Introduction
Sepsis is a complex clinical syndrome that results
in the widespread activation and dysfunction of innate and adaptive
immunity (1,2). In the development of sepsis, several
lines of evidence have suggested that T cells are involved in the
maintenance of peripheral homeostasis and regulation of the immune
response, and are considered not only the effector cells but also
the modulator cells in the immune response during sepsis (3–5).
Subjection to acute insult alters T cells by inducing an imbalance
in T helper (Th) cell functions, caused by a phenotypic imbalance
in the regulation of the Th1 and Th2 immune response.
Tumor necrosis factor-α (TNF-α)-induced protein 8
(TNFAIP8), which is also known as SCC-S2, GG2-1 and MDC-3.13, was
the first identified member of the TNFAIP8 family. TNFAIP8 was
originally detected in a primary human head and neck squamous cell
carcinoma (HNSCC) cell line and its matched metastatic
HNSCC-derived cell line from the same patient, as determined by
expression profile analysis (6).
TNFAIP8 is associated with enhanced cell survival and inhibition of
proapoptotic enzymes, including caspase-8 and caspase-3, and
depends on the activation of nuclear factor-κB and TNF-α in human
cancer cells (7). Since T
lymphocytes are also essential in cell-mediated immunity in the
setting of acute injury, it was hypothesized that TNFAIP8 may be
associated with the immune regulation mediated by cluster of
differentiation (CD)4+ T cells. Therefore, the present
study aimed to investigate the potential effects of TNFAIP8 on T
cell-mediated immunity in cecal ligation and puncture (CLP)-induced
sepsis.
Materials and methods
Ethics statement
The present study was approved by the 309th Hospital
of Chinese People's Liberation Army Medical Research Ethical
Committee (Beijing, China). Male C57BL/6 mice (n=100) were
purchased from Shandong University Animal Ethical Committee (Jinan,
China), and were 8–10 weeks old at the time of entry into the
present study.
Medium and reagents
Triton X-100 and MTT were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640 medium
was purchased from Hyclone; GE Healthcare Life Sciences, (Logan,
UT, USA), supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) The
CD4+ T cell Isolation kit was purchased from Miltenyi
Biotec GmbH (Bergisch-Gladbach, Germany. Anti-CD3 and anti-CD28
monoclonal antibodies were purchased from BD Biosciences
(Affymetrix, San Jose, CA, USA; cat. nos. 16-0022 and 16-0281,
respectively). The enhanced chemiluminescence (ECL) plus
chemiluminescence kit was purchased from GE Healthcare Life
Sciences (Uppsala, Sweden). ELISA kits for interleukin IL-2 (cat.
no. EM002-96), IL-4 (cat. no. EM003-96) and interferon IFN-γ (cat.
no. EM007-96) were purchased from Shanghai ExCell Biology, Inc.
(Shanghai, China).
Murine CLP model
Male C57BL/6 mice used in the present study (weight,
18–22 g) were provided by the Shandong University Animal Ethical
Committee. All animals were housed in separate cages in a
temperature-controlled room at 26°C under a 12-h light/dark cycle.
All animals had free access to food and water. Polymicrobial sepsis
was induced by the CLP procedure, as described by Wichterman et
al (8). Briefly, anesthesia
was induced through the intraperitoneal administration of
thiopental (25 mg/kg), the mice were placed in a supine position
and their abdomens were shaved. An abdominal midline incision of 1
cm was made to expose the cecum. According to Rittirsch et
al (9), the cecum was ligated
at the middle and punctured twice with a 21-gauge (0.73-mm) needle
to induce sepsis of moderate severity. Sham-operated mice underwent
the same laparotomy procedure with the exception of ligation and
perforation.
Experimental design
In the present study, 100 mice were randomly divided
into four groups as follows: Sham injury group (n=30), CLP group
(n=30), CLP with lentivirus-RNA-TNFAIP8 group (n=20) and CLP with
negative control group (n=20). Mice in all groups were sacrificed
24 h following CLP, and spleen samples were harvested for the
isolation of CD4+ T cells.
TNFAIP8 RNA lentivirus generation and
infection
Small RNA specific to TNFAIP8 was synthesized by
GenchemBiotetchnology Company (Shanghai, China), the sequence was
as follows:
5′-CCGGCATGGAGAAGTTCAAGAAGAATTCAAGAGATTCTTCTTGAACTTCTCCATGTTTTT-3′.
To induce overexpression of TNFAIP8, recombinant lentiviruses
[pFH-L lentiviral vector (Shanghai GeneChem Co., Ltd., Shanghai,
China)], carrying TNFAIP8-RNAwere injected intraperitoneally at a
multiplicity of infection (MOI) of 109 TU/ml into mice
14 days prior to CLP injury. Concomitantly, the same concentration
of recombinant lentiviruses that carried the negative control RNA
(empty vector; Shanghai GeneChem Co., Ltd.) was injected at an MOI
of 5×108 TU/ml into the mice, which served as a wild
type control. TNFAIP8 and negative control RNA lentivirus
generation was performed according to the lentivirus vector
particle manufacturer's protocol. The efficiency of overexpression
was determined by western blot analysis of TNFAIP8 expression.
