Introduction
Cerebrovascular diseases are common conditions
caused by impairments in the oxygen supply to the brain, among
which stroke is characterized by a high incidence rate, and is one
of the most common causes of morbidity and mortality worldwide
(1,2). Comorbidities associated with stroke
include visual impairments, loss of speech, paralysis and mental
disorders (3). Stroke severely
degrades the health and quality of life of surviving patients, and
it poses a heavy economic and psychological burden for the patients
and their families, and a major health concern for society
(3).
Stroke is an acute disorder caused by brain hypoxia
or ischemia, as a result of blood vessel blockage or cerebral blood
vessel rupture and subsequent hemorrhage (4). Strokes may thus be divided into
ischemic strokes and hemorrhagic strokes, with ischemic strokes
accounting for ~87% of all stroke cases. Cerebral ischemia can
rapidly cause the necrosis of cerebral tissue in the ischemic
nucleus, followed by tardive neuronal death in ischemic and
surrounding tissues, which ultimately results in brain damage
(5). The primary challenge to the
treatment of ischemic stroke is the short time window for
successful intervention; as a result, a large number of stroke
victims fail to be treated in a timely and effective manner, with
severe consequences to the restoration of brain function (6).
Disturbances in energy homeostasis are among the
main causes of cerebral ischemia/reperfusion injury (CIRI)
(7). Oxidative stress is involved
in CIRI, as the dysregulation of mitochondrial oxidative
phosphorylation leads to a decrease in ATP production and an
increase in reactive oxygen species (ROS) generation (7). In addition to the decrease in ATP
levels and the increased production of free radicals, which can
directly damage cellular components, adaptive regulation mechanisms
of CIRI also participate in the molecular mechanisms underlying the
pathology of the disorder (8).
The area of the ischemic event is composed of a
necrotic area in the infarct focus, and a surrounding ischemic
penumbra (9). Cells in the center
of the infarction are largely necrotic, whereas the ischemic
penumbra is mainly characterized by cellular apoptosis; however,
penumbral cells may be salvaged following reperfusion (9). Cellular apoptosis is a physiological
process that can be activated by internal and external factors, and
is regulated by several genes, enzymes and signal transduction
pathways (9). The
mitogen-activated protein kinase (MAPK) cascade communicates
signals from the cell surface to the nucleus and is involved in
numerous physiological cellular processes, including adaptation,
proliferation, differentiation, survival and apoptosis (10). Therefore, the implication of the
MAPK pathway in the molecular mechanisms of CIRI has garnered
considerable attention.
p38 MAPK is a critical member of the MAPK family,
which can be phosphorylated in response to various extracellular
stimuli, thus activating the MAPK signaling pathway (11). P38 MAPK has been implicated in the
regulation of inflammatory responses, and has been associated with
cellular differentiation, cell cycle progression and apoptosis
(12). During CIRI, p38/MAPK
signaling has been reported to be activated, as p38 MAPK has been
demonstrated to translocate from the cytoplasm into the nucleus,
activate downstream kinases and transcription factors, and modulate
the expression of apoptosis-associated genes, thus promoting
cellular apoptosis (13).
The extracts of the Chinese medicinal herb
Artemisia carvifolia have demonstrated diverse
pharmacological properties, including immune-regulatory,
anti-inflammatory, antitumor and anti-angiogenetic effects
(14). Artesunate
(dihydroartemisinin 1,2 α succinic acid monoester) is a
semi-synthetic sesquiterpene lactonic derivative of the
antimalarial drug artemisinin, which is one of the main active
ingredients of Artemisia carvifolia (15). Artesunate is a novel antimalarial
drug, characterized by high efficacy, fast action, low toxicity and
low tolerance (15). In addition,
artesunate has exhibited anti-tumor activity accompanied by low
toxicity and has been used successfully for the treatment of
patients with metastatic melanoma in clinical practice (15). Previous studies have reported that
artesunate increased the intracellular production of oxygen free
radicals, and intervened in nuclear factor (NF)-κΒ- and
phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt-mediated
signaling pathways, thus causing DNA damage, interfering with cell
cycle regulation, and preventing metastasis and multidrug
resistance (15–17). Therefore, the present study
investigated the effects of artesunate during CIRI and the possible
molecular mechanisms underlying its actions.
