Introduction
Osteoarthritis (OA) is a common disease of the
joints that is characterized by chronic and progressive loss of
articular cartilage. Articular cartilage is a type of hyaline
cartilage comprising highly differentiated chondrocytes and
extracellular matrix (ECM). The major structural components of ECM
include type II collagen and aggrecan. Aggrecanases belong to the a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS) family, comprising ADAMTS-1, ADAMTS-4, ADAMTS-5, ADAMTS-8,
ADAMTS-9 and ADAMTS-15. ADAMTS-4 and ADAMTS-5 mediate the
degradation of aggrecan. In addition, matrix metalloproteinase
(MMP)-3, MMP-9 and MMP-13 cleave the telopeptide region of type II
collagen, which significantly influences OA development. Although
the mechanisms of cartilage degradation have been widely studied,
the pathogenesis of OA is unclear (1). The etiology of OA involves
mechanical, biochemical and genetic factors (2).
Vascular endothelial growth factor (VEGF) is an
influential angiogenic factor and a crucial regulator of
angiogenesis during skeletal development and bone remodeling. A
number of previous studies have reported on the possible role of
VEGF in OA and have demonstrated that OA chondrocytes produce VEGF
and VEGF receptor (3–5), and it is hypothesized that VEGF
exerts considerable influence on the pathogenesis of OA (6,7). As
previously reported, VEGF affects endochondral bone formation by
inducing endochondral angiogenesis (8), which also leads to the upregulated
expression levels of MMPs and other catabolic mediators that may
degrade the cartilage matrix and to the downregulated expression
levels of tissue inhibitors of metalloproteinases (TIMPs) in
immortalized chondrocytes (9).
VEGF expression is associated with the Mankin score and the degree
of cartilage destruction (10);
injection of VEGF into knee joints was reported to induce OA in
mice (11). Our previous study
demonstrated an increase in VEGF and VEGFR-2 mRNA expression in the
cartilage of a rabbit OA model (12).
To the best of our knowledge, no systematic study
has been conducted on the biological activity of exogenous VEGF in
articular cartilage degeneration, and the potential molecular
mechanisms are still under investigation. As a result, the present
study aimed to investigate the effects of local intra-articular
injection of exogenous VEGF and the subsequent changes in
expression levels of catabolic mediators in the cartilage of OA
model rats. The results findings of the present study revealed the
macroscopic- and microscopic-morphology changes in rat
osteoarthritis model following surgery and injection treatment. The
changes were validated and illustrated by reverse
transcription-quantitative polymerase chain reaction analysis and
western blotting. The results may provide new ideas for the roles
of VEGF in cartilage degeneration in OA progression, and offer
valuable diagnostic indicators and therapeutic targets for the
treatment of the disease.
Materials and methods
Animals
This study was approved by the Institutional Animal
Care and Use Committee of Wuhan University (Wuhan, China) and
followed the 1996 Guide for the Care and Use of Laboratory Animals.
A total of 24 male Sprague-Dawley rats (age, 8–10 weeks; weight,
250±20 g), were obtained from the Center of Experimental Animals of
Wuhan University Medicine College (Wuhan, China). Rats were housed
under specific pathogen-free (SPF) conditions at a temperature of
20–26°C, 50±10% humidity under a 12 h light/dark cycle. The noise
is below 60 decibels, and the concentration of ammonia is below 14
mg/m3. Ventilation is greater than or equal to 15 times
per hour. Then rats were provided access to conventional chow and
tap water ad libitum. With the aim to minimize suffering, all rats
were anesthetized with ketamine (30 mg/kg) intraperitoneally,
maintained with 1% isoflurane and placed on a homoeothermic table
to retain a body temperature of 37°C during surgery.
Materials
Recombinant murine VEGF165 was purchased from
PeproTech (Rocky Hill, NJ, USA) and dissolved in normal saline (NS;
Baxter, Shanghai, China) to reach a final concentration of 0.1
mg/ml. Mouse anti-collagen II monoclonal antibody (sc-52658) and
rabbit anti-aggrecan polyclonal antibody (sc-166951) were purchased
from Santa Cruz Biotechnology. Inc. (Dallas, TX, USA); rabbit
anti-MMP-3 polyclonal antibody (ab53015), rabbit anti-MMP-9
monoclonal antibody (ab76003) and rabbit anti-ADAMTS-4 polyclonal
antibody (ab185722) were purchased from Abcam (Cambridge, MA, USA);
and mouse anti-GAPDH monoclonal antibody (60004) was purchased from
ProteinTech Group, Inc. (Chicago, IL, USA).
Experimental design
Rats were randomly assigned to one of three groups
(n=8/group): i) Control group; ii) NS-injected group; and iii)
VEGF-injected group. Prior to surgery, animals in NS- and
VEGF-treated groups were anesthetized with ketamine (30 mg/kg body
weight), and thereafter maintained with 1% isoflurane. Animals were
placed on a homoeothermic table to maintain the body temperature at
37°C, and bilateral anterior cruciate ligament (ACL) transection
was conducted in SPF conditions. Briefly, medial arthrotomy was
performed using a medial parapatellar approach that incised the
skin. Following dislocation of the patella and positioning the knee
in full flexion, the ACL was visualized and carefully transected
without damaging the articular cartilage. The knee was irrigated
with NS, followed by separate closure of the capsule and skin.
At 4 weeks post-surgery, rats in the NS and the VEGF
groups were anesthetized as aforementioned, the knees were shaved
to expose the patellar ligament and access from the lateral side of
the knee was visualized using a microsyringe. A 100 µl solution
containing either 9 mg/ml NS or 0.1 mg/ml VEGF was injected into
the intra-articular space beneath the patellar ligament through the
microinjection needle. This injection was performed every week for
a total of four weeks in the NS and the VEGF groups. The rats were
maintained under SPF conditions for an addition four weeks without
any disposal. Conversely, rats in the Control group did not undergo
any surgical procedures nor received any treatment. At the
termination of the study, 12 weeks post-surgery, all the animals
were sacrificed with an overdose injection of pentobarbital sodium
(200 mg/kg). Subsequently, both knee joints of each rat were
excised and fixed in 4% paraformaldehyde, or immediately frozen and
stored at −80°C until subsequent analyses.
