Introduction
The endoplasmic reticulum (ER) is one of the most
important organelles in a cell; ER regulates calcium storage, lipid
synthesis and protein folding. A physiological imbalance between
the cellular demand for protein folding and the capacity of the ER
to promote protein maturation leads to the accumulation of unfolded
proteins in the ER lumen (1,2).
This can cause ER stress (ERS), which includes three aspects: i)
Increase in the transcription and expression of ER proteins; ii)
reduction in the speed of unfolded protein (UP) translation,
preventing too many UPs from entering the ER; and iii) degradation
of UPs by ER-associated degradation (3). This process is termed the unfolded
protein response (UPR). The UPR is initiated by three ER
transmembrane proteins: Basic leucine-zipper transcription factor
activation of transcription factor 6 (ATF6), PKR-like ER-associated
kinase (PERK) and endonuclease inositol-requiring enzyme 1 (IRE1)
(4–6). When unfolded proteins aggregate in
the ER lumen, they activate the ER membrane proteins and initiate
the UPR. A moderate level of ERS is protective, however, when ERS
becomes irreversible and normal functions cannot be restored, an
apoptotic signal is initiated; in this way, the cells ultimately
induce programmed cell death, or apoptosis. ERS-induced apoptosis
includes the expression/activation of ERS-associated pro-apoptotic
molecules, including C/EBP homologous protein (CHOP) and caspase
12. These can activate apoptotic proteins downstream of ERS,
including B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) and
caspase 3 (7). Inflammation is one
of the most common reactions in several diseases, and is a major
factor leading to local environmental changes and the regulation of
ERS in cells.
Melatonin
(C13H16N2O2; MLT) is
predominantly secreted by the pineal gland; however, the retina,
tear ducts and skin can also produce low levels of melatonin in
mammals (8–10). Melatonin is considered a compound
with numerous potential applications due to its several biological
functions, including improvement of sleep, combating aging,
regulating immunity and suppressing tumors (11,12).
In addition, MLT can exhibit anti-oxidant and anti-inflammatory
activities. Several studies have indicated that the nuclear factor
(NF)-κB pathway is important in inflammatory diseases. The
administration of exogenous MLT causes anti-inflammatory effects
via the NF-κB pathway (13), and
studies have found that MLT can interfere with the NF-κB pathway in
the lung tissues of an asthma rat model (14). This effect also occurs in stress
ulcers (15) and inflammatory
bowel disease (16).
However, whether MLT exerts its anti-inflammatory
effect via ERS remains to be elucidated. The present study examined
the potential role of MLT in controlling the ERS-associated
signaling pathway to reduce inflammation in macrophages.
Materials and methods
Cell culture
The RAW264.7 macrophage cell line, purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA), was
cultured in DMEM/high glucose with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were
incubated at 37°C under a humidified atmosphere of 5%
CO2. The nutrient solution was refreshed approximately
every 48 h, and the cells were passaged every 3–4 days.
Cell Counting Kit-8 (CCK-8) assay
Drug toxicity was assayed using a CCK-8 assay
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay. A
RAW264.7 cell suspension was seeded into 96-well plates (100
µl/well; 1×104 cells/well). Following culture for 24 h,
the plating medium was replaced. The MLT was dissolved in fresh
medium at different concentrations (125, 250 and 500 µmol/l). A
control was supplemented in the culture medium. Each group
consisted of three parallel wells. Following incubation for 24 h,
the CCK-8 was added to the culture media, and a plate reader
(Infinite® 200 PRO NanoQuant; Tecan Austria GmbH,
Grödig, Austria) was used to measure the supernatant in each well
at a wavelength of 450 nm. Each experiment was performed in
triplicate. Cell viability in each group was calculated from the
absorbance measured.
Cell treatment
A RAW264.7 cell suspension was seeded into 6-well
plates (2 ml/well; 5×105 cells/well). Following culture
for 24 h, the plating medium was replaced with DMEM without 10%
FBS. After 24 h, MLT (Sigma-Aldrich; Merck KGaA) was dissolved in
fresh medium at different concentrations (125, 250 and 500 µmol/l),
whereas the cells in the control and lipopolysaccharide (LPS) group
were incubated in culture medium. After 1 h, LPS (Sigma-Aldrich;
Merck KGaA) was added at a concentration of 1 µg/ml to the cells in
the LPS and MLT (125, 250 and 500 µmol/l) groups for 6 h. To
investigate the effects of LPS on the ERS pathway for different
durations of functional treatment, the cells were treated with 1
µg/ml LPS for 0, 3, 6, 12 and 24 h.
