Introduction
Colorectal cancer (CRC) is a frequently occurring
type of cancer and is a primary contributor to morbidity and
mortality rates worldwide (1). The
incidence rates of CRC have gradually increased in developing
countries, which previously showed a decrease in cases of CRC
(2). Although methods have become
available for the diagnosis and treatment of CRC during the last
few decades, the overall five-year survival rate remains at 40–45%
due to the limited ability to detect cancer at an early stage and
provide prognostic predictions (3). At present, the molecular and
functional mechanisms of CRC remain to be fully elucidated.
Therefore, investigations aimed at determining biomarkers and
effective molecular targets for CRC are urgently required.
MicroRNAs (miRNAs), which are non-coding RNAs of ~20
nucleotides, are involved in post-transcriptional regulation by
targeting the 3′untranslated region (UTR) of target genes (4–6).
There have been an increasing number of studies showing that miRNAs
are vital in the occurrence and development of various diseases,
including cancer in humans (4,7–9).
Increasing evidence indicates that miRNAs are involved in the
regulation of various biological processes, including
proliferation, differentiation, apoptosis, migration and invasion
(10). Several studies have
indicated that certain miRNAs are involved in the developmental
process of CRC, including miR-135b (11), miR-34a (12), miR-25 (13) and miR-21 (14). However, the biological functions
and molecular mechanisms of miR-205-5p in CRC remain to be fully
elucidated.
Protein-tyrosine kinase 7 (PTK7) belongs to the
defective receptor protein-tyrosine kinases, and includes an
extracellular domain, a transmembrane domain and a tyrosine kinase
domain (15,16). PTK7 is involved in the development
of planar cell polarity, functioning as a regulator. Studies have
indicated that PTK7 is involved in the Wnt pathway and PCP
signaling pathway (17–19). Studies have also demonstrated that
PTK7 is expressed at high levels in various types of cancer,
including colon cancer (20), lung
cancer (21), gastric cancer
(22) and acute myeloid leukemia
(23). Studies have also shown
that PTK7 can affect the proliferation and invasion abilities of
liposarcoma cells (24), and the
migration and invasion abilities mediated by vascular endothelial
growth factor (25). However, the
interaction and association between miR-205-5p and PTK7, and the
effects of miR-205-5p on the proliferation, migration and invasion
abilities of CRC through PTK7 remain to be elucidated.
The present study investigated the potential role of
miR-205-5p in the development of CRC through PTK7. The miRNA target
sites in the sequence of the PTK7 3′UTR were predicted, which
revealed that the expression of miR-205-5p was low in CRC. The
correlation between miR-205-5p and PTK7 was examined in CRC tissues
and the regulatory association between miR-205-5p and PTK7 was
examined in CRC cells. The study also aimed to measure the effects
of miR-205-5p on the proliferation, apoptosis, migration and
invasion abilities of CRC cells through PTK7.
Materials and methods
Cell lines and transfection
HT29 and SW480 human CRC cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
HT29 and SW480 cells were cultured at 37°C in an appropriate
incubator containing 5% CO2 in RPMI 1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), penicillin (100 U/ml) and streptomycin (100 µg/ml). For
treatment, 2×105 HT29 and SW480 cells were seeded into
6-well plates and then transfected with 200 µl mature hsa-miR-NC
(negative control) and the predicted miRNAs [predicted using
TargetScan (http://www.targetscan.org/); miRBase (http://www.mirbase.org/), and MISIM (http://cmbi.bjmu.edu.cn/misim)], including
hsa-miR-409-5p, hsa-miR-205-5p, hsa-miR-495-3p, hsa-miR-5688, and
hsa-miR-503-5p (GenePharma Co., Ltd., Shanghai, China),
respectively, for 72 h. The miR-205-5p mimic sequence was
5′-UCCUUCAUUCCACCGGAGUCUG-3′, the miR-control sequence was
5′-ACUACUGAGUGACAGUAGA-3′, the inhibitor NC sequence was
5′-CAGUACUUUUGUGUAGUACAA-3′, and the miR-205-5p inhibitor sequence
was 5′-CAGACUCCGGUGGAAUGAAGGA-3′. The HT29 and SW480 cells were
then transfected with miR-205-5p (50 nM), miR-control (50 nM),
inhibitor NC (50 nM), and miR-205-5p inhibitor (50 nM),
respectively. Finally, HT29 and SW480 cells were transfected with
miR-control, miR-205-5p, miR-205-5p and vector, and miR-205-5p and
PTK7, respectively, for 72 h. All transfections were performed with
Lipofectamine™ 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Clinical specimens
In the present study, tissue samples were collected
from 46 patients (age 50.34±5.87 years, range 29–91;
man/woman=25/21) with CRC in The People's Hospital of Tianjin
(Tianjin, China) between January 2015 and November 2016. All tissue
samples were verified by a trained pathologist and were immediately
preserved at −80°C until further use. The tumor grades were defined
according to the criteria of World Health Organization (26). Written informed consent was
provided by all patients. The present study was approved by the
institutional ethics committee at the People's Hospital of
Tianjin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
As described previously (27), total RNA was extracted from the CRC
tissues, the matched adjacent noncancerous tissues, and the treated
HT29 and SW480 cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Tissues (100 mg) were treated with liquid nitrogen, and then ground
into powder; the cell suspensions were centrifuged (1,000 × g, 5
min, 4°C). Trizol (1 ml) was added and ground for 5 min on ice, and
then 0.2 ml chloroform was added and centrifuged (12,000 × g, 15
min, 4°C). The supernatant solution was obtained, precipitated by
alcohol, and then centrifuged (7,500 × g, 5 min, 4°C). RNA purity
can be detected using the NanoDrop (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). The optical density260/280 ratio
is used as indicator for RNA purity. A ratio >1.8 is regarded as
suitable for gene expression measurements. Total RNA (1 µg) was
reverse transcribed using the RevertAid First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were 25°C, 5 min; 42°C, 60 min; 70°C, 10 min. A
SYBR-Green PCR Master Mix kit (Takara Bio, Inc., Otsu, Japan) was
used to detect the mRNA expression levels of PTK7 and miR-205-5p.