Isolation of splenic CD4+ T
cells
Spleens were obtained from the sham mice and CLP
mice, and were cultured in 5 ml RPMI-1640 medium. Mononuclear cells
were isolated with the use of Ficoll-Paque density gradient
centrifugation at 1,500 × g for 15 min at 4°C, according to the
manufacturer's protocol, and CD4+ T cells were isolated
from them using the CD4+ T cell isolation kit according
to the manufacturer's protocol. Mononuclear cells (10
µl/107 total cells) were stained with a biotin-antibody
cocktail (MiltenyiBiotec GmbH, BergischGladbach, Germany; cat. no.
130049201) and incubated for 10 min at 4°C. They were then
magnetically labeled with anti-biotin MACS microbeads (20
µl/107 total cells; MiltenyiBiotec GmbH), incubated for
15 min at 4°C, and harvested through a negative selection LS column
(MiltenyiBiotec GmbH).
Western blot analysis
Western blotting was performed to determine the
expression of TNFAIP8 in the extracted CD4+ T cells.
Cells were lysed by radioimmunoprecipiation lysis buffer [25 mM
Tris-HCL (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate,
0.1% SDS] at the temperature of 0°C for 30 min. Following
sonication for 5 sec, the lysed cells were then centrifuged at
12,000 × g for 30 min at 4°C. Protein levels were quantified using
a bicinchoninic acid protein assay kit. Protein extracts (50 µg)
were separated by 8% SDS-PAGE, and the products were electro
transferred to an immobilon polyvinylidene difluoride membrane.
Following blocking with 10% skim milk overnight at 4°C, the
membrane was incubated for 4 h at room temperature with
anti-TNFAIP8 polyclonal antibody (cat. no. ab64988; 1:500 dilution;
Abcam, Cambridge, MA, USA) or control anti-β-actin antibody (cat.
no. sc-130201; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). The membrane was then washed three times with PBS and
incubated with peroxidase-labeled mouse IgG secondary antibody
(cat. no. A0548; 1:5,000; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. As aforementioned, the membrane was washed with
PBS three times, and blots were then developed using the ECL plus
chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.). The
protein bands were detected using an ECL detection system (Pierce;
Thermo Fisher Scientific, Inc.).
Confocal microscopy analysis
CD4+ T cells with density of
106/ml in the normal group were washed with PBS three
times, fixed with 4% paraformaldehyde in PBS for 20 min, then
permeabilized with 0.2% Triton X-100 for 20 min both at room
temperature. Sections were pre-blocked with 1% FBS in PBS for 30
min, and stained with anti-TNFAIP8 antibody (cat. no. ab64988;
1:200; Abcam) overnight at 4°C. Following washing three times in
PBS, CD4+ T cells were stained with a fluorescein
isothiocyanate-conjugated goat anti-immunoglobulin G secondary
antibody (cat. no. C1309; 1:5,000; Applygen Technologies Inc.,
Beijing, China) for 1 h at room temperature followed by three
further washes in PBS. Following washing, the nuclei were stained
with 4′,6-diamidino-2-phenylindole for 5 min at room temperature.
The cells were observed under a laser scanning confocal
microscope.
T cell proliferation assay and
cytokine measurements
Purified CD4+ T cells were cultured in
RPMI-1640 medium supplemented with 10% heat-inactivated FBS, and
were stimulated for 24 h for CD4+ activation with a
combination of 1 µg/ml soluble anti-CD3 and 1 µg/ml soluble
anti-CD28 monoclonal antibodies at 37°C. The T cells were then
plated in 96-well plates at a density of 1×105
cells/well, and were incubated at 37°C in 5% CO2 for 68
h. Subsequently, modified MTT solution (5 mg/ml, 10 µl/well)
(10–12) was added and the cells were
incubated for a further 4 h, after which 100 µg acid isopropanol
was added to dissolve the MTT crystals. The MTT crystal suspension
was repeatedly mixed with a pipette, and the optical density was
measured using a microplate reader at a wavelength of 540 nm.
To analyze the secretion of IL-2, IL-4 and IFN-γ
into the culture medium of the cells, the supernatants obtained
from CD4+ T cells were analyzed, and the supernatants
were removed by pipetting. The levels of IL-2, IL-4 and IFN-γ were
measured using commercially available ELISA kits according to the
manufacturer's protocols.
