Introduction
Phenotypic switch is a major characteristic of
vascular smooth muscle cells (VSMCs), which respond to
environmental stimuli via downregulation of contractile marker
genes, upregulation of proliferative marker genes, migration
ability and synthesis of the cellular matrix (1). Phenotypic transfer of VSMCs from a
contractile to a synthetic phenotype is demonstrated to be
essential in various vascular diseases, including hypertension,
restenosis and atherosclerosis (2,3).
Increasing evidence has indicated that as a major peptide hormone
of the rennin-angiotensin system, angiotensin II (Ang II) is
significant in cardiovascular disease pathogenesis (4). Chronic inflammatory and immunity is
significantly associated with atherosclerosis process; according to
a previous study, Ang II could generate inflammation in VSMCs via
activating Toll-like receptor 4 via the nuclear factor (NF)-κB
pathway (5).
Bcl-2-associated athanogene 3 (BAG3), a 576-amino
acid anti-apoptotic protein, has been reported to be constitutively
expressed in the skeletal muscle, myocardial cells and various
tumors, and is associated with multiple pathological process, such
as cell survival, cell invasion, and adhesion (6–8).
Growing evidence has demonstrated that BAG3 may serve as a key
mediator in cardiovascular diseases. Homma et al (9) demonstrated that mice with a BAG3 gene
defect exhibited myofibrillar degeneration without inflammation,
Z-disk architecture disruption, early postnatal apoptotic features
and death by 4 weeks of age. Recently, clinical studies have
confirmed that BAG3 is associated with cardiomyopathy, such as
Takotsubo and dilated cardiomyopathy (10,11).
Growing evidence has confirmed that BAG3 serves a
key mediated role in the pathogenesis of cardiovascular damage;
however, to the best of our knowledge, there are currently no
studies that have investigated whether BAG3 is involved in Ang
II-induced VSMC proliferation of VSMCs. Furthermore, the potential
underlying mechanism of Ang II inducing BAG3 expression in VSMCs
remains unclear. Therefore, the present study aimed to investigate
the anti-proliferative effects of BAG3 on Ang II-induced VSMCs, and
to elucidate the potential underlying mechanisms.
Materials and methods
Reagents
Fetal bovine serum (FBS) was purchased from Abgent,
Inc. (San Diego, CA, USA). Dulbecco's modified Eagle's medium
(DMEM) was obtained from Hyclone; GE Healthcare Life Sciences
(Logan, CT, America). A Cell Counting kit-8 (CCK-8) was purchased
from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Ang
II, pyrrolidine dithiocarbamate (PDTC), penicillin and streptomycin
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Smooth muscle protein 22α (SM22α; cat. no. PAC781Ra01) antibody was
purchased from Cloud-Clone Corp (Katy, TX, USA). GAPDH (cat. no.
sc-25778), BAG3 (cat. no. sc-136467), proliferating cell nuclear
antigen (PCNA; cat. no. sc-56), NF-κB p65 (cat. no. sc-372),
phosphorylated (p)-NF-κB p65 (Ser 536; sc-33020) antibodies were
all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz,
Dallas, TX, USA). Toll-like receptor 4 (TLR4; cat. no. WL00196)
antibody was purchased from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China).
Cell culture
The A7r5 rat VMSC line was purchased from the China
Center for Type Culture Collection (Wuhan, China). Cells were
cultured in 25-cm2 culture flasks (Corning lncorporated,
Corning, NY, USA). The cells were developed in DMEM with 100 U/ml
streptomycin, 100 U/ml penicillin and 10% fetal bovine serum, and
were stored in a humidified incubator with 5% CO2 at
37°C. Cells at 80% confluence in culture wells were grown and
arrested by serum-starvation for 1 day for subsequent experiments.
Cell proliferation and cell number count experiments were conducted
as previously described (12). A
VMSC suspension (0.5×105 cells/ml) was incubated for 1
day without or with Ang II (10−7 M) for 0, 8, 16, 24 or
48 h. Light microscopy was used to count cells in a
hemocytometer.
At the density of 5,000 cells per well, CCK-8 assay
cells were cultivated in 96-well plates.
2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,
4-disulfophenyl]-2H-tetrazolium, monosodium salt (10 µl solution)
was added to every well after the treatments. Plates were incubated
at 37°C for 4 h. A microplate reader was used to evaluate the
absorbance at 450 nm.