The reaction volumes for RT-qPCR were 5.0 µl SYBR®
Premix Ex Taq™ II (2 X), 0.4 µl PCR Forward Primer (10
µM), 0.4 µl PCR Reverse Primer (10 µM), 0.2 µl ROX Reference Dye
(50X), 1.0 µl cDNA template and 3.0 µl ddH2O. Reaction steps: 95°C
for 30 sec as the first step in a loop; 95°C for 5 sec, 60°C for 34
sec as the second step, a total of 40 cycles. The primer sequences
were as follows: GAPDH, forward 5′-CCTCGTCTCATAGACAAGATGGT-3′ and
reverse 5′-GGGTAGAGTCATACTGGAACATG-3′ (internal control); PTK7,
forward 5′-CAGTTCCTGAGGATTTCCAAGAG-3′ and reverse
5′-TGCATAGGGCCACCTTC-3′; hsa-miR-205-5p,
5′-TCCTTCATTCCACCGGAGTCTG-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′
and reverse 5′-AACGCTTCACGAATTTGCGT-3′. All the primers above were
synthesized by IDT (Coralville, IA, USA). The fold change in
expression was determined using the 2−∆∆Cq method
(28).
Western blot analysis and
antibodies
The treated HT29 and SW480 cells were lysed in lysis
buffer containing a protease inhibitor cocktail (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). The concentrations of proteins
were measured using a bicinchoninic acid Protein Assay kit (Thermo
Fisher Scientific, Inc.). Equivalent quantities of protein were
separated by 10% SDS-PAGE on gels and then transferred onto
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). The PVDF membranes were blocked in 5% skim
milk (BD Biosciences, Franklin Lakes, NJ, USA) for 2 h at room
temperature. The PVDF membranes were then incubated with anti-PTK7
antibody (1:1,000; cat. no. MAB4499; R&D Systems, Inc.,
Minneapolis, MN, USA); anti-GAPDH antibody (1:4,000; cat. no.
12255; Cell Signaling Technology, Inc., Beverly, MA, USA) at 4°C
overnight, and were then incubated with HRP-conjugated secondary
antibodies (goat anti-mouse; 1:5,000; cat. no. SC-2005, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; goat anti-rabbit; 1:5,000;
cat. no. SC-2004, Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Finally, the proteins were detected using an enhanced
chemiluminescence detection kit (EMD Millipore, Billerica, MA,
USA). The signals were detected using a chemiluminescence detection
system with Super Signal West Pico Chemiluminescent Substrate
(Thermo Fisher Scientific, Inc., cat. no. 34080). Anti-GAPDH
antibody was used as an internal control.
Luciferase reporter assays
The sequences of the wild-type (WT) and mutant type
(Mut) PTK7-3′UTR were amplified by PCR using human genomic DNA of
the HT29 cell line and cloned into the pGL3-promoter vector
(Promega Corporation, Madison, WI, USA; cat. no. E1751). The HT29
and SW480 cells (5×104 cells/well) were cultured in
24-well plates and co-transfected with 50 nM miR-205-5p,
miR-control, inhibitor NC, or 100 nM miR-205-5p inhibitor,
respectively with 15 ng of WT pGL3-promoter-PTK7-3′UTR or 15 ng Mut
type pGL3-promoter-PTK7-3′UTR, and the Renilla plasmid
(RL-SV40) using Lipofectamine 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
According to the manufacturer's protocol, the luciferase activity
of PTK7 was detected using a Dual-Luciferase reporter assay system
(Promega Corporation). The duration was 10 h between activity
measurement and transfection and the results were normalized to
pRL-CMV Renilla.
Colony formation assay
The HT29 and SW480 cells were transfected with
miR-control, miR-205-5p, miR-205-5p and vector, and miR-205-5p and
PTK7, respectively, for 72 h. The treated HT29 and SW480 cells were
incubated in complete medium for 14 days. The colonies were fixed
with methanol for 15 min at room temperature, and dyed with giemsa
dye solution for 10 min at room temperature. The colonies were then
identified and counted under a light microscope (BX51; Olympus
Corporation, Tokyo, Japan).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The treated HT29 and SW480 cells (2,000 cells/well)
were seeded in 96-well plates with complete medium for 0, 24 and 48
h, respectively. Each group consisted of five wells and each well
was treated with MTT (20 µl/well) solution (5 mg/ml; Sigma-Aldrich;
Merck KGaA) at 0, 12, 24 and 48 h. After 4 h, 100 µl dimethyl
sulfoxide (Sigma-Aldrich, Merck KGaA) was added to dissolve the
crystal. The absorbance (optical density) was detected using a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at
570 nm.
Flow cytometric analysis of cell
apoptosis
According to the manufacturer's protocol, the
treated HT29 and SW480 cells were stained with Annexin
V-fluorescein isothio-cyanate (FITC)/propidium iodide (PI) kit
(cat. no. 4830-01-K; R&D systems, Inc.). Samples were analyzed
for apoptosis using a FACSCalibur flow cytometer (BD Biosciences).
FlowJo software 7.6.5 (Tree Star Inc., Ashland, OR, USA) was used
to analyze the results of the flow cytometry.
Migration and invasion assays
For the migration assay, the treated HT29 and SW480
cells (1×105 cells/well) were seeded in the top of each
well containing serum-free medium, and 600 µl complete medium was
added to the lower chamber. After 24 h, the migrated cells were
fixed with 4% paraformaldehyde for 30 min at room temperature and
stained with 0.1 % crystal violet solution (Sigma-Aldrich; Merck
KGaA) for 20 mins at room temperature. The migrated cells were
identified and counted using a light microscope (BX51; Olympus
Corporation). For the invasion assay, the diluted Matrigel (BD
Biosciences,) was added to the Transwell chamber for 1 h at 37°C,
and the remaining steps were similar to those of the migration
assay.
Statistical analysis
The data were analyzed using SPSS 18.0 version
(SPSS, Inc. Chicago, IL, USA). The results were compared using
one-way analysis of variance followed by Dunnett's posttest for
multiple comparisons. All results are expressed as the mean ±
standard deviation from three replicates. P<0.05 was considered
to indicate a statistically significant difference.