ELISA
Following treatment, the culture supernatants of the
cells were collected with the centrifugation (2,500 × g for 10 min
at 4°C). The concentrations of tumor necrosis factor (TNF)-α,
interleukin (IL)-1 and IL-6 in the culture supernatants were
assessed using ELISA (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's instructions. The analyses were
performed in triplicate in 96-well plates (Nunc; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), which had been coated
with 100 µl aliquots of either anti-mouse TNF-α (cat. no. MAB4101),
IL-1 (cat. no. MAB401) or IL-6 (cat. no. MAB406) (all from R&D
Systems, Inc.) monoclonal antibodies (dilutions for all 1:500) in
phosphate-buffered saline (PBS) overnight at 4°C. The plates were
washed in PBS containing 0.05% Tween-20 and blocked with PBS
containing 10% FBS for 2 h. Following washing, the standards and
the serum were added into the plates and incubated at room
temperature for 3 h. Following incubation, the wells were washed
and 0.2 µg/ml the biotinylated anti-mouse TNF-α, IL-1 or IL-6 was
added to each well. Incubation was continued at room temperature
for 1 h. The wells were washed, avidin-peroxidase was added, and
the plates were incubated for 30 min at room temperature. The
3,5,3′,5′-tetramethyl benzidine substrate was added following
washing. To each well, sulfuric acid (2 mol/l) was added to
terminate the reaction. The optical density of each well was read
at a wavelength of 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Following treatment with different concentrations of
MLT and LPS for different durations, total RNA of the cells was
isolated using TRIzol™ reagent, and synthesized to cDNA
using a reverse transcription kit (both from Invitrogen; Thermo
Fisher Scientific, Inc.). The primers were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China) and the oligonucleotide
sequences were as follows: Glucose-regulated protein 78 (GRP78)
forward, 5′-AGCGACAAGCAACCAAAGAT-3′ and reverse,
5′-CCCAGGTCAAACACAAGGAT-3′; CHOP forward,
5′-ACAGAGGTCACACGCACATC-3′ and reverse, 5′-CTCCTGCTCCTTCTCCTTCA-3′;
caspase 12 forward, 5′-CAATCTACAAGATCAAAGGTTTGGC-3′ and reverse,
5′-CAAACTTTTTGTTGCAGATGATGAG-3′; TNF-α forward,
5′-ACGGCATGGATCTCAAAGAC-3′ and reverse, 5′-GTGGGTGAGGAGCACGTAGT-3′;
β-actin forward, 5′-GTGCTATGTTGCTCTAGACTTCG-3′ and reverse,
5′-ATGCCACAGGATTCCATACC-3′. The qPCR procedure (10 min at 95°C
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min) was
performed using a qPCR system (ABI 7500 system with SDS software
1.4; Applied Biosystems; Thermo Fisher Scientific, Inc.) and ABI
Power SYBR-Green PCR Master mix (cat. no. 4367659). All reactions
were repeated a minimum of three times. The mRNA expression levels
were normalized against the housekeeping gene (β-actin) using the
ABI 7500 system with SDS 1.4 software and fold changes were
calculated using the 2−ΔΔCq normalization method
(17).
Western blot analysis
The cultured cells were homogenized in ice-cold RIPA
buffer and phenylmethane sulfonyl fluoride (both from Beyotime
Institute of Biotechnology, Shanghai, China) for 30 min on ice. The
cells were scraped off the plate, and the extracts were transferred
to a microcentrifuge tube and centrifuged at 1.2×104 x g
at 4°C for 20 min. The protein concentration was determined using
the BCA assay (Beyotime Institute of Biotechnology). Equal
quantities of total protein (40 µg) were subjected to 12% SDS-PAGE
and then transferred onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 170 mA for 1–2
h. The membranes were blocked at room temperature for 2 h in
blocking buffer (TBS with 0.1% Tween-20 and 5% non-fat milk) and
then immunoblotted overnight at 4°C with primary antibodies
targeted against the following: GRP78 (cat. no. ab108615), CHOP
(cat. no. ab179823), caspase 12 (cat. no. ab10455), TNF-α (cat. no.
