Introduction
Atherosclerosis (AS) is a complex multifactorial
disease characterized by the concentration of large scale lipids,
inflammatory cells and fibrous elements. Evidence is accumulating
to suggest that damage to vascular endothelial cells initiates AS
(1). Endothelial cells form a
permeable barrier, which maintains the internal environment by
manufacturing and excreting various cytokines and by regulating
cellular cholesterol, lipid homeostasis and inflammation in the
vascular wall (2). NO is a potent
vasodilator factor and signal regulator in endothelial cells, and
is induced by eNOS through catalyzing L-arginine. The NO/eNOS
system is crucial in endothelium-dependent diastolic function and
damage to endothelial function generally always follows a decrease
in eNOS activity (3). Caveolae
consist of caveolin, cholesterin and sphingomyelin, and are thus
termed from their flask-shaped, invaginated structures observed in
the cytoplasmic membrane. CAV-1 is an essential structural protein
for the formation of caveolae, and is expressed in endothelial
cells, macrophages and smooth muscle cells (4). It has been demonstrated that CAV-1
may serve as a target site for AS by restraining eNOS activity by
combining with eNOS (5–7).
All-trans retinoic acid (ATRA), a derivative of
vitamin A, is involved in inducing cell differentiation, reducing
inflammation and suppressing cell proliferation and metastasis
(8). Previous studies (9,10)
have demonstrated that ATRA is effective in delaying the process of
AS, although the exact mechanism remains to be elucidated. The
majority of studies exploring the function of ATRA focus on
vascular smooth muscle (VSMC), however, few (11–14)
have demonstrated that ATRA regulates the activity of eNOS to
affect NO concentration in endothelial cells.
The present study attempted to investigate the
therapeutic effect of ATRA in AS rabbits, in addition to the
potential mechanisms of ATRA-attenuated AS.
Materials and methods
Reagents
ATRA was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Anti-occludin (cat. no. sc-8144),
anti-CAV-1 (cat. no. sc-70516) and anti-β-actin (cat. no. sc-47778)
were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). All secondary antibodies were purchased from EMD Millipore
(Billerica, MA, USA). HRP-conjugated secondary antibodies,
including rabbit-anti-goat (1:1,000, cat. no. AP106P),
goat-anti-mouse (1:1,000, cat. no. AP127P) and goat-anti-mouse
(1:2,000, cat. no. AP127P) were purchased from EMD Millipore
(Billerica, MA, USA). Histostain-plus kits and
3,3′-diaminobenzidine (DAB) horseradish peroxidase (HRP) color
development kits were obtained from OriGene Technologies, Inc.
(Beijing, China). eNOS and NO kits were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China).
Sulfo-NHS-LC-biotin was obtained from Pierce (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Animal experimental procedures
The present study was approved by the Ethics
Committee of Anhui Medical University (Hefei, China). All rabbits
were bred humanely in compliance with the ‘Principles of Laboratory
Animal Care’ formulated by the USA National Society for Medical
Research and the Guide for the Care and Use of Laboratory Animals.
A total of 36 4-month-old and weight 1.8±0.2 kg male New Zealand
white rabbits were obtained from Nanjing Anlimo Technology Co.,
Ltd. (Nanjing, China), and maintained in individual cages under
moderate temperature and a 12-h light/dark cycle, with ad
libitum access to food and clean water. Following a 7-day
acclimation period, they were randomly divided into the control
group (n=10), an AS group (AS group, n=10) and an ATRA treatment
group (ATRA group, n=10). Rabbits in the control group were fed a
normal diet (150 g/day), the AS group were fed a high-cholesterol
diet (1% cholesterol and 5% lard, 150 g/day), and the ATRA group
received a high-cholesterol diet and 10 mg/kg/day ATRA. The control
and the AS groups received the same volume of medium by gavage. All
the rabbits were euthanized at the end of week 12.
Tissue collection
Animals were anaesthetized by 3% pentobarbital
sodium (Sigma-Aldrich; Merck KGaA; 1 ml/kg) and then sacrificed by
rapid exsanguination. The aortic arch, the thoracic and abdominal
aorta, and the arteria iliaca were removed. The aortic arch was cut
longitudinally and fixed in 4% paraformaldehyde for pathological
and immunohistochemical assays. The upper section of the abdominal
aorta was placed in a dish containing ice-cold Krebs solution
(composition in mmol/l: NaCl, 120; KCl, 4.7;
KH2PO4, 1.18; CaCl2, 2.25;
NaHCO3, 24.5; MgSO4·7H2O, 1.2;
glucose, 11.1; EDTA, 0.03) and continuously aerated with 95%
O2 and 5% CO2; the samples were then cut into
rings (3 mm in length) for the measurement of isometric contractile
tension. The remaining arteries were frozen in liquid nitrogen and
stored at −80°C for western blotting.
Measurement of isometric contractile
tension
Individual aortic rings were vertically suspended
between two stainless steel wire hooks in a jacketed organ bath
containing 25 ml Krebs solution (as described above), which was
replaced at 15-min intervals. The bathing solution was aerated
continuously with a mixture of 95% O2 and 5%
CO2 at 37°C. Isometric contractile tension was
continuously recorded using a BL-420F experimental system of
biological function (Chengdu Tai Meng Science and Technology Co.,
Ltd., Chengdu, China). Resting tension was increased stepwise to
reach a final tension of 2 g that was applied to the aortic rings;
then, they were equilibrated for 45 min. Following equilibration,
rings were precontracted with 1×10−6 mol/l phenylephrine
(Phe) and, once a stable contraction plateau was obtained,
1×10−9-1×10−4 mol/l acetylcholine (ACh) or
1×10−9-1×10−4 mol/l sodium nitroprusside
(SNP) was cumulatively added to the organ bath until a maximal
vasodilator response was achieved. Cumulative vasodilator response
data were expressed as the percentage of relaxation relative to the
Phe-induced precontraction.
