Introduction
Sepsis is defined as life-threatening organ
dysfunction caused by a dysregulated host response to infection
(1). It is a complex pathological
process that leads to an inflammatory cascade within the host, and
it is triggered by pathogen-associated molecular patterns caused by
bacteria or other pathogens. Monocytes play an essential role in
the initiation and maintenance of host responses in systemic
inflammation (2). When an
inflammatory reaction occurs, monocytes can aggregate at the site
of inflammation and release a large number of inflammatory factors
and chemokines such as interleukin (IL)-1b, IL-6 and tumor necrosis
factor (TNF)-α, which further intensify the inflammatory response.
High mobility group box-1 protein (HMGB1) is a late mediator that
is extensively involved in systemic and local inflammatory
responses through receptor pathways such as the receptor for
advanced glycation end products (RAGE) and toll-like receptors
(TLR). HMGB1 promotes monocyte recruitment by activating the
RAGE/nuclear factor (NF)-κB signaling pathway, and it inhibits
monocyte apoptosis by activating the TLR4/mitogen-activated protein
kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling
pathway (3). Monocyte
chemoattractant protein-1 (MCP-1) is a chemokine involved in the
initiation and maintenance of the inflammatory response. Myeloid
cell leukemia 1 (Mcl-1) is an anti-apoptotic protein. These two
cytokines both play an important role in inflammation.
Glycyrrhizin (GL), also called glycyrrhizic acid, is
an effective component of licorice. It possesses many
pharmacological properties including antiviral, anti-inflammatory,
anti-tumor, and hepatoprotective effects. In East Asia, GL has been
clinically used for over two decades as an anti-inflammatory factor
and for the treatment of chronic hepatitis (4–6). GL
was reported to interact directly with HMGB1, thereby reducing the
concentration of HMGB1 and inhibiting its biological activities
(7). In addition, GL can weaken
the pro-inflammatory effect of HMGB1 through different signaling
pathways. Zhao et al (8)
showed that interaction between cell receptors and HMGB1 could be
blocked by GL, leading to the inactivation of downstream
MAPKs/NF-κB signaling pathways. A recent study showed that an
appropriate decrease in the number of neutrophils and monocytes is
beneficial for the survival of cecal ligation and puncture (CLP)
mice (9). Although the important
role of monocytes in sepsis is known, the effect of GL on the
recruitment and apoptosis of monocytes to reduce the HMGB1-mediated
inflammatory cascade remains unclear. In addition, the effects on
monocytes in relation to the concentration of GL remain
unexplored.
In this study, we first examined the effects of
HMGB1 on promoting the migration and inhibiting apoptosis of
monocytes. Next, we investigated the effects of GL treatment on
HMGB1-affected monocytes using THP-1 cells. Finally, the mechanism
underlying the effect of GL treatment on monocytes through the
interplay between HMGB1 and downstream signaling pathways was
investigated.
Materials and methods
Cell culture
THP-1 cells were purchased from the cell bank of the
Chinese Academy of Sciences (Shanghai, China) and cultured in
RPMI1640 medium (HyClone, Logan, UT, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 10×104 U/ml penicillin/streptomycin
(HyClone). Cells were cultured in an incubator at 37°C with 5%
CO2 and 95% O2.
Transwell assay
Transwell chambers used were ECM550 (Chemicon,
Temecula, CA, USA). THP-1 cells with good growth conditions were
made into single cell suspensions and 1×105 (total
volume of 200 µl) cells were added to the upper chamber. A volume
of 300 µl of serum-containing culture medium with different
concentrations of recombinant human HMGB1 (R&D Systems, Inc.,
Minneapolis, MN, USA) was added to the lower chamber and incubated
for 24 h at 37°C. Then, the medium in the lower chamber was
aspirated, sodium chloride-alcohol was added, and washed after
fixing for 10 min. Then, 0.1% crystal violet (Sigma-Aldrich, St.
Louis, MO, USA) was added to the lower chamber and stained for 10
min. Cells on the membrane of the upper chamber were carefully
wiped, while cells on the basement membrane of the lower chamber
were counted in 6 view fields randomly selected under a ×200
optical microscope. The average was recorded as the number of THP-1
cells passing through the artificial basement membrane. Different
concentrations of GL (Sigma-Aldrich) were added to the culture
fluid in the lower chamber as treatment before the 24 h
culture.