ab6671) (all from Abcam, Cambridge, MA, USA), rabbit monoclonal
Bcl-2 (D55G8; cat. no. 4223; Cell Signaling Technology, Inc.,
Beverly, MA, USA), Bax (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), caspase 3 (cat. no. 9662; Cell Signaling Technology, Inc.),
GAPDH (Santa Cruz Biotechnology, Inc.) and β-actin (Abcam) at
dilutions of 1:1,000. Following washing three times with TBST for
10 min, the membranes were incubated for 1 h at room temperature
with goat anti-rabbit or goat anti-mouse secondary IgG conjugated
to horseradish peroxidase (1:5,000; Bioworld Technology, Inc., St.
Louis Park, MN, USA) and then washed with TBST as previously.
Finally, a Western Bright ECL detection kit (Advansta, Inc., Menlo
Park, CA, USA) was used and the bands were detected. The density of
specific bands was quantified using Image Lab software 4.1 (Bio-Rad
Laboratories, Inc.) with an imaging densitometer (Bio-Rad ChemiDoc
MP; Bio-Rad Laboratories, Inc.). The blots were subjected to
densitometry using GAPDH or β-actin as internal controls.
Statistical analysis
All results were analyzed using SPSS software 13.0
(SPSS, Inc., Chicago, IL, USA). The data are reported as the mean ±
standard deviation. One-way analysis of variance was used for data
comparisons. The Newman-Keuls post hoc test was used to compare the
data in the presence of a significant difference. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Toxicity of MLT towards RAW264.7
cells
The toxicity of MLT was assayed using a CCK-8 assay.
The RAW264.7 cells were treated with MLT at different
concentrations (125, 250 and 500 µmol/l). These concentrations were
selected according to previous experiments (18). The results indicated that, at these
concentrations, MLT was not toxic towards the RAW264.7 cells
(Fig. 1A). No significant
differences were observed between the groups (P>0.05).
 | Figure 1.Toxicity of MLT towards RAW264.7 cells
and its effects on attenuating the inflammatory reaction. (A)
RAW264.7 cells were treated with various concentrations of MLT (0,
125, 250 and 500 µmol/l). The Cell Counting Kit-8 assay results
indicated that MLT at these concentrations was not toxic towards
RAW264.7 cells with no statistically significant differences
between groups (P>0.05). Cells were pre-treated with MLT at
concentrations of 125, 250 and 500 µmol/l for 1 h in the M125,
M250, and M500 groups, respectively. The LPS, M125, M250 and M500
groups were then incubated with 1 µg/ml LPS for 6 h. Culture
supernatants were collected and analyzed for (B) TNF-α, (C) IL-1
and (D) IL-6 using ELISA. aP<0.05 vs. control group;
bP<0.05 vs. LPS group. MLT, melatonin; LPS,
lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
Effects of MLT on attenuation of the
inflammatory reaction
ELISA was used to evaluate the inflammatory indices
of the differently treated groups. As shown in Fig. 1B-D, compared with the control, LPS
induced inflammation (P<0.05). The inflammation in the
MLT-treated groups was between that of the control and the
LPS-treated groups (P<0.05). However, there were no
statistically significant differences between the concentrations of
MLT (125, 250 and 500 µmol/l).