Macroscopic morphological
assessment
Animals were sacrificed 12 weeks post-surgery as
aforementioned. Blinded assessment of macroscopic cartilage injury
was conducted, and the degree of cartilage injury was evaluated
according to a well-defined semi-quantitative grading table
comprising a five-grade scale: 0, surface smooth with normal color;
1, surface rough with minimal fibrillation or a slight yellowish
discoloration; 2, cartilage erosion extending into the superficial
or middle layers; 3, cartilage erosion extending into the deep
layers; 4, complete cartilage erosion with subchondral bone exposed
(13).
Microscopic morphology
Following evaluation of macroscopic morphology, knee
joint tissues were excised and fixed in neutral buffered solution
(4% formaldehyde in 0.1 M PBS pH 7.4) for 48 h, followed by
decalcification in 10% EDTA for 3–4 weeks at room temperature,
dehydration in graded ethanol and embedding in paraffin. Paraffin
sections (5 µm) were mounted on glass slides and stained with
hematoxylin and eosin (H&E) for general morphological
evaluation. Tissue sections were also stained with Safranin-O and
Fast Green at a concentration of 0.1% to illustrate sulfated
proteoglycans. For either staining method, two sections from each
animal in all groups were used. The stained slides were analyzed
using a computerized morphometric system (cellSens, Olympus
Corporation, Tokyo, Japan) connected to OLYMPUS BX53 upright
microscope; ≥10 fields of view per slide were analyzed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cartilage tissues from the medial femoral condyle
were harvested from rats in each group and frozen in liquid
nitrogen. The samples were ground into a powder in liquid nitrogen
based on the hand milling technique. Total RNA was extracted using
the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol; RNA
samples were quantitated at an absorbance of A260. Subsequently,
RNA was reverse-transcribed to cDNA using an PrimeScript One Step
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol; cDNA was used immediately
or stored at −20°C. qPCR was conducted using the SYBR Green
Realtime PCR Master Mix (Toyobo Life Science, Osaka, Japan) and an
ABI 7900HT Fast Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.); primer sequences are listed in Table I. The final volume of the qPCR
reaction was 10 µl, which comprised 2X SYBR Premix Ex Taq (5 µl),
primer solution (0.2 µl; 10 µmol/l), 50X ROX Reference Dye (0.2
µl), cDNA template (1 µl), adjusted to 10 µl with distilled water.
qPCR thermocycling conditions included an initial denaturing step
at 95°C (10 sec) followed by 40 cycles of 95°C (5 sec), 60°C (30
sec) and 72°C (30 sec). A standard curve was established using
5-fold decrements of synthesized oligonucleotides that resembled
cDNA fragments as a template. GAPDH expression was used as an
endogenous control and to normalize target gene expression, from
which the fold-change in gene expression level was determined.
Specificity of each reaction was controlled by melting curve
analysis. Data were analyzed by 2−ΔΔCq quantitation
method (14). A blank PCR control
containing water rather than cDNA was also carried out. RT-qPCR was
conducted on three independent biological replicates.
 | Table I.Gene-specific primer sequences. |
Table I.
Gene-specific primer sequences.
| Genes | Sequence (5′ →
3′) |
|---|
| MMP-3 | F:
GGCCATCTCTTCCTTCAG |
|
| R:
GTCACTTTCTTTGCATTTGG |
| MMP-9 | F:
CTTCTGGCGTGTGAGTTTCC |
|
| R:
GCACGGTTGAAGCAAAGA |
| MMP-13 | F:
CCTGGACAAGTAGTTCCAAAGG |
|
| R:
AGGGATAAGGAAGGGTCACAT |
| ADAMTS-4 | F:
GCAACGTCAAGGCTCCTCTT |
|
| R:
CTCCACAAATCTACTCAGTG |
| ADAMTS-5 | F:
CCTGCCCACCCAATGGTAAATC |
|
| R:
CGGCCTACATTCAGTGCCATC |
| Type III
collagen | F:
ATGGTGGCTTTCAGTTCACC |
|
| R:
TGGGGTTTCAGAGAGTTTGG |
| TGF-β1 | F:
TGAGTGGCTGTCTTTTGACG |
|
| R:
GTITGGGACTGATCCCATTG |
| ADAM-12 | F:
GCTGATGAAGTTGTCAGTGC |
|
| R:
GAGACTGACTGCTGAATCAG |
| GAPDH | F:
ATCACTGCCACCCAGAAGAC |
|
| R:
ATGAGGTCCACCACCCTGTT |
Western blotting
Protein was extracted from cartilage tissues using
the M-PER Mammalian Protein Extraction Reagent (Pierce; Thermo
Fisher Scientific, Inc.) and the Protease Inhibitor Cocktail Set
III (EMD Millipore, Billerica, MA, USA) with 5 mmol/l EDTA. A total
of 20 µg protein [quantitated by a Bicinchoninic Acid Protein Assay
kit (Beyotime Institute of Biotechnology, Haimen, China)] was
separated by 10% SDS-PAGE and subsequently transferred to a
polyvinylidene difluoride membrane. The membranes were blocked with
5% non-fat milk in TBS containing 0.05% Tween-20 (TBST) for 1 h at
room temperature, and incubated with primary antibodies (1:1,000)
in TBST overnight at 4°C. The membranes were washed three times
with TBST and incubated with horseradish peroxidase-labeled goat
anti-mouse immunoglobulin G (IgG; 1:10,000; A0216, Beyotime
Institute of Biotechnology) or goat anti-rabbit IgG (1:10,000,
A0208, Beyotime Institute of Biotechnology) in TBST for 1 h at room
temperature. Protein bands were visualized with an Enhanced
Chemiluminescence System kit (Pierce; Thermo Fisher Scientific,
Inc.) and densitometric analysis was performed with ImageJ software
(v1.48u, National Institutes of Health, Bethesda, MD, USA). The
GAPDH from the cartilage tissues were applied as endogenous control
and the relative expression levels of the target gene mRNA were
calculated based on the determination of its copy numbers. The
associated fold changes were determined, with the value set equal
to one for each experiment; all tests were repeated in
triplicate.
Statistical analysis
All values were expressed as the mean ± standard
deviation of at least three independent experiments. SPSS 17.0
software (SPSS Inc., Chicago, IL, USA) was used to conduct
statistical analyses, and significant differences were analyzed by
one-way analysis of variance followed by Student-Newman-Keuls post
hoc q-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
VEGF increases articular cartilage
injury
To evaluate the extent of articular cartilage
injury, pathological changes of the femoral condylar cartilage were
examined macroscopically, and the degree of damage was evaluated
and scored according to a semi-quantitative five-grade scale. The
condylar cartilage of rats in the Control group was macroscopically
normal, without any signs of destruction (Fig. 1A). Conversely, different degrees of
degenerative lesions of the cartilage, such as ulceration, erosion,
fibrocartilage proliferation and osteophyte formation, were
observed in NS-injected and in VEGF-injected OA model rats
(Fig. 1B and C, respectively).
