoptosis
regulator Bax (Bax; sc-6236; 1:500), TLR4 (sc-10741; 1:500),
myeloid differentiation primary response protein MyD88 (MyD88;
sc-11356; 1:500), nuclear factor (NF)-κB (sc-109; 1:500) and GAPDH
(sc-25778; 1:500; all from Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at 4°C overnight. The membrane was washed with TBST and
incubated with goat anti-rabbit or anti-mouse IgG-horseradish
peroxidase conjugated secondary antibody (sc-2004 and sc-2005;
1:5,000; Santa Cruz Biotechnology, Inc.) at room temperature for 1
h with agitation. The membrane was observed using BeyoECL Plus
(Beyotime Institute of Biotechnology, Haimen, China) and analyzed
using Image Lab Software version 3.0 (Bio-Rad Laboratories,
Inc.).
Caspase-3 activity
Tissue samples (20 mg) were homogenized and
dissociated in radioimmunoprecipitation assay lysis buffer (BestBio
Biotechnology) for 30 min on ice. The supernatants were collected
following centrifugation at 10,000 × g for 10 min at 4°C and used
to measure total protein using BCA buffer (BestBio Biotechnology).
Protein (10–20 µg) was incubated with Ac-DEVD-pNA (2 mM; BestBio
Biotechnology) for 1 h at 37°C. Absorbance values for caspase-3
activity were measured at 405 nm.
Immunohistochemistry
Following treatment with sparstolonin B, rats were
sacrificed using decollation and the spinal cord acquired as above.
The spinal cords were fixed in 4% paraformaldehyde for 24 h at room
temperature. Tissue samples were cryoprotected in 30% sucrose in
0.1 M phosphate buffer with 0.01% sodium azide and sectioned at 15
µm. Tissue samples were then immersed in phosphate buffered saline
with 0.2% Triton X-100 (PBST) and 5% normal goat serum in PBST for
1 h. Then incubated overnight at 4°C with primary mouse anti-TLR4
(ab22048; Abcam, 1:1,000) and anti-NF-κB (ab16502; Abcam, 1:1,000).
The samples were then incubated for 2 h at room temperature in a
moist environment with secondary antibody Alexa Fluor fluorescent
568 anti-mouse (ab175471; 1:200; Abcam).
Statistical analysis
All data are presented as the mean ± standard
deviation. Differences between groups were assessed using one-way
analysis of variance and followed by Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sparstolonin B attenuates SCI-induced
Batto, Beattie and Bresnahan (BBB) score and water content in
rats
Following treatment with sparstolonin B, the effects
of sparstolonin B on the BBB score and water content of SCI rats
were analyzed. As presented in Fig.
2, there were significant decrease in BBB score and an increase
in the water content of spinal cord in the SCI model group,
compared with the control group. Treatment with sparstolonin B
significantly recovered the decrease in BBB score and the increase
in the water content of the spinal cord in SCI rats, compared with
the SCI model group (Fig. 2).
Sparstolonin B attenuates the mRNA
expression of proinflammatory cytokines interleukin (IL-18, IL-6,
IL-1β and IL-23 in SCI rats
Subsequently, the anti-inflammatory effects of
sparstolonin B were analyzed in SCI rats. The expression levels of
IL-18, IL-6, IL-1β and IL-23 were measured using RT-qPCR analysis.
The IL-18, IL-6, IL-1β, and IL-23 levels in SCI rats were increased
compared with the control group (Fig.
3). Treatment with sparstolonin B significantly decreased
IL-18, IL-6, IL-1β and IL-23 levels in SCI rats, compared with the
SCI model group (Fig. 3).
Sparstolonin B attenuates the levels
of TNF-α and IFN-γ in SCI rats
We determined TNF-α and IFN-γ levels in SCI rat
treated by Sparstolonin B using ELISA kits. As presented in
Fig. 4, a significant increase was
observed in TNF-α and IFN-γ levels in the SCI model group, compared
with the control group. Sparstolonin B significantly inhibited
TNF-α and IFN-γ expression in SCI rats, compared with the SCI model
group (Fig. 4).