Immunofluorescence
Arteries were unfolded on freezing optimal cutting
temperature compound and treated with Sulfo-NHS-LC-biotin for 30
min, then preserved at −80°C. The frozen sections were washed in
PBS 3 times (10 min each), blocked with 5% non-fat milk overnight
at 4°C, then incubated with Rhodamin (1:20) for 2 h at 4°C, then
washed three times prior to mounting and coverslipping. The
sections were observed using a Leica immunofluorescence microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
Immunohistochemistry
The expression levels of CAV-1 and occludin in the
aortas were measured by immunohistochemistry. The sections were
dewaxed in xylene, rehydrated in graded ethanol solutions and
subjected to antigen retrieval in citrate-buffered solution at 95°C
for 15 min. Endogenous peroxidase activity was blocked by 3%
hydrogen peroxide. The specimens were blocked by normal goat serum
and incubated with the anti-CAV-1 or anti-occludin overnight. After
being washed in PBS, the slides were incubated with
streptavidin-biotin horseradish peroxidase complex after
biotin-conjugated secondary antibody. Then the sections were
treated with DAB for 5 min and, after thorough washing, were
mounted on slide glasses with Resinene (Guo Yao Chemical Reagent
Co., Ltd., Shanghai, China).
Western blot analysis
The aortas were washed with PBS for 3 times, and
lysed in RIPA buffer (Tris-HCl, pH 7.14, 150 mmol/l NaCl, 1 mmol/l
EDTA, 1% Triton X-100, 0.1% SDS, 5 mg/ml leupeptin and 1 mmol/l
PMSF). The lysates were centrifuged at 14,000 × g for 30 min at
4°C. The protein concentration of each sample was measured with
Micro-BCA Protein Assay Reagent kit (Beyotime Institute of
Biotechnology, Haimen, China). Protein extracts were blended with
SDS sample buffer and boiled for 8 min, separated through 12%
SDS-PAGE, and then transferred onto polyvinylidene difluoride
membranes. The membranes were blocked with 5% fat-free milk in PBST
(PBS, 0.1% Tween-20) for 2 h at room temperature, and then
incubated overnight with anti-CAV-1 (1:500), anti-occludin (1:500)
or anti-β-actin (1:1,000) at 4°C, followed by the appropriate
HRP-conjugated secondary antibody with CAV-1, occludin or β-actin,
and detected with enhanced chemiluminescence (Beyotime Institute of
Biotechnology). Protein bands were visualized by exposing the blots
to Kodak X-ray film (Kodak, Rochester, NY, USA).
Statistical analysis
Statistical analyses were conducted using SPSS,
version 19.0 (IBM SPSS, Armonk, NY, USA). All data are expressed as
the mean ± standard deviation. One-way analysis of variance was
used to evaluate the statistical significance of the differences
between multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ATRA improved endothelium-dependent
relaxation (EDR) function in AS rabbits
EDR of thoracic aorta was induced by ACh ranging
from 10−9-10−4 mol/l. As presented in
Table I, the maximum relaxation
induced by ACh (10−9 mol/l) in the normal group was
95.68% compared with 53.34% in the AS group. This suggested that
the EDR function in the AS group was seriously impaired compared
with the normal group (P<0.05). Treatment with ATRA markedly
ameliorated this damage and restored the relaxation to 73.98%
compared with the AS group, indicating that ATRA makes a
contribution to EDR in AS rabbits. There was no notable difference
on SNP-induced non-endothelium-dependent relaxation (NEDR) in the
thoracic aortic rings (Table
II).
 | Table I.Endothelium-dependent relaxation of
thoracic aorta produced by ACh (%, n=6, mean ± SD). |
Table I.
Endothelium-dependent relaxation of
thoracic aorta produced by ACh (%, n=6, mean ± SD).
| ACh (mol/l) | Normal | Model | ATRA |
|---|
| 10‒9 |
4.83±0.26 |
1.65±0.19a |
1.99±0.19a,b |
|
10‒8 |
14.66±1.23 |
3.98±0.45a |
9.48±1.31a,b |
|
10‒7 |
52.52±1.92 |
22.79±2.98a |
34.56±2.12a,b |
|
10‒6 |
77.45±2.97 |
44.06±5.76a |
55.99±3.67a,b |
|
10‒5 |
84.52±3.19 |
50.88±1.63a |
68.51±3.14a,b |
|
10‒4 |
95.68±3.57 |
53.34±2.11a |
73.98±3.08a,b |
 | Table II.Non-endothelium-dependent relaxation
of thoracic aorta produced by SNP (%, n=6, mean ± SD). |
Table II.