However, the extent and grade of cartilage damage in the
VEGF-treated group were obvious and more severe than those in the
NS-treated group. The 16 condyles examined from the 8 rats in
Control group presented at Grade 0 (surface smooth with normal
color). All 16 specimens within each of the NS and VEGF groups
exhibited complete transection of the ACL, which indicated
successful establishment the OA model. In the NS group, 2 out of 16
condyles were classified as Grade 1 (surface rough with minimal
fibrillation or a slight yellowish discoloration), whereas none of
the VEGF-treated rats presented Grade 1. In addition, 6 out of 16
rats in the NS group and 3 out of 16 rats in the VEGF group were
classified as Grade 2; 5 out of 16 rats in the NS group and 6 out
of 16 in the VEGF group were Grade 3 (cartilage ulceration
extending into the deep layers); and 3 out of 16 in the NS group
and 7 out of 16 in the VEGF group were Grade 4 (cartilage depletion
with subchondral bone exposed). The statistical frequency of
distribution of grade classifications in the different groups were
calculated (Fig. 1D; Table II), and the average scores of the
NS and VEGF injection groups were 2.56 and 3.25, respectively.
 | Table II.Classification of condyles in three
groups. |
Table II.
Classification of condyles in three
groups.
|
| Grade |
|
|---|
|
|
|
|
|---|
| Group (n) | 0 | 1 | 2 | 3 | 4 | Meana |
|---|
| Control (16) | 16 | 0 | 0 | 0 | 0 | 0 |
| NS (16) | 0 | 2 | 6 | 5 | 3 | 2.56 |
| VEGF (16) | 0 | 0 | 3 | 6 | 7 | 3.25 |
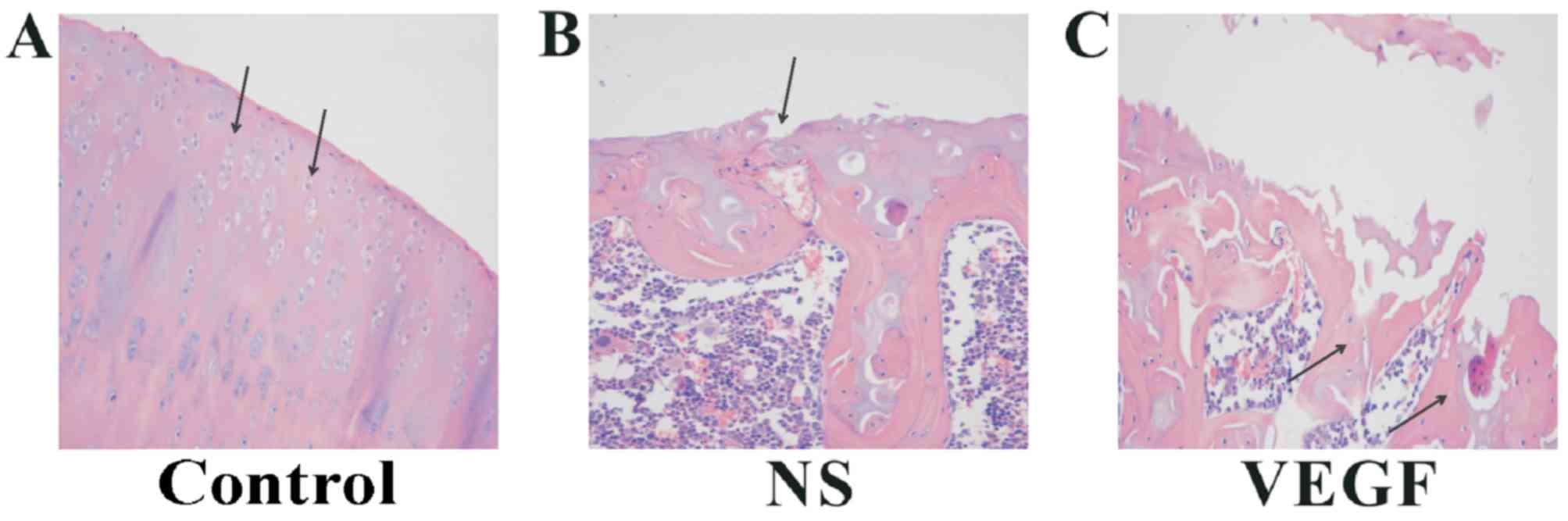
VEGF induces structural component loss
in articular cartilage
A series of microscopic morphology tests were
conducted to examine the effects of VEGF on articular cartilage in
the OA rat model. H&E staining was used to assess general
morphology, and the condylar cartilage of rats in the Control group
was healthy, as indicated by the clear and ordered arrangement of
the transitional layer, the surface layer the calcified layer and
the radiation layer, as well as round, regularly distributed and
evenly dyed chondrocytes (Fig.
2A). Cartilage tissues exhibited obvious degenerated changes in
the NS and VEGF-injected group (Fig.
2B and C, respectively). In particular, the layer structure is
absent, and fibrillations and fissures were observed in most the
examined cartilage samples. At the cellular level, chondrocytes
were irregularly distributed, swollen, and uneven staining. Notable
differences in H&E staining were observed among these groups,
and more severe injury to cartilage structures were noted in the
VEGF-injected group compared with rats in the NS and Control
groups. In the VEGF-injected group, there was a notable reduction
in average thickness, including calcified cartilage layers,
subchondral bone, as well as the total joint thickness. Notably,
vessels invaded the tidemark in NS-injected and in VEGF-injected
groups, but not in normal joints.