Results
Identification of PTK7-integrated
miRNAs
To identify miRNAs, which were potential target
sites in the sequence of the PTK7 3′UTR. TargetScan (http://www.targetscan.org/) was used. It was found
that there were five potential miRNAs, including hsa-miR-409-5p,
hsa-miR-205-5p, hsa-miR-495-3p, hsa-miR-5688 and hsa-miR-503-5p
(Fig. 1A). The HT29 and SW480
cells were then transfected with hsa-miR-NC (negative control) and
the predicted miRNAs (miR-409-5p, miR-205-5p, miR-495-3p, miR-5688,
and miR-503-5p, respectively). The results revealed that the mRNA
expression level of PTK7 was decreased in HT29 cells transfected
with miR-205-5p, compared with that in the NC cells (P<0.05;
Fig. 1B). Similarly, the mRNA
expression level of PTK7 was decreased in SW480 cells transfected
with miR-205-5p, compared with that in the NC cells (P<0.05;
Fig. 1C). To investigate whether
miR-205-5p was physically associated with PTK7 in CRC tissues
(n=46), the correlation between the expression of miR-205-5p and
PTK7 in CRC tissues was detected using RT-qPCR analysis. The result
showed that there was a negative correlation between the gene
expression of PTK7 and miR-205-5p and in the CRC tissues (Fig. 1D).
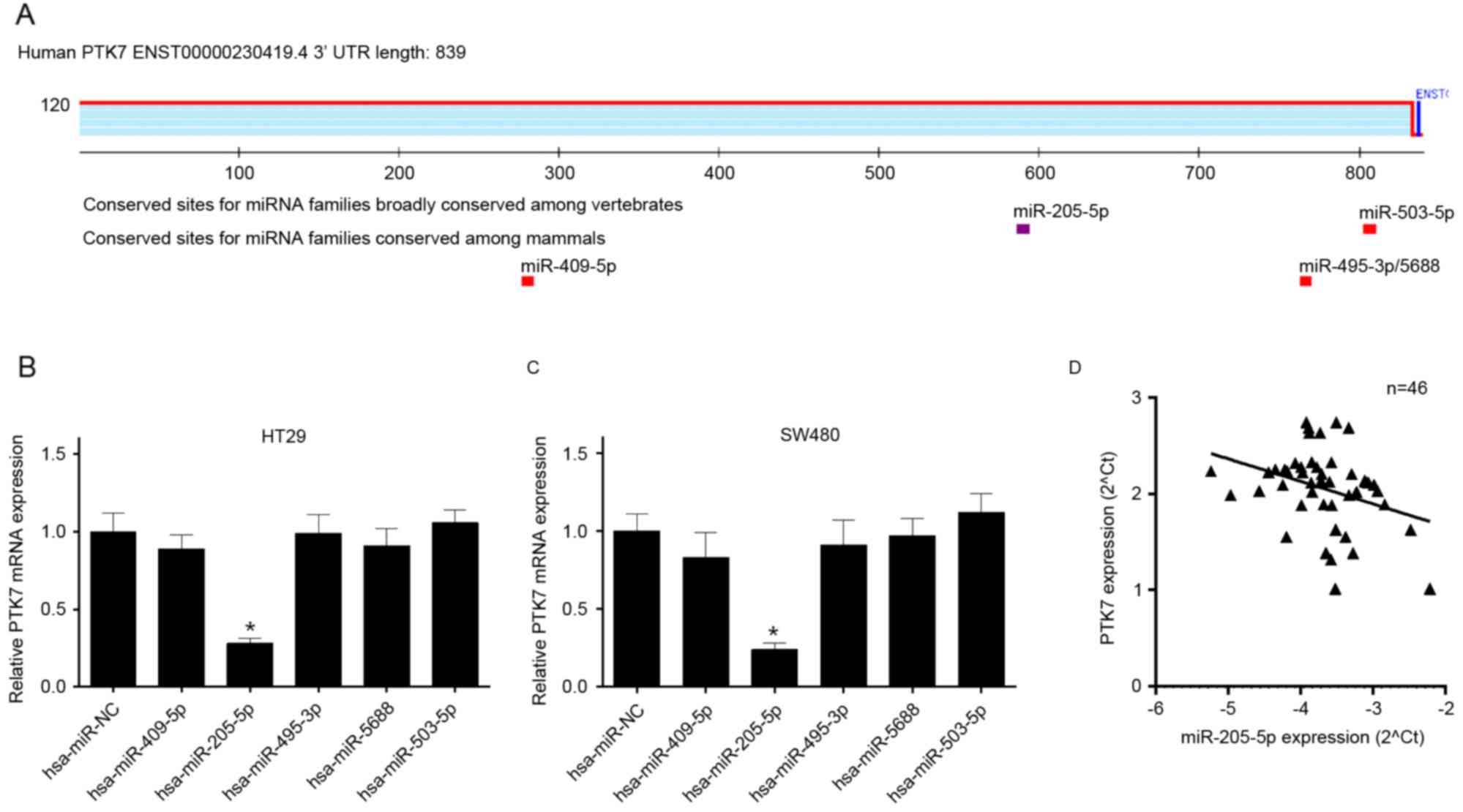 | Figure 1.Identification of PTK7-integrated
miRNAs. (A) miRNA target sites in the PTK7 3′UTR sequence were
predicted using TargetScan. (B) RT-qPCR analysis was used to
determine the mRNA expression level of PTK7 in HT29 cells
transfected with hsa-miR-NC and the predicted miRNAs, including
hsa-miR-409-5p, hsa-miR-205-5p, hsa-miR-495-3p, hsa-miR-5688 and
hsa-miR-503-5p, respectively. (C) The mRNA expression level of PTK7
was detected using RT-qPCR analysis in SW480 cells treated as B.
(D) Negative correlation between miR-205-5p and PTK7, analyzed
using RT-qPCR analysis in colorectal cancer tissues (n=46).
*P<0.05 vs. hsa-miR-NC group. miRNA/miR, microRNA; PTK7,
protein-tyrosine kinase 7; NC, negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; UTR,
untranslated region. |
miR-205-5p regulates the gene
transcription of PTK7 and suppresses the expression of PTK7 in
human CRC
The present study further demonstrated that
miR-205-5p may directly regulate the transcriptional level of PTK7.
According to the binding site of the miR-205-5p targeting
PTK7-3′UTR, the promoter region of PTK7-3′UTR was designed and
cloned into pMIR-report, including a WT (PTK7-3′UTR-WT) vector and
Mut type (PTK7-3′UTR-Mut) vector (Fig.
2A). The PTK7-3′UTR-WT or PTK7-3′UTR-Mut luciferase reporter
was co-transfected into HT29 and SW480 cells with the miR-control,
miR-205-5p, inhibitor NC, and miR-205-5p inhibitor, respectively.