Statistical analysis
All data in the present study are presented as the
mean ± standard deviation of 3 independent experiments. Continuous
data were examined by one-way analysis of variance followed by a
post hoc Dunnett's test for multiple comparisons. SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA) was used to perform these
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
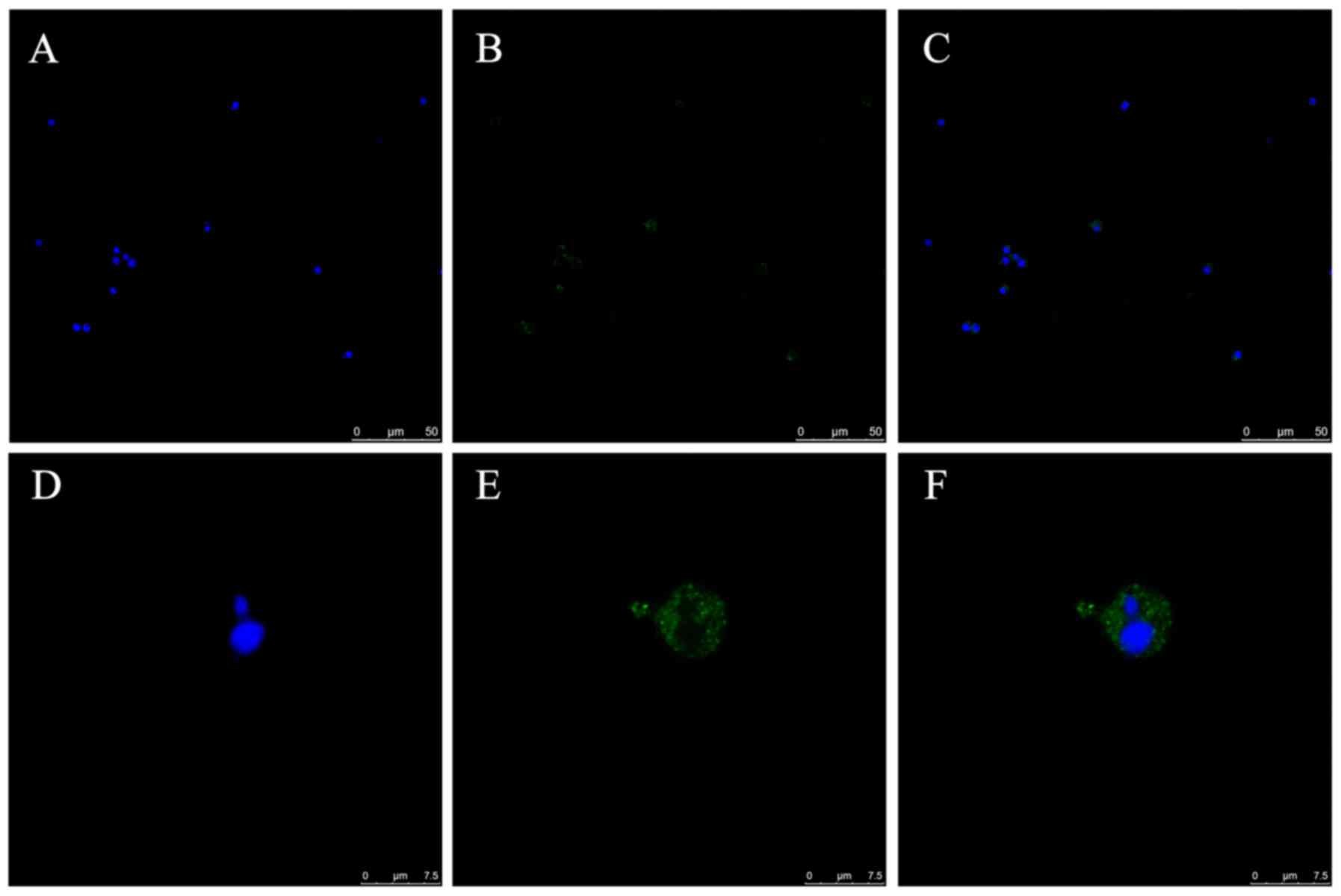
TNFAIP8 expression in CD4+
T cells
The expression and distribution of TNFAIP8 protein
in CD4+ T cells isolated from normal mice was
investigated by means of confocal laser scanning microscopy. Green
fluorescence was observed in the cytoplasm of the CD4+ T
cells, with their nuclei stained blue (Fig. 1A-F).
