Introduction
An increasing imbalance between the number of donor
organs and potential liver transplant recipients has led to the
development of novel strategies to increase the pool of organ
donors (1). The declaration of
brain death (BD) as a point of no return has been accepted by most
societies, and organs derived from BD donors currently represent
the main source of organs used in transplantation (1,2).
Since the first BD donor organ transplantation in the 1960s,
clinical transplant results of donor organs have significantly
improved (3). To date, the grafts
obtained from BD donors have yielded positive outcomes. However, BD
is a dynamic and rather unphysiological course of events that
influences a number of physiological processes in the human body,
whereby potential grafts are damaged before liver transplantation
(4). Several experiments
demonstrated that systemic and hormonal changes occur immediately
under BD conditions, followed by oxidative stress, an inflammatory
response, and tissue ischemia reperfusion, all of which result in
liver damage (5–8). BD itself has a complicated influence
on the liver, thus limiting the number of suitable organs for liver
transplantation (6–9). The mechanism underlying the
deteriorating effect of BD on organs has not been fully
established. Therefore, it is necessary to investigate the
characterization of liver injury under conditions of BD to improve
the outcome of liver transplantation.
As the resident macrophages in the liver, Kupffer
cells (KCs) express key renin angiotensin system (RAS) components,
and RAS activity potentially participates in pathology and
physiology (10). The activation
of donor KCs is closely correlated with intense phagocytosis, a
high expression of membranous molecules, antigen presentation, and
the secretion of numerous cytokines, which all participate in the
immune and pro-inflammatory or anti-inflammatory reactions
(11). However, the exact role of
KCs in liver injury under conditions of BD remains unknown. The
purpose of this study was to explore the role of KCs in
inflammation and apoptosis in liver injury under conditions of BD
in a rat model.
Materials and methods
Experimental animals and
treatment
Sprague-Dawley (SD) rats weighing 300–350 g were
purchased from Henan Provincial Experimental Animal Center
(Zhengzhou, China). The Ethics Committee of the First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) approved this
study protocol. All animals were provided humane care in compliance
with governmental regulations and institutional guidelines. In
total, 24 rats were randomly divided into four groups as follows:
Rats that underwent sham operations (Sham group); rats subjected to
BD (BD group); rats subjected to BD plus gadolinium chloride
(GdCl3) treatment (Gd group) with GdCl3
solution (7 mg/kg of body weight delivered intraperitoneally;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) administered
continuously for two days before the operation (12); and rats subjected to BD plus normal
saline (Saline group). All operative procedures were the same as
those described for the Gd group. Rats were sacrificed 6 h after
BD, and 6 rats per time point were assessed.
Construction of BD models
The BD model was established according to previously
reported methods (13–15). Rats were anesthetized via
intraperitoneal injection of pentobarbital sodium (50 mg/kg body
weight). The BD model was induced by gradually increasing
intra-cranial pressure by slow inflation through a No. 3 Fogarty
catheter balloon (Edwards Lifesciences Corp., Irvine, CA, USA)
(13). Sham-operated rats
underwent the same surgical procedures but without the induction of
BD; this group served as BD controls. The rats were humanely
sacrificed by cervical dislocation at the indicated time after BD
as assessed by respiratory and circulatory parameters. Blood from
the caudal vein and liver samples were harvested in situ and
stored in liquid nitrogen until further analyses.
Analysis of histopathological changes
and plasma markers of liver function
The liver tissues were fixed in 100 g/l of neutral
formalin solution and embedded in paraffin wax. The sections were
stained with hematoxylin and eosin to assess morphological changes.
Portal inflammation, periportal/bridging necrosis, intralobular
degeneration/focal necrosis and fibrosis were evident pathological
changes in the injured livers as scored by the Knodell histological
activity index (16). The sections
were evaluated in a blinded manner under light microscopy by two
investigators. The necro-inflammatory score (HAI-NI) was analyzed
to evaluate the severity of hepatic damage. Changes in plasma
markers of liver function, serum alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) were detected using an automatic
biochemical analyzer. The AST/ALT ratio (AAR) was measured to
appraise liver damage following BD. Blood was collected through the
caudal vein to measure ALT and AST levels.
ELISA for the analysis of liver
cytokines
Total protein was extracted from hepatic tissue, and
protein levels were normalized to measure the expression levels of
tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10. These
protein levels were determined using rat TNF-α, IL-1β and IL-10
ELISA kits (Wuhan Boster Biological Technology, Ltd., Wuhan, China)
following the manufacturer's instructions. Sham-operated rats
served as BD controls. The results are presented at each time
point.