Transfection
A BAG3 mRNA silencing plasmid, short hairpin
(sh)BAG3 (GenBank no. NM_001011936) was designed and synthesized by
Shanghai GeneChem (Shanghai, China). Three different shBAG3
(shRNA-N1, shRNA-N2, and shRNA-N3) targeting the BAG3 gene, or
nonspecific shRNA [negative control (NC)-shRNA-N] were transfected
into VSMCs for 6 h using Lipofectamine 2000™ (Life
Technologies, Carlsbad, CA, USA), according to the manufacturer's
protocol. Following this, VMSCs were treated in the presence or
absence of 10−7 M Ang II for 24 h. Plasmid sequences are
presented in Table I.
 | Table I.Primer sequences for Bcl-2-associated
athanogene 3. |
Table I.
Primer sequences for Bcl-2-associated
athanogene 3.
| Plasmid | Sequence |
|---|
| shBAG3#1 |
5′-TGCTGAGAAAGTGGAAGTGAA-3′ |
| shBAG3#2 |
5′-GAAGGCAAGAAGACTGATAAA-3′ |
| shBAG#3 |
5′-AGGATAAGAAAGGTCCTGAAA-3′ |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In the present study, TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
extract total cellular RNA. The integrity and purity of the
extracted total RNA was evaluated. Reverse transcription was
conducted using a Prime Script RT reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China) with 1 µg RNA. A 25-µl reaction system
was adopted containing 2 µl cDNA products and 12.5 µl SYBR Premix
primer mixture (Takara Biotechnology Co., Ltd., Dalian, China).
Sangon Biotechnology was assigned to design and synthesize the
primers used in the present study, which are presented in Table II. A Thermal Cycler Dice Real Time
system (Takara Biotechnology, Co., Ltd.) and a
two-step-plus-melting curve program was used to run all the
reactions in the following conditions: 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec and 60°C for 30 sec. Finally, a Thermal
Cycle Dice Real Time system was used to analyze gene expression,
and the ΔCq method in reference to GAPDH was adopted to
analyze the data. The 2−ΔΔCq method was performed to
calculate the data based on the measure of the quantitation cycle
(13). Three independent
experiments were repeated under the same conditions.
 | Table II.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table II.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Primer | Sequences |
|---|
| BAG3 |
|
|
Forward |
5′-TAGCTGGACCAGATCTCCCTCCTG-3′ |
|
Reverse |
5′-CCTTCACTTCCACTTTCTCAGCAG-3′ |
| PCNA |
|
|
Forward |
5′-TAGAGATGAATGAGCCAGTTCAGC-3′ |
|
Reverse |
5′-GGGTACATCTGCAGACATACTGAG-3′ |
| SM22α |
|
|
Forward |
5′-ATCCAAGCCAGTGAAGGTGC-3′ |
|
Reverse |
5′-CCTCTGTTGCTGCCCATTTG-3′ |
| GAPDH |
|
|
Forward |
5′-GCCTGGAGAAACCTGCCAAGTATG-3′ |
|
Reverse |
5′-GAGACAACCTGGTCCTCAGTGTAG-3′ |
Protein extraction and western blot
analysis
VSMCs were lysed in cold radioimmunoprecipitation
lysis buffer with protease inhibitors. Based on the standard of
bovine serum albumin (BSA), a Bicinchoninic Acid assay was used to
measure protein concentrations (Beyotime Institute of
Biotechnology, Haimen, China). Samples were boiled in 5X sample
buffer for 5 min at 100°C. Equal amounts of protein (50 µg/sample)
were separated by 8–12% SDS-PAGE and subsequently transferred to a
polyvinylidene difluoride membrane with constant current of 100 V.
Membranes were blocked with 5% BSA at room temperature for 120 min
in TBS with Tween-20 (pH 7.6) and incubated with primary antibodies
against TLR4, BAG3, p-NF-κB p65, NF-κB p65, SM22α, PCNA (1:500) and
GAPDH (1:1,000). Following incubation with the corresponding
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibodies or anti-mouse IgG secondary antibodies (SA00001-2 or
SA00001-1 respectively; 1:5,000; Wuhan Sanying Biotechnology,
Wuhan, China), proteins were detected by the Microchemi 4.2
Bio-imaging system with Enhanced Chemiluminescent-Plus HRP reagents
(Beyotime Institute of Biotechnology). Gelpro32 software (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA) was used to
compare the relative grey value of immunoreactive bands. To
evaluate protein activation, the densitometry of phosphorylated
protein was normalized to total protein. Under the same
experimental conditions, the experiments were repeated 3 times.