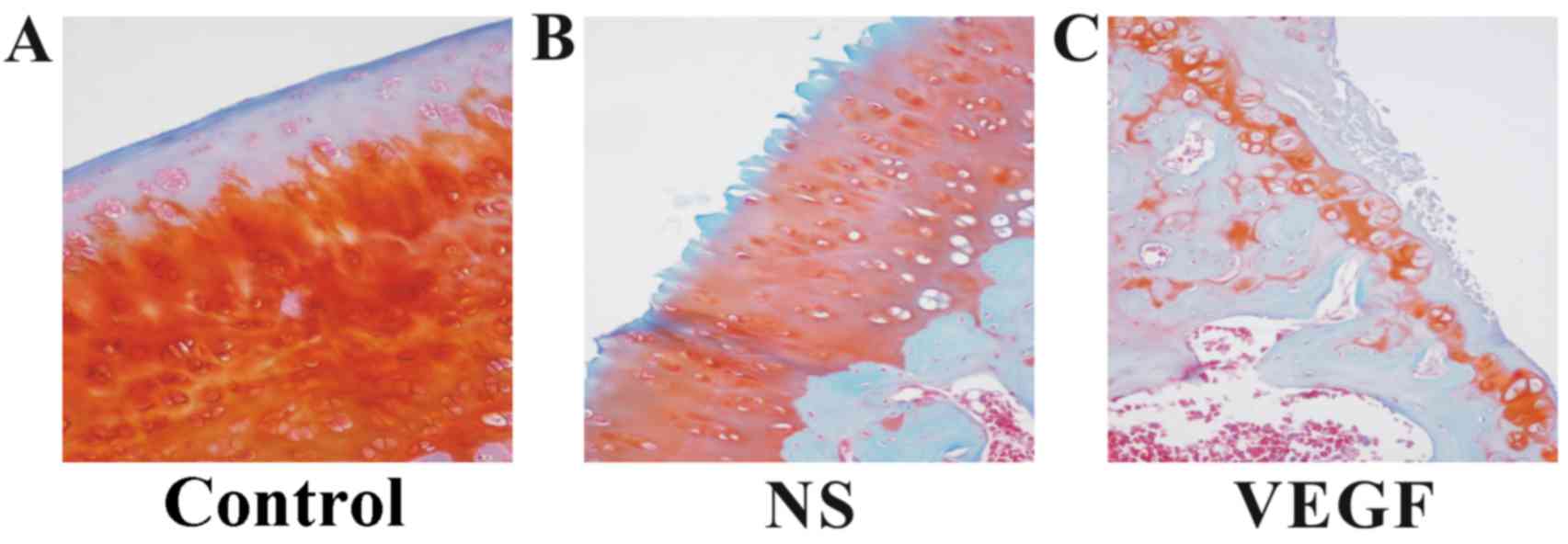
In addition, Safranin-O/Fast Green staining
indicated a loss of Safranin-O stain in the cartilage tissues from
rats in the NS-treatment group and in the VEGF-treatment group
compared with the Control group (Fig.
3). The number of chondrocytes labeled with Safranin-O/Fast
Green was notably different in the three groups. Similar to the
results from H&E staining, more severe injuries to the
cartilage structures were observed in the VEGF-injected group
compared with the NS-injected and the Control groups.
Results from these two staining methods indicated
that more severe lesions were observed in the condylar cartilage
from rats in the VEGF-injected group compare with rats in the NS
and Control groups, which was mostly due to a decline in
chondrocyte number as well as the loss of structural
components.
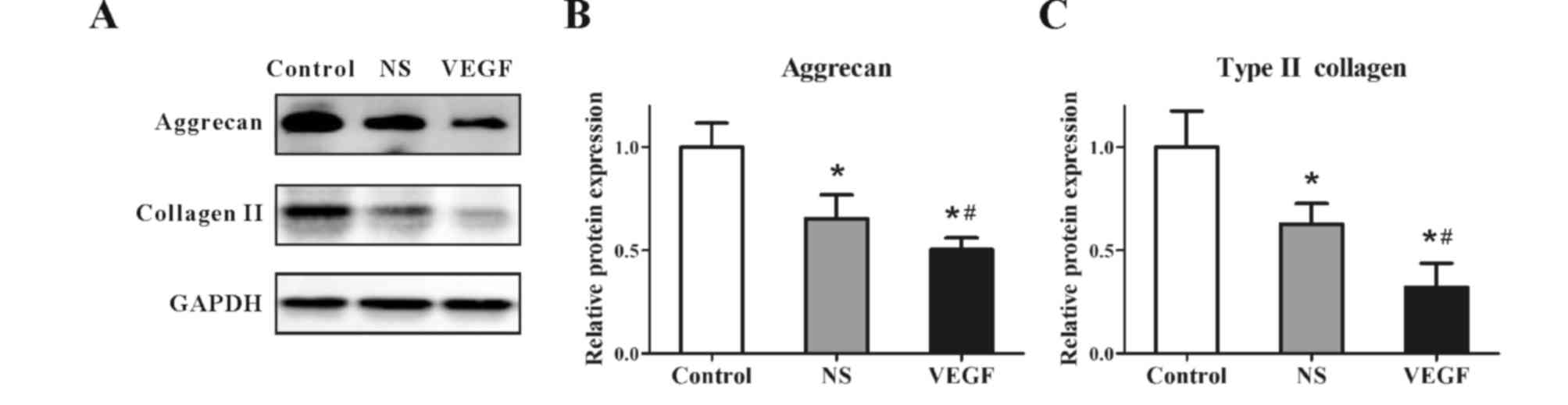
VEGF downregulates aggrecan and type
II collagen expression
Protein expression levels of aggrecan and type II
collagen were examined by western blotting (Fig. 4A). GADPH was used as an internal
reference, and densitometric analyses of the bands were conducted
by computerized laser densitometry, which demonstrated that the
protein expression levels of aggrecan (Fig. 4B) and type II collagen (Fig. 4C) were significantly reduced in the
NS group compared with the respective expression levels in the
Control group (0.65±0.14 vs. 1.00±0.11 and 0.61±0.09 vs. 1.00±0.16,
respectively; P<0.05). In addition, protein expression levels
were further reduced in model rats treated with VEGF compared with
rats in the NS-treated group (aggrecan at 0.48±0.05 vs. 0.65±0.14
and type II collagen at 0.32±0.11 vs. 0.61±0.09; P<0.05). These
results indicated that the protein expression levels of aggrecan
and type II collagen were significantly inhibited by VEGF injection
in the rat model of OA.
VEGF upregulates the expression of
cartilage-degradation and fibrogenic factors
In a previous study, eight cartilage-degradation and
fibrogenic factors were examined to be associated with
collagenase-mediated OA pathogenesis (15). Therefore, the present study
examined the mRNA expression levels of eight related
cartilage-degradation and fibrogenic proteins to see whether they
were influenced by exogenous VEGF in our model. RT-qPCR was used to
determine the relative mRNA expression levels of cartilage
degradation factors, including MMP-3, MMP-9, MMP-13, ADAMTS-4 and
ADAMTS-5 and of fibrogenic factors, including type III collagen,
transforming growth factor (TGF)-β1 and ADAM-12, which were
normalized to the relative mRNA expression of the housekeeping gene
GAPDH (Fig. 5). The relative mRNA
expression levels were significantly increased in the NS-injected
group compared with the Control group (MMP-3, 1.46±0.11 vs.