Apoptosis measurements
The concentration of apoptotic cells was determined
using the terminal deoxynucleotidyl transferase dUTP nick
end-labeling (TUNEL) assay. Apoptotic cells in liver tissue
sections (4 µm) were identified using the in situ cell death
detection kit (Fluorescein; Roche Diagnostics GmbH, Mannheim,
Germany) according to the manufacturer's protocol. The apoptotic
index (AI) results are expressed as the percentage of apoptotic
cells among the total number of cells.
Western blot analysis of liver
tissue
Liver tissue samples were used to measure the
expression levels of apoptosis-related proteins (cleaved caspase-3,
caspase-3 and Bcl-2) using selective polyclonal antibodies. Protein
concentrations of homogenates were determined using the Bradford
technique. Specific bands were detected using an enhanced
chemiluminescence system and captured on X-ray film. β-actin was
used as a loading control. The density of the bands on the membrane
was analyzed using Quantity One software v4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Data analysis
All values are expressed as the mean ± standard
deviation. The means of two continuous normally distributed
variables were compared by independent samples Student's t-test.
Multigroup comparisons of the means were performed using one-way
analysis of variance with post hoc Student-Newman-Keuls test.
Values were analyzed using the statistical package SPSS for Windows
version 15.0 (SPSS, Inc., Chicago, IL, USA). Statistical
significance was set at an α value of P=0.05.
Results
KC depletion aggravates liver injury
under conditions of BD
Histopathological changes in the
liver
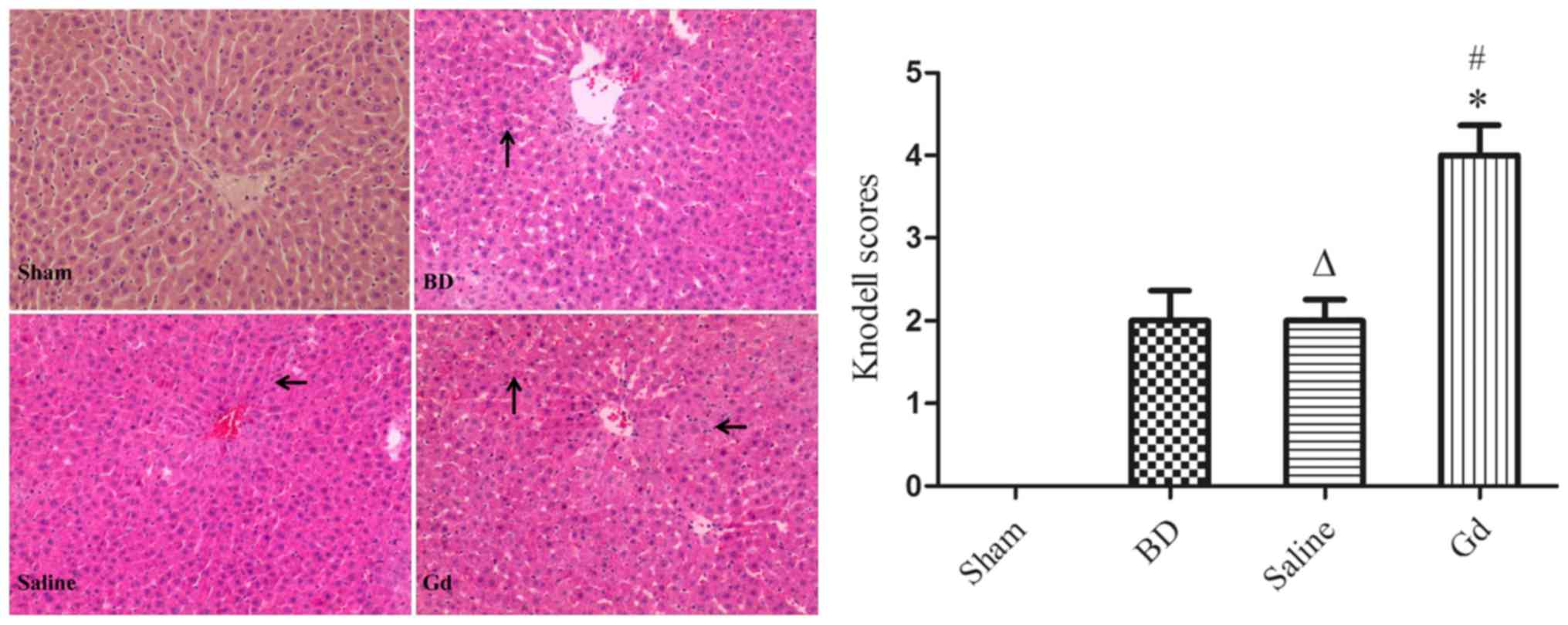
Liver histological sections were stained with
H&E, and liver injury was graded according to the degree of
necro-inflammation (HAI-NI) using the Knodell score system
(16,17). We observed that the zones of
necrosis were located around pericentral areas in the rats, with
the exception of rats undergoing sham operation. The hepatocytes in
the GdCl3 group exhibited aggravated vacuolar
degeneration and edema, and a greater number of inflammatory cells
infiltrated the portal area compared with the B and S groups.