Flow cytometric detection of cell
apoptosis
Staurosporine (STS) (300 nM) with or without
different concentrations of HMGB1 was added to the THP-1 cell
culture medium and incubated for 24 h. After centrifugation and
resuspension in PBS, 5–10×104 cells were stained with
200 µl Annexin V-fluorescein isothiocyanate (FITC) and 10 µl
propidium iodide (PI) following secondary centrifugation. Flow
cytometry was performed by using a FACSCalibur system (Becton
Dickinson, Franklin Lakes, NJ, USA) after incubation at room
temperature in the dark for 10–20 min and ice bath. The STS and
apoptosis detection kit Annexin V-FITC were obtained from Beyotime
Institute of Biotechnology (Shanghai, China). The scatter plot was
divided into four quadrants: The left lower quadrant was defined as
viable cells (‘low’ FITC and ‘low’ PI signal), the right upper
quadrant was defined as necrotic cells (‘high’ FITC and ‘high’ PI
signal), and the right lower quadrant was defined as apoptotic
cells (‘high’ FITC and ‘low’ PI signal). The percentage of Annexin
V-positive cells in the total cells of the scatter plot was used to
determine the results. Different concentrations of GL were added to
the samples prior to the one day incubation for the intervention
experiment.
Western blotting
Cellular proteins were extracted using lysis buffer,
and the concentrations of protein were measured using a BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.). For the detection of
NF-κB level in nucleus, nuclear protein was extracted using nuclear
extraction kit (Epigentek, Farmingdale, NY, USA) in accordance with
the manufacturer's instructions. Equal protein amounts were
fractionated in 5–8% SDS-PAGE gels and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA). After blocking with 5% skimmed milk powder, the membranes
were incubated with the following primary antibodies at 1:1,000
dilution: Cleaved caspase-3, PARP, phosphorylation of IκBα
(p-IκBα), IκBα, NF-κB p65, phosphorylation level of ERK1/2
(p-ERK1/2), ERK1/2, and Mcl-1 (all from Cell Signaling Technology,
Beverly, MA, USA), and HMGB1, β-actin and Lamin B1 (all from Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. The
membranes were then incubated with HRP-conjugated rabbit or mouse
secondary antibodies (Cell Signaling Technology) at room
temperature for 2 h after washing. Immunoblots were detected by
using an enhanced chemiluminescence reagent (Tiangen Biotech, Co.,
Ltd., Beijing, China) and imager (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Gray value was analyzed with the software
ImageJ, and the gray coefficient ratio was calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from THP-1 cells using the
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription was performed using a First Strand cDNA
Synthesis kit (Fermentas, ON, Canada). PCR amplification was
performed using a One Step SYBR-Green I Quant qRT-PCR kit (Tiangen
Biotech, Co., Ltd.) on an ABI 7000 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Table I shows the primers used for MCP-1
and β-actin synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.) for PCR. PCR parameters were 5 min at 94°C, followed by 40
cycles of 30 sec at 94°C, 30 sec annealing at 60°C for MCP-1 or
55°C for β-actin, and 30 sec at 72°C, ending at 72°C for 10 min.
The 2−∆∆Cq method (10)
was used to calculate the relative levels of MCP-1 mRNA, and
β-actin expression was used for normalization.
 | Table I.Primer sequences for polymerase chain
reactions. |
Table I.
Primer sequences for polymerase chain
reactions.
| Primer | Strand | Sequence (5′-3′) |
|---|
| MCP-1 | Sense |
CAGCCAGATGCAATCAATGCC |
|
| Antisense |
TGGAATCCTGAACCCACTTCT |
| β-actin | Sense |
CATGTACGTTGCTATCCAGGC |
|
| Antisense |
CTCCTTAATGTCACGCACGAT |
Statistical analysis
Data are expressed as the mean ± SEM. Differences
between groups were evaluated using one-way ANOVA followed by the
Newman-Keuls test, using SPSS version 16.0 software (SPSS, Inc.,
Chicago, IL, USA). Statistical significance was established when
P<0.05.