NF-κB blocking assay
To evaluate if TLR4/NF-κB mediated the upregulation
of BAG3, a selective inhibitor of NF-κB (PDTC; cat. no. S1808;
Beyotime Institute of Biotechnology) was dissolved in dimethyl
sulfoxide and was added to the medium at a final concentration of
25 µmol/l. A VMSC suspension (0.5×105 cells/ml) was
incubated for 1 day prior to pre-treatment with PDTC for 1 h and
subsequent culture with or without Ang II for 24 h. Following this,
western blot analysis was performed as described above.
Statistical analysis
The experiments were repeated in triplicate. To
analyze data, statistical software package of SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA) was used. Data are
expressed as the mean ± standard error of three independent
experiments. One-way analysis of variance was used to analyze
differences between the groups, followed by the Least Significant
Difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
VSMC proliferation is induced by Ang
II in a dose- and time-dependent manner
Ang II induced cell proliferation in VSMCs in a
dose-dependent manner (Figs. 1 and
2). Furthermore, following
treatment with 10−7 M Ang II, the proliferation of VSMCs
increased in a time-dependent manner (Figs. 1 and 2).
Ang II-induced upregulation of BAG3
mRNA and protein expression in VSMCs
As determined by RT-qPCR, BAG3 mRNA expression
levels increased in a concentration-dependent manner. PCNA mRNA
expression levels peaked following 10−7 M Ang II
treatment. No significant differences were observed in SM22α mRNA
expression levels after 10−8 and 10−6 M Ang
II treatment; however, 10−7 resulted in a decrease in
expression levels (Fig. 3A).
Similar effects were observed at the protein level, except
10−6 M Ang II treatment resulted in a significant
downregulation in SM22α expression (Fig. 3B and C). Furthermore, Ang II
stimulation increased BAG3 and PCNA and decreased SM22α mRNA and
protein expression levels in a time-dependent manner (Fig. 4).
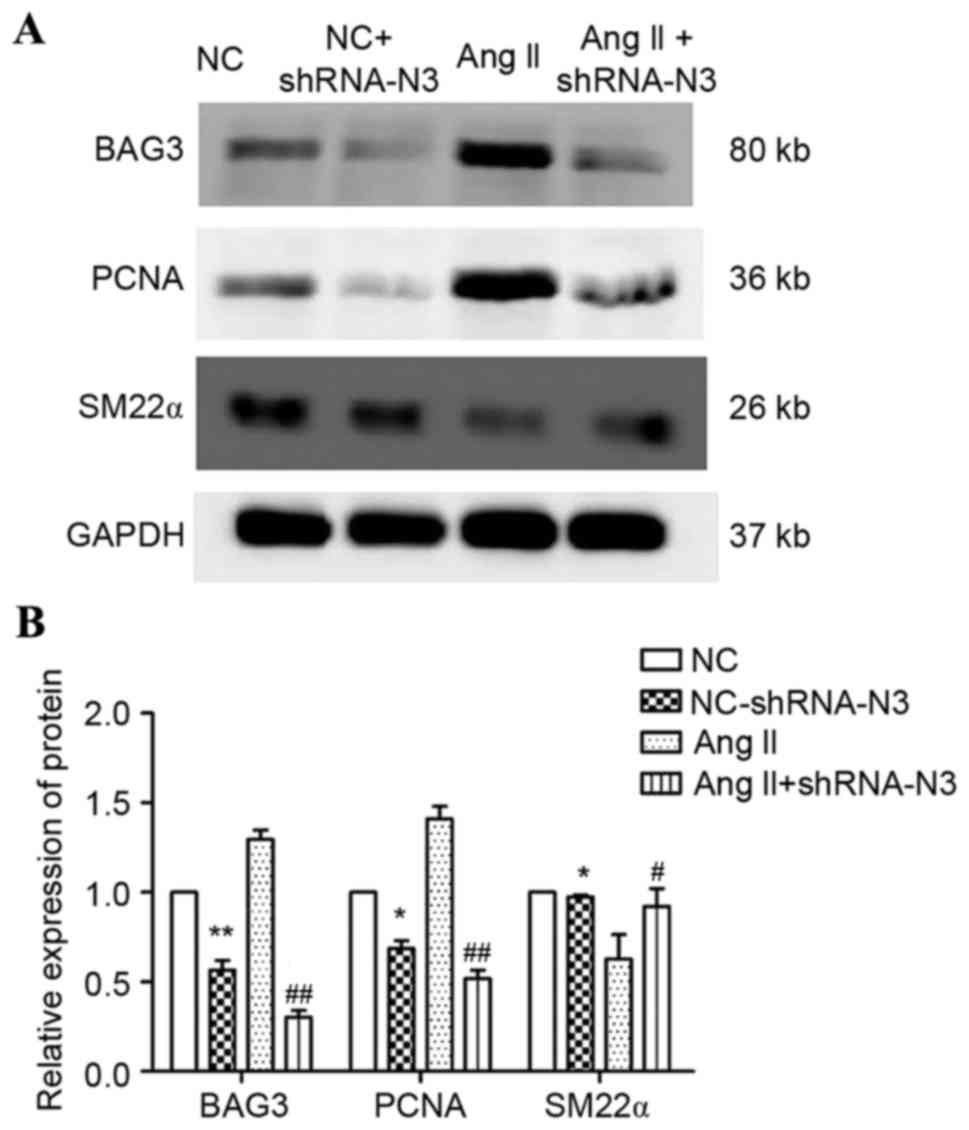
Knockdown BAG3 expression attenuates