However, there were no differences in injury between the saline
group and the BD group. Saline had no effect on hepatocyte injury
under conditions of BD (Fig. 1).
The pathological Knodell scores of the livers exposed to
GdCl3 treatment were significantly increased compared
with those of the B and S groups (P<0.05). However, no
significant difference was noted between the B and S groups
(P>0.05).
Plasma liver function markers
After 6 h of BD, ALT levels increased 4-fold
compared with those in the sham operation group. AST levels
followed the same trend as ALT levels. Six h after BD, pretreatment
with GdCl3 caused increases in ALT and AST levels
compared with those in the saline group (P<0.05). However,
pretreatment with saline had no effect on ALT and AST levels.
Statistically significant differences in serum ALT and AST levels
were noted between the saline and BD groups. GdCl3
treatment prior to BD increased AST/ALT ratio without significant
statistical difference (P>0.05; Fig. 2). These results reveal that
GdCl3 treatment significantly aggravated hepatocyte
injury as assessed by ALT and AST levels and necrotic cell death
without significant statistical difference of AAR.
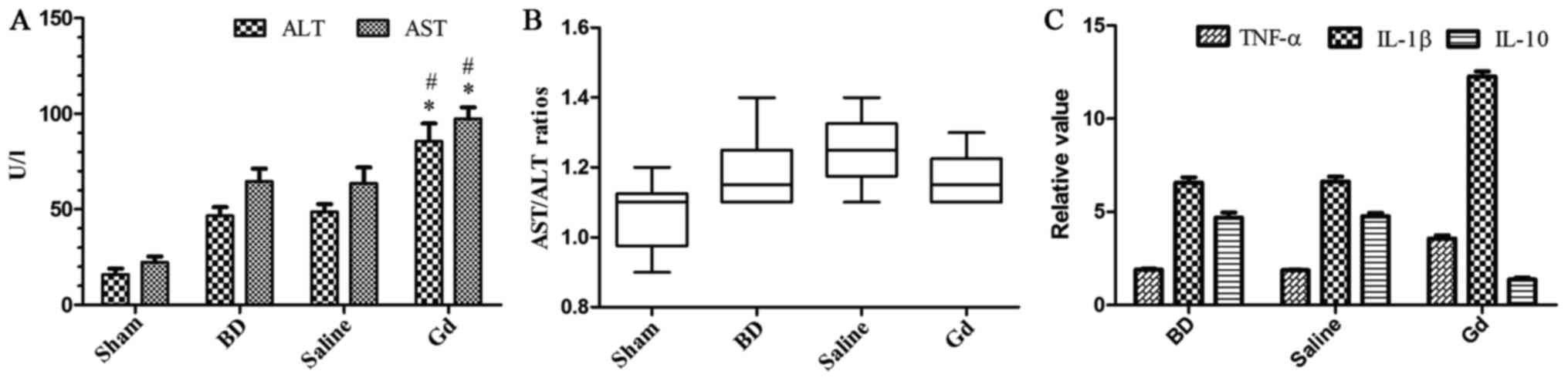 | Figure 2.(A) ALT and AST levels in each group.
GdCl3 treatment prior to BD increased hepatocellular
damage. The data are expressed as the means ± standard deviation
(SD). #and *P<0.05 vs. the BD group, saline group and
sham group. (B) AST/ALT ratios in each group. GdCl3
treatment prior to BD increased AST/ALT ratio without significant
statistical difference (P>0.05). (C) Inflammatory cytokine
expression in liver. The results are presented as the fold change
after normalization to baseline of the sham operation group, and
the data are expressed as the means ± SD. TNF-α, IL-1β and IL-10
levels were upregulated in each liver group after BD. TNF and IL-1β
levels were upregulated in GdCl3-treated animals vs.
diluent-treated controls and the BD group, whereas IL-10 levels
were significantly downregulated in GdCl3-treated
animals vs. diluent-treated controls and the BD group. No
significant differences were noted between the diluent-treated
controls and the BD group. ALT, alanine aminotransferase; AST,
aspartate aminotransferase; IL, interleukin; BD, brain dead; GdCl3,
gadolinium chloride; TNF-α, tumor necrosis factor-α. |
Effect of KCs on the regulation of liver
inflammation
ELISA results of liver
pro-inflammatory cytokines
To more fully investigate the damaged phenotype
displayed in GdCl3-treated animals subjected to BD, the
expression levels of liver pro-inflammatory cytokines were
measured. In terms of the ELISA analyses of TNF-α, IL-1β and IL-10,
the concentrations of TNF-α and IL-1β in the GdCl3 group
were markedly increased compared with those in the saline and BD
groups (P<0.05). TNF-α and IL-1β levels were upregulated at 6 h
in GdCl3-treated animals (Fig. 2).