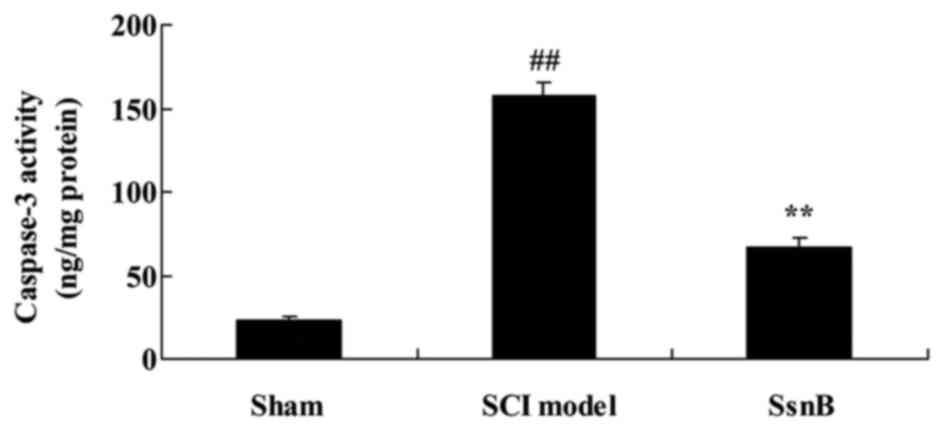
Sparstolonin B attenuates caspase-3
activity in SCI rats
The anti-apoptotic effects of Sparstolonin B were
examined in SCI rats. Compared with the control group, caspase-3
activity in the SCI model group was significantly promoted
(Fig. 5). The promotion of
caspase-3 activity in SCI rats was significantly decreased by
Sparstolonin B, compared with the SCI model group (Fig. 5).
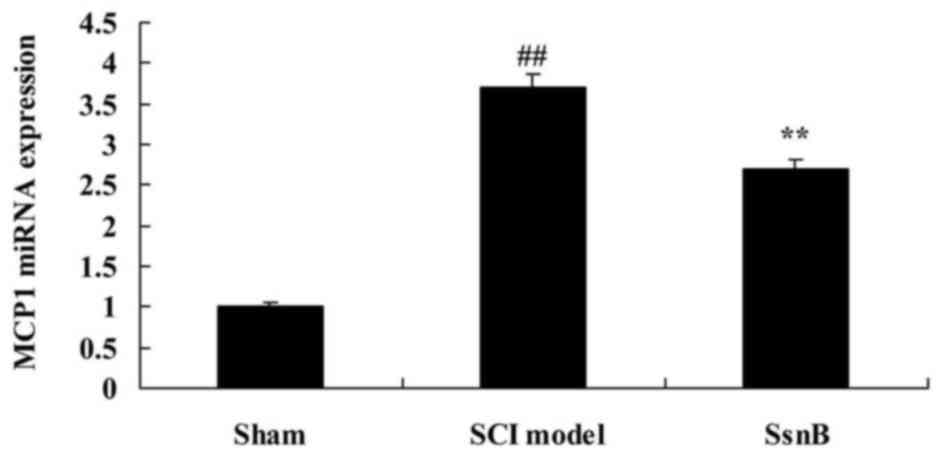
Sparstolonin B attenuates the monocyte
chemoattractant protein 1 (MCP1) mRNA level in SCI rats
The present study investigated the effects of
Sparstolonin B on MCP1 mRNA expression in SCI rats, using RT-qPCR
analysis. Fig. 6 demonstrates that
the MCP1 mRNA level in the SCI model group was significantly
increased compared with the control group. The increased MCP1 mRNA
level in SCI rats was reversed by Sparstolonin B, compared with the
SCI model group (Fig. 6).