Splenic T lymphocyte proliferation
following CLP-induced sepsis
In the present study, the effects of TNFAIP8 on
proliferative activity of CD4+ T cells in a CLP mouse
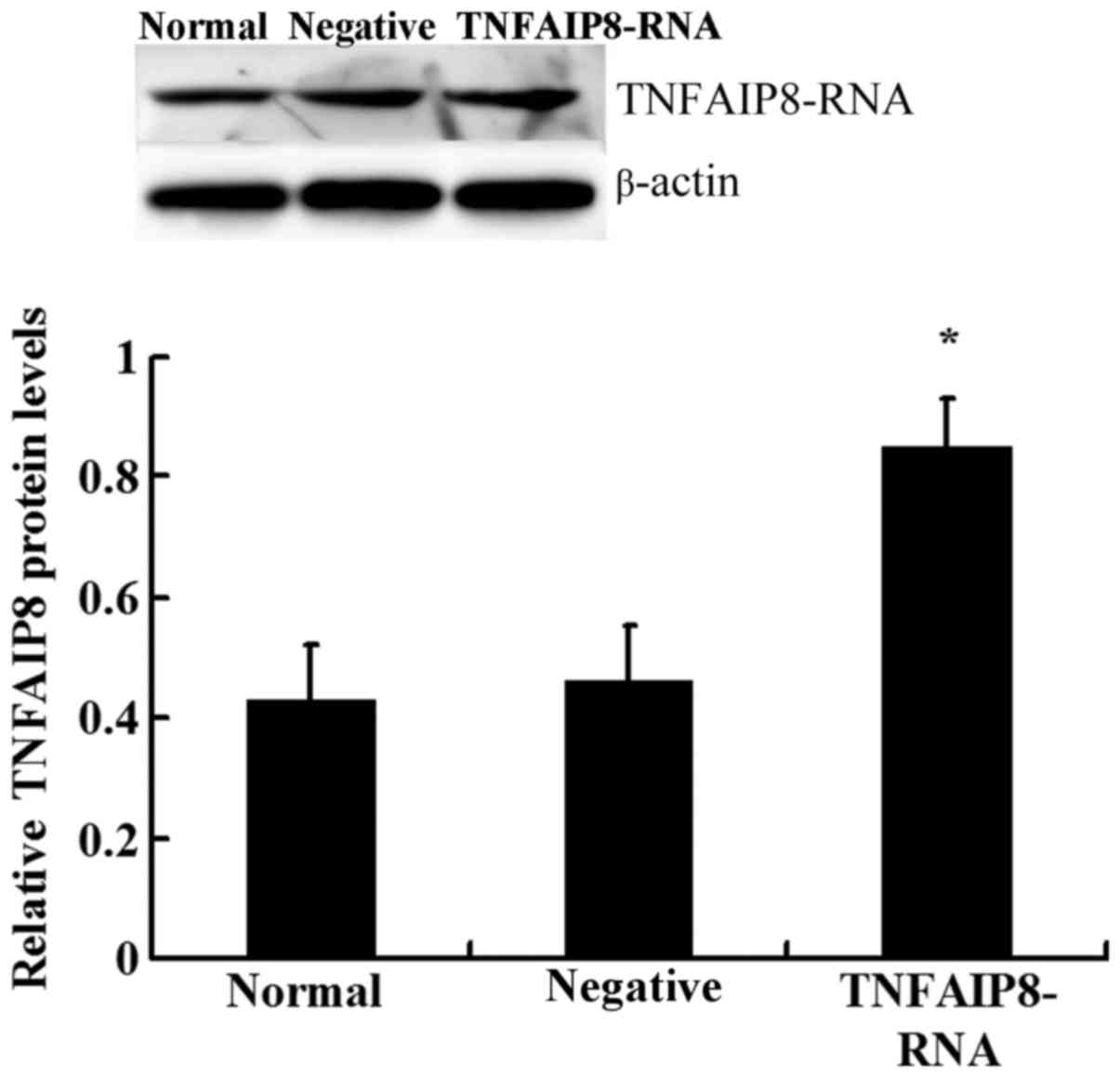
model were observed. TNFAIP8 was successfully upregulated following
injection of mice with lentivirus-RNA-TNFAIP8 (Fig. 2). Western blot analysis revealed
that the protein expression levels of TNFAIP8 were significantly
upregulated in the TNFAIP8 overexpression group compared with the
control groups (Fig. 2). The
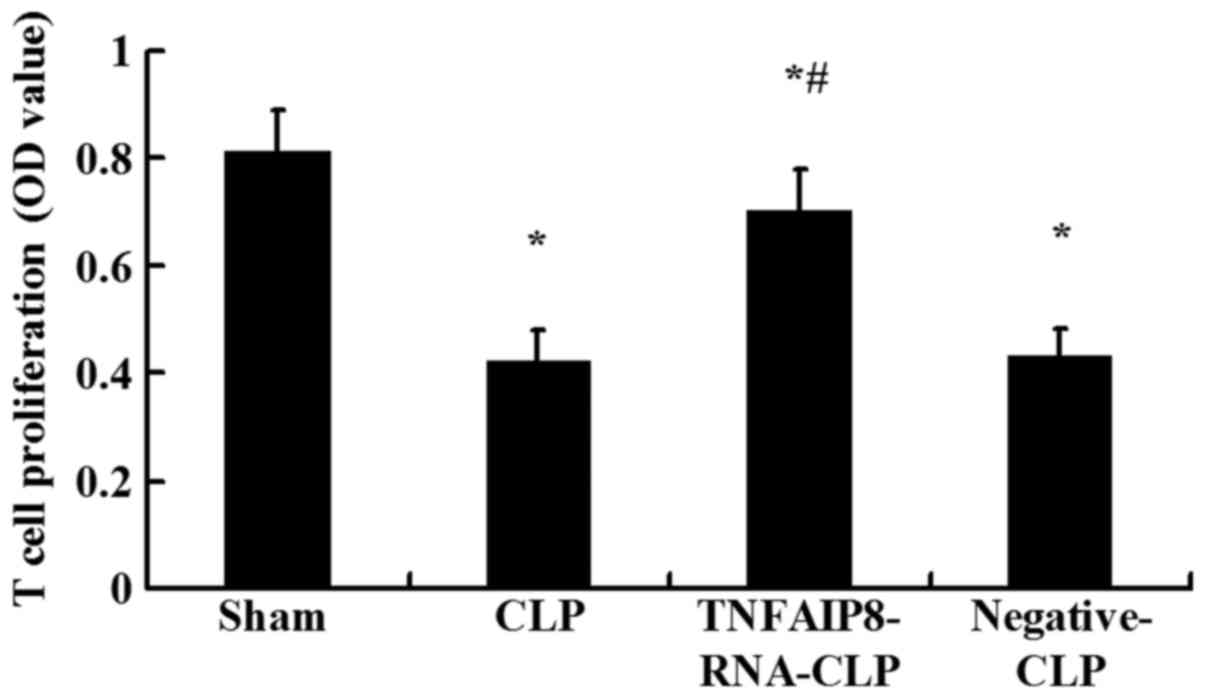
proliferative activity of splenic CD4+ T cells was
significantly suppressed 24 h following CLP compared with the sham