Ang II-induced VSMC proliferation
To assess the potential effect of BAG3 on VSMCs
pretreated with Ang II in vitro, a BAG3 silencing plasmid
was used to knock down BAG3 expression.
As presented in Fig.
5, shRNA-N2 and shRNA-N3 significantly decreased the expression
of BAG3, whereas shRNA-N1 appeared to upregulate BAG3 expression
(P>0.05). The shRNA-N3 had the greatest downregulation effect on
both mRNA (40.61%; P<0.001; Fig.
5A) and protein (57.47%; P<0.01; Fig. 5B and C) expression levels.
Therefore, shRNA-N3 was used in subsequent experiments.
Furthermore, no significant differences were observed between BAG3
mRNA and protein expression levels between the NC and NC-shRNA-N
groups (Fig. 5); therefore,
transfection efficacy was determined.
Compared with the NC group, Ang II significantly
increased cell viability (Fig. 6).
Furthermore, Ang II upregulated the protein expression levels of
BAG3 and PCNA, and decreased SM22α expression (Fig. 7). However, shBAG3 significantly
ameliorated these effects (Figs. 6
and 7), indicating that regulation
of Ang II may be partially mediated by BAG3 activity, and may be
based on upregulation of proliferative factors.
Ang II induces BAG3 production via the
NF-κB p65 signaling pathway
As the NF-κB p65 signaling pathway may be linked to
the proliferative, migratory and invasion effects of BAG3, the
present study further analyzed the TLR4/NF-κ P65 pathway activity
in the process of Ang II-treated VSMCs. When treated with Ang II,
the TLR4/NF-κ p65 pathway was activated (Fig. 8). To further estimate whether
TLR4/NF-κ p65 mediates the upregulation of BAG3, VSMCs were
pretreated with PDTC and were subsequently cultured with or without
Ang II for 24 h (14). The results
suggested that PDTC pretreatment markedly decreases BAG3 expression
in response to the treatment of Ang II (P<0.001); however, no
such effect was observed on the basic expression under regular
conditions (Fig. 9). Therefore,
Ang II may regulate BAG3 expression via the TLR 4/NF-κ p65
signaling pathway in VSMCs.