The results of luciferase reporter gene assays indicated that
miR-205-5p decreased the promoter activity of PTK7 in HT29 cells
(P<0.05; Fig. 2B); and
miR-205-5p decreased the promoter activity of PTK7 in SW480 cells
(P<0.05; Fig. 2C). The results
of the RT-qPCR analysis also showed that miR-205-5p decreased the
mRNA expression level of PTK7 in the HT29 and SW480 cells; the
inhibition of miR-205-5p by the inhibitor increased the mRNA
expression level of PTK7 in the HT29 and SW480 cells (Fig. 2D). The results of the western blot
analysis indicated that miR-205-5p also decreased the protein
expression levels of PTK7 in the HT29 and SW480 cells; the
inhibition of miR-205-5p by the inhibitor increased the protein
expression level of PTK7 in HT29 and SW480 cells (Fig. 2E).
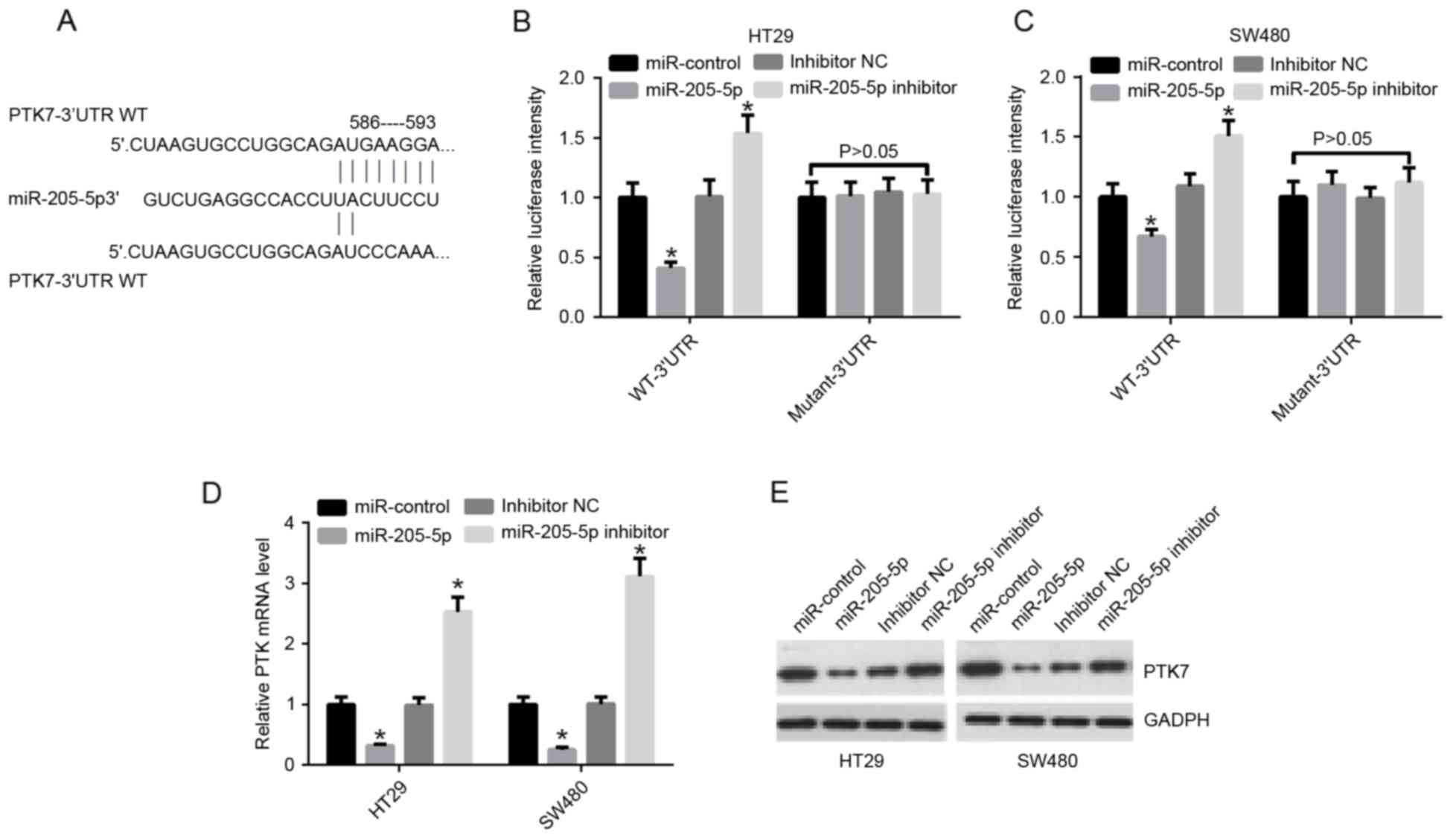 | Figure 2.miR-205-5p regulates the gene
transcription of PTK7 and suppresses the expression of PTK7 in
human colorectal cancer. (A) Binding sites of miR-205-5p (586–593)
targeting PTK7-3′UTR-WT and its mutant sequences (PTK7-3′UTR-Mut)
are shown. The sites without connections indicate the mutation
position. (B) PTK7-3′UTR-WT or PTK7-3′UTR-Mut luciferase reporter
was co-transfected into HT29 cells with miR-control, miR-205-5p,
inhibitor NC, and miR-205-5p inhibitor, respectively, for 48 h.
Luciferase activity of the PTK7 3′UTR was detected using a
luciferase reporter gene assay. (C) PTK7-3′UTR-WT or PTK7-3′UTR-Mut
luciferase reporters was co-transfected into SW480 cells with
miR-control, miR-205-5p, inhibitor NC, and miR-205-5p inhibitor,
respectively, for 48 h. Luciferase activity of the PTK7 3′UTR was
detected using the luciferase reporter gene assay. (D) Reverse
transcription-quantitative polymerase chain reaction analysis of
the mRNA expression levels of PTK1 in HT29 and SW480 cells
transfected with miR-control, miR-205-5p, inhibitor NC, and
miR-205-5p inhibitor, respectively. (E) Protein expression levels
of PTK7, measured using western blot analysis, in HT29 and SW480
cells treated as in D. *P<0.05 vs. miR-control. miR, microRNA;
PTK7, protein-tyrosine kinase 7; WT, wild-type; Mut, mutant; UTR,
untranslated region; NC, negative control. |
miR-205-5p accelerates cell
proliferation and inhibits apoptosis through PTK7 in CRC cells
The effects of miR-205-5p on the proliferation and
apoptosis capacities of the CRC cells were investigated. The HT29
and SW480 cells were transfected with miR-control, miR-205-5p,
miR-205-5p and vector, and miR-205-5p and PTK7, respectively. The
mRNA expression level of PTK7 was detected using RT-qPCR analysis.