Results
GL treatment suppresses HMGB1-induced
monocyte migration
THP-1 cells were treated with different
concentrations of HMGB1 (0.1, 0.5, and 1 µg/ml). The results of
Transwell assays showed that HMGB1 significantly increased the
number of migrated cells, especially at higher concentrations
(Fig. 1A). GL at all
concentrations tested (10, 50, and 100 µg/ml) suppressed the
HMGB1-induced monocyte migration, particularly at 50 and 100 µg/ml
(Fig. 1B).
GL treatment increases monocyte
apoptosis
THP-1 cells were treated with STS and HMGB1 (0.1,
0.5, and 1 µg/ml). Fig. 2A shows
that the control group had a lower apoptosis rate, and STS
significantly induced apoptosis. HMGB1 at 0.5 and 1 µg/ml
suppressed STS-induced apoptosis, and GL at 50 and 100 µg/ml
significantly improved the inhibition of apoptosis by HMGB1
(Fig. 2B). Furthermore, we
explored effects of HMGB1 and GL on key factors in apoptotic
pathway. As shown in Fig. 2C and
D, STS significantly increase the level of cleaved caspase-3
and PARP. HMGB1 suppressed the STS-induced cleavage of caspase-3
and PARP, and GL significantly increased the cleavage at 50 and 100
µg/ml.
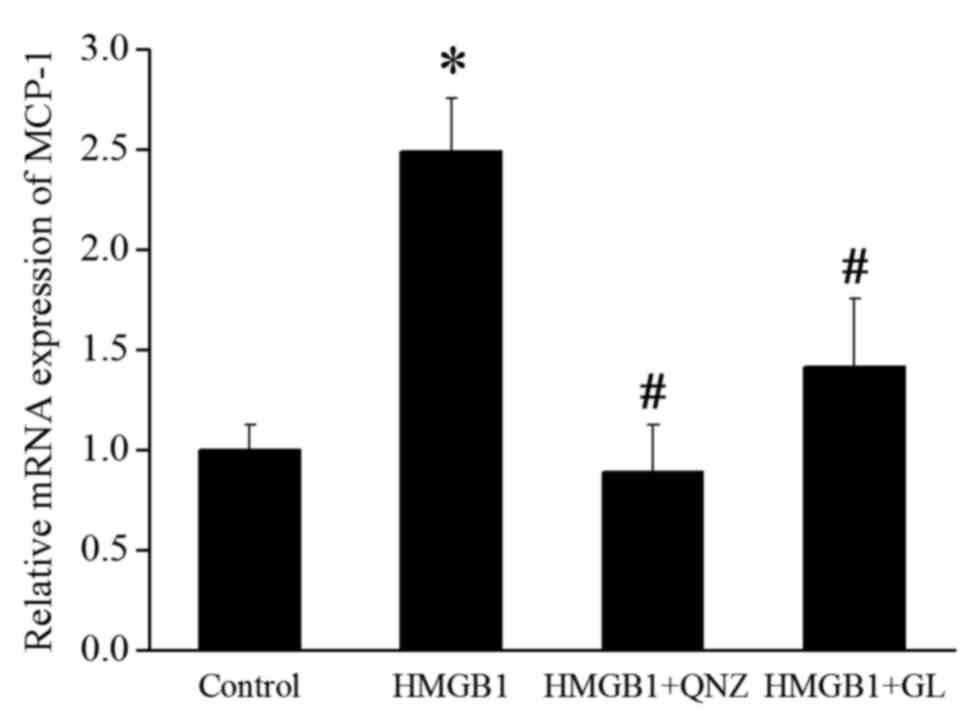
GL treatment inhibits HMGB1 induced
activation of NF-κB and downregulates MCP-1
HMGB1 promotes monocyte recruitment by activating
the RAGE/NF-κB signaling pathway (3). p-IκBα results in the activation of
nuclear translocation of NF-κB. The levels of p-IκBα and nuclear
NF-κB p65 were detected to evaluate the effect of GL treatment on
the activation of NF-κB. Fig. 3
shows that HMGB1 increased the level of p-IκBα and nuclear NF-κB
p65, whereas GL decreased this level by suppressing the function of
HMGB1. MCP-1 has the ability to recruit and activate particular
leukocytes and is a potential downstream effector of NF-κB
(11). RT-qPCR was performed to
detect the effect of HMGB1, the NF-κB inhibitor QNZ (100 nM;
Selleck Chemicals, Houston, TX, USA), and GL on the expression of
MCP-1. HMGB1 significantly increased the mRNA expression of MCP-1,
whereas QNZ and GL remarkably suppressed the induction of MCP-1
expression by HMGB1 (Fig. 4).