ELISA results of liver
anti-inflammatory cytokines
IL-10 was one of the most potent anti-inflammatory
cytokines produced in the liver; therefore, IL-10 liver expression
levels under BD conditions were measured. Regarding the ELISA
analysis, a marked increase in IL-10 levels was noted in the saline
and BD groups 6 h after BD compared with those in the sham
operation group (P<0.05; Fig.
2). In contrast, IL-10 levels in the GdCl3-treated
rats remained at baseline levels compared with those in the sham
operation group 6 h after BD. These results reveal the lack of
IL-10 production in GdCl3-treated animals. IL-10
expression in the liver was significantly suppressed in the
GdCl3-treated groups compared with the saline and BD
groups (P<0.05; Fig. 2).
Effect of KC depletion on liver cell
apoptosis under BD conditions
Liver apoptosis
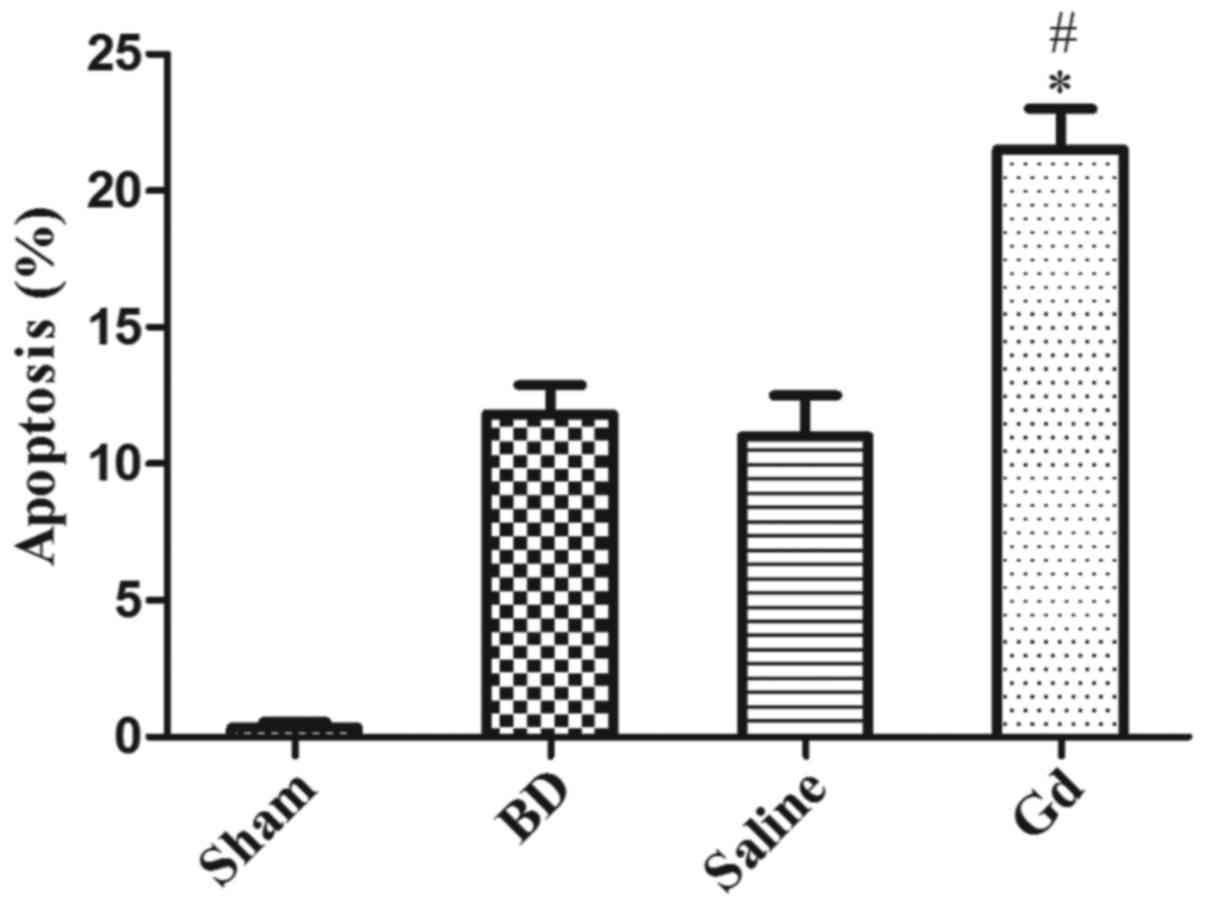
Apoptotic cells were quantified in each field. In
situ labeling of cell nuclei using the TUNEL assay revealed
that BD significantly increased hepatic cell apoptosis in the
saline and BD groups 6 h after BD compared with that in the sham
operation group. The AI increased 6 h after BD. However, hepatic
cell apoptosis was dramatically aggravated 6 h after
GdCl3 treatment (P<0.05; Fig. 3).