Non-endothelium-dependent relaxation
of thoracic aorta produced by SNP (%, n=6, mean ± SD).
| SNP (mol/l) | Normal | Model | ATRA |
|---|
|
10‒9 |
7.34±0.33 |
7.22±0.59 |
7.16±0.37 |
|
10‒8 |
32.64±1.03 |
34.00±1.80 |
33.32±2.34 |
|
10‒7 |
64.38±1.96 |
62.61±1.88 |
62.55±3.57 |
|
10‒6 |
87.35±2.62 |
86.54±2.72 |
88.29±3.95 |
|
10‒5 |
97.01±2.28 |
96.96±3.97 |
98.14±4.60 |
|
10‒4 |
106.55±3.93 |
106.20±4.22 |
107.99±4.68 |
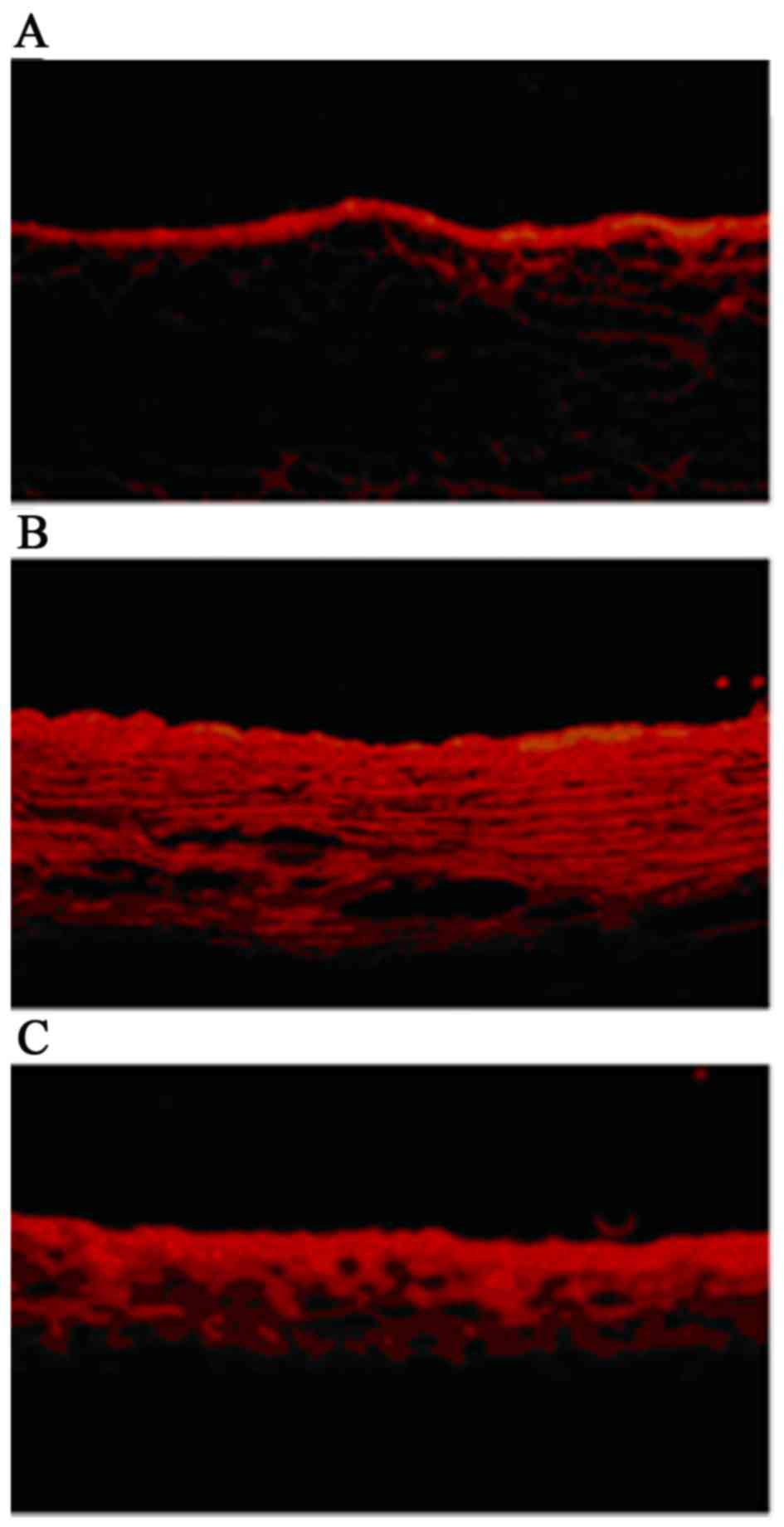
ATRA improves the permeability of the
arterial wall in AS rabbits
The permeability of endothelial cells was detected
by immunofluorescence (surface biotinylation technique).
Concentration profiles of NHS-LC-biotin were acquired according to
the radial distance through the media of the arterial wall. There
was a little paracellular leakage of NHS-LC-biotin in the normal
arterial walls (Fig. 1A) in
contrast, all the arterial layers were biotinylated in the AS
rabbits (Fig. 1B). As presented in
Fig. 1C, leakage of NHS-LC-biotin,
which permeates through arterial intima, was reduced markedly in AS
rabbits following treatment with ATRA, suggesting that ATRA was
able to restore the permeability of damaged endothelial to some
extent.