The results demonstrated that miR-205-5p decreased the mRNA
expression level of PTK7 in the HT29 and SW480 cells;
PTK7-transfection increased the mRNA expression level of PTK7
mediated by miR-205-5p (P<0.05; Fig. 3A). Simultaneously, the results of
the western blot analysis showed that miR-205-5p inhibited the
protein expression level of PTK7 in the HT29 and SW480 cells;
PTK7-transfection increased the protein expression level of PTK7
mediated by miR-205-5p (Fig. 3B).
Subsequently, the MTT assays indicated that miR-205-5p inhibited
the proliferation ability of HT29 cells; the overexpression of PTK7
promoted the proliferation ability of HT29 cells mediated by
miR-205-5p (P<0.05; Fig. 3C).
It was also indicated that miR-205-5p inhibited the proliferation
ability of SW480 cells; the overexpression of PTK7 promoted the
proliferation ability of SW480 cells mediated by miR-205-5p
(P<0.05; Fig. 3D). The
apoptosis of cells was detected using Annexin V-FITC/PI staining.
It was found that apoptosis was increased in HT29 and SW480 cells
transfected with miR-205-5p, compared with those transfected with
miR-control; the apoptosis was deceased in HT29 and SW480 cells
transfected with miR-205-5p and PTK7, compared with those
transfected with miR-205-5p and vector (P<0.05; Fig. 3E). A colony formation assay was
also performed to detect the proliferation ability. The results
also indicated that miR-205-5p inhibited proliferation ability, and
PTK7 promoted the proliferation ability mediated by miR-205-5p in
the SW480 and HT29 cells (Fig.
3F).
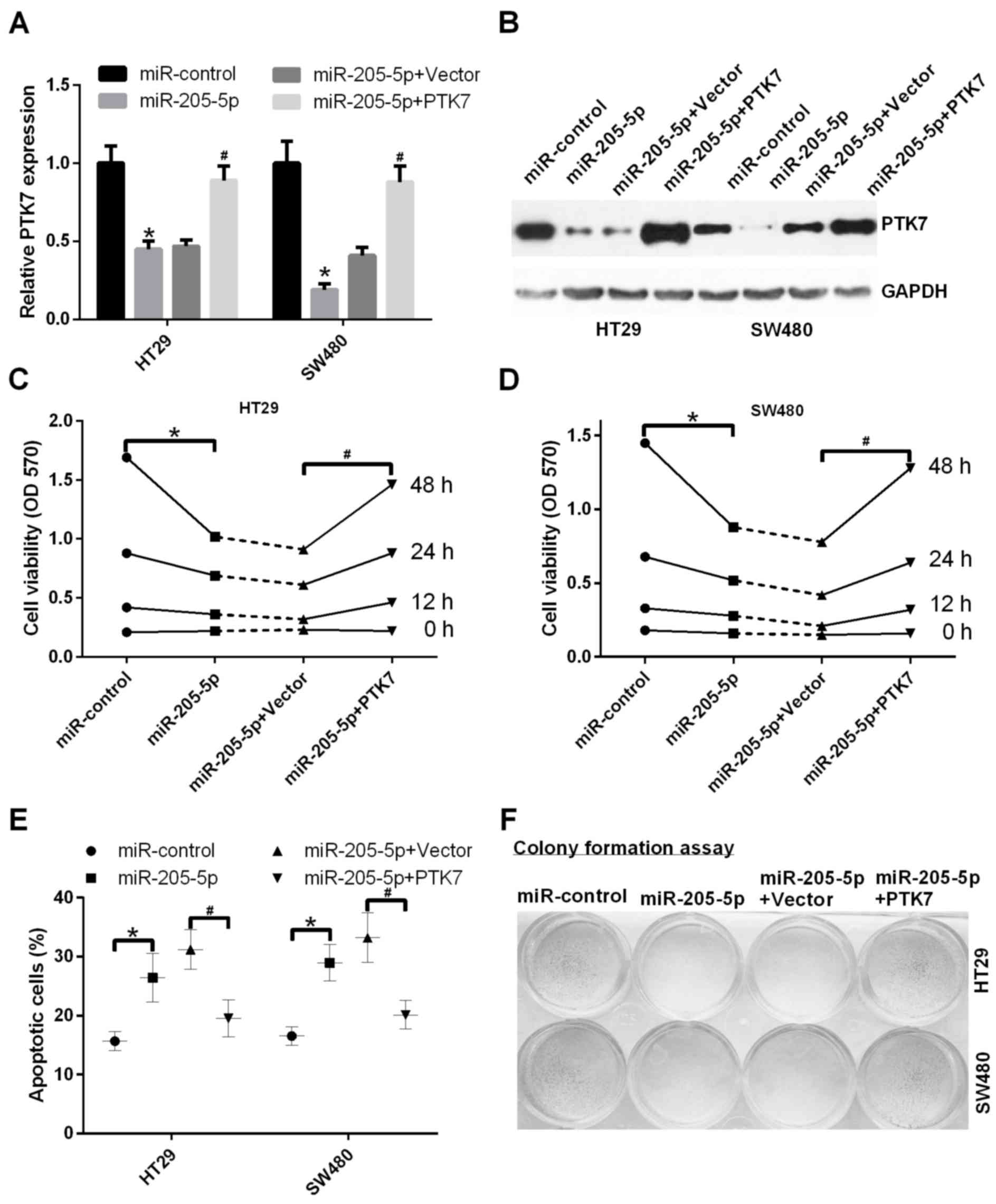 | Figure 3.miR-205-5p accelerates cell
proliferation, and inhibits apoptosis through PTK7 in CRC cells.