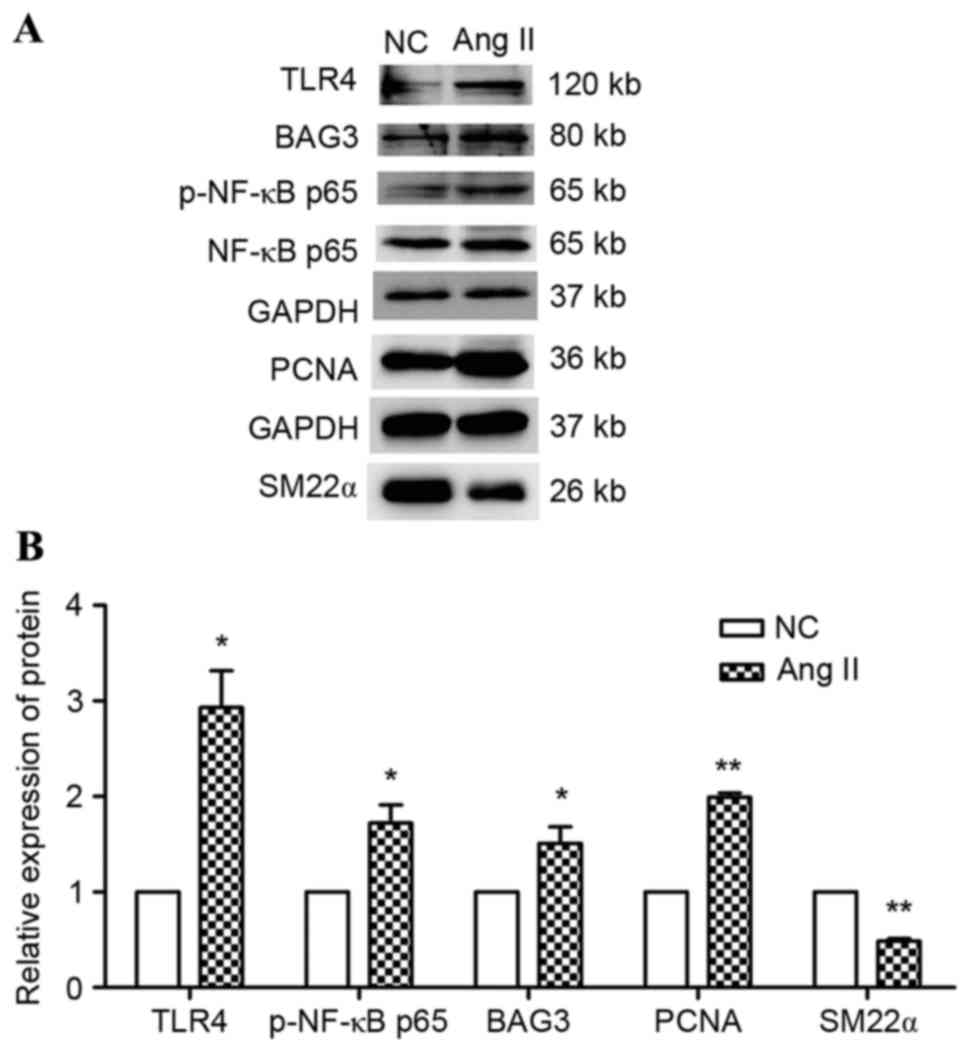 | Figure 8.Effects of Ang II on TLR4, p-NF-κB p65
and BAG3 protein expression. VSMCs were incubated with or without
Ang II for 24 h. (A) Representative western blot images and (B)
quantification of TLR4, p-NF-κB p65 and BAG3 protein expression.
Data are expressed as the mean ± standard error of three
independent experiments. *P<0.05, **P<0.01 vs. NC. NC,
negative control; TLR4, toll-like receptor-4; NF-κB p65, nuclear
factor-κB p65; p, phosphorylated; Ang II, angiotensin II; BAG3,
Bcl-2-associated athanogene 3; SM22α, smooth muscle protein α;
PCNA, proliferating cell nuclear antigen. |
Discussion
The present study demonstrated that Ang II induced
cell proliferation and upregulated BAG3 expression in VSMCs.
Similarly, a previous study reported that Ang II regulates BAG3
mRNA and protein expression in cultured renal fibroblast (15). Notably, the results of the present
study demonstrated that regulation of Ang II on BAG3 expression may
be partially mediated via the TLR4/NF-κB p65 signaling pathway in
VSMCs. This research firstly presents a potential link between Ang
II and BAG3 in VSMCs in vitro.
Previous studies have reported that in various tumor
diseases, BAG3 may be significant in sustaining cellular survival,
proliferation, migration and invasion, and resistance to therapy
(16,17). Therefore, BAG3 has been identified
as a novel anti-cancerous target in humans (18). However, BAG3 has been reported to
only highly expressed in limited cell types, including myocytes and
skeletal muscle cells. BAG3 is associated with cell resistance to
mechanical stress (6). Du et
al (15,19) demonstrated in a rat unilateral
ureteral obstruction model that BAG3 expression is associated with
the renal fibrosis level. Furthermore, they verified that BAG3
contributes to renal fibrosis via increasing the synthesis and
deposition of extracellular matrix protein in vitro
(15,19). Similarly, BAG3 mutations have been
reported to be associated with familial cardiomyopathy.