Western blot analysis of
apoptosis-related proteins in liver tissue
To determine the importance of hepatic cell
apoptosis in KC-mediated protective mechanisms and BD-induced
hepatic injury, western blot analysis of apoptosis-related proteins
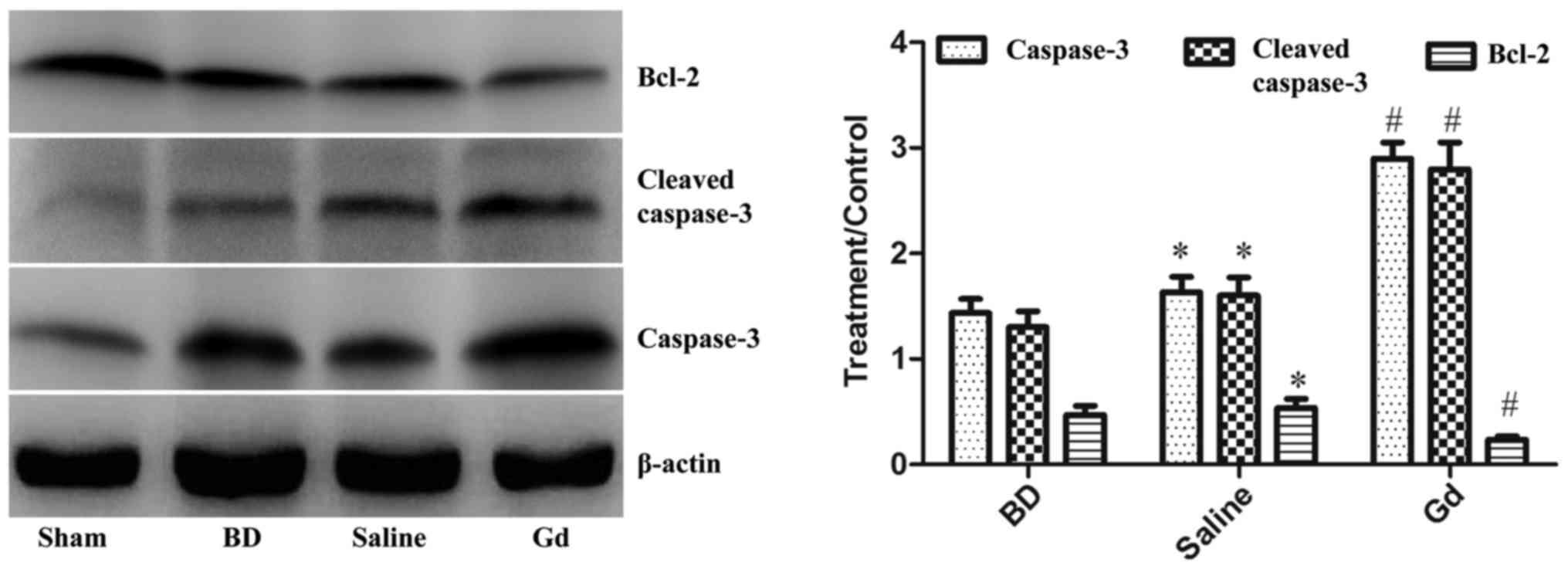
was performed on total hepatic protein. Caspase-3, cleaved
caspase-3 and Bcl-2 expression levels were examined by western blot
analysis. Caspase-3 and cleaved caspase-3 expression levels were
significantly increased, whereas Bcl-2 expression was significantly
decreased 2 and 6 h after BD compared with those in the sham
operation group (P<0.05). After GdCl3 treatment,
caspase-3 and cleaved caspase-3 expression levels were
significantly decreased, whereas Bcl-2 expression was significantly
increased compared with those in the saline and BD groups
(P<0.05; Fig. 4).
Discussion
As resident macrophages of the liver, KCs excrete
significant amounts of pro-inflammatory and anti-inflammatory
cytokines and play a central role in the detrimental effects of
liver transplantation. Studies have demonstrated that inhibition of
KC activation through pharmacological mechanisms improved the
outcomes of liver transplantation. Although different categories of
hepatic macrophages were suppressed by liposomal clodronate to
varying degrees, KCs were most susceptible to suppression (12,18).
Therefore, GdCl3 was used to inhibit KC function in our
study. Several scholars reported that KCs were protective in liver
tissues subjected to total ischemia/reperfusion (I/R) in
transplantation (19). KCs are
directly involved in inducing liver transplantation tolerance and
hepatic I/R injury. Furthermore, KCs are activated during the onset
of BD induction in animals (6).