Effects of concentrations of MLT on
the ERS pathway
Western blot analysis and RT-qPCR analyses were used
to determine the effects of MLT on ERS for the period of 6 h. As
shown in Figs. 2 and 3A-D, LPS provoked inflammation and
activated ERS (P<0.05). Re-treatment with MLT relieved the
inflammation and reduced the ERS (P<0.05). The extent of the
inhibition was likely to be dependent on the concentration of
MLT.
 | Figure 2.Effects of various concentrations of
MLT on the endoplasmic reticulum stress pathway, determined using
western blot analysis. Following treatment for 6 h, cell lysates
were subjected to SDS-PAGE followed by western blot analysis with
anti-GRP78, anti-CHOP, anti-caspase12, anti-TNF-α and anti-GAPDH
antibodies. The expression levels of all the above proteins were
highest in the LPS group, and higher in the MLT groups, compared
with the control group. The expression levels decreased as the
concentration of MLT increased. LPS, lipopolysaccharide; MLT,
melatonin; M125, 125 µmol/l MLT; M250, 250 µmol/l MLT; M500, 500
µmol/l MLT; GRP78, glucose-regulated protein 78; CHOP, C/EBP
homologous protein; TNF-α, tumor necrosis factor-α. |
 | Figure 3.Effects of various concentrations of
melatonin on the endoplasmic reticulum stress pathway, determined
using RT-qPCR analysis. Total RNA from cells was isolated for
RT-qPCR analysis of (A) GRP78, (B) CHOP, (C) caspase 12 and (D)
TNF-α. aP<0.05 vs. control cells;
bP<0.05 vs. LPS. LPS, lipopolysaccharide; M125, 125
µmol/l melatonin; M250, 250 µmol/l melatonin; M500, 500 µmol/l
melatonin; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; GRP78, glucose-regulated protein 78; CHOP, C/EBP
homologous protein; TNF-α, tumor necrosis factor-α. |
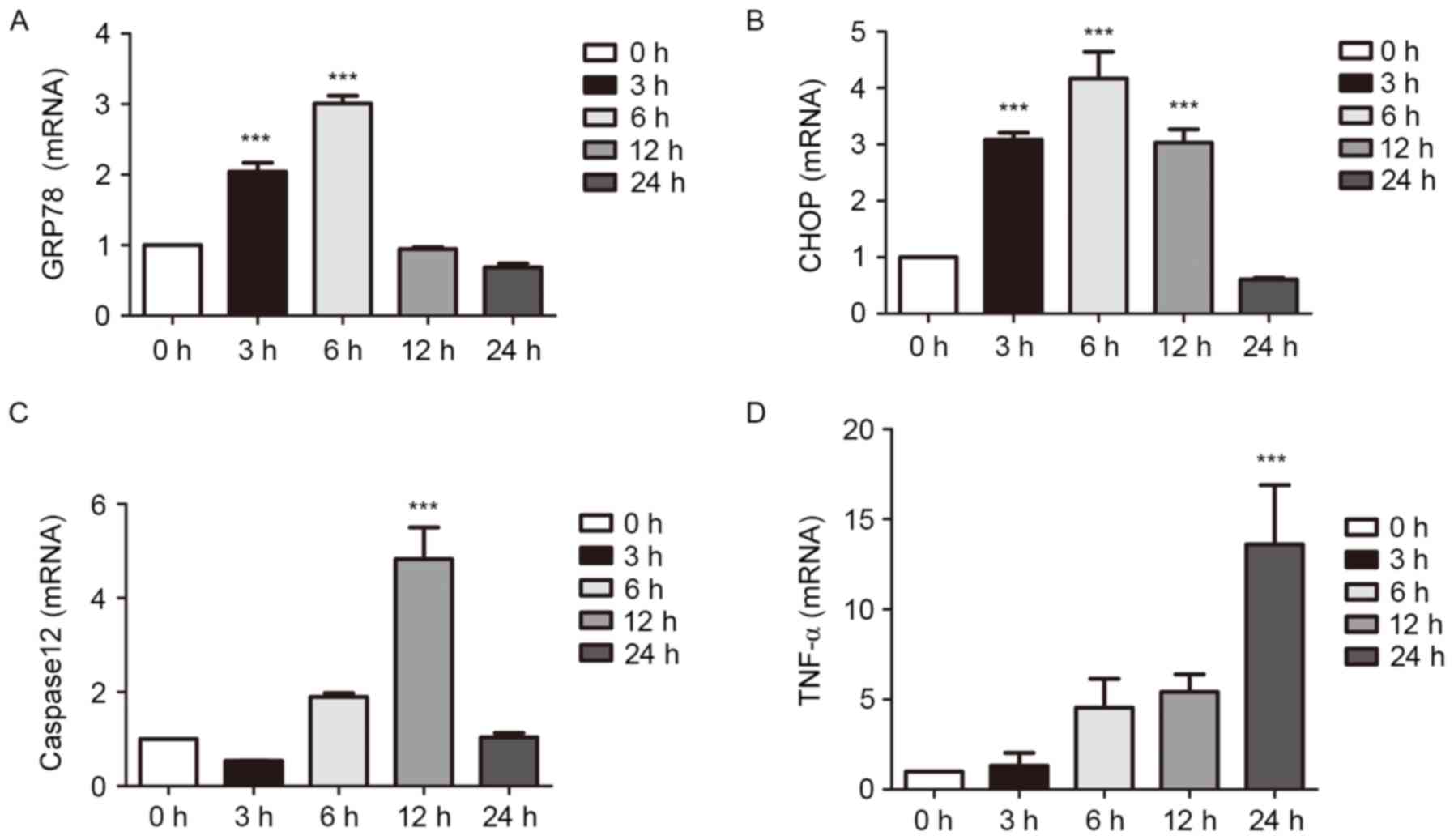
Effects of LPS on the ERS pathway at
different durations of treatment
The expression levels of proteins associated with
ERS were examined using RT-qPCR and western blot analyses of cells
cultured with LPS for 0, 3, 6, 12 or 24 h. The results showed that
inflammation became more pronounced as duration was extended. The
expression of TNF-α increased slowly as LPS was at a relatively low
concentration, however, the increased mRNA expression of TNF-α was
apparent at 6 h. The expression levels of the ERS-associated
molecules were increased significantly in the early stage of
inflammation. Following an extended period of time, the levels
decreased (P<0.001; Figs. 4 and
5).
 | Figure 4.Effects of LPS on the ERS pathway at
different durations of treatment, determined using western blot
analysis. The cells were treated with 1 µg/ml LPS for 0, 3, 6, 12
and 24 h. The cultured cells were collected to extract proteins for
western blot analysis. The expression levels of ERS-associated
molecules were increased in the early stage of inflammation. At a