Furthermore, in end-stage heart failure without a familial history,
the level of BAG3 is significantly reduced (20,21).
A previous study demonstrated that BAG3 regulates contractility and
Ca2+ homeostasis in adult mouse ventricular myocytes
(22). However, to the best of our
knowledge, there is no evidence to clarify whether BAG3 is involved
in the pathogenic process of VSMCs. Therefore, the present study
aimed to estimate the potential effects of Ang II on BAG3
expression in VSMCs in vitro.
A dose- and time-dependent increase in BAG3 mRNA and
protein expression levels were observed in the Ang II-induced VSMC
proliferative process. Therefore, Ang II may cause vascular
remodeling partially by modulation of BAG3 expression. It is well
demonstrated that Ang II has a significantly regulative effect on
cell proliferation in VSMCs, and serves as a key regulator in the
cell proliferative process. A previous study demonstrated that in
serum-deprived HK2 cells, the expression of BAG3 mRNA is relatively
low. However, transforming growth factor-β1 markedly upregulates
this expression (15). Consistent
with this, the present study demonstrated relatively low levels of
BAG3 under normal conditions. However, treatment with Ang II
significantly upregulated BAG3 expression levels, accompanied by
increased VMSC proliferation in a dose- and time-dependent manner.
This suggested a key regulative role of BAG3 in the proliferative
process of Ang II stimulation.
Ang II is significantly associated with the
pathophysiological action of atherosclerosis through its
pro-inflammatory effect. It has been demonstrated to produce
inflammation in VSMCs via the TLR4/NF-κB p65 signaling pathway
(5). A previous study demonstrated
that suppression of NF-κB p65 activity may prevent proliferation in
VSMCs induced by high glucose (23). Furthermore, Rapino et al
(24) revealed that NF-κB
activation is necessary in BAG3 induction following ST80/Bortezomib
treatment in rhabdomyosarcoma cells. Therefore, it was hypothesized
that Ang II may induce BAG3 upregulation via activation of the
TLR4/NF-κB p65 signaling pathway. The results of the present study
suggested that the TLR4/NF-κB p65 pathway was stimulated by Ang II.
When VSMCs were pretreated with PDTC, an antagonist of the NF-κB
p65 signaling pathway, BAG3 protein expression levels were
decreased. Therefore, Ang II may BAG3 expression partially via the
NF-κB p65 signaling pathway.
In conclusion, to the best of our knowledge, the
present study demonstrated that Ang II induces VSMC proliferation,
accompanied with an elevation of BAG3 expression. Furthermore, Ang
II elevated BAG3 expression partially via activation of the
TLR4/NF-κB p65 pathway in VSMCs. To the best of our knowledge, the
present study provides novel evidence to explain the pathological
mechanism of Ang II-induced VSMC proliferation, demonstrating that
BAG3 may act as a mediator of this mechanism and may be a viable
target in the development of future therapies for vascular
disease.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81470417).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langheinrich AC and Bohle RM:
Atherosclerosis: Humoral and cellular factors of inflammation.
Virchows Arch. 446:101–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:S419–S420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berk BC, Vekshtein V, Gordon HM and Tsuda
T: Angiotensin II-stimulated protein synthesis in cultured vascular
smooth muscle cells. Hypertension. 13:305–314. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji Y, Liu J, Wang Z and Liu N: Angiotensin
II induces inflammatory response partly via toll-like receptor
4-dependent signaling pathway in vascular smooth muscle cells. Cell
Physiol Biochem. 23:265–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosati A, Graziano V, De Laurenzi V,
Pascale M and Turco MC: BAG3: A multifaceted protein that regulates
major cell pathways. Cell Death Dis. 2:e1412011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki M, Iwasaki M, Sugio A, Hishiya A,
Tanaka R, Endo T, Takayama S and Saito T: BAG3 (BCL2-associated
athanogene 3) interacts with MMP-2 to positively regulate invasion
by ovarian carcinoma cells. Cancer Lett. 303:65–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kassis JN, Guancial EA, Doong H, Virador V
and Kohn EC: CAIR-1/BAG-3 modulates cell adhesion and migration by
downregulating activity of focal adhesion proteins. Exp Cell Res.