ATRA attenuated CAV-1 protein and
enhanced occludin expression level in AS rabbits
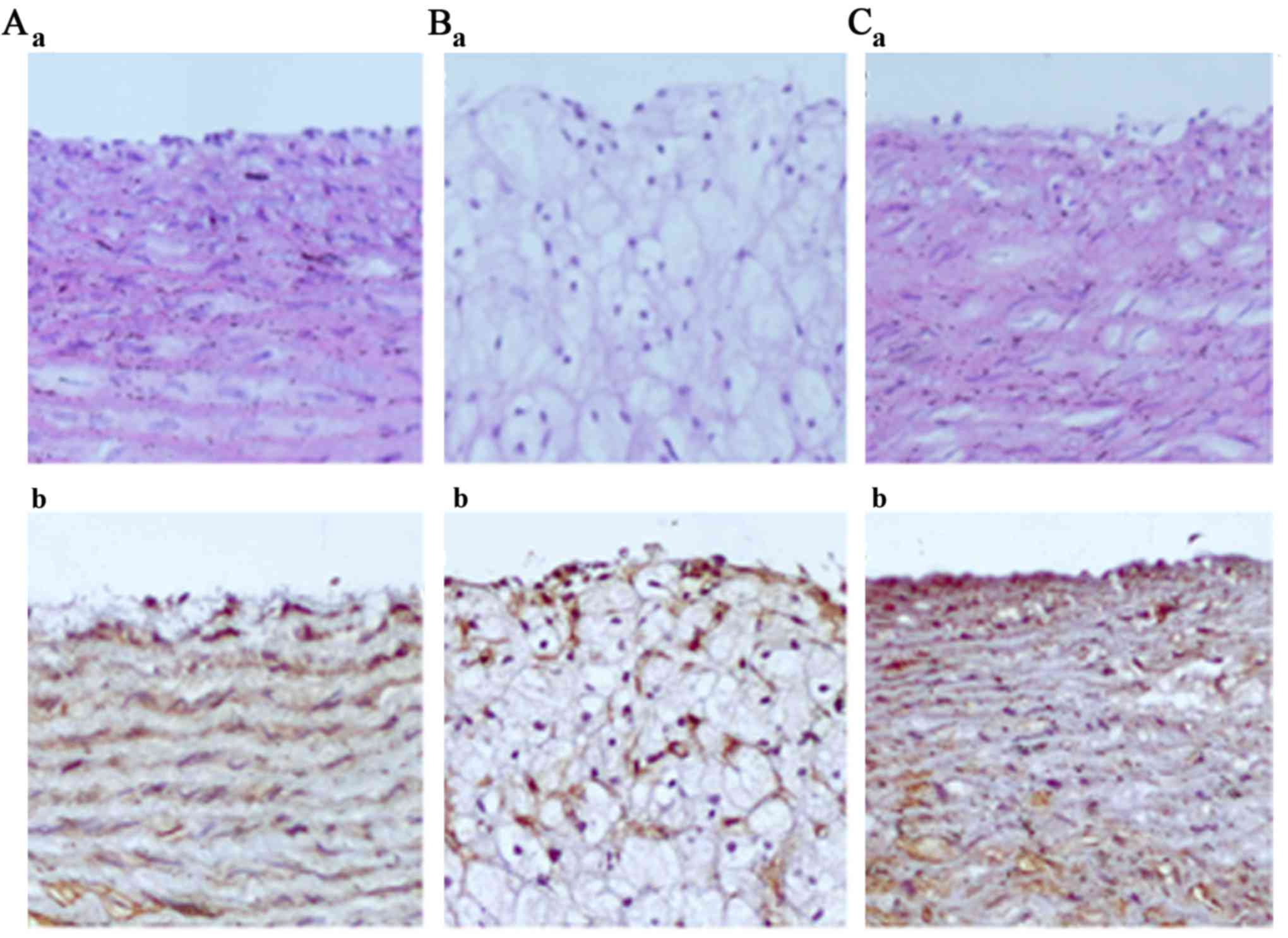
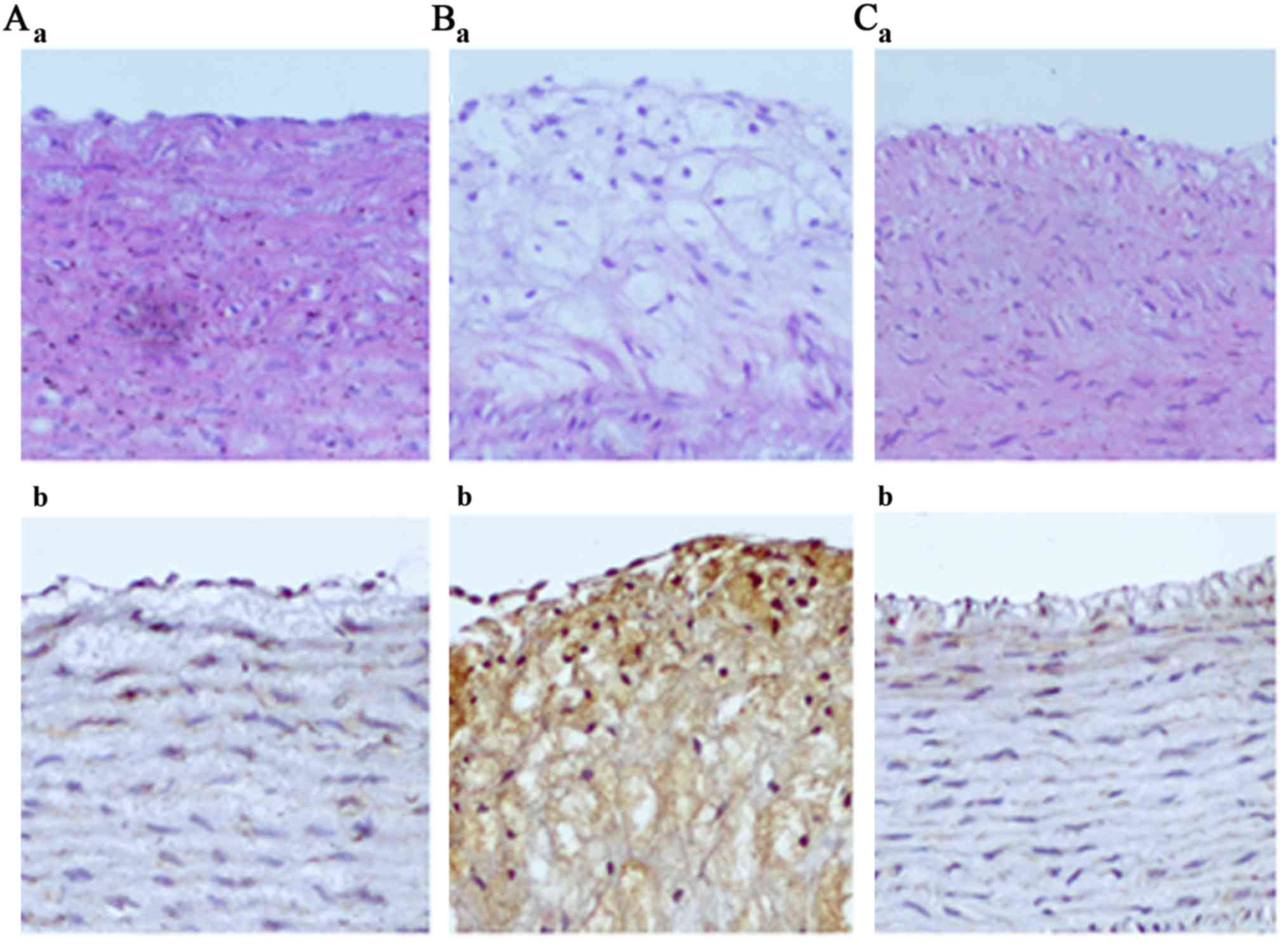
The features of the arterial lesions were examined
by hematoxylin and eosin (H&E) staining. The arterial intima
was clear and intact in the normal group and the endothelial cell
cores were stained and evenly arranged (Figs. 2A and 3A). However broken arterial intima,
increased intercellular space, numerous foam cells and fibrous
plaques were observed in the AS group (Figs. 2B and 3B). Treatment with ATRA resulted in fewer
foam and inflammatory cells, and no fibrous plaques (Figs. 2C and 3C). Immunohistochemical analysis
demonstrated that the expression of occludin was lower in the AS
rabbits compared with the normal rabbits, while the level of
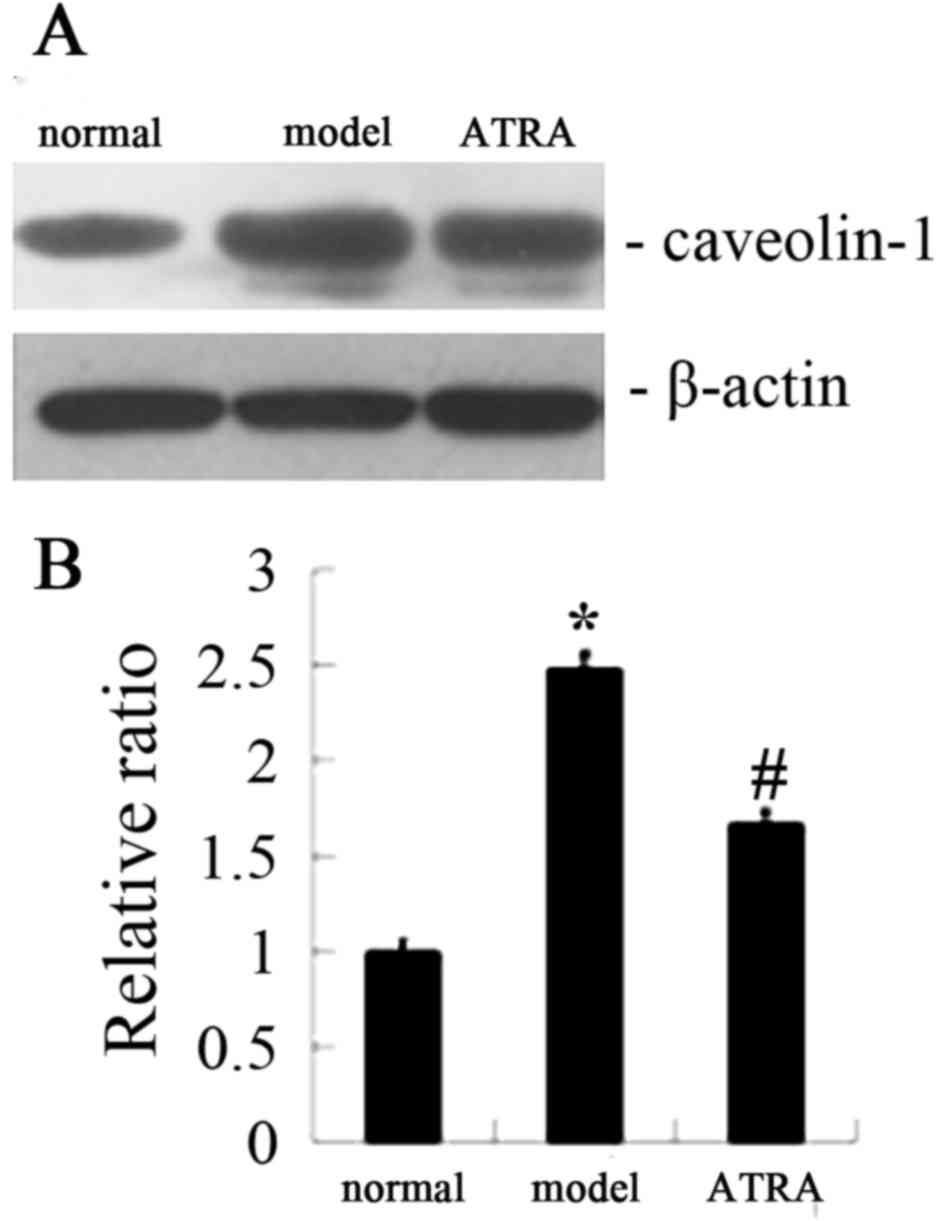
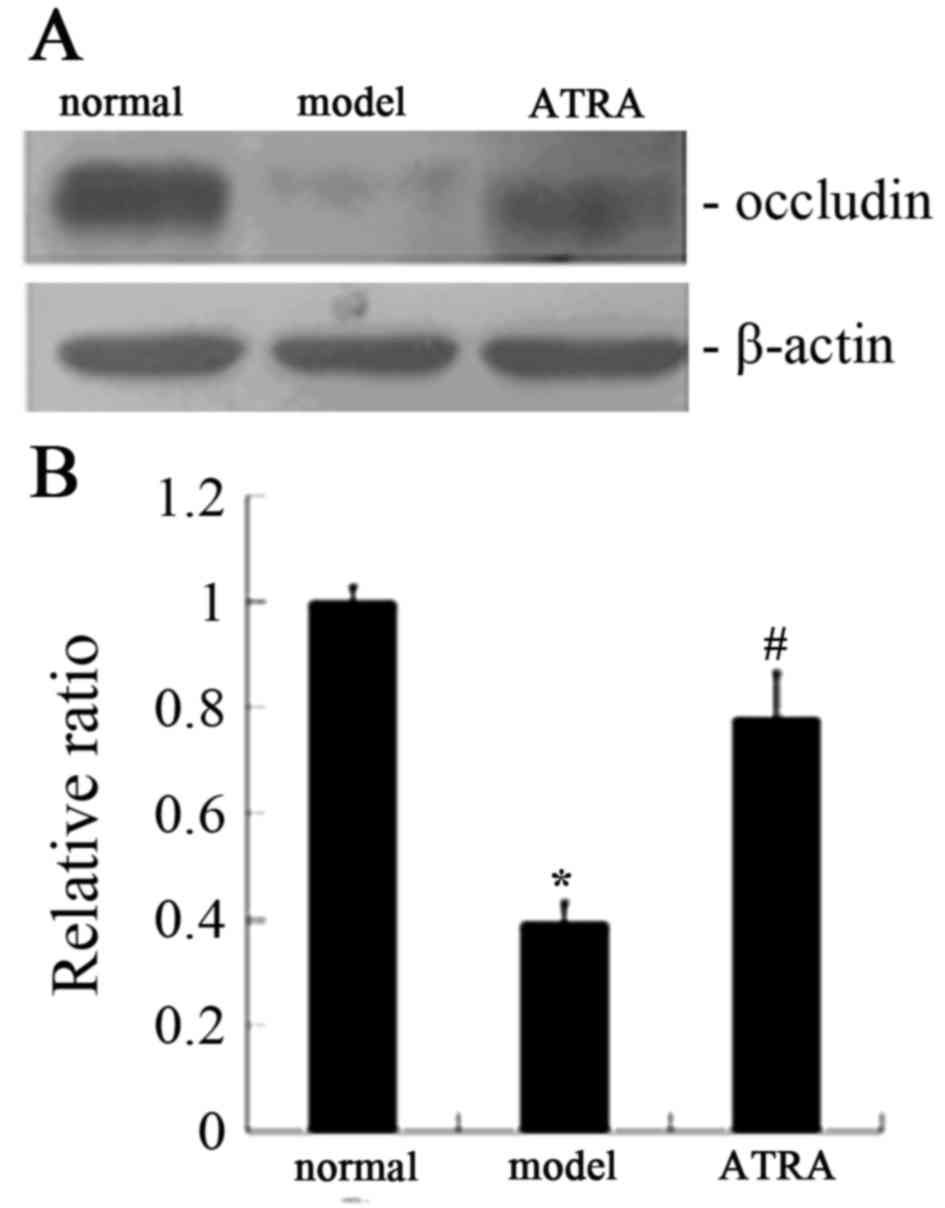
occludin was markedly increased following ATRA treatment (Fig. 2). In addition, western blot