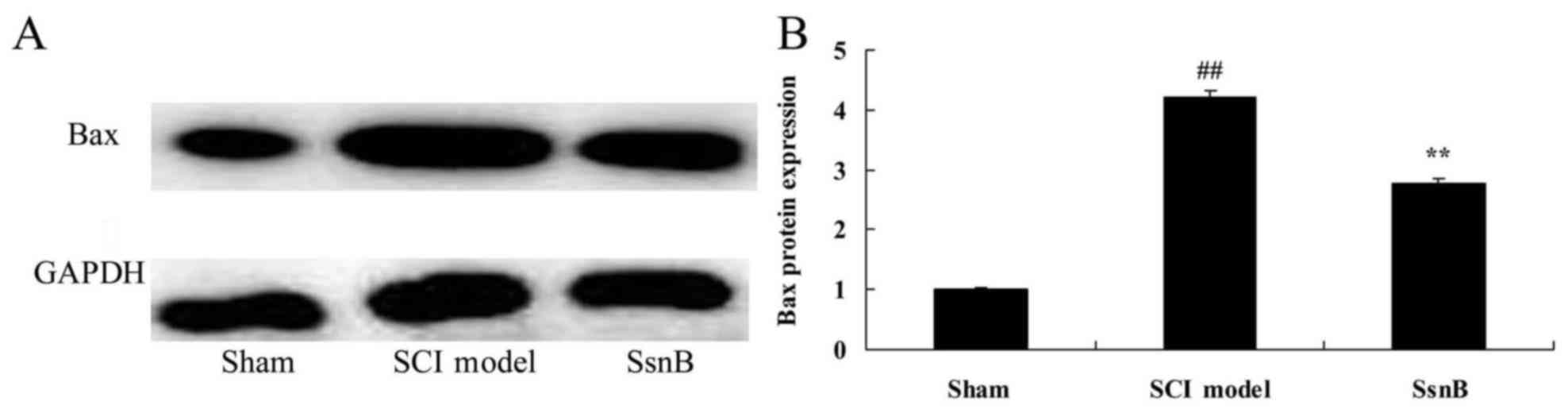
Sparstolonin B attenuates Bax protein
expression in SCI rats
The present study investigated the anti-apoptotic
mechanism of Sparstolonin B in SCI; the Bax protein level was
measured using western blot analysis. The results of the western
blot analysis demonstrated that the Bax protein level in the SCI
model group was significantly upregulated, compared with the
control group (Fig. 7). However,
treatment with Sparstolonin B significantly suppressed Bax protein
expression in SCI rats compared with the SCI model group (Fig. 7).
Sparstolonin B attenuates TLR4 MyD88
and NF-κB protein levels in SCI rat
In order to investigate the anti-inflammatory
mechanism of Sparstolonin B in SCI, the TLR4 MyD88 and NF-κB
signaling pathway was analyzed using western blot analysis and
immunohistochemistry. As presented in Fig. 8, TLR4, MyD88 and NF-κB expression
was significantly induced in the SCI model group, compared with the
control group. Treatment with Sparstolonin B suppressed TLR4, MyD88
and NF-κB protein levels in SCI tissue compared with the SCI model
group (Fig. 8).
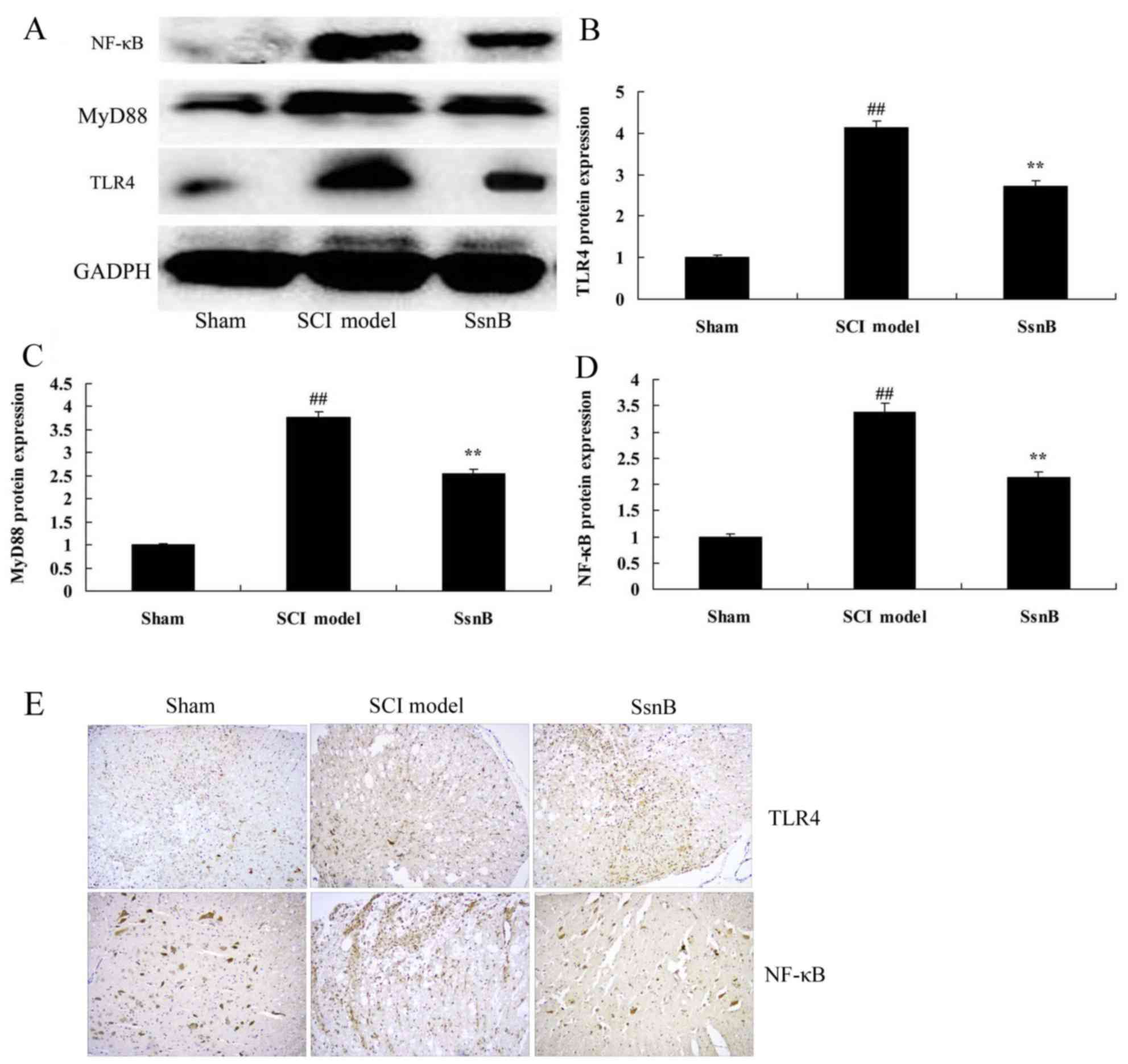 | Figure 8.Sparstolonin B attenuates TLR4, MyD88
and NF-κB protein levels in SCI rats. Sparstolonin B attenuated the
TLR4, MyD88 and NF-κB protein expression level, as demonstrated
using (A) western blotting and statistical analysis of (B) TLR4,
(C) MyD88 and (D) NF-κB protein level. (E) TLR4 and NF-κB protein
levels were additionally analyzed using immunohistochemistry