312:2962–2971. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Homma S, Iwasaki M, Shelton GD, Engvall E,
Reed JC and Takayama S: BAG3 deficiency results in fulminant
myopathy and early lethality. Am J Pathol. 169:761–773. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chami N, Tadros R, Lemarbre F, Lo KS,
Beaudoin M, Robb L, Labuda D, Tardif JC, Racine N, Talajic M and
Lettre G: Nonsense mutations in BAG3 are associated with
early-onset dilated cardiomyopathy in French Canadians. Can J
Cardiol. 30:1655–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Avenia M, Citro R, De Marco M, Veronese
A, Rosati A, Visone R, Leptidis S, Philippen L, Vitale G, Cavallo
A, et al: A novel miR-371a-5p-mediated pathway, leading to BAG3
upregulation in cardiomyocytes in response to epinephrine, is lost
in Takotsubo cardiomyopathy. Cell Death Dis. 6:e19482015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao F, Puddefoot JR, Barker S and Vinson
GP: Mechanism for aldosterone potentiation of angiotensin
II-stimulated rat arterial smooth muscle cell proliferation.
Hypertension. 44:340–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu C, Lv C, Chen F, Ma X, Shao Y and Wang
Q: The function of miR-199a-5p/Klotho regulating TLR4/NF-κB
p65/NGAL pathways in rat mesangial cells cultured with high glucose
and the mechanism. Mol Cell Endocrinol. 417:84–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du F, Li S, Wang T, Zhang HY, Li DT, Du
ZX, Wang HQ and Wang YQ: BAG3 regulates ECM accumulation in renal
proximal tubular cells induced by TGF-β1. Am J Transl Res.
7:2805–2814. 2015.PubMed/NCBI
|
|
16
|
Shi H, Xu H, Li Z, Zhen Y, Wang B, Huo S,
Xiao R and Xu Z: BAG3 regulates cell proliferation, migration, and
invasion in human colorectal cancer. Tumour Biol. 37:5591–5597.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong DH, Li S, Du ZX, Liu C, Liu BQ, Li C,
Zong ZH and Wang HQ: BAG3 elevation inhibits cell proliferation via
direct interaction with G6PD in hepatocellular carcinomas.
Oncotarget. 7:700–711. 2016.PubMed/NCBI
|
|
18
|
Zhu H, Liu P and Li J: BAG3: A new
therapeutic target of human cancers? Histol Histopathol.
27:257–261. 2012.PubMed/NCBI
|
|
19
|
Du F, Li S, Wang T, Zhang HY, Li DT, Du ZX
and Wang HQ: Implication of Bcl-2-associated athanogene 3 in
fibroblast growth factor-2-mediated epithelial-mesenchymal
transition in renal epithelial cells. Exp Biol Med (Maywood).
240:566–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feldman AM, Begay RL, Knezevic T, Myers
VD, Slavov DB, Zhu W, Gowan K, Graw SL, Jones KL, Tilley DG, et al:
Decreased levels of BAG3 in a family with a rare variant and in
idiopathic dilated cardiomyopathy. J Cell Physiol. 229:1697–1702.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Marco M, D'Auria R, Rosati A, Vitulano
G, Gigantino A, Citro R, Piscione F, Zilinski J, Januzzi JL Jr and
Turco MC: BAG3 protein in advanced-stage heart failure. JACC Heart
Fail. 2:673–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feldman AM, Gordon J, Wang J, Song J,
Zhang XQ, Myers VD, Tilley DG, Gao E, Hoffman NE, Tomar D, et al:
BAG3 regulates contractility and Ca(2+) homeostasis in adult mouse
ventricular myocytes. J Mol Cell Cardiol. 92:10–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong IK, Oh DH, Park SJ, Kang JH, Kim S,
Lee MS, Kim MJ, Hwang YC, Ahn KJ, Chung HY, et al: Inhibition of
NF-κB prevents high glucose-induced proliferation and plasminogen
activator inhibitor-1 expression in vascular smooth muscle cells.
Exp Mol Med. 43:684–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rapino F, Abhari BA, Jung M and Fulda S:
NIK is required for NF-κB-mediated induction of BAG3 upon
inhibition of constitutive protein degradation pathways. Cell Death
Dis. 6:e16922015. View Article : Google Scholar : PubMed/NCBI
|