injury group (P<0.05; Fig. 3).
To further clarify the involvement of TNFAIP8 in the decreased cell
proliferation observed following CLP-induced sepsis, the effects of
upregulated TNFAIP8 were determined in vivo 24 h following
CLP. Compared with the CLP group, proliferative activity was
significantly increased in the CLP with TNFAIP8 overexpression
group (P<0.05; Fig. 3),
indicating that TNFAIP8 may be closely associated with the immune
functions of CD4+ T cells.
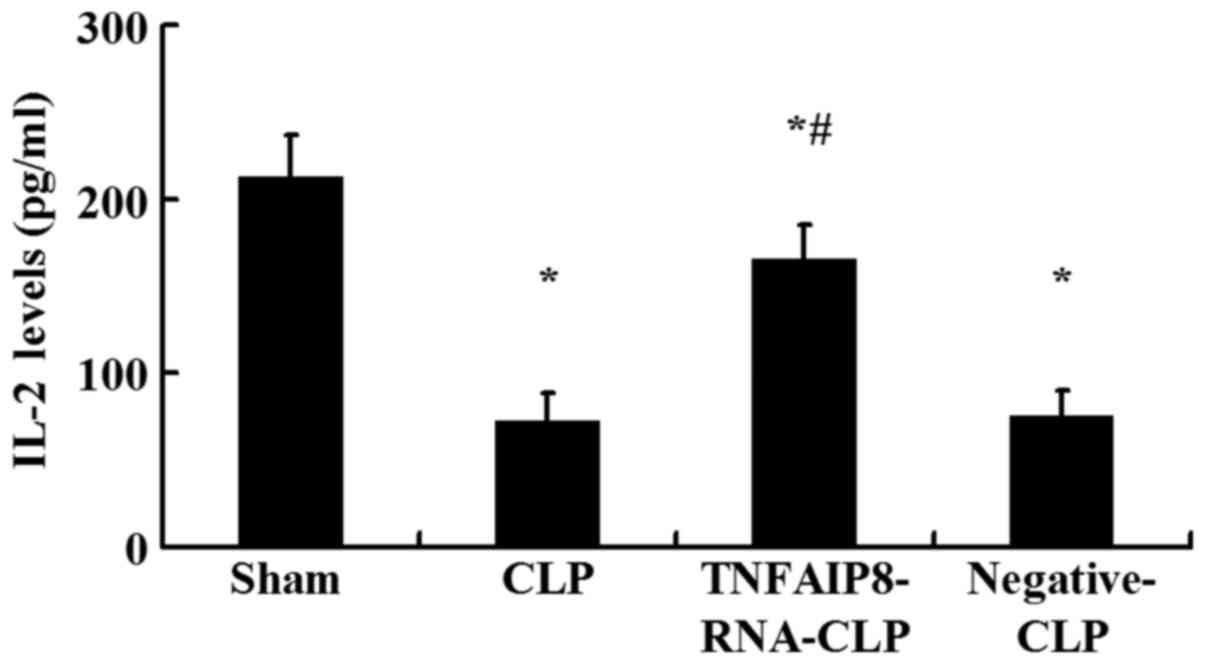
IL-2 production
IL-2 is a potent T cell growth factor that acts upon
itself in an autocrine fashion. The levels of IL-2 in culture
supernatants of CD4+ T cells were measured by ELISA.