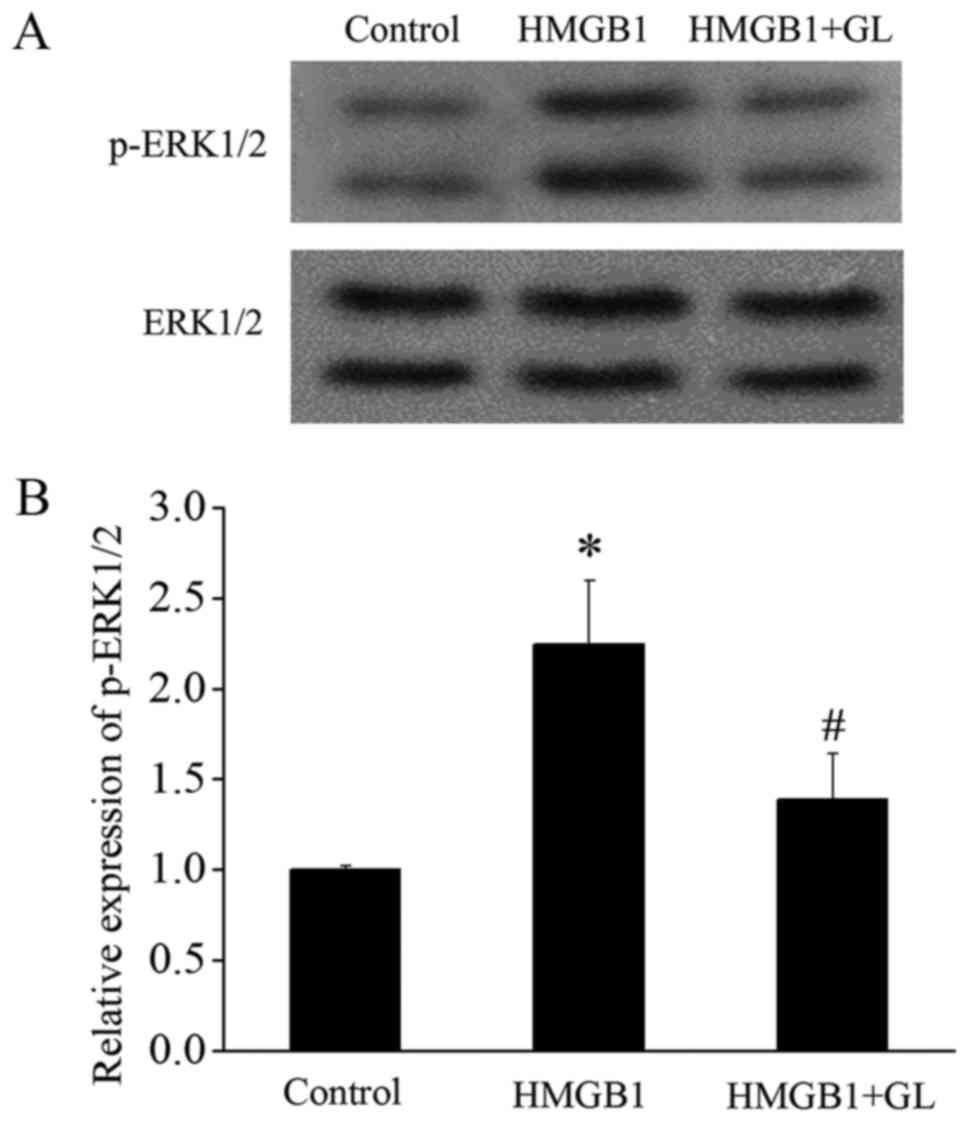
GL treatment inhibits HMGB1-induced
activation of MAPK/ERK and downregulates Mcl-1
Since HMGB1 inhibits apoptosis of monocytes by
activating the TLR4/MAPK/ERK signaling pathway (3), we further investigated the effect of
GL on this pathway. HMGB1 significantly increased the p-ERK1/2,
whereas GL suppressed the HMGB1-induced activation of ERK1/2
(Fig. 5). It has been reported
that the protein degradation of Mcl-1 is inhibited by ERK (12). Therefore, activation of the ERK
pathway could prevent the protein degradation of Mcl-1. We
speculated that HMGB1 may inhibit the degradation of Mcl-1 via the
MAPK/ERK pathway, and further antagonize apoptosis. Fig. 6 shows that HMGB1 significantly
increased the level of Mcl-1 compared to that in the STS treated
group. The level of Mcl-1 increased by HMGB1 was remarkably
decreased by the ERK1/2 inhibitor SCH772984 (SCH) (10 nM;
Medchemexpress LLC, Princeton, NJ, USA) and GL.