1.00±0.04; MMP-9, 1.52±0.11 vs. 1.00±0.11; MMP-13, 2.75±0.63 vs.
1.00±0.25; ADAMTS-4, 1.47±0.09 vs. 1.00±0.10; type III collagen,
2.35±0.55 vs. 1.00±0.29; TGF-β1, 1.34±0.23 vs. 1.00±0.19; and
ADAM-12, 2.22±0.47 vs. 1.00±0.13; all P<0.05), whereas no
significant difference was identified for the mRNA expression
levels of ADAMTS-5 between the NS-treated group and the untreated
Control group (1.14±0.11 vs. 1.00±0.12; P>0.05). There was a
significant increase in the mRNA expression levels in the
VEGF-injected group compared with the NS-injected group (MMP-3,
2.38±0.42 vs. 1.46±0.11; MMP-9, 3.08±0.38 vs. 1.52±0.11; MMP-13,
5.50±1.13 vs. 2.75±0.63; ADAMTS-4, 3.43±0.75 vs. 1.47±0.09;
ADAMTS-5, 1.39±0.18 vs. 1.14±0.11; type III collagen, 3.62±0.57 vs.
2.35±0.55; TGF-β1, 1.88±0.31 vs. 1.34±0.23; and ADAM-12, 4.67±1.01
vs. 2.22±0.47; all P<0.05). The data demonstrated that the mRNA
expression levels of these eight genes were significantly promoted
by VEGF injection in OA model rats.
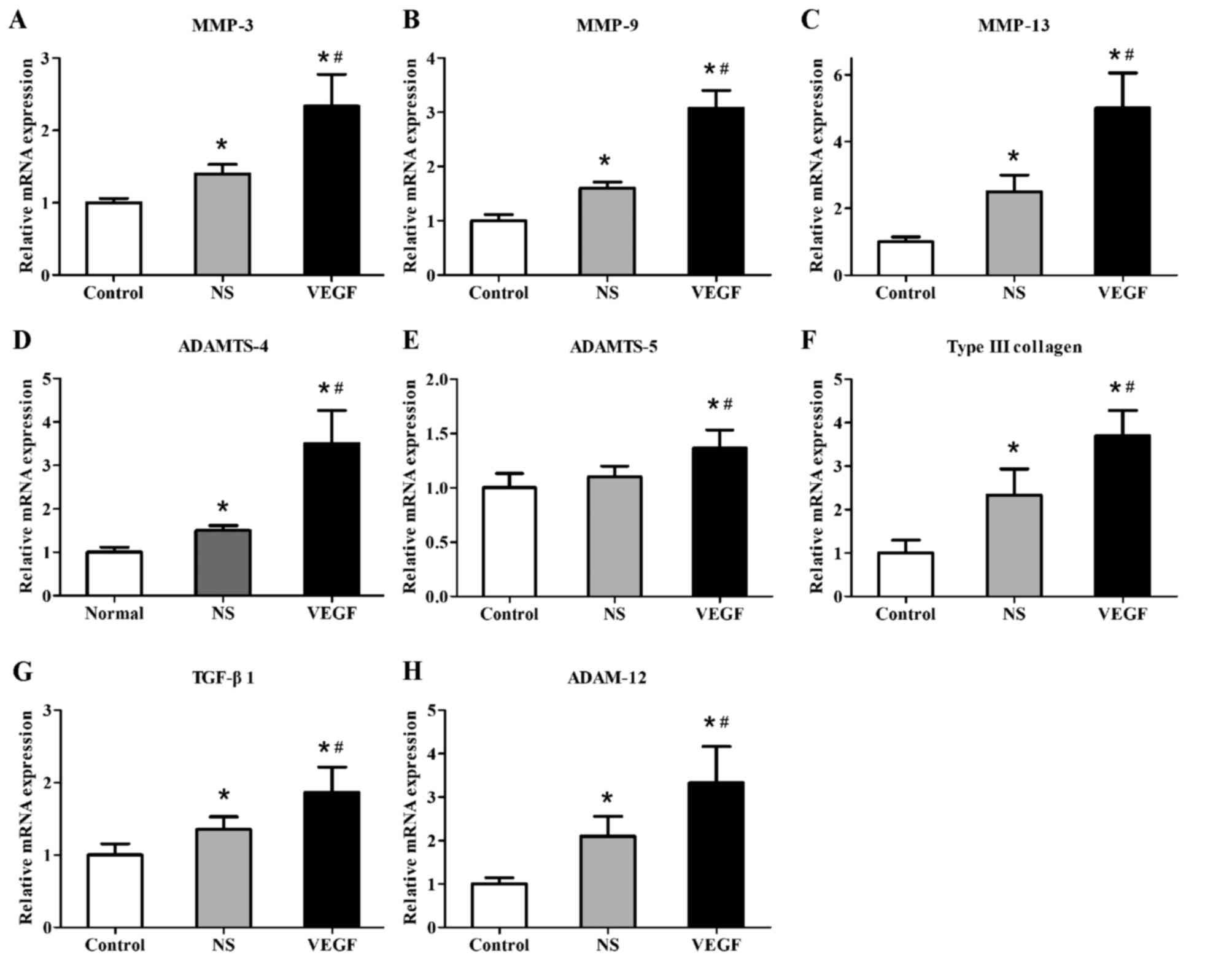 | Figure 5.mRNA expression levels of cartilage
degradation factors and fibrogenic factors in rat condylar
cartilage at 12 weeks following anterior cruciate ligament
transection. Reverse transcription-quantitative polymerase chain
reaction was used to detect the mRNA expression levels of cartilage
degradation factors (A) MMP-3, (B) MMP-9, (C) MMP-13, (D) ADAMTS-4
and (E) ADAMTS-5, and the expression levels of fibrogenic factors
(F) type III collagen, (G) TGF-β1 and (H) ADAM-12. Data are
presented as the mean ± standard deviation; *P<0.05 vs. Control;
#P<0.05 vs. NS. ADAMTS, a disintegrin and
metalloproteinase with thrombospondin motifs; MMP, matrix
metalloproteinase; NS, normal saline; TGF, transforming growth
factor; VEGF, vascular endothelial growth factor. |
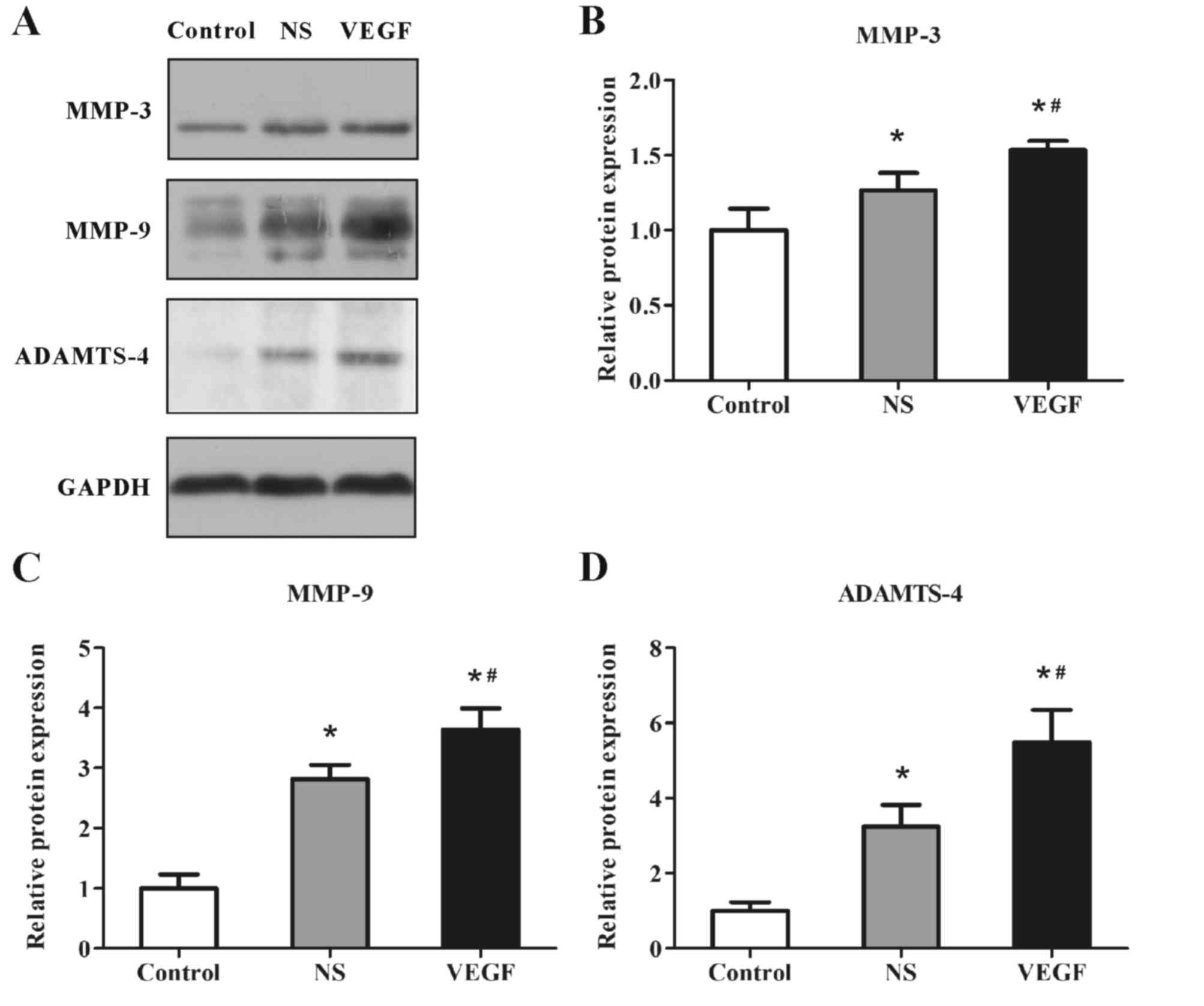
To further validate the influence of VEGF on these
deleterious factors at the translation level, MMP-3, MMP-9 and
ADAMTS-4 were selected as the representatives, since the three
proteins have been greatly studied as representative cartilage
degradation-associated factors (16–18),
and protein expression levels were examined by western blotting
(Fig. 6A); GADPH was used as an
internal reference and densitometric analyses of the bands were
conducted by computerized laser densitometry. The results revealed