Upon GdCl3 treatment to deplete KCs, we demonstrated
that extensive KC elimination aggravated liver injury after BD in
rats. The levels of the pro-inflammatory cytokines TNF-α and IL-1β
increased, whereas expression of the anti-inflammatory cytokine
IL-10 in the liver decreased. In addition, liver cell apoptosis was
accelerated by suppression of KCs. Our finding suggested that KCs
play a protective role in liver injury after BD potentially based
on IL-10 expression and liver apoptosis suppression.
Secondary to BD, inflammation is driven by both the
innate and adaptive immune systems in addition to numerous
cytokines (19). Studies have
demonstrated that BD is associated with significant upregulation of
inflammatory cytokines through several mechanisms, thus promoting
severe injury after liver transplantation (20,21).
Upon methylprednisolone treatment, soluble ILs and TNF-α were
significantly decreased, which significantly ameliorated liver
injury post-transplantation (22).
IL-1β is an important mediator of the
pro-inflammatory response and is produced by activated macrophages
as a proprotein (6,23). Research has demonstrated that BD
promotes the induction of IL-1β and TNF-α based on the activation
of non-parenchymal cells in the liver (6,24).
IL-10 suppresses the production of pro-inflammatory cytokines and
upregulates inhibitors of IL-1β and TNF-α, leading to impairment or
reversal of the effects of pro-inflammatory mediators (25). In the liver under BD conditions,
IL-10 was primarily produced by KCs, as reported previously
(26). We observed that IL-1β and
IL-10 were significantly increased in the liver tissues of BD rats.
IL-1β and TNF-α expression in the liver increases 6 h after BD,
whereas GdCL3 treatment increased IL-1β and TNF-α
levels. However, IL-10 was significantly decreased upon treatment
with GdCL3. The data indicate that in the absence of
KCs, the production of liver cytokines was significantly
imbalanced. We suggest that the absence of KCs and their production
of IL-10 lead to a pro-inflammatory response under BD conditions,
which aggravates liver injury after BD in rats.
Previous studies have demonstrated that BD induces
hepatocellular apoptosis and liver dysfunction (9,12,27).
Therefore, we examined the effect of KCs on liver apoptosis upon
treatment with GdCl3 under BD conditions. Caspase-3 is a
major executioner caspase that is cleaved at an aspartate residue
to become activated. Cleaved caspase-3 degrades multiple cellular
proteins and is responsible for morphological changes in cells
during apoptosis. Previous research has demonstrated that apoptosis
was obviously regulated by cleaved caspase-3 and Bcl-2 protein
expression. Further studies revealed that the administration of the
pan-caspase inhibitor IDN-6556 during liver transplantation offers
local therapeutic protection against hepatocellular apoptosis and
liver injury (28). Our present
supporting evidence demonstrates that KC function is inhibited by
GdCl3 treatment, shifting the balance of pro-apoptotic
and anti-apoptotic molecules to favor cell death. We observed that
BD induces hepatocellular apoptosis, upregulation of cleaved
caspase-3 expression and suppression of Bcl-2 expression. Under
conditions of GdCl3 pretreatment, increased liver
apoptosis was noted with increased cleaved caspase-3 and caspase-3
expression and decreased Bcl-2 expression. KC elimination by
GdCl3 aggravated liver injury in a manner dependent on
the regulation of apoptosis under BD conditions in rats by shifting
the balance of cleaved caspase-3 and Bcl-2 molecules to favor cell
death. Therefore, KCs may protect against BD by inhibiting
hepatocyte apoptosis. Similarly, caspase-3 and cleaved caspase-3
expression increased, and Bcl-2 expression decreased. Given that
caspase-3 promotes apoptosis and Bcl-2 impedes apoptosis, KCs may
regulate cleaved caspase-3 and Bcl-2 to fight against apoptosis in
BD. In summary, these results provide strong evidence that KCs
protect liver function under BD conditions through the regulation
of a pro-inflammatory state and apoptosis.
Murine KCs are protective in total hepatic I/R
injury (19,29), and these results were similar to
those presented in our research. KCs are exceptionally plastic
cells that can polarize to specific activation states and perform
various functions in different microenvironments. It has been
reported that alternatively activated M2 KCs can promote
caspase-3-dependent apoptosis of classically activated M1 KCs, thus
providing a protective mechanism. The M2-mediated apoptosis of M1
KCs was shown to be arginase dependent by way of IL-10 (30). Another study demonstrated that
GdCl3 played an important protective role in early I/R
injury and suppressed bile duct cell apoptosis during liver
transplantation (31). During BD,
liver-specific parameters of plasma endotoxin levels and the
endotoxin-neutralizing capacity were compromised independent of
hemodynamic status (32). Despite
varying inflammatory profiles in organs after BD, BD does not
accelerate I/R injury in transplantation (33). This discrepant finding is likely
due to the predominant inflammatory component resulting from plasma
endotoxin levels in our BD model, which differs from I/R injury. In
this study, we demonstrated that KCs appear to play a protective
role in liver subjected to BD. No single method is available to
inhibit apoptosis and apoptosis genes to verify the role of KCs in
liver injury protection in the context of BD. Therefore, the
protective effects of KCs in liver injury observed in this study
may only represent one aspect of the overall protective mechanism.