(A) miR-205-5p inhibited the mRNA expression levels of PTK7 in HT29
and SW480 cells. The mRNA expression levels of PTK7 were detected
using reverse transcription-quantitative polymerase chain reaction
analysis in HT29 and SW480 cells transfected with miR-control,
miR-205-5p, miR-205-5p and vector, miR-205-5p and PTK7,
respectively (*P<0.05 miR-205-5p, vs. NC; #P<0.05
miR-205-2p+PTK7 vs. miR-205-5p+vector). (B) Western blot analysis
was used to measure the protein expression levels of PTK7 in HT29
and SW480 cells treated as in A. (C) An MTT assay was performed to
determine the proliferation ability of HT29 cells transfected with
miR-control, miR-205-5p, miR-205-5p and vector, and miR-205-5p and
PTK7, respectively, at 0, 12, 24 and 48 h *P<0.05 miR-205-5p,
vs. NC; #P<0.05 miR-205-2p+PTK7 vs.
miR-205-5p+vector. (D) MTT assay was performed to detect
proliferation ability of SW480 cells treated as in C. *P<0.05
miR-205-5p, vs. NC; #P<0.05 miR-205-2p+PTK7 vs.
miR-205-5p+vector). (E) HT29 and SW480 cells were transfected with
miR-control, miR-205-5p, miR-205-5p and vector, and miR-205-5p and
PTK7, respectively, and cell apoptosis was detected using Annexin
V-FITC/PI staining. *P<0.05 miR-205-5p, vs. NC;
#P<0.05 miR-205-2p+PTK7 vs. miR-205-5p+vector. (F)
Cell proliferation was detected using colony formation assays in
HT29 and SW480 cells transfected with miR-control, miR-205-5p,
miR-205-5p and vector, and miR-205-5p and PTK7, respectively. miR,
microRNA; PTK7, protein-tyrosine kinase 7; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PI,
propidium iodide. |
miR-205-5p promotes the migration and
invasion abilities of CRC cells through PTK7
The effects of the expression of miR-205-5p on the
migration and invasion abilities of CRC cells were detected in HT29
and SW480 cells transfected with miR-control, miR-205-5p,
miR-205-5p and vector, and miR-205-5p and PTK7, respectively. The
results showed that the invasion capacities of the HT29 and SW480
cells transfected with miR-205-5p were significantly decreased
compared with those transfected with miR-control (P<0.05); the
invasion capacities of the HT29 and SW480 cells transfected with
miR-205-5p and PTK7 were significantly increased, compared with the
cells transfected with miR-205-5p and vector (P<0.05; Fig. 4A and B). Similarly, the results
showed that the migration capacities of the HT29 and SW480 cells
transfected with miR-205-5p were significantly decreased, compared
with those transfected with miR-control (P<0.05); the cell
migration capacities of the HT29 and SW480 cells transfected with
miR-205-5p and PTK7 were significantly increased, compared with
those transfected with miR-205-5p and vector (P<0.05; Fig. 4C and D).
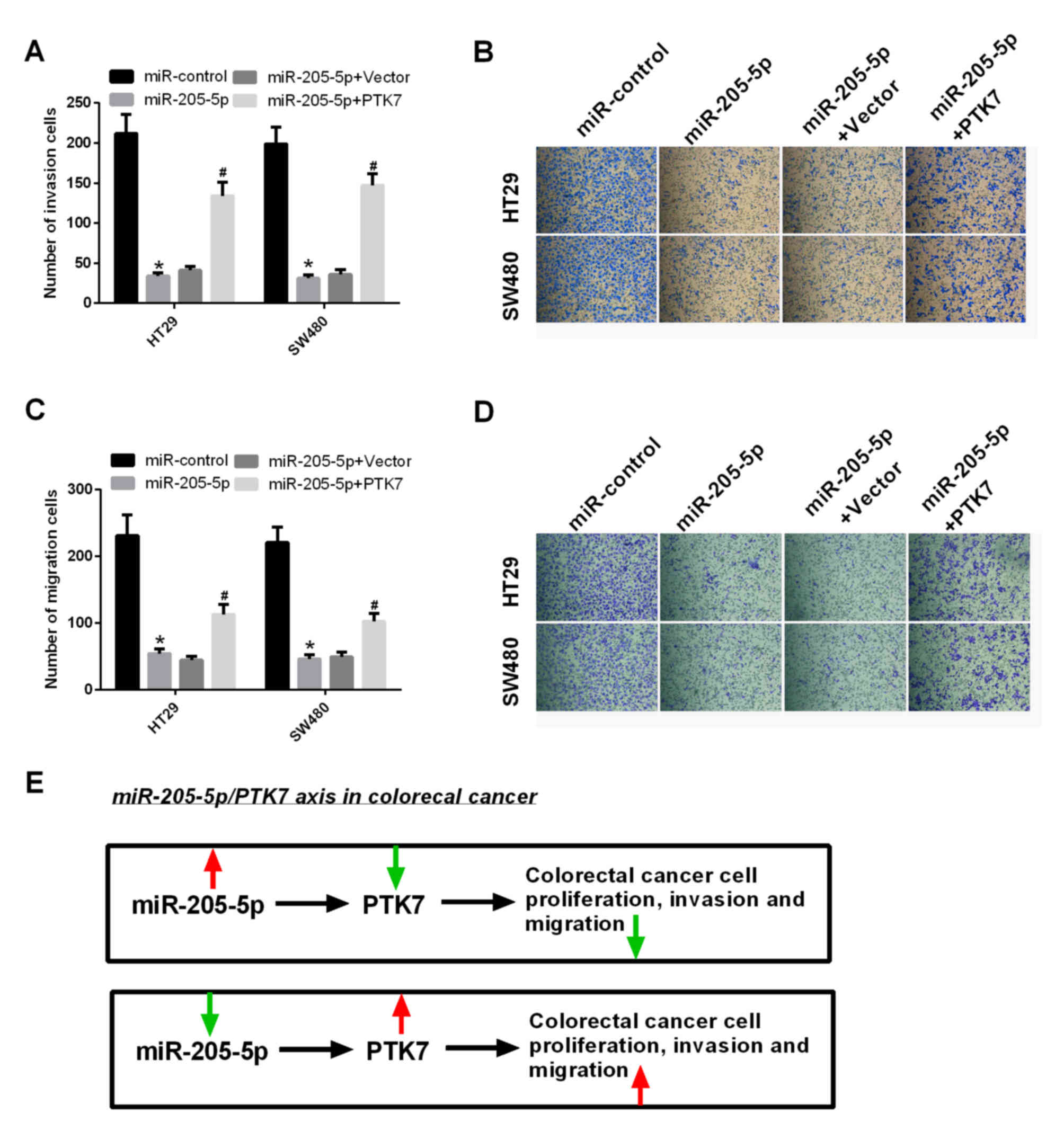 | Figure 4.miR-205-5p promotes migration and
invasion abilities of CRC cells through PTK7. (A) Invasion assays
were performed in HT29 and SW480 cells transfected with
miR-control, miR-205-5p, miR-205-5p and vector, and miR-205-5p and
PTK7, respectively. Quantification of invasive cells was
calculated. *P<0.05 miR-205-5p, vs. NC; #P<0.05
miR-205-2p+PTK7 vs. miR-205-5p+vector. (B) Images of invasive cells
(magnification, ×200). (C) Migration assays were performed in HT29
and SW480 cells transfected with miR-control, miR-205-5p,
miR-205-5p and vector, and miR-205-5p and PTK7, respectively.