that the protein expression levels of MMP-3 (Fig. 6B), MMP-9 (Fig. 6C) and ADAMTS-4 (Fig. 6D) were significantly increased in
the NS group compared with expression levels in the Control group
(MMP-3, 1.24±0.10 vs. 1.00±0.13; MMP-9, 2.71±0.16 vs. 1.00±0.17;
ADAMTS-4, 3.50±0.55 vs. 1.00±0.19; all P<0.05). The expression
levels of these proteins were further enhanced in the VEGF-injected
group compared with the NS group (MMP-3, 1.55±0.06 vs. 1.24±0.10;
MMP-9, 3.43±0.28 vs. 2.71±0.16; ADAMTS-4, 5.75±1.05 vs. 3.50±0.55;
all P<0.05). Consistent with the mRNA expression results, the
protein expression levels of MMP-3, MMP-9 and ADAMTS-4 from
cartilage tissue were significantly promoted by VEGF injection in
OA model rats.
Discussion
OA is a degenerative joint lesion that progresses to
the articular cartilage and causes intra-articular inflammation, as
characterized by synovitis (8).
The pathogenesis and pathophysiology of OA remain unclear, which
creates obstacles in studying the development and progression of OA
(9). Numerous studies have
reported on the involvement of VEGF in the degenerative progression
of cartilage (19–21). Angiogenic inhibitors are produced
by normal articular cartilage, which, consequently, lack vascular
tissue (4). VEGF is a well-studied
angiogenic factor that serves an important role in skeletal bone
growth and remodeling by regulating angiogenesis, and is
hypothesized to be involved in pathogenesis of OA (22). Neve's research suggested the
involvement of osteoblast-derived VEGF in the pathogenesis of bone
diseases (23). Hence, the present
study speculated that VEGF may be a potent biomarker for diagnosis
and a potential therapeutic target of OA.
Previous studies have reported an elevated
expression level of VEGF in osteoarthritic cartilage compared with
the normal tissue, which had a direct association with the Mankin
score for OA (3,5,10).
Our previous studies demonstrated a strong association between the
levels of VEGF expression and OA pathogenesis (12,24,25).
VEGF is a proangiogenic factor in numerous tissues and affects
angiogenesis associated with cartilage (8). Its expression is regulated by
interleukin (IL)-1β, hypoxia-inducible factor-1α, tumor necrosis
factor (TNF)-α and IL-6, as well as growth factors under
inflammatory environment associated with OA (26–28).