certain time-point, expression levels declined. ERS, endoplasmic
reticulum stress; LPS, lipopolysaccharide; GRP78, glucose-regulated
protein 78; CHOP, C/EBP homologous protein; TNF-α, tumor necrosis
factor-α. |
Effects of melatonin on apoptosis in
the inflammatory response
The expression of proteins associated with apoptosis
was detected using western blot analysis with antibodies against
Bcl-2, Bax, pro-caspase 3 and cleaved-caspase 3. As shown in
Fig. 6, the levels of Bax and
cleaved-caspase 3 were increased significantly in the LPS-treated
group, whereas the levels of Bcl-2 and pro-caspase 3 were
decreased, compared with those in the control or MLT-treated
groups. Therefore, inflammation promoted apoptosis, whereas
treatment with MLT reduced apoptosis, compared with treatment with
LPS.
Discussion
In the present study, it was demonstrated that MLT
had an anti-inflammatory effect via ERS in RAW264.7 macrophages.
ERS was activated in the early stages of inflammation induced by
LPS. Treatment with MLT significantly inhibited the expression of
inflammatory cytokines and ERS-associated molecules, and exerted a
protective effect by suppressing apoptosis of the cells.
Oztekin et al (19) found elevated levels of TNF-α
following resection of the pineal gland and MLT inhibited the
expression of TNF-α. However, in the present study, the trend was
less marked in the results of the western blot analysis, compared
with that using ELISA. Therefore, it was hypothesized that MLT may
have an effect on the release of TNF-α rather than its expression
levels. This may be a focus of direction in future
investigations.
Stimulation by external factors, including oxidative
stress, calcium ion imbalance and lecithin synthesis disorder, can
lead to numerous unfolded proteins within the ER, triggering ERS,
which is an early self-protection mechanism against exogenous
stress within cells. When the damage to the ER becomes irreversible
and the cell cannot return to its normal function, the apoptotic
signaling pathway is initiated, and the cell eventually triggers
programmed cell death, or apoptosis. The apoptosis induced by ERS
occurs through various events involving ERS-associated molecules,
including CHOP and caspase 12, which promote apoptosis, and the
expression of survival molecules, including activation of growth
arrest and DNA damage protein 34 and binding immunoglobulin
protein. There are several mechanisms involved in the interaction
between inflammation and ERS. In pathological conditions, activated
macrophages can secrete various inflammatory molecules and
stimulate the activation of other cells. In mammals, however, the
stress reaction, induced by the expression of stress proteins, is
reversible and protects the cells from the effects of the external
environment (20).