analysis gave the similar results for occludin expression (Fig. 4). As demonstrated in Figs. 3 and 5, immunohistochemistry gave the same
indications of the expression of CAV-1 as did western blot
analysis, unlike occludin under the same conditions.
ATRA increased eNOS activity and NO
concentration in AS rabbits
As presented in Table
III, eNOS activity and NO concentration of the AS group were
markedly decreased compared with the normal group (P<0.05),
nevertheless they were notably increased in the AS rabbits
following treatment with ATRA.
 | Table III.Comparison of eNOS activity and NO
concentration in three groups (n=10, x±s). |
Table III.
Comparison of eNOS activity and NO
concentration in three groups (n=10, x±s).
| Group | eNOS activity
(U/mgprot) | NO concentration
(µmol/gprot) |
|---|
| Normal |
6.53±0.86 |
18.56±9.77 |
| Model |
0.82±0.62a |
1.52±0.42a |
| ATRA |
3.75±2.57a,b |
7.72±2.81a,b |
Discussion
ATRA is part of a family of signaling molecules that
are derived from vitamin A, and emergent studies have concentrated
on its antitumor effects (15,16).
Now it has been demonstrated that ATRA may serve an important
function in AS (17). AS is
identified as a common pathological basis of cardio-cerebrovascular
disease, which involves a variety of risk factors and etiological
mechanisms. Although the etiological factors and pathological
mechanisms remain to be fully elucidated, the widely accepted
hypothesis postulates that the endothelial damage is regarded as
the initiation of AS (18,19). To further elucidate the potential
mechanisms of ATRA in AS was the aim of the present study.
Endothelial cells, located between blood and tissue,
form a cellular barrier to circulating blood and have an important
role in maintaining vascular function, regulating the permeability
and balancing the coagulation and fibrinolysis systems (20,21).