Discussion
The results of the present study suggested that GL
attenuates the HMGB1-induced inflammatory reaction by suppressing
the migration of monocytes and inducing apoptosis. Furthermore,
HMGB1 might promote the migration and suppress apoptosis of
monocytes through the NF-κB/MCP-1 and MAPK/ERK/Mcl-1 signaling
pathways. Both of these signaling pathways could be blocked by GL
treatment.
Consistent with a previous study (3), HMGB1 promoted the migration, an event
accompanying the recruitment of monocytes (13), and suppressed the apoptosis of
monocytes. We used THP-1 cells as a research model because of its
wide application in the study of monocyte function (14). GL treatment suppressed the
HMGB1-induced migration of monocytes and anti-apoptosis effects in
a dose-dependent manner. The anti-inflammatory effect of GL on
monocytes was related to the concentration of GL.
A previous study showed that HMGB1 could activate
the RAGE/NF-κB pathway and promote the recruitment of monocytes
(3). In addition, HMGB1 suppressed
the apoptosis of monocytes by activating the TLR4/MAPK/ERK pathway.
The cell receptor signaling pathways involved suggested a potential
anti-inflammatory mechanism underlying the effect of GL. Our
previous study showed that GL inhibits the MAPK/NF-κB pathway by
blocking the interaction of HMGB1 with TLR4 and RAGE, as determined
by co-immunoprecipitation assays using rat NR8383 alveolar
macrophages (8). In the present
study in THP-1 cells, GL probably functions through the same
signaling pathway in monocytes.
Different properties of monocytes and macrophages
affect cell behavior, which is affected by the downstream proteins
of signaling pathways. MCP-1 is a small proinflammatory factor and
a member of the CC chemokine family that is secreted by a variety
of cells, such as epithelial cells, endothelial cells, smooth
muscle cells, fibroblasts, and monocytes. Some specific leukocytes
including monocytes, lymphocytes, and mast cells can be recruited
and activated by MCP-1 (15). A
previous study showed that MCP-1 could be produced via the NF-κB
signaling pathway to mediate the migration of amoeboid microglia in
rats (11). Our study showed that
HMGB1 could increase the expression of MCP-1, whereas both an NF-κB
inhibitor and GL significantly suppressed MCP-1 expression induced
by HMGB1. These results indicated that GL might act through the
RAGE/NF-κB signaling pathway and suppress the production of MCP-1.
Further research is necessary to confirm this hypothesis.
Dysregulation of apoptosis in specific leukocytes
may increase the duration and severity of the systemic inflammatory
response (16). Anti-apoptotic
monocytes release cytokines and cytotoxic products, which recruit
additional leukocytes and damage host cells, with the end result of
the amplification of the inflammatory cascade. Mcl-1 is a unique
member of the Bcl-2 family, which plays an important role in
apoptosis. It has been widely investigated in the field of cancer.
Because of its anti-apoptotic function, Mcl-1 levels are inversely
correlated with neutrophil apoptosis (17,18).
Although Mcl-1 has a short half-life of <5 h, the ERK pathway
can delay protein degradation and prolong its lifespan (12), resulting in a decrease in
apoptosis. We found that the level of Mcl-1 was significantly
increased because of HMGB1, and further remarkably decreased by
both the ERK1/2 inhibitor and GL. This suggested that HMGB1
suppressed the degradation of Mcl-1 via the MAPK/ERK pathway. GL
might restore Mcl-1 to normal half-life by blocking this signaling
pathway, and promote the recovery of apoptosis in monocytes. This
signaling pathway mechanism merits further investigation.
In conclusion, GL inhibited the effect of HMGB1 on
monocyte migration and apoptosis, probably via the NF-κB/MCP-1
pathway and ERK/Mcl-1 pathway. GL restored the normal function of
monocytes, resulting in the reduction of the systemic inflammatory
response.