A previous study found that UPR signaling pathways
can activate NF-κB. Kaneko et al (21) showed that the activation of IRE1a
in the cytoplasm was associated with TNF receptor-2, and activated
the inhibitor of NF-κB (IκB) kinase (IKK), which phosphorylated
IκB. The phosphorylation of IκB targeted it for ubiquitination and
protease degradation. This caused the release of NF-κB, which
relocated into the nucleus and initiated the transcription of
associated genes. When inflammation and infection activate ERS, the
PERK pathway is activated, the transcription of IκB is reduced, and
nuclear NF-κB increases and regulates transcription (22). Similarly, ATF6 can also activate
the NF-κB-IKK signaling pathways. Unfolded proteins are transferred
from the ER to the Golgi apparatus, where SP1 and SP2 divide ATF6
into ATF6a and ATF6b, resulting in the activation of these nuclear
transcription factors (23).
Protein kinase B (AKT) exists in the ER during ERS, and treatment
with ATF6 small interfering RNA inhibits the phosphorylation of
AKT, which affects the expression of downstream targets, including
NF-κB. This shows that ATF6 can activate the inflammatory response
through the AKT-NF-κB signaling pathway (24). There is also evidence that ER
overload, rather than Ca2+ or reactive oxygen species
(ROS) stress, can lead to activation of NF-κB in the classic
UPR.
The action of heat shock proteins (HSPs) can also
relieve inflammation. In cells, HSPs are important in the process
of recovery to reduce irritation and damage. HSPs promote the
synthesis of proteins in the ER, and their folding and
translocation in the cell membrane (25). GRP78 and other GRPs are important
partners of ER proteins, and they belong to the HSP protein family.
GRPs, particularly GRP78, are essential in the condition of ERS
(26).
The association between ERS and inflammation is not
unilateral, and inflammatory cytokines can also activate ERS. The
processing of TNF-α in fibrosarcoma cells from mice can cause UPR
activation, as indicated by the increased expression of X-box
binding protein 1, GRP78 and the phosphorylation of eukaryotic
initiation factor 2 (eIF2) (27).
TNF-α, IL-1β and IL-6 can also lead to ERS in liver cells and cause
the activation of cAMP-responsive element-binding protein H, thus
mediating the URP. This mechanism may be associated with
inflammatory factors promoting calcium ion release and the
accumulation of ROS in the ER, protein misfolding and mitochondrial
metabolism imbalance (28). By
contrast, ERS is also involved in the regulation of the
inflammatory response. The activation of CHOP, induced by the
PERK/eIF-2olved in, has been shown to have a negative regulatory
role in inflammation (29). A
study by Ho et al showed that LPS elicited inducible nitric
oxide synthase (iNOS) in murine RAW264.7 cells, and tunicamycin and
brefeldin A, two ER stressors, attenuated it. This indicated that
multiple mechanisms are involved in the inhibition of LPS-induced
gene expression of iNOS by ER stressors (30). In the present study, MLT attenuated
the inflammatory response by inhibiting the activation of ERS in
RAW264.7 macrophages. Therefore, the two conclusions are consistent
and mutually supportive.
In conclusion, increasing the protein folding
ability in the ER can prevent the accumulation of misfolded and
toxic proteins. When the UPR cannot restore the protein folding in
the ER, the cells use the three branches of the UPR to activate
apoptosis. CHOP cannot directly induce apoptosis, but the
degradation of anti-apoptotic proteins, including Bcl-2, and the
increased expression of apoptosis-promoting proteins, including
Bax, activate the caspase cascade and lead to apoptosis. In the
present study, ERS was activated in RAW264.7 macrophages under the
condition of LPS-induced inflammation. At the LPS concentration of
1 µg/ml, irreversible cell damage led to apoptosis through a
reduction in the levels of Bcl-2 and increases in the levels of Bax
and caspase 3. MLT decreases the expression of Toll-like receptor 3
(TLR3)-mediated inflammatory factor via inhibiting the activation
of NF-κB, and modulates TLR4-mediated inflammatory genes through
the TIR-domain-containing adapter-inducing interferon β- and
MyD88-dependent signaling pathways in LPS-stimulated RAW264.7 cells
(31,32). However, the mechanism of action
underlying MLT and ERS remains to be elucidated. In the present
study, MLT reduced the inflammatory response, decreased the
expression of ERS-associated proteins and inhibited cell apoptosis.