Following 24 h of CLP, IL-2 production in the culture supernatant
was significantly downregulated compared with in the sham-injured
group (P<0.05; Fig. 4).
Conversely, IL-2 expression levels were significantly higher
following TNFAIP8 upregulation by lentivirus-RNA-TNFAIP8 infection
in vivo compared with the CLP group, whereas no significant
difference was observed between the negative control CLP and CLP
groups (Fig. 4). It was noted that
excessive TNFAIP8 expression was associated with the increased IL-2
levels secreted by CD4+ T cells.
Polarization of T cells following
sepsis
It is possible for an immune response to become
polarized towards either Th1 or Th2 production over time;
therefore, one subtype or the other dominates. It is well known
that Th1 cells produce IFN-γ and Th2 cells produce IL-4; therefore,
ELISA was used in the present study to detect these T cell-produced
cytokines, in order to identify the polarization of naive T cells.
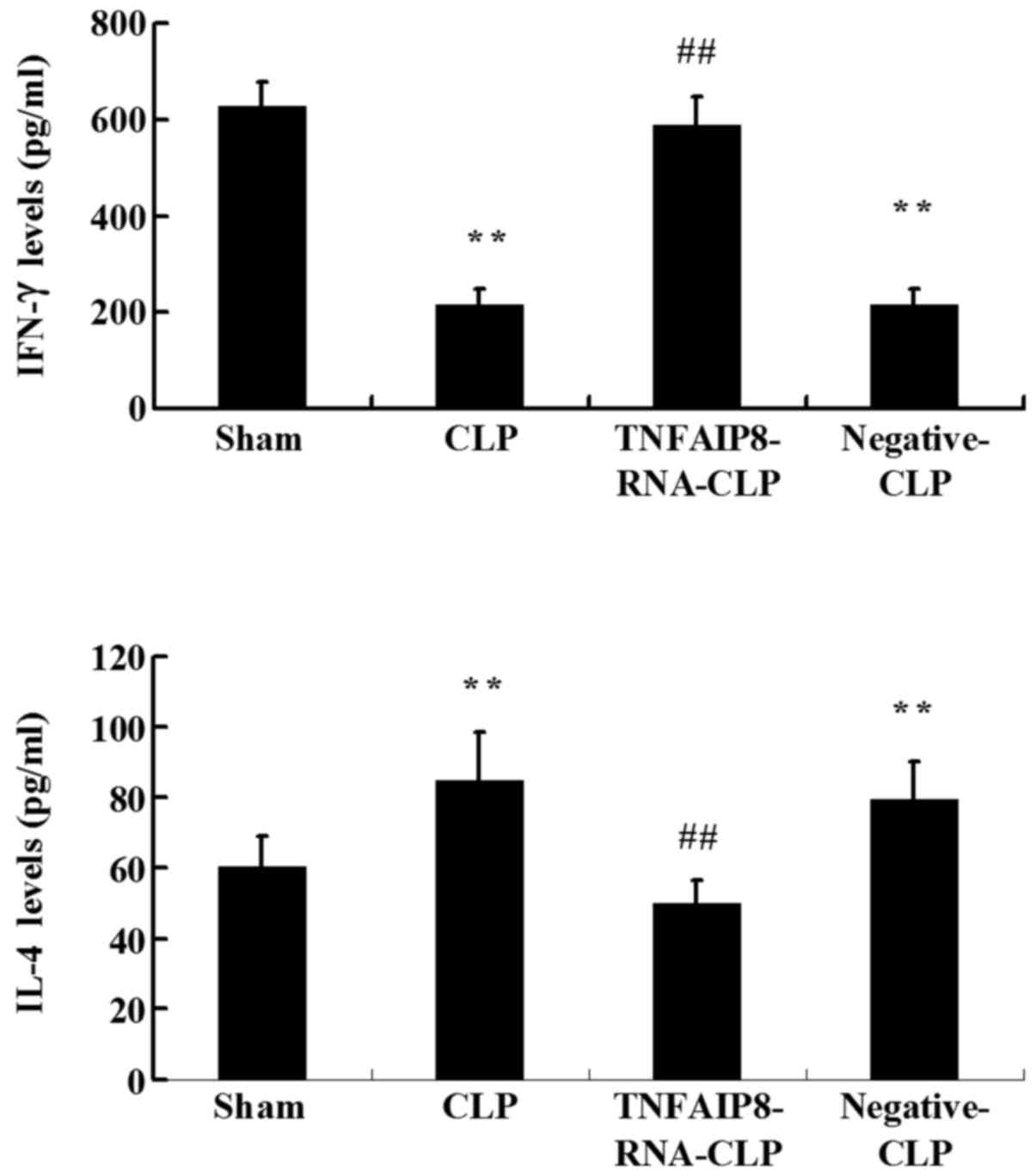
The results revealed that the levels of IFN-γ produced by
CD4+ T cells were significantly reduced, and that the
levels of IL-4 were significantly increased at 24 h in the CLP
group compared with the sham group (P<0.01; Fig. 5). Furthermore, a significant
increase in IFN-γ and a significant decrease in IL-4 were detected
in CD4+ T cells following TNFAIP8 overexpression in the
CLP-induced sepsis group (P<0.01; Fig. 5). Taken together, these results
suggested that TNFAIP8 may affect T cell polarization following
sepsis.