(magnification, ×5). ##P<0.01 vs. sham control group;
**P<0.01 vs. SCI model group. SsnB, sparstolonin B group; SCI,
spinal cord injury; NF-κB, nuclear factor-κB; MyD88, myeloid
differentiation primary response protein MyD88; TLR4, Toll-like
receptor 4. |
Discussion
SCI is a common injury observed in spinal surgery.
Generally, SCI is caused by transport accidents, falling,
construction and industrial accidents, sports injuries and combat
injury. In recent years, with the development of urban construction
and the transportation industry, the incidence of acute SCI has
been increasing annually (20). A
previous epidemiological study demonstrated that ~11,000 new cases
of acute SCI were reported in the USA each year (21). Acute SCI frequently causes a
variety of complications, including respiratory dysfunction,
respiratory failure, pneumonia, pulmonary edema, pulmonary embolism
and other respiratory complications, which are the most commonly
detected complications of SCI with an incidence rate of 67%
(22). In addition, respiratory
system complications contribute to the early mortality of patients,
with a proportion >20–50% (23). Although pre-hospital first aid and
clinical therapy has received continuous innovation and
improvement, early mortality from acute SCI caused by respiratory
failure remains at a high level (23). The results of the present study
indicated that sparstolonin B significantly recovered the decrease
in BBB score and the increase in the water content of the spinal
cord in SCI rat.