Quantification of invasive cells was calculated. *P<0.05
miR-205-5p, vs. NC; #P<0.05 miR-205-2p+PTK7 vs.
miR-205-5p+vector. (D) Images of migrated cells (magnification,
×200). (E) Functional mechanism of PTK7-integrated miR-205-5p in
CRC. The red arrows represent an increase and the green arrows
represent a decrease. miR, microRNA; PTK7, protein-tyrosine kinase
7; CRC, colorectal cancer. |
miR-205-5p/PTK7 axis in CRC
The results of the present study confirmed the
functional mechanism of PTK7-integrated miR-205-5p in CRC. The
overexpression of miR-205-5p inhibited the proliferation, migration
and invasion abilities of CRC through inhibition of the expression
of PTK7. The decreased expression of miR-205-5p accelerated the
proliferation, migration and invasion abilities of CRC through
activation of the expression of PTK7 (Fig. 4E).
Discussion
Several studies have demonstrated that PTK7 is
expressed at high levels in various types of cancer, including CRC,
liposarcoma, esophageal cancer, and gastric cancer (24,29–31).
High expression levels of PTK7 have been associated with poor
prognosis in esophageal cancer. PTK7 is involved in the
proliferation, survival, invasion and migration of tumors,
including CRC (32), esophageal
cancer (30), and lung cancer
(33). Furthermore, is have been
shown that PTK7 may be a potential oncogene (30,31,34).
However, the regulatory association between PTK7 and miRNA remains
to be fully elucidated.
miRNAs, a class of small non-coding RNAs of 19–24
nucleotides, are differentially expressed in various types of
cancer suggesting the important function of miRNAs in tumorigenesis
(35–37). An increasing number of studies have
indicated that miRNAs can act as oncogenes or tumor suppressors
(36,37), and can affect the proliferation,
metastasis, angiogenesis and inflammation of tumors by targeting
mRNAs (38,39). There is evidence that various
miRNAs are associated with the occurrence and development of CRC.
For example, miR-21 has been found to stimulate invasion,
intravasation and metastasis in CRC by downregulating Pdcd4
(40); miR-135b, as a downstream
effector of oncogenic pathways, also promotes the progression of
CRC (11) and, as a potential
tumor suppressor, miR-25 has been found to be involved in CRC by
targeting small mothers against decapentaplegic 7 (13).
miR-218 inhibits cell cycle and promotes apoptosis
of CRC cells (41); miR-498 is
downregulated in CRC and affects the functions of CRC cells
(42). Furthermore, the expression
of miRNAs can provide biomolecular and prognostic characteristics
(43,44). However, the function of miR-205-5p
in CRC remains to be fully elucidated.
In the present study, five potential miRNAs,
including hsa-miR-409-5p, hsa-miR-205-5p, hsa-miR-495-3p,
hsa-miR-5688 and hsa-miR-503-5p, were identified as potential
target sites in the sequence of PTK7 3′UTR. It was indicated that
the mRNA expression levels of PTK7 were decreased in HT29 and SW480
cells transfected with miR-205-5p, compared with levels in NC
cells. In addition, there was a negative correlation between the
gene expression of PTK7 and miR-205-5p in CRC tissues. It was
confirmed that miR-205-5p directly regulated the transcriptional
level of PTK7, with miR-205-5p simultaneously decreasing the mRNA
and protein expression levels of PTK7 in HT29 and SW480 cells. It
was also revealed that miR-205-5p accelerated the proliferation,
migration and invasion abilities of CRC cells, and inhibited
apoptosis through PTK7.
In conclusion, the results of the present study
confirmed the functional mechanism of PTK7-integrated miR-205-5p in
CRC. It was found that there was a negative correlation between
miR-205-5p and PTK7 in CRC tissues. Furthermore, miR-205-5p was
indicated to be involved in the processes of proliferation,
apoptosis, migration and invasion in CRC through the regulation of
PTK7.
Acknowledgements
The present study was supported by the Science
Foundation of Chinese and Western Integrative Medicine of Zhejiang
Province, China (No. 2014LYZ0017).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khair G, Monson JR and Greenman J:
Epithelial molecular markers in the peripheral blood of patients
with colorectal cancer. Dis Colon Rectum. 50:1188–1203. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancerMicroRNA Cancer Regulation. Springer;
New York, NY: pp. 1–20. 2013, View Article : Google Scholar
|
|
5
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasinski AL and Slack FJ: MicroRNAs en
route to the clinic: Progress in validating and targeting microRNAs
for cancer therapy. Nat Rev Cancer. 11:849–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baer C, Claus R and Plass C: Genome-wide
epigenetic regulation of miRNAs in cancer. Cancer Res. 73:473–477.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zaman MS, Maher DM, Khan S, Jaggi M and
Chauhan SC: Current status and implications of microRNAs in ovarian
cancer diagnosis and therapy. J Ovarian Res. 5:442012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Leva G and Croce CM: The role of
microRNAs in the tumorigenesis of ovarian cancer. Front Oncol.
3:1532013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valeri N, Braconi C, Gasparini P, Murgia
C, Lampis A, Paulus-Hock V, Hart JR, Ueno L, Grivennikov SI, Lovat
F, et al: MicroRNA-135b promotes cancer progression by acting as a
downstream effector of oncogenic pathways in colon cancer. Cancer
Cell. 25:469–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bu P, Chen KY, Chen JH, Wang L, Walters J,
Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al: A
microRNA miR-34a-regulated bimodal switch targets Notch in colon
cancer stem cells. Cell Stem Cell. 12:602–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H,
Wang W, Zhang L, Zhang X, Tang Q, et al: MicroRNA-25 functions as a
potential tumor suppressor in colon cancer by targeting Smad7.
Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal
J, Sarkar FH and Majumdar AP: MicroRNA-21 induces stemness by
downregulating transforming growth factor beta receptor 2 (TGFβR2)
in colon cancer cells. Carcinogenesis. 33:68–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SK, Lee HS and Lee ST:
Characterization of the human full-length PTK7 cDNA encoding a
receptor protein tyrosine kinase-like molecule closely related to
chick KLG. J Biochem. 119:235–239. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee ST, Strunk KM and Spritz RA: A survey
of protein tyrosine kinase mRNAs expressed in normal human
melanocytes. Oncogene. 8:3403–3410. 1993.PubMed/NCBI
|
|
17
|
Shnitsar I and Borchers A: PTK7 recruits
dsh to regulate neural crest migration. Development. 135:4015–4024.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu X, Borchers AG, Jolicoeur C, Rayburn H,
Baker JC and Tessier-Lavigne M: PTK7/CCK-4 is a novel regulator of
planar cell polarity in vertebrates. Nature. 430:93–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puppo F, Thomé V, Lhoumeau AC, Cibois M,
Gangar A, Lembo F, Belotti E, Marchetto S, Lécine P, Prébet T, et
al: Protein tyrosine kinase 7 has a conserved role in Wnt/β-catenin
canonical signalling. EMBO Rep. 12:43–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saha S, Bardelli A, Buckhaults P,
Velculescu VE, Rago C, St Croix B, Romans KE, Choti MA, Lengauer C,
Kinzler KW and Vogelstein B: A phosphatase associated with
metastasis of colorectal cancer. Science. 294:1343–1346. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Endoh H, Tomida S, Yatabe Y, Konishi H,
Osada H, Tajima K, Kuwano H, Takahashi T and Mitsudomi T:
Prognostic model of pulmonary adenocarcinoma by expression
profiling of eight genes as determined by quantitative real-time
reverse transcriptase polymerase chain reaction. J Clin Oncol.
22:811–819. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorringe KL, Boussioutas A and Bowtell DD:
Melbourne Gastric Cancer Group, Peter Mac Micro ArrayFacility:
Novel regions of chromosomal amplification at 6p21, 5p13, and 12q14
in gastric cancer identified by array comparative genomic
hybridization. Genes Chromosomes Cancer. 42:247–259. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller-Tidow C, Schwäble J, Steffen B,
Tidow N, Brandt B, Becker K, Schulze-Bahr E, Halfter H, Vogt U,
Metzger R, et al: High-throughput analysis of genome-wide receptor
tyrosine kinase expression in human cancers identifies potential
novel drug targets. Clinical Cancer Res. 10:1241–1249. 2004.
View Article : Google Scholar
|
|
24
|
Gobble RM, Qin LX, Brill ER, Angeles CV,
Ugras S, O'Connor RB, Moraco NH, Decarolis PL, Antonescu C and
Singer S: Expression profiling of liposarcoma yields a multigene
predictor of patient outcome and identifies genes that contribute
to liposarcomagenesis. Cancer Res. 71:2697–2705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin WS, Maeng YS, Jung JW, Min JK, Kwon
YG and Lee ST: Soluble PTK7 inhibits tube formation, migration, and
invasion of endothelial cells and angiogenesis. Biochem Biophys Res
Commun. 371:793–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World health organization
classification of lung tumors: Impact of Genetic, clinical and
radiologic advances since the 2004 Classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang L, Lai YK, Zhang J, Wang H, Lin MC,
He ML and Kung HF: Targeting S100P inhibits colon cancer growth and
metastasis by Lentivirus-mediated RNA interference and proteomic
analysis. Mol Med. 17:709–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mossie K, Jallal B, Alves F, Sures I,
Plowman GD and Ullrich A: Colon carcinoma kinase-4 defines a new
subclass of the receptor tyrosine kinase family. Oncogene.
11:2179–2184. 1995.PubMed/NCBI
|
|
30
|
Shin WS, Kwon J, Lee HW, Kang MC, Na HW,
Lee ST and Park JH: Oncogenic role of protein tyrosine kinase 7 in
esophageal squamous cell carcinoma. Cancer Sci. 104:1120–1126.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Zhang LH, Wang XH, Xing XF, Cheng
XJ, Dong B, Hu Y, Du H, Li YA, Zhu YB, et al: PTK7 as a novel
marker for favorable gastric cancer patient survival. J Surg Oncol.
106:880–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Na HW, Shin WS, Ludwig A and Lee ST: The
cytosolic domain of PTK7, generated from sequential cleavage by
ADAM17 and γ-secretase, enhances cell proliferation and migration
in colon cancer cells. J Biol Chem. 287:25001–25009. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JH, Kwon J, Lee HW, Kang MC, Yoon HJ,
Lee ST and Park JH: Protein tyrosine kinase 7 plays a tumor
suppressor role by inhibiting ERK and AKT phosphorylation in lung
cancer. Oncol Reports. 31:2708–2712. 2014. View Article : Google Scholar
|
|
34
|
Meng L, Sefah K, O'Donoghue MB, Zhu G,
Shangguan D, Noorali A, Chen Y, Zhou L and Tan W: Silencing of PTK7
in colon cancer cells: Caspase-10-dependent apoptosis via
mitochondrial pathway. PLoS One. 5:e140182010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Paris PL, Chen J, Ngo V, Yao H,
Frazier ML, Killary AM, Liu CG, Liang H, Mathy C, et al: Next
generation sequencing of pancreatic cyst fluid microRNAs from low
grade-benign and high grade-invasive lesions. Cancer Lett.
356:404–409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stahlhut C and Slack FJ: MicroRNAs and the
cancer phenotype: Profiling, signatures and clinical implications.
Genome Med. 5:1112013. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He X, Dong Y, Wu CW, Zhao Z, Ng SS, Chan
FK, Sung JJ and Yu J: MicroRNA-218 inhibits cell cycle progression
and promotes apoptosis in colon cancer by downregulating BMI1
polycomb ring finger oncogene. Mol Med. 18:1491–1498. 2012.
View Article : Google Scholar :
|
|
42
|
Gopalan V, Smith RA and Lam AK:
Downregulation of microRNA-498 in colorectal cancers and its
cellular effects. Exp Cell Res. 330:423–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roldo C, Missiaglia E, Hagan JP, Falconi
M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A and
Croce CM: MicroRNA expression abnormalities in pancreatic endocrine
and acinar tumors are associated with distinctive pathologic
features and clinical behavior. J Clin Oncol. 24:4677–4684. 2006.
View Article : Google Scholar : PubMed/NCBI
|


