Discussion
Sepsis represents a complex clinical condition that
results from a damaging host response to infection. Considerable
data have demonstrated that acute insults, including major burns,
trauma and hemorrhage, may result in T cell immune suppression,
which is associated with the loss of function of the Th1 lymphocyte
phenotype (13–17). TNFAIP8 has been demonstrated to be
associated with enhanced survival and inhibition of proapoptotic
enzymes, including caspase-8 and caspase-3, and participates in
cell death, transcriptional regulation, migration, proliferation
and apoptosis (7,18). In addition to high expression in
tumor tissue, TNFAIP8 is also highly expressed in immune organs and
lymphoid tissues; however, its function in the immune system
remains unclear. In the present study, experiments were conducted
to verify the potential effect of TNFAIP8 upregulation on the
CD4+ T cell-mediated immune response in a CLP murine
model
During experimental and clinical sepsis, T cells are
critical cellular components of immunity, which are essential for
an effective immune response to acute insults or septic challenge.
A previous study demonstrated that TNFAIP8 was expressed in
CD4+ and CD8+ T cells, and the mRNA and
protein levels were significantly decreased in tumor-infiltrating
CD4+ and CD8+ T cells compared with
peripheral CD4+ and CD8+ T cells (19). In the present study, confocal laser
scanning microscopy was used to orientate TNFAIP8 protein
expression, and TNFAIP8 protein was revealed to be expressed in the
cytosol of CD4+ T cells, thus suggesting that TNFAIP8
may be involved in the immune response. The present study suggested
that TNFAIP8 expression may be involved in the pathogenesis of
CD4+ T cell immune dysfunction in mice during sepsis,
whereas TNFAIP8 overexpression significantly improved the immune
function of CD4+ T cells. The results demonstrated that
the proliferation of splenic CD4+ T cells was
significantly inhibited 24 h following CLP and that overexpression
of TNFAIP8 in vivo attenuated the suppression of splenic T
lymphocyte proliferative activity 24 h following CLP. Therefore,
TNFAIP8 may be involved in development of the impairment of immune
function of T lymphocytes in CLP-induced sepsis.
IL-2 is a key regulator of the immune response,
which is secreted by activated T lymphocytes and is essential to
activate T lymphocyte proliferation (20,21).
IL-2 production was significantly inhibited in CD4+ T
cells from CLP mice compared with in those from sham-injured mice.
However, this suppression was ameliorated by TNFAIP8 upregulation
in vivo. Therefore, TNFAIP8 may affect IL-2 secretion by T
lymphocytes in the setting of acute insult and further modulate
activation of T lymphocytes.
It has been reported that proliferation of T
lymphocytes is suppressed and modulation of Th1, as well as Th2, is
shifted in sepsis (22–25). Notably, in animal models of injury,
the release of IL-2 and IFN-γ produced by Th1, and IL-4 produced by
Th2 was altered (25). In the
present study, splenic CD4+ T cells were demonstrated to
develop into Th2 cells in animals subjected to CLP. To further
clarify the potential effect of TNFAIP8 on CD4+ T cells,
TNFAIP8 was upregulated. TNFAIP8 overexpression was demonstrated to
initiate a CD4+ T cell to shift to Th1 following CLP.
These results indicated that increased expression of TNFAIP8 may
influence the polarization of splenic T cells. Nevertheless, the
present study has limitations. Nuclear factor of activated T cells,
which was the first characterized transcription factor that binds
to the IL-2 promoter, should be used to study the effects of
TNFAIP8 on the immune functions of CD4+ T cells.
Furthermore, different time points following CLP should be
studied.
In conclusion, based on the results of the present
in vivo study, TNFAIP8 was demonstrated to be associated
with the development of the splenic T lymphocyte immune response in
mice following CLP-induced sepsis. However, numerous key issues
remain to be resolved. It remains unclear how TNFAIP8 signaling
controls the immune function of T lymphocytes, or what the
association is between TNFAIP8 and other molecules in cell-mediated
immunity. Further studies investigating the precise mechanism of
action of TNFAIP8 are therefore required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470043) and the
Department Military Medicine and Geriatric Diseases Research Fund
(grant no. ZCWS14B04).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
MC conceived the study. BY and LX designed the
process of this study. LX, BY and DZ performed the experiments. SL
analyzed the experimental results. BY and LX wrote the paper. DZ
and SL reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the 309th Hospital
of Chinese People's Liberation Army Medical Research Ethical
Committee (Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Oberholzer A, Oberholzer C and Moldawer
LL: Sepsis syndromes: Understanding the role of innate and acquired
immunity. Shock. 16:83–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber GF and Swirski FK:
Immunopathogenesis of abdominal sepsis. Langenbecks Arch Surg.
399:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gatewood MO, Wemple M, Greco S, Kritek PA
and Durvasula R: A quality improvement project to improve early
sepsis care in the emergency department. BMJ Qual Saf. 24:787–795.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khakpour S, Wilhelmsen K and Hellman J:
Vascular endothelial cell Toll-like receptor pathways in sepsis.
Innate Immun. 21:827–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delano MJ and Ward PA: Sepsis-induced
immune dysfunction: can immune therapies reduce mortality. J Clin
Invest. 126:23–31. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar D, Whiteside TL and Kasid U:
Identification of a novel tumor necrosis factor-alpha-inducible
gene, SCC-S2, containing the consensus sequence of a death effector
domain of fas-associated death domain-like
interleukin-1beta-converting enzyme-inhibitory protein. J Biol
Chem. 275:2973–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You Z, Ouyang H, Lopatin D, Polver PJ and
Wang CY: Nuclear factor-kappa B-inducible death effector
domain-containing protein suppresses tumor necrosis factor-mediated
apoptosis by inhibiting caspase-8 activity. J Biol Chem.
276:26398–26404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wichterman KA, Baue AE and Chaudry IH:
Sepsis and septic shock-a review of laboratory models and a
proposal. J Surg Res. 29:189–201. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh MP, Rai AK and Singh SM: Gender
dimorphism in the progressive in vivo growth of a T cell lymphoma:
Involvement of cytokines and gonadal hormones. J Reprod Immunol.
65:17–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang LF, Yao YM, Zhang LT, Dong N, Yu Y
and Sheng ZY: The effect of high-mobility group box 1 protein on
activity of regulatory T cells after thermal injury in rats. Shock.
31:322–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Yao YM, Huang LF, Dong N, Yu Y
and Sheng ZY: The potential effect and mechanism of high-mobility
group box 1 protein on regulatory T cell-mediated
immunosuppression. J Interferon Cytokine Res. 31:249–257. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shinkai K, Mohrs M and Locksley RM: Helper
T cells regulate type-2 innate immunity in vivo. Nature.
420:825–829. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller AC, Rashid RM and Elamin EM: The
‘T’ in trauma: The helper T-cell response and the role of
immunomodulation in trauma and burn patients. J Trauma.
63:1407–1417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma T, Han L, Gao Y, Li L, Shang X, Hu W
and Xue C: The endoplasmic reticulum stress-mediated apoptosis
signal pathway is involved in sepsis-induced abnormal lymphocyte
apoptosis. Eur Surg Res. 41:219–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lang JD and Matute-Bello G: Lymphocytes,
apoptosis and sepsis: Making the jump from mice to humans. Crit
Care. 13:1092009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kasten KR, Tschop J, Adediran SG, Hildeman
DA and Caldwell CC: T cells are potent early mediators of the host
response to sepsis. Shock. 34:327–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valmiki MG and Ramos JW: Death effector
domain-containing proteins. Cell Mol Life Sci. 66:814–830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Song Y and Men X: Variance of
TNFAIP8 expression between tumor tissues and tumor-infiltrating
CD4+ and CD8+ T cells in non-small cell lung
cancer. Tumour Biol. 35:2319–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y, Zhang Y, et al: Tissue-specific expression
of TIPE2 provides insights into its function. Mol Immunol.
47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma A, Yang WL, Matsuo S and Wang P:
Differential alterations of tissue T-cell subsets after sepsis.
Immunol Lett. 168:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abboushi N, El-Hed A, El-Assaad W, Kozhaya
L, El-Sabban ME, Bazarbachi A, Badreddine R, Bielawska A, Usta J
and Dbaibo GS: Ceramide inhibits IL-2 production by preventing
protein kinase C-dependent NF-kappaB activation: Possible role in
protein kinase Ctheta regulation. J Immunol. 173:3193–3200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cuenca AG, Delano MJ, Kelly-Scumpia KM,
Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA and Moldawer
LL: A paradoxical role for myeloid-derived suppressor cells in
sepsis and trauma. Mol Med. 17:281–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diosa-Toro MA, Jaimes BFA, Rugeles LMT and
Velilla HPA: Cells with immunoregulatory properties and their
impact in the pathogenesis of sepsis. Rev Chilena Infectol.
28:572–578. 2011.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Condotta SA, Cabrera-Perez J, Badovinac VP
and Griffith TS: T-cell-mediated immunity and the role of TRAIL in
sepsis-induced immunosuppression. Crit Rev Immunol. 33:23–40. 2013.
View Article : Google Scholar : PubMed/NCBI
|