Inflammatory reactions are the primary cause of
sequential damage associated with SCI, and inflammation is an
important part of the acute SCI pathophysiological mechanism
(24). SCI promotes a series of
molecular biological events which lead to inflammatory cell
activation, arising from circulatory system infiltration in spinal
cord tissue, pro-inflammatory factors and neurotoxin release, and
the generation of oxygen free radical and nitroso compounds which
lead to cellular lesions (25). In
the present study, it was demonstrated that sparstolonin B
significantly decreased IL-18, IL-6, IL-1β, and IL-23 levels, and
TNF-α and IFN-γ levels, in SCI rats. Liu et al (18) reported that sparstolonin B
decreased vascular smooth muscle cell proliferation, migration and
inflammatory responses.
Inflammatory reactions are predominantly regulated
by gene expression. The NF-κB family is the major regulatory factor
for inflammatory gene expression, regulating the expression of
numerous cytokines in central nervous system injury and controlling
the inflammatory reactions (26).
Abnormally-activated NF-κB may induce neuronal apoptosis (26). A previous study demonstrated that
following SCI, abnormal activation of NF-κB, colocalization of
activated NF-κB and its target gene product inducible nitric oxide
synthase (iNOS) may be observed (27). In traumatic SCI, the expression of
a number of genes regulated by NF-κB has been detected, including
proinflammatory cytokines TNF-α, IL-1β and IL-6, MCP-1, adhesion
molecules intercellular adhesion molecule 1 and vascular cell
adhesion protein 1, cyclooxygenase-2, iNOS, and matrix
metalloproteinases (28). Direct
inhibition of NF-κB activation may reduce the expression of such
genes associated with inflammatory reactions following SCI, thereby
relieving inflammation and improving functional recovery (29). Previous studies have demonstrated
that the inhibition of NF-κB alleviates inflammatory reaction in
SCI (30,31). The results of the present study
demonstrated that sparstolonin B attenuated caspase-3 activity and
Bax protein expression, and inhibited MCP1 mRNA expression, in SCI
rats.
TLRs are type I transmembrane proteins. TLRs belong
to a highly conservative pattern recognition receptor family, and
are widely distributed on the surface of macrophages, monocytes,
dendritic cells, natural killer cells and lymphocytes (32). The TLR family includes ≥12 members,
including TLR4 (33). TLR4 has
been extensively studied due to its involvement in and mediation of
inflammatory reactions (34). A
previous study demonstrated that when the spinal cord is damaged,
microglial cell are activated and inflammatory factors released
(35); the expression of TLR4 and
downstream signaling pathways serve roles in this process. During
SCI, necrotic neurons release 60 kDa heat shock protein
mitochondrial and other endogenous ligands (36). In addition to the TLR4 on the cell
surface and microglial cell activation, TLR4 is able to activate
downstream signal transduction through the MyD88-dependent and
MyD88 independent pathways, activate interferon regulatory factor
3, NF-κB and other transcription factors, induce spinal cord
inflammation, provoke an immune response, release an inflammatory
medium and lead to spinal cord sequential injury, necrosis and
apoptosis of nerve cells (34).
Necrotic or apoptotic nerve cells release more endogenous ligands
and continuously activate microglial cells, thus causing a cyclical
effect. TLR4 signal pathway activation may lead to the activation
of microglial cells. It has been observed that the MyD88
dependent-NF-κB signaling pathway of microglial cells and the
mitogen-activated protein kinase signaling pathway may be activated
(36). Downstream inflammatory
mediators may be released, leading to marked neuronal death
(37). A previous study indicated
that the activation of the microglial TLR4 signaling pathway lead
to inflammatory reactions in the central nervous system, thereby
causing apoptosis and necrosis of nerve cell (35). Additionally, the expression of
microglial TLR4 may be upregulated (37).