Injury of the endothelial cells leads to an alteration in EDR in
response to ACh. In the present study, the maximal relaxation
induced by ACh (10−9 mol/l) was only 53.34% in the AS
group, suggesting that the EDR function was seriously impaired in
the AS rabbits compared with the normal rabbits. After treatment
with ATRA, the relaxation was restored to 73.98%, indicating that
ATRA makes a contribution to EDR in AS rabbits (Table I). In the present study,
experiments on isolated thoracic aorta rings demonstrated that
there was no notable difference on SNP-induced NEDR (Table II).
It has been demonstrated that the exchange of
substances between the inside and outside of the blood vessel,
including signaling molecules and structural proteins, depends on
endothelial permeability. That permeability is regulated by the
adhesive force maintained by cell-cell junctions and cell-matrix
contacts (22,23). The present study demonstrated that
the permeability of the arterial wall was increased after the
rabbits were provided with a high-fat diet for 12 weeks, while
endothelial permeability had recovered following treatment with
ATRA (Fig. 1). The results
indicated ATRA as a regulator to adjust the damaged endothelial
permeability in the AS rabbits.
Tight junctions (TJs), which consist of occludin,
the claudin protein family and ZO-1, are vital structures generated
in epithelial and endothelial cells, regulating the paracellular
permeation of ions and macromolecules (24). Acting as an important constituent
of TJs, occludin restricts the flow of fluid from the vascular
lumen to the intercellular space (25). Although it is disputed that
occludin maintains the integrity of TJs, a study demonstrated that
TJs may be damaged following a deficiency of occludin (26), causing the migration of hemameba
and lipids, the increase of endothelial permeability and ultimately
leading to AS formation. The present study noted that the
expression of occludin was lower in AS rabbits compared with normal
rabbits (Fig. 2Ab and Bb)
according to immunohistochemical analysis, while occludin
expression level was markedly increased after ATRA treatment
(Fig. 2Cb). Western blot analysis
yielded the same results as immunohistochemical analysis (Fig. 4). These results indicated that ATRA
improves the permeability of the aorta intima by upregulating
occludin expression.
The results of the present study demonstrated that
the anti-AS effects of ATRA were associated with NO, an
endothelium-derived relaxing factor that is released by endothelial
cells. NO is one of the most important vasoactive compounds in
vivo, restraining VSMC migration and proliferation, platelet
aggregation and leukocyte adhesion (27). Endothelial dysfunction, a
precondition of AS, is always accompanied by the decrease of NO
synthesis and release, in addition to NO activity and
bioavailability (28). It has been
established that ATRA can increase NO concentration via two ways:
i) Changing asymmetric dimethylarginine, the inhibitor of eNOS
system and ii) enhancing the phosphorylation of eNOS to increase NO
formation (29). The present study
demonstrated that eNOS activation and NO concentration in the AS
group were significantly reduced compared with the normal group,
while they were clearly increased after treatment with ATRA
(Table III).
The phosphorylation of eNOS at serine 1177 by the
phosphoinositide 3-kinase-protein kinase B pathway is associated
with NO release (30), while a
previous study (31) demonstrated
that CAV-1 is able to inhibit eNOS activity by forming an
eNOS-CAV-1 complex in endothelial cells. When cells are stimulated,
eNOS is displaced from the eNOS-CAV-1 complex and activated by
combining with calmodulin, making a contribution to the increase of
NO concentration (32). Here,
immunohistochemical analysis indicated that the expression of CAV-1
was increased in AS rabbits compared with normal rabbits (Fig. 3Ab and Bb), while the level of CAV-1
was notably decreased following ATRA treatment (Fig. 3Cb). Western blot analysis confirmed
the results of immunohistochemical analysis (Fig. 5).
In summary, ATRA has a promising effect in the
amelioration of high-fat-induced AS in rabbits, and it may produce
its protective effects by activating eNOS, in addition to
downregulating CAV-1 expression. However, the exact and detailed
mechanism of ATRA function remains to be elucidated.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570419 and
81270372), Key Project of Chinese Ministry of Education (grant no.
212077) and Grants for Scientific Research of BSKY (grant nos.
XJ201107 and XJ2008015) from Anhui Medical University.
References
|
1
|
Fernández-Hernando C, Yu J, Dávalos A,
Prendergast J and Sessa WC: Endothelial-specific overexpression of
caveolin-1 accelerates atherosclerosis in apolipoprotein
E-Deficient mice. Am J Pathol. 177:998–1003. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sima AV, Stancu CS and Simionescu M:
Vascular endothelium in atherosclerosis. Cell Tissue Res.
335:191–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu D, He Z, Wu L and Fang Y: Effects of
induction/inhibition of endogenous heme oxygenase-1 on lipid
metabolism, endothelial function, and atherosclerosis in rabbits on
a high fat diet. J Pharmacol Sci. 118:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zulli A, Buxton BF, Black MJ, Ming Z,
Cameron A and Hare DL: The immunoquantification of caveolin-1 and
eNOS in human and rabbit diseased blood vessels. J Histochem
Cytochem. 54:151–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Buikema H, van Gilst WH and Henning
RH: Caveolae and endothelial dysfunction: Filling the caves in
cardiovascular disease. Eur J Pharmacol. 585:256–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MH, Chen SJ, Tsao CM and Wu CC:
Perivascular adipose tissue inhibits endothelial function of rat
aortas via caveolin-1. PLoS One. 9:e999472014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo DX, Cheng J, Xiong Y, Li J, Xia C, Xu
C, Wang C, Zhu B, Hu Z and Liao DF: Static pressure drives
proliferation of vascular smooth muscle cells via caveolin-1/ERK1/2
pathway. Biochem Biophys Res Commun. 391:1693–1697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siddikuzzaman, Guruvayoorappan C and
Berlin Grace VM: All trans retinoic acid and cancer.