Acknowledgements
Not applicable.
Funding
The present study was supported by a general project
from Shanghai Municipal Commission of Health and Family Planning
(grant nos. 201540043 and 2016ZB0202-01), and The Scientific
Research Project supported by Huashan Hospital, Fudan University
(grant no. 2014QD15).
Availability of data and materials
The analyzed data sets generated during the study
are available upon reasonable request for non-commercial purposes,
without breaching participant confidentiality.
Authors' contributions
JT, WW and YG conceived and designed the
experiments. JT, FZ and SD performed the experiments, and JT and HZ
analyzed the data. JT, WW and YG wrote the paper. YG approved the
final version to be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhodes A, Evans LE, Alhazzani W, Levy MM,
Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally
ME, et al: Surviving sepsis campaign: international guidelines for
management of sepsis and septic shock: 2016. Crit Care Med.
45:486–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Carr B, Goyal M and Gaieski DF:
Sepsis: The inflammatory foundation of pathophysiology and therapy.
Hosp Pract (1995). 39:99–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogel S, Rath D, Borst O, Mack A, Loughran
P, Lotze MT, Neal MD, Billiar TR and Gawaz M: Platelet-derived
high-mobility group box 1 promotes recruitment and suppresses
apoptosis of monocytes. Biochem Biophys Res Commun. 478:143–148.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi JR, Mao LG, Jiang RA, Qian Y, Tang HF
and Chen JQ: Monoammonium glycyrrhizinate inhibited the
inflammation of LPS-induced acute lung injury in mice. Int
Immunopharmacol. 10:1235–1241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asl MN and Hosseinzadeh H: Review of
pharmacological effects of Glycyrrhiza sp. and its bioactive
compounds. Phytother Res. 22:709–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korenaga M, Hidaka I, Nishina S, Sakai A,
Shinozaki A, Gondo T, Furutani T, Kawano H, Sakaida I and Hino K: A
glycyrrhizin-containing preparation reduces hepatic steatosis
induced by hepatitis C virus protein and iron in mice. Liver Int.
31:552–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mollica L, De Marchis F, Spitaleri A,
Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L,
Musco G and Bianchi ME: Glycyrrhizin binds to high-mobility group
box 1 protein and inhibits its cytokine activities. Chem Biol.
14:431–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao F, Fang Y, Deng S, Li X, Zhou Y, Gong
Y, Zhu H and Wang W: Glycyrrhizin protects rats from sepsis by
blocking HMGB1 signaling. Biomed Res Int. 2017:97196472017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber GF, Chousterman BG, He S, Fenn AM,
Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, et al:
Interleukin-3 amplifies acute inflammation and is a potential
therapeutic target in sepsis. Science. 347:1260–1265. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng YY, Lu J, Ling EA and Kaur C:
Monocyte chemoattractant protein-1 (MCP-1) produced via NF-kappaB
signaling pathway mediates migration of amoeboid microglia in the
periventricular white matter in hypoxic neonatal rats. Glia.
57:604–621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Namgaladze D, Kollas A and Brüne B:
Oxidized LDL attenuates apoptosis in monocytic cells by activating
ERK signaling. J Lipid Res. 49:58–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerhardt T and Ley K: Monocyte trafficking
across the vessel wall. Cardiovasc Res. 107:321–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin Z: The use of THP-1 cells as a model
for mimicking the function and regulation of monocytes and
macrophages in the vasculature. Atherosclerosis. 221:2–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oppenheim JJ, Zachariae CO, Mukaida N and
Matsushima K: Properties of the novel proinflammatory supergene
‘intercrine’ cytokine family. Annu Rev Immunol. 9:617–648. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jimenez MF, Watson RW, Parodo J, Evans D,
Foster D, Steinberg M, Rotstein OD and Marshall JC: Dysregulated
expression of neutrophil apoptosis in the systemic inflammatory
response syndrome. Arch Surg. 132:1263–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Craig RW: MCL1 provides a window on the
role of the BCL2 family in cell proliferation, differentiation and
tumorigenesis. Leukemia. 16:444–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leuenroth SJ, Grutkoski PS, Ayala A and
Simms HH: The loss of Mcl-1 expression in human polymorphonuclear
leukocytes promotes apoptosis. J Leukoc Biol. 68:158–166.
2000.PubMed/NCBI
|