The oxidative activity of neutrophil granulocytes
and other inflammatory cells in the serum of patients with acute
SCI has been demonstrated to be markedly increased (38). In addition, free radicals have been
observed to be increased, NF-κB may be upregulated and the
enzymatic activity of myeloperoxidase may be increased (39). In addition, a previous study
demonstrated that the neuronal damage caused by SCI may lead to the
generation and release of certain inflammatory factors or other
proteins (40). These proteins may
enter the circulation through the injured blood brain barrier to
mediate systemic inflammatory responses and lead to SCI (40). Wang et al (33) suggested that sparstolonin B may
decreased high fat diet-induced obesity in rats and inhibit
lipopolysaccharide-induced cytokine production via TLR4 and NF-κB
expression in 3T3-L1 adipocytes. The results of the present study
demonstrated that sparstolonin B markedly suppressed TLR4, MyD88
and NF-κB protein expression in SCI tissues. Liang et al
(31) reported that sparstolonin B
may act as a selective TLR2 and TLR4 antagonist by blocking early
intracellular events.
In conclusion, the present study demonstrated that
sparstolonin B attenuated spinal cord injury-induced inflammation
and apoptosis in rats by modulating TLR4 trafficking. The results
of the present study provided initial evidence that sparstolonin B
exhibits the potential to serve as a therapeutic agent for
protection against SCI.
References
|
1
|
Choi C, Rim B and Kim J: Development and
evaluation of a assistive computer interface by SEMG for
individuals with spinal cord injuries. IEEE Int Conf Rehabil Robot.
2011:pp. 59753862011; PubMed/NCBI
|
|
2
|
Nussbaum EL, Flett H, Hitzig SL,
McGillivray C, Leber D, Morris H and Jing F: Ultraviolet-C
irradiation in the management of pressure ulcers in people with
spinal cord injury: A randomized, placebo-controlled trial. Arch
Phys Med Rehabil. 94:650–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allison DJ and Ditor DS: Targeting
inflammation to influence mood following spinal cord injury: A
randomized clinical trial. J Neuroinflammation. 12:2042015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang ML, Li JJ, Gao F, Du LJ, Zhao HP,
Wang YM, Yang DG, Chen L, Liu HW, Yang HD, et al: A preliminary
evaluation of the surgery to reconstruct thoracic breathing in
patients with high cervical spinal cord injury. Spinal Cord.
52:564–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ancha HR, Spungen AM, Bauman WA, Rosman
AS, Shaw S, Hunt KK, Post JB, Galea M and Korsten MA: Clinical
trial: The efficacy and safety of routine bowel cleansing agents
for elective colonoscopy in persons with spinal cord injury- a
randomized prospective single-blind study. Aliment Pharmacol Ther.
30:1110–1117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Celik EC, Erhan B, Gunduz B and Lakse E:
The effect of low-frequency TENS in the treatment of neuropathic
pain in patients with spinal cord injury. Spinal Cord. 51:334–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Apostolidis A, Thompson C, Yan X and
Mourad S: An exploratory, placebo-controlled, dose-response study
of the efficacy and safety of onabotulinumtoxinA in spinal cord
injury patients with urinary incontinence due to neurogenic
detrusor overactivity. World J Urol. 31:1469–1474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grossman RG, Fehlings MG, Frankowski RF,
Burau KD, Chow DS, Tator C, Teng A, Toups EG, Harrop JS, Aarabi B,
et al: A prospective, multicenter, phase I matched-comparison group
trial of safety, pharmacokinetics, and preliminary efficacy of
riluzole in patients with traumatic spinal cord injury. J
Neurotrauma. 31:239–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang ML, Li JJ, So KF, Chen JY, Cheng WS,
Wu J, Wang ZM, Gao F and Young W: Efficacy and safety of lithium
carbonate treatment of chronic spinal cord injuries: A
double-blind, randomized, placebo-controlled clinical trial. Spinal
Cord. 50:141–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Straaten MG, Cloud BA, Morrow MM,
Ludewig PM and Zhao KD: Effectiveness of home exercise on pain,
function, and strength of manual wheelchair users with spinal cord
injury: A high-dose shoulder program with telerehabilitation. Arch
Phys Med Rehabil. 95:1810–1817 e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lobenwein D, Tepeköylü C, Kozaryn R,
Pechriggl EJ, Bitsche M, Graber M, Fritsch H, Semsroth S, Stefanova
N, Paulus P, et al: shock wave treatment protects from neuronal
degeneration via a toll-like receptor 3 dependent mechanism:
Implications of a first-ever causal treatment for ischemic spinal
cord injury. J Am Heart Assoc. 4:e0024402015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freria CM, Bernardes D, Almeida GL, Simões
GF, Barbosa GO and Oliveira AL: Impairment of toll-like receptors 2
and 4 leads to compensatory mechanisms after sciatic nerve axotomy.