Therefore, it was concluded that MLT attenuated the inflammatory
response by inhibiting the activation of ERS and suppressing
apoptosis of the RAW264.7 macrophages. These results provide a
novel line of investigation for the anti-inflammatory effect of
MLT, which uses the ERS pathway to reduce the effects of
inflammation. Clinically, it is essential to intervene in the ERS
pathway to enhance the therapeutic effect of anti-inflammatory
drugs. However, the present study had limitations; it did not
explain whether ERS was a cause or an effect of inflammation, nor
did it identify the optimal concentration of MLT. Therefore,
subsequent investigations aim to focus on answering the above
questions.
Acknowledgements
All the authors would like to thank Mr. Weimin Li at
Department of Gastroenterology (The Affiliated Hospital of Hangzhou
Normal University, Hangzhou, China) for technical support.
Funding
The present study was supported by The Science and
Technology Bureau of Wenzhou, Zhejiang Province, China (grant no.
2014S0192) and the Public Projects of Zhejiang Province (grant no.
2016C33215).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YC performed the research and wrote this study; JW
designed this study; JZ analyzed the data, revised and translated
the study; QZ and YS collected information, and YJ advised on assay
performance.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schröder M: Endoplasmic reticulum stress
responses. Cell Mol Life Sci. 65:862–894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen X, Zhang K and Kaufman RJ: The
unfolded protein response-a stress signaling pathway of the
endoplasmic reticulum. J Chem Neuroana. 28:79–92. 2004. View Article : Google Scholar
|
|
3
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: Coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori K: Tripartite management of unfolded
proteins in the endoplasmic reticulum. Cell. 101:451–454. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ron D: Translational control in the
endoplasmic reticulum stress response. J Clin Invest.
110:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slominski A, Tobin DJ, Zmijewski MA,
Wortsman J and Paus R: Melatonin in the skin: Synthesis, metabolism
and functions. Trends Endocrinol Metab. 19:17–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hemadi M, Saki G, Shokri S and Ghasemi FM:
Follicular dynamics in neonate vitrified ovarian grafts after host
treatment with melatonin. Folia Morphol (Warsz). 70:18–23.
2011.PubMed/NCBI
|
|
10
|
do Carmo Buonfiglio D, Peliciari-Garcia
RA, do Amaral FG, Peres R, Nogueira TC, Afeche SC and Cipolla-Neto
J: Early-stage retinal melatonin synthesis impairment in
streptozotocin-induced diabetic wistar rats. Invest Ophthalmol Vis
Sci. 52:7416–7422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen Engler A, Hadash A, Shehadeh N and
Pillar G: Breastfeeding may improve nocturnal sleep and reduce
infantile colic: Potential role of breast milk melatonin. Eur J
Pediatr. 171:729–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carpentieri A, Díaz de Barboza G, Areco V,
Peralta López M and Tolosa de Talamoni N: New perspectives in
melatonin uses. Pharmacol Res. 65:437–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forman K, Vara E, García C, Kireev R,
Cuesta S, Acuña-Castroviejo D and Tresguerres JA: Beneficial
effects of melatonin on cardiological alterations in a murine model
of accelerated aging. J Pineal Res. 49:312–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YT, Chen SL and Xu SY: Effect of
melatonin on the expression of nuclear factor-kappa B and airway
inflammation in asthmatic rats. Zhonghua Er Ke Za Zhi. 42:94–97.