In addition, it may be concluded that the effect of KCs in liver
injury under conditions of BD involves a combined action requiring
the participation of numerous mechanisms. Further studies may also
be necessary to understand the exact mechanism.
In summary, our study indicates that KCs play a
protective role in livers subjected to BD, which appears to be due
to KC secretion of the potent anti-inflammatory cytokine IL-10. We
also demonstrated that GdCl3 efficiently inhibits the
activity of KCs, which participate in the onset of liver injury
through its effect on pro-inflammatory and anti-inflammatory
activation. All of these results suggest that KCs are a potential
target of GdCl3 for the prevention and treatment of
liver injury under BD conditions in rats.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China
Postdoctoral Science Foundation (grant no. 2015M582209) and the Key
Project of the Science and Technology Research Education Department
of Henan Province (grant no. 16A320030).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
RZ designed research and wrote the paper; WG, HF, SC
and BY performed the rat experiments; SC, KZ and SZ analyzed the
data. All the authors approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) approved this
study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
KCs
|
Kupffer cells
|
|
GdCl3
|
gadolinium chloride
|
|
BD
|
brain death
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
IL-1β
|
interleukin-1β
|
|
IL-10
|
interleukin-10
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Tacke F, Kroy DC, Barreiros AP and Neumann
UP: Liver transplantation in Germany. Liver Transplant.
22:1136–1142. 2016. View
Article : Google Scholar
|
|
2
|
Van der Hoeven JA, Lindell S, van
Schilfgaarde R, Molema G, Ter Horst GJ, Southard JH and Ploeg RJ:
Donor brain death reduces survival after transplantation in rat
livers preserved for 20 hr. Transplantation. 72:1632–1636. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neuberger J: Liver transplantation in the
United Kingdom. Liver Transpl. 22:1129–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Westendorp WH, Leuvenink HG and Ploeg RJ:
Brain death induced renal injury. Curr Opin Organ Transplant.
16:151–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pratschke J, Wilhelm MJ, Kusaka M, Basker
M, Cooper DK, Hancock WW and Tilney NL: Brain death and its
influence on donor organ quality and outcome after transplantation.
Transplantation. 67:343–348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olinga P, van der Hoeven JA, Merema MT,
Freund RL, Ploeg RJ and Groothuis GM: The influence of brain death
on liver function. Liver Int. 25:109–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Novitzky D, Mi Z, Videla LA, Collins JF
and Cooper DK: Thyroid hormone therapy and procurement of livers
from brain-dead donors. Endocr Res. 41:270–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leithead JA, Armstrong MJ, Corbett C,
Andrew M, Kothari C, Gunson BK, Muiesan P and Ferguson JW: Hepatic
ischemia reperfusion injury is associated with acute kidney injury
following donation after brain death liver transplantation. Transpl
Int. 26:1116–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Der Hoeven JA, Moshage H, Schuurs T,
Nijboer M, Van Schilfgaarde R and Ploeg RJ: Brain death induces
apoptosis in donor liver of the rat. Transplantation. 76:1150–1154.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen SW, Ager EI, Neo J and Christophi C:
The renin angiotensin system regulates Kupffer cells in colorectal
liver metastases. Cancer Biol Ther. 14:720–727. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsutsui H and Nishiguchi S: Importance of
Kupffer cells in the development of acute liver injuries in mice.
Int J Mol Sci. 15:7711–7730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Yang Z, Wang L, Lu Y, Tang B, Miao
H, Xu Q and Chen X: Combination of sorafenib and gadolinium
chloride (GdCl3) attenuates dimethylnitrosamine(DMN)-induced liver
fibrosis in rats. BMC Gastroenterol. 15:1592015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao S, Wang T, Yan B, Lu Y, Guo W and
Zhang S: Protective effects of SP600125 in brain death-induced
liver injury. Clin Res Hepatol Gastroenterol. 38:577–582. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Cao S, Wang T, Yan B, Lu Y and
Zhao Y: Modified brain death model for rats. Exp Clin Transplant.
12:469–473. 2014.PubMed/NCBI
|
|
15
|
Pratschke J, Wilhelm MJ, Kusaka M,
Laskowski I and Tilney NL: A model of gradual onset brain death for
transplant-associated studies in rats. Transplantation. 69:427–430.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knodell RG, Ishak KG, Black WC, Chen TS,
Craig R, Kaplowitz N, Kiernan TW and Wollman J: Formulation and
application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis.
Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desmet VJ: Knodell RG, Ishak KG, Black WC,
Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation
and application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis
[Hepatology 1981;1:431-435]. J Hepatol. 38:382–386. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Wang Y, Li M, Yang X, Gong J and
Zhang W: Gadolinium chloride suppresses acute rejection and induces
tolerance following rat liver transplantation by inhibiting
Kupffer-cell activation. Exp Ther Med. 8:1777–1782. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ellett JD, Atkinson C, Evans ZP, Amani Z,
Balish E, Schmidt MG, van Rooijen N, Schnellmann RG and Chavin KD:
Murine Kupffer cells are protective in total hepatic
ischemia/reperfusion injury with bowel congestion through IL-10. J
Immunol. 184:5849–5858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barklin A: Systemic inflammation in the
brain-dead organ donor. Acta Anaesthesiol Scand. 53:425–435. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weiss S, Kotsch K, Francuski M,
Reutzel-Selke A, Mantouvalou L, Klemz R, Kuecuek O, Jonas S,
Wesslau C, Ulrich F, et al: Brain death activates donor organs and
is associated with a worse I/R injury after liver transplantation.
Am J Transplant. 7:1584–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kotsch K, Ulrich F, Reutzel-Selke A,
Pascher A, Faber W, Warnick P, Hoffman S, Francuski M, Kunert C,
Kuecuek O, et al: Methylprednisolone therapy in deceased donors
reduces inflammation in the donor liver and improves outcome after
liver transplantation: A prospective randomized controlled trial.
Ann Surg. 248:1042–1050. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu C, Li J, Zhang G, Zhang Y, Zhai W, Shi
J, Li Z, Li J and Zhang S: Brain death disrupts structure and
function of pig liver. Transplant Proc. 42:pp. 733–736. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuecuek O, Mantouvalou L, Klemz R, Kotsch
K, Volk HD, Jonas S, Wesslau C, Tullius S, Neuhaus P and Pratschke
J: Significant reduction of proinflammatory cytokines by treatment
of the brain-dead donor. Transplant Proc. 37:pp. 387–388. 2005;
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li JQ, Qi HZ, He ZJ, Hu W, Si ZZ, Li YN
and Li DB: Cytoprotective effects of human interleukin-10 gene
transfer against necrosis and apoptosis induced by hepatic cold
ischemia/reperfusion injury. J Surg Res. 157:e71–e78. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olinga P, Merema MT, de Jager MH, Derks F,
Melgert BN, Moshage H, Slooff MJ, Meijer DK, Poelstra K and
Groothuis GM: Rat liver slices as a tool to study LPS-induced
inflammatory response in the liver. J Hepatol. 35:187–194. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao S, Wang T, Yan B, Lu Y, Zhao Y and
Zhang S: Brain death is associated with endoplasmic reticulum
stress and apoptosis in rat liver. Transplant Proc. 46:pp.
3297–3302. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baskin-Bey ES, Washburn K, Feng S,
Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van
Vilsteren FG, Oliver LK, et al: Clinical trial of the pan-caspase
inhibitor, IDN-6556, in human liver preservation injury. Am J
Transplant. 7:218–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sutter AG, Palanisamy AP, Ellet JD,
Schmidt MG, Schnellmann RG and Chavin KD: Intereukin-10 and Kupffer
cells protect steatotic mice livers from ischemia-reperfusion
injury. Eur Cytokine Netw. 25:69–76. 2014.PubMed/NCBI
|
|
30
|
Wan J, Benkdane M, Teixeira-Clerc F,
Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat
A, et al: M2 Kupffer cells promote M1 Kupffer cell apoptosis: A
protective mechanism against alcoholic and nonalcoholic fatty liver
disease. Hepatology. 59:130–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang B, Zhang Q, Zhu B, Cui Z and Zhou J:
Protective effect of gadolinium chloride on early warm
ischemia/reperfusion injury in rat bile duct during liver
transplantation. PLoS One. 8:e527432013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Golling M, Mehrabi A, Blum K, Jahnke C,
Kellner H, Bud O, Hashemi B, Breitkreutz R, Becker-Brandenburg K,
Schemmer P, et al: Effects of hemodynamic instability on brain
death-induced prepreservation liver damage. Transplantation.
75:1154–1159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ritschl PV, Ashraf MI, Oberhuber R,
Mellitzer V, Fabritius C, Resch T, Ebner S, Sauter M, Klingel K,
Pratschke J and Kotsch K: Donor brain death leads to differential
immune activation in solid organs but does not accelerate
ischaemia-reperfusion injury. J Pathol. 239:84–96. 2016. View Article : Google Scholar : PubMed/NCBI
|