J Neuroinflammation. 13:1182016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li XQ, Lv HW, Tan WF, Fang B, Wang H and
Ma H: Role of the TLR4 pathway in blood-spinal cord barrier
dysfunction during the bimodal stage after ischemia/reperfusion
injury in rats. J Neuroinflammation. 11:622014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang Q, Yu F, Cui X, Duan J, Wu Q,
Nagarkatti P and Fan D: Sparstolonin B suppresses
lipopolysaccharide-induced inflammation in human umbilical vein
endothelial cells. Arch Pharm Res. 36:890–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang Q, Dong S, Lei L, Liu J, Zhang J, Li
J, Duan J and Fan D: Protective effects of Sparstolonin B, a
selective TLR2 and TLR4 antagonist, on mouse endotoxin shock.
Cytokine. 75:302–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng X, Zhang Y, Jiang F, Chen R, Peng P,
Wen B and Liang J: The Chinese herb-derived Sparstolonin B
suppresses HIV-1 transcription. Virol J. 12:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou NS, Liang QL, Li P, Liu J, Liu X, Kang
A and Deng HS: Determination of sparstolonin B by ultra-high
performance liquid chromatography coupled with triple quadrupole
mass spectrometry: Application to pharmacokinetic study of
sparstolonin B in rat plasma. Biomed Chromatogr. 29:1486–1491.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q, Li J, Liang Q, Wang D, Luo Y, Yu F,
Janicki JS and Fan D: Sparstolonin B suppresses rat vascular smooth
muscle cell proliferation, migration, inflammatory response and
lipid accumulation. Vascul Pharmacol 67–69. 1–66. 2015.
|
|
19
|
Darouiche RO, Al Mohajer M, Siddiq DM and
Minard CG: Short versus long course of antibiotics for
catheter-associated urinary tract infections in patients with
spinal cord injury: A randomized controlled noninferiority trial.
Arch Phys Med Rehabil. 95:290–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wadsworth BM, Haines TP, Cornwell PL,
Rodwell LT and Paratz JD: Abdominal binder improves lung volumes
and voice in people with tetraplegic spinal cord injury. Arch Phys
Med Rehabil. 93:2189–2197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DI, Lee H, Lee BS, Kim J and Jeon JY:
Effects of a 6-week indoor hand-bike exercise program on health and
fitness levels in people with spinal cord injury: A randomized
controlled trial study. Arch Phys Med Rehabil. 96:2033–2040 e1.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim Y, Jo SH, Kim WH and Kweon OK:
Antioxidant and anti-inflammatory effects of intravenously injected
adipose derived mesenchymal stem cells in dogs with acute spinal
cord injury. Stem Cell Res Ther. 6:2292015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ak H, Gülşen İ, Karaaslan T, Alaca İ,
Candan A, Koçak H, Atalay T, Çelikbilek A, Demir İ and Yılmaz T:
The effects of caffeic acid phenethyl ester on inflammatory
cytokines after acute spinal cord injury. Ulus Travma Acil Cerrahi
Derg. 21:96–101. 2015.PubMed/NCBI
|
|
24
|
Hsieh SM, Wang YH, Chang SC and Huang TS:
Low dose HIV-1 Tat improves the defective nuclear factor
(NF)-kappaB activity of dendritic cells from persons with spinal
cord injury. Cell Immunol. 257:105–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu LW, Chen JY, Wu PC and Wu BN:
Atorvastatin prevents neuroinflammation in chronic constriction
injury rats through nuclear NFκB downregulation in the dorsal root
ganglion and spinal cord. ACS Chem Neurosci. 6:889–898. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chengke L, Weiwei L, Xiyang W, Ping W,
Xiaoyang P, Zhengquan X, Hao Z, Penghui Z and Wei P: Effect of
infliximab combined with methylprednisolone on expressions of
NF-κB, TRADD, and FADD in rat acute spinal cord injury. Spine
(Phila Pa 1976). 38:E861–E869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang N, Hai Y, Yang J, Liang F and Gao CJ:
Hyperbaric oxygen intervention reduces secondary spinal cord injury
in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int J
Clin Exp Pathol. 8:1141–1153. 2015.PubMed/NCBI
|
|
28
|
Kuang X, Huang Y, Gu HF, Zu XY, Zou WY,
Song ZB and Guo QL: Effects of intrathecal epigallocatechin
gallate, an inhibitor of Toll-like receptor 4, on chronic
neuropathic pain in rats. Eur J Pharmacol. 676:51–56. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YK, Liu JT, Peng ZW, Fan H, Yao AH,
Cheng P, Liu L, Ju G and Kuang F: Different TLR4 expression and
microglia/macrophage activation induced by hemorrhage in the rat
spinal cord after compressive injury. J Neuroinflammation.