2004.(In Chinese). PubMed/NCBI
|
|
15
|
Wu JS, Wu JM, Wang D, Huang QK, Chen XR,
Huang ZM, Lin XF, Chen MX and Han QX: Protective effect of
melatonin on stress ulcer in rats by nuclear factor-κB. Chin J
Digest. 25:107–109. 2005.(In Chinese).
|
|
16
|
Mei Q, Xu JM, Zhao ZH, Wu J, Hu YM and Xu
XH: Effect of melatonin on expression of inflammatory cytokines in
immune colitis. Chin J Microbiol Immunol. 24:711–714. 2004.(In
Chinese).
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao BL, Zhang YX, Wang KJ, Ying F, Sun XC
and Wu JS: Melatonin reduces the production of NO and ROS in
peritoneal macrophages by inhibiting p38 pathway. J Med Res.
44:63–67. 2015.(In Chinese).
|
|
19
|
Oztekin E, Mogulkoc R, Baltaci AK and
Tiftik AM: The influence of estradiol and progesterone and
melatonin supplementation on TNF-alpha levels in ovariectomized and
pinealectomized rats. Acta Biol Hung. 57:275–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayakawa K, Hiramatsu N, Okamura M,
Yamazaki H, Nakajima S, Yao J, Paton AW, Paton JC and Kitamura M:
Acquisition of anergy to proinflammatory cytokines in nonimmune
cells through endoplasmic reticulum stress response: A mechanism
for subsidence of inflammation. J Immunol. 182:1182–1191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaneko M, Niinuma Y and Nomura Y:
Activation signal of nuclear factor-kappa B in response to
endoplasmic reticulum stress is transduced via IRE1 and tumor
necrosis factor receptor-associated factor 2. Biol Pharm Bull.
26:931–935. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng J, Lu PD, Zhang Y, Scheuner D,
Kaufman RJ, Sonenberg N, Harding HP and Ron D: Translational
repression mediates activation of nuclear factor kappa B by
phosphorylated translation initiation factor 2. Mol Cell Biol.
24:10161–10168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haze K, Yoshida H, Yanagi H, Yura T and
Mori K: Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to
endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakajima S, Hiramatsu N, Hayakawa K, Saito
Y, Kato H, Huang T, Yao J, Paton AW, Paton JC and Kitamura M:
Selective abrogation of BiP/GRP78 blunts activation of NF-κB
through the ATF6 branch of the UPR: Involvement of C/EBPβ and
mTOR-dependent dephosphorylation of Akt. Mol Cell Biol.
31:1710–1718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartl FU and Hayer-Hartl M: Molecular
chaperones in the cytosol: From nascent chain to folded protein.
Science. 295:1852–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee AS: The glucose-regulated proteins:
Stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang K, Shen X, Wu J, Sakaki K, Saunders
T, Rutkowski DT, Back SH and Kaufman RJ: Endoplasmic reticulum
stress activates cleavage of CREBH to induce a systemic
inflammatory response. Cell. 124:587–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin W, Harding HP, Ron D and Popko B:
Endoplasmic reticulum stress modulates the response of myelinating
oligodendrocytes to the immune cytokine interferon-gamma. J Cell
Biol. 169:603–612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garg AD, Kaczmarek A, Krysko O,
Vandenabeele P, Krysko DV and Agostinis P: ER stress-induced
inflammation: Does it aid or impede disease progression? Trends Mol
Med. 18:589–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho HJ, Huang DY, Ho FM, Lee LT and Lin WW:
Inhibition of lipopolysaccharide-induced inducible nitric oxide
synthase expression by endoplasmic reticulum stress. Cell Signal.
24:2166–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang SH, Cao XJ and Wei W: Melatonin
decreases TLR3-mediated inflammatory factor expression via
inhibition of NF-kappa B activation in respiratory syncytial
virus-infected RAW264.7 macrophages. J Pineal Res. 45:93–100. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia MZ, Liang YL, Wang H, Chen X, Huang
YY, Zhang ZH, Chen YH, Zhang C, Zhao M, Xu DX and Song LH:
Melatonin modulates TLR4-mediated inflammatory genes through MyD88-
and TRIF-dependent signaling pathways in
lipopolysaccharide-stimulated RAW264.7 cells. J Pineal Res.
53:325–334. 2012. View Article : Google Scholar : PubMed/NCBI
|