Immunopharmacol Immunotoxicol. 33:241–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Axel DI, Frigge A, Dittmann J, Runge H,
Spyridopoulos I, Riessen R, Viebahn R and Karsch KR: All-trans
retinoic acid regulates proliferation, migration, differentiation,
and extracellular matrix turnover of human arterial smooth muscle
cells. Cardiovasc Res. 49:851–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herdeg C, Oberhoff M, Baumbach A,
Schroeder S, Leitritz M, Blattner A, Siegel-Axel DI, Meisner C and
Karsch KR: effects of local all-trans-retinoic acid delivery on
experimental atherosclerosis in the rabbit carotid artery.
Cardiovasc Res. 57:544–553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uruno A, Sugawara A, Kanatsuka H,
Kagechika H, Saito A, Sato K, Kudo M, Takeuchi K and Ito S:
Upregulation of nitric oxide production in vascular endothelial
cells by all-trans retinoic acid through the phosphoinositide
3-kinase/Akt pathway. Circulation. 112:727–736. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho DH, Choi YJ, Jo SA, Nam JH, Jung SC
and Jo I: Retinoic acid decreases nitric oxide production in
endothelial cells: A role of phosphorylation of endothelial nitric
oxide synthase at Ser(1179). Biochem Biophys Res Commu.
326:703–710. 2005. View Article : Google Scholar
|
|
13
|
Drolet MC, Plante E, Battistini B, Couet J
and Arsenault M: Early endothelial dysfunction in cholesterol-fed
rabbits: A non-invasive in vivo ultrasound study. Cardiovasc
Ultrasound. 2:102004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang AL, Yeh CK, Su CT, Lo CW, Lin KL and
Lee SD: Aerobic exercise acutely improves insulin and insulin-like
growth factor-1-mediated vasorelaxation in hypertensive rats. Exp
Physiol. 95:622–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bushue N and Wan YJ: Retinoid pathway and
cancer therapeutics. Adv Drug Deliv Rev. 62:1285–1298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye Xf, Wu Q, Liu S, Lin Xf, Zhang B, Wu
Jf, Cai Jh, Zhang Mq and Su Wj: Distinct role and functional mode
of TR3 and RARalpha in mediating ATRA-induced signalling pathway in
breast and gastric cancer cells. Int J Biochem Cell Biol.
36:98–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou B, Pan Y, Hu Z, Wang X, Han J, Zhou
Q, Zhai Z and Wang Y: All-trans-retinoic acid ameliorated high fat
diet-induced atherosclerosis in rabbits by inhibiting platelet
activation and inflammation. J Biomed Biotechnol. 2012:2596932012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansson GK, Robertson AK and
Söderberg-Nauclér C: Inflammation and atherosclerosis. Annu Rev
Pathol. 1:297–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sitia S, Tomasoni L, Atzeni F, Ambrosio G,
Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P,
Camici P, et al: From endothelial dysfunction to atherosclerosis.
Autoimmun Rev. 9:830–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simionescu M and Antohe F: Functional
ultrastructure of the vascular endothelium: Changes in various
pathologies. Handb Exp Pharmacol. 41–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mehta D and Malik AB: Signaling mechanisms
regulating endothelial permeability. Physiol Rev. 86:279–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan SY: Protein kinase signaling in the
modulation of microvascular permeability. Vascul Pharmacol.
39:213–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shepro D: The american microcirculatory
society landis award lecture: Endothelial cells, inflammatory
edema, and the microvascular barrier: Comments by a ‘free radical’.
Microvasc Res. 35:246–264. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki H, Nishizawa T, Tani K, Yamazaki Y,
Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O and Fujiyoshi
Y: Crystal structure of a claudin provides insight into the
architecture of tight junctions. Science. 344:304–307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McKenzie JA and Ridley AJ: Roles of
Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial
morphology and permeability. J Cell Physiol. 213:221–228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao J, Hao J, Fei X, Wang X, Hou Y and
Deng C: Isoflurane inhibits occludin expression via up-regulation
of hypoxia-inducible factor 1α. Brain Res. 1562:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dias RG, Negrão CE and Krieger MH: Nitric
oxide and the cardiovascular system: Cell activation, vascular
reactivity and genetic variant. Arq Bras Cardiol. 96:68–75.
2011.PubMed/NCBI
|
|
28
|
Verma S, Buchanan MR and Anderson TJ:
Endothelial function testing as a biomarker of vascular disease.
Circulation. 108:2054–2059. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Achan V, Tran CT, Arrigoni F, Whitley GS,
Leiper JM and Vallance P: All-trans-retinoic acid increases nitric
oxide synthesis by endothelial cells: A role for the induction of
dimethylarginine dimethylaminohydrolase. Circ Res. 90:764–769.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Figueroa XF, González DR, Puebla M,
Acevedo JP, Rojas-Libano D, Durán WN and Boric MP: Coordinated
endothelial nitric oxide synthase activation by translocation and
phosphorylation determines flow-induced nitric oxide production in
resistance vessels. J Vasc Res. 50:498–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trane AE, Pavlov D, Sharma A, Saqib U, Lau
K, van Petegem F, Minshall RD, Roman LJ and Bernatchez PN:
Deciphering the binding of caveolin-1 to client protein endothelial
nitric-oxide synthase (eNOS): Scaffolding subdomain identification,
interaction modeling, and biological significance. J Biol Chem.
289:13273–13283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du YH and Chen AF: A new role for
caveolin-1: Regulation of guanosine triphosphate cyclohydrolase I
and tetrahydrobiopterin in endothelial cells. Hypertension.
53:115–117. 2009. View Article : Google Scholar : PubMed/NCBI
|