10:1122013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Impellizzeri D, Ahmad A, Di Paola R,
Campolo M, Navarra M, Esposito E and Cuzzocrea S: Role of Toll like
receptor 4 signaling pathway in the secondary damage induced by
experimental spinal cord injury. Immunobiology. 220:1039–1049.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Q, Wu Q, Jiang J, Duan J, Wang C,
Smith MD, Lu H, Wang Q, Nagarkatti P and Fan D: Characterization of
sparstolonin B, a Chinese herb-derived compound, as a selective
Toll-like receptor antagonist with potent anti-inflammatory
properties. J Biol Chem. 286:26470–26479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu JM, Tao LJ, Fu D, Lv ZP, Li L and Dai
RP: Activation of interleukin-1 beta (IL-1 beta) signaling in the
spinal cord in the rats with experimental cardiac injury. Int J
Cardiol. 128:413–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang M, Xiu L, Diao J, Wei L and Sun J:
Sparstolonin B inhibits lipopolysaccharide-induced inflammation in
3T3-L1 adipocytes. Eur J Pharmacol. 769:79–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu C, Wu F, Mao C, Wang X, Zheng T, Bu L,
Mou X, Zhou Y, Yuan G, Wang S and Xiao Y: Excess iodine promotes
apoptosis of thyroid follicular epithelial cells by inducing
autophagy suppression and is associated with Hashimoto thyroiditis
disease. J Autoimmun. 75:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raad H, Eskalli Z, Corvilain B, Miot F and
De Deken X: Thyroid hydrogen peroxide production is enhanced by the
Th2 cytokines, IL-4 and IL-13, through increased expression of the
dual oxidase 2 and its maturation factor DUOXA2. Free Radic Biol
Med. 56:216–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee J, Yi S, Kang YE, Chang JY, Kim JT,
Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM, et al: Defective
ciliogenesis in thyroid hurthle cell tumors is associated with
increased autophagy. Oncotarget. 7:79117–79130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koc A, Batar B, Celik O, Onaran I, Tasan E
and Sultuybek GK: Polymorphism of the NFKB1 affects the serum
inflammatory levels of IL-6 in Hashimoto thyroiditis in a Turkish
population. Immunobiology. 219:531–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu P, Liao LY, Zhao TT, Mo XM, Chen GG
and Liu ZM: GPER/ERK&AKT/NF-κB pathway is involved in
cadmium-induced proliferation, invasion and migration of
GPER-positive thyroid cancer cells. Mol Cell Endocrinol. 442:68–80.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pringle DR, Vasko VV, Yu L, Manchanda PK,
Lee AA, Zhang X, Kirschner JM, Parlow AF, Saji M, Jarjoura D, et
al: Follicular thyroid cancers demonstrate dual activation of PKA
and mTOR as modeled by thyroid-specific deletion of Prkar1a and
Pten in mice. J Clin Endocrinol Metab. 99:E804–E812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xian H, Wang F, Teng W, Yang D and Zhang
M: Thyroid hormone induce a p53-dependent DNA damage through
PI3K/Akt activation in sperm. Gene. 615:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|