Articular cartilage, of hyaline nature, comprises
ECM and highly differentiated chondrocytes (29). ECM is made of type II collagen and
aggrecan, the former of which is major structural component,
constituting 30–60% of the dry weight of the cartilage ECM.
Collagen fibrils interact with other components of ECM, especially
aggrecans in articular cartilage (30). Aggrecans expanded due to water and
alter their structure formation to confront compressive force.
Hence, a lack of type II collagen as well as aggrecan may induce
articular cartilage degeneration. MMP-3 is demonstrated to involve
in the cleavage of telopeptide region of type II collagen. Besides,
ADAMTS-4, a type of aggrecanase, is capable to induce aggrecan
degradation. Altogether, these two proteins interplay with OA
development.
MMP expression levels are enhanced, whereas the
secretion of TIMPs is blocked by VEGF in immortalized chondrocytes
(9). In addition, the expression
of IL-6, IL-1β, nitric oxide, and TNF-α were reported to be induced
by VEGF, and subsequently stimulate the proliferation of
chondrocytes, which may lead to accelerative articular cartilage
degeneration (9). VEGF production,
in turn, is activated by TNF-α in osteoarthritic chondrocytes
(31). OA in the temporomandibular
joint was reported to be induced by VEGF, which destroyed
subchondral bone and cartilage (32). In a mouse model, OA was induced
following VEGF injection into knee joints (11). Articular cartilage degeneration in
OA model rats was delayed following local injections of the
anti-VEGF monoclonal antibody bevacizumab (22).
Collectively, VEGF may be considered as a promising
biomarker to assess disease severity in osteoarthritis, and may be
the basis for targeted treatments in the future; however, further
investigation regarding the detailed mechanism is required.
However, it was not clear if exogenous VEGF served a
role in joint degeneration. The expression of cartilage-degradation
and fibrogenic factors as well as histological changes of
osteoarthritic joint tissues were assessed by the present study
following injections of exogenous VEGF. The results suggested that
exogenous VEGF may accelerate OA progression: Cartilage degradation
was more severe in rats in the VEGF-injected group compared with
the NS-injected group, as defined by erosive cartilage, deep
fibrillation and increased loss of Safranin O.
Similar to a previous report (24), results from the present study
suggested that exogenous VEGF may induce degeneration of articular
cartilage by blocking the synthesis of and inhibiting the
expression of type II collagen and aggrecan. In immortalized
chondrocytes, MMPs were reported to be upregulated, whereas TIMPs
were downregulated by VEGF (9). To
the best of our knowledge, the present study was the first to
reveal the effects of locally injected, exogenous VEGF on the
expression levels of ADAMTS-4, MMP-3 and MMP-9 in OA joints. The
results demonstrated that mRNA and protein expression levels of
ADAMTS-4, MMP-3 and MMP-9 were elevated in VEGF-injected and
NS-injected OA model rats, and these increased in expression levels
were highest in the VEGF-injected group. These data suggested a
potential role for ADAMTS-4, MMP-3 and MMP-9 in cartilage
degeneration in response to exogenous VEGF, which may degrade type
II collagen and aggrecan as well. In addition, MMP-3 was involved
in pathological degradation of collagen and MMP-1 activation
(33). Increases in MMP-3 and
MMP-9 expression levels have been reported to be related with
decreasedTIMP-1 and TIMP-2 (9).
The role of VEGF in articular cartilage destruction has been
confirmed by various studies, which, induces ADAMTS-4 (15), ADAMTS-5 (34) and MMPs expressions (32) in return, and degrades major ECM
molecules, such as type II collagen (24), aggrecan (22). Type III collagen, TGF-β1 and
ADMS-12 have been associated with synovial fibrosis in OA knees
(15). However, little is known
about these fibrosis-related proteins in the progression of
cartilage degeneration. In the present study, the increased
expression levels of type III collagen, TGF-β1 and ADAM-12 have
positive association with the severity of cartilage degeneration as
demonstrated by macroscopic-morphological assessments and analyses
of mRNA expression levels. It remains to be elucidated whether
these proteins are involved in cartilage degeneration through
fibrosis or through some other mechanism.
In conclusion, the present study demonstrated the
effects of VEGF in OA progression through cartilage degeneration,
which indicated that VEGF may be a valuable candidate as a
diagnostic biomarker as well as therapeutic target. Additional
studies are warranted to reveal the mechanism of OA.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (grant no. 81301592).
Glossary
Abbreviations
Abbreviations:
|
ACL
|
anterior cruciate ligament
|
|
ADAMTS
|
a disintegrin and metalloproteinase
with thrombospondin motifs
|
|
MMP
|
matrix metalloproteinase
|
|
TGF
|
transforming growth factor
|
|
ADAM
|
a disintegrin and
metalloproteinase
|
|
OA
|
osteoarthritis
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Goldring MB: Anticytokine therapy for
osteoarthritis. Expert Opin Biol Ther. 1:817–829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang LZ, Zheng HA, Jiang Y, Tu YH, Jiang
PH and Yang AL: Mechanical and biologic link between cartilage and
subchondral bone in osteoarthritis. Arthritis Care Res. 64:960–967.
2012. View Article : Google Scholar
|
|
3
|
Pufe T, Petersen W, Tillmann B and
Mentlein R: The splice variants VEGF121 and VEGF189 of the
angiogenic peptide vascular endothelial growth factor are expressed
in osteoarthritic cartilage. Arthritis Rheum. 44:1082–1088. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horner A, Bord S, Kelsall AW, Coleman N
and Compston JE: Tie2 ligands angiopoietin-1 and angiopoietin-2 are
coexpressed with vascular endothelial cell growth factor in growing
human bone. Bone. 28:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfander D, Kortje D, Zimmermann R, Weseloh
G, Kirsch T, Gesslein M, Cramer T and Swoboda B: Vascular
endothelial growth factor in articular cartilage of healthy and
osteoarthritic human knee joints. Ann Rheum Dis. 60:1070–1073.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue K, Masuko-Hongo K, Okamoto M and
Nishioka K: Induction of vascular endothelial growth factor and
matrix metalloproteinase-3 (stromelysin) by interleukin-1 in human
articular chondrocytes and synoviocytes. Rheum Int. 26:93–98. 2005.
View Article : Google Scholar
|
|
7
|
Kim WU, Kang SS, Yoo SA, Hong KH, Bae DG,
Lee MS, Hong SW, Chae CB and Cho CS: Interaction of vascular
endothelial growth factor 165 with neuropilin-1 protects rheumatoid
synoviocytes from apoptotic death by regulating Bcl-2 expression
and Bax translocation. J Immunol. 177:5727–5735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerber HP, Vu TH, Ryan AM, Kowalski J,
Werb Z and Ferrara N: VEGF couples hypertrophic cartilage
remodeling, ossification and angiogenesis during endochondral bone
formation. Nat Med. 5:623–628. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pufe T, Harde V, Petersen W, Goldring MB,
Tillmann B and Mentlein R: Vascular endothelial growth factor
(VEGF) induces matrix metalloproteinase expression in immortalized
chondrocytes. J Pathol. 202:367–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enomoto H, Inoki I, Komiya K, Shiomi T,
Ikeda E, Obata K, Matsumoto H, Toyama Y and Okada Y: Vascular
endothelial growth factor isoforms and their receptors are
expressed in human osteoarthritic cartilage. Am J Pathol.
162:171–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ludin A, Sela JJ, Schroeder A, Samuni Y,
Nitzan DW and Amir G: Injection of vascular endothelial growth
factor into knee joints induces osteoarthritis in mice.
Osteoarthritis Cartilage. 21:491–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou JL, Liu SQ, Qiu B, Hu QJ, Ming JH and
Peng H: Effects of hyaluronan on vascular endothelial growth factor
and receptor-2 expression in a rabbit osteoarthritis model. J
Orthop Sci. 14:313–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Zhang Y, Chen C, Zhang H, Ma C and
Xia Y: Establishment of a rabbit model to study the influence of
advanced glycation end products accumulation on osteoarthritis and
the protective effect of pioglitazone. Osteoarthritis Cartilage.
24:307–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ko JY, Lee MS, Lian WS, Weng WT, Sun YC,
Chen YS and Wang FS: MicroRNA-29a counteracts synovitis in knee
osteoarthritis pathogenesis by targeting VEGF. Sci Rep. 7:35842017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wimsey S, Lien CF, Sharma S, Brennan PA,
Roach HI, Harper GD and Górecki DC: Changes in immunolocalisation
of beta-dystroglycan and specific degradative enzymes in the
osteoarthritic synovium. Osteoarthritis Cartilage. 14:1181–1188.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yik JH, Hu Z, Kumari R, Christiansen BA
and Haudenschild DR: Cyclin-dependent kinase 9 inhibition protects
cartilage from the catabolic effects of proinflammatory cytokines.
Arthritis Rheumatol. 66:1537–1546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsarouhas A, Soufla G, Katonis P, Pasku D,
Vakis A and Spandidos DA: Transcript levels of major MMPs and
ADAMTS-4 in relation to the clinicopathological profile of patients
with lumbar disc herniation. Eur Spine. 20:781–790. 2011.
View Article : Google Scholar
|
|
19
|
Yu J, Liang F, Huang H, Pirttiniemi P and
Yu D: Effects of loading on chondrocyte hypoxia, HIF-1α and VEGF in
the mandibular condylar cartilage of young rats. Orthod Craniofac
Res. 2017.(Epub ahead of print).
|
|
20
|
Fay J, Varoga D, Wruck CJ, Kurz B,
Goldring MB and Pufe T: Reactive oxygen species induce expression
of vascular endothelial growth factor in chondrocytes and human
articular cartilage explants. Arthritis Res Ther. 8:R1892006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lingaraj K, Poh CK and Wang W: Vascular
endothelial growth factor (VEGF) is expressed during articular
cartilage growth and re-expressed in osteoarthritis. Ann Acad Med.
39:399–403. 2010.
|
|
22
|
Nagai T, Sato M, Kobayashi M, Yokoyama M,
Tani Y and Mochida J: Bevacizumab, an anti-vascular endothelial
growth factor antibody, inhibits osteoarthritis. Arthritis Res
Ther. 16:4272014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neve A, Cantatore FP, Corrado A, Gaudio A,
Ruggieri S and Ribatti D: In vitro and in vivo angiogenic activity
of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF
and vitamin D3 treatment. Regul Pept. 184:81–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen XY, Hao YR, Wang Z, Zhou JL, Jia QX
and Qiu B: The effect of vascular endothelial growth factor on
aggrecan and type II collagen expression in rat articular
chondrocytes. Rheumatol Int. 32:3359–3364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jian-lin Z, Hong-song F, Hao P, Shuang D,
Shen C, Jian-ping L, Bo Q, Jin-qing W and Feng L: The relationship
between HIF-2α and VEGF with radiographic severity in the primary
osteoarthritic knee. Yonsei Med J. 57:735–740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jackson JR, Minton JA, Ho ML, Wei N and
Winkler JD: Expression of vascular endothelial growth factor in
synovial fibroblasts is induced by hypoxia and interleukin 1beta. J
Rheumatol. 24:1253–1259. 1997.PubMed/NCBI
|
|
27
|
Harada S, Nagy JA, Sullivan KA, Thomas KA,
Endo N, Rodan GA and Rodan SB: Induction of vascular endothelial
growth factor expression by prostaglandin E2 and E1 in osteoblasts.
J Clin Invest. 93:2490–2496. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Distler JH, Wenger RH, Gassmann M,
Kurowska M, Hirth A, Gay S and Distler O: Physiologic responses to
hypoxia and implications for hypoxia-inducible factors in the
pathogenesis of rheumatoid arthritis. Arthritis Rheum. 50:10–23.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van der Rest M and Garrone R: Collagen
family of proteins. FASEB J. 5:2814–2823. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hardingham T: Proteoglycans: Their
structure, interactions and molecular organization in cartilage.
Biochem Soc Trans. 9:489–497. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Honorati MC, Cattini L and Facchini A:
VEGF production by osteoarthritic chondrocytes cultured in
micromass and stimulated by IL-17 and TNF-alpha. Connect Tissue
Res. 48:239–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen P, Jiao Z, Zheng JS, Xu WF, Zhang SY,
Qin A and Yang C: Injecting vascular endothelial growth factor into
the temporomandibular joint induces osteoarthritis in mice. Sci
Rep. 5:162442015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rose BJ and Kooyman DL: A tale of two
joints: The role of matrix metalloproteases in cartilage biology.
Dis Markers. 2016:48950502016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oh JS, Youm YS, Cho SD, Choi SW and Cho
YJ: The expression of vascular endothelial growth factor and
Syndecan-4 in cartilage from osteoarthritic knees. Bone Joint J.
96-B:1319–1324. 2014. View Article : Google Scholar : PubMed/NCBI
|