Materials and methods
Animals and experimental protocol
The experimental procedures used in the present
study were reviewed and approved by the Animal Experiment Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China). Male
adult Kunming mice (weight, 21–23 g; age, 6–7 weeks; n=30) were
purchased from the Experimental Animal Center of Cangzhou Central
Hospital and were allowed to acclimate to lab conditions for 1 week
prior to the commencement of experiments. Mice were housed at a
temperature of 21–23°C and 55–60% relative humidity, under a 12-h
light/dark cycle, with free access to food and water.
Mice were randomly assigned to the following 5
groups: Sham-operated group (n=6); CIRI model group (n=6), mice
were subjected to middle cerebral artery occlusion for 2 h,
followed by 22 h reperfusion, and treated with normal saline for 7
days; CIRI + low artesunate group (n=6), CIRI mice were treated
with 10 mg/kg/day artesunate (intraperitoneal administration,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 7 days; CIRI +
medium artesunate group (n=6), CIRI mice were treated with 20
mg/kg/day artesunate (intraperitoneal administration) for 7 days;
and CIRI + high artesunate group (n=6), CIRI mice were treated with
40 mg/kg/day artesunate (intraperitoneal administration) for 7
days.
Establishment of CIRI mouse model
Mice were anesthetized with 35 mg/kg sodium
pentobarbital. A midline incision was conducted, and the right
common, external and internal carotid arteries were exposed. The
right common and external carotid arteries were ligated using 6-0
sutures, and the internal carotid artery was subsequently occluded
using a clamp. A silicone-coated 6-0 nylon monofilament was
inserted into the internal carotid artery from a small incision on
the right common carotid artery, until the tip blocked the origin
of the middle cerebral artery. The surgical incisions were closed
with 6-0 sutures, mice were maintained at 37°C for 2 h, and the
clamp was subsequently removed for reperfusion. Subsequently, mice
were treated with artesunate.
Neurological evaluation
Following treatment with artesunate, the
neurological function of mice was examined as previously described
(18). The following grading
system was used for evaluation: 0, No neural function deficit; 1,
left forepaw weakness; 2, constantly turning left; 3, mouse falling
to the contralateral side; 4, mouse unable to walk spontaneously or
comatose.
Histological examination
Following treatment with artesunate, the brains were
isolated and sectioned into 4 mm coronal slices. To evaluate
cerebral infarct volume, the brain tissue sections were incubated
with 1% 2,3,5-triphenyl-tetrazolium chloride for 30 min at 37°C in
the dark and then fixed with 10% formalin at room temperature for
24 h. Photographs of stained brain tissue samples were captured
using a digital camera (Nikon Corporation, Tokyo, Japan) and
analyzed using ImageJ software (version 3.1; National Institutes of
Health, Bethesda, MD, USA). For histopathological examination,
hippocampi were isolated, fixed in 10% formalin at room temperature
for 24 h, embedded in paraffin and sliced into 4-µm sections.
Subsequently, tissue sections were deparaffinized and stained with
hematoxylin and eosin (H&E) at room temperature for 10–15 min
and observed with a fluorescence microscope (magnification, ×10;
Zeiss GmbH, Jena, Germany).
Evaluation of proinflammatory factors, oxidative
stress and caspase-3 activity. Blood samples were collected and the
supernatants were isolated after 2,000 × g for 10 min at 4°C to
measure the activity of interleukin (IL)-1β (cat. no. H002), IL-6
(cat. no. H007), IL-10 (cat. no. H009), tumor necrosis factor
(TNF)-α (cat. no. H052), glutathione (GSH; cat. no. A006-2) and
superoxide dismutase (SOD; cat. no. A001-2) using ELISA kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The
absorbance of the samples was measured at 450 nm using a Model 680
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ROS production was evaluated using a Reactive Oxygen Species Assay
kit (Beyotime Institute of Biotechnology, Haimen, China) with a
Model 680 microplate reader (Bio-Rad Laboratories, Inc.), using an
excitation wavelength of 488 nm and an emission wavelength of 525
nm. Caspase-3 activity was measured using a Caspase-3 Activity
Assay kit (Beyotime Institute of Biotechnology). The absorbance of
the samples was measured at 405 nm using a Model 680 microplate
reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
The hippocampus and cortex of the injured hemisphere
were isolated and homogenized in cold lysis buffer containing
phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology)
at 4°C for 30 min. The supernatants were collected following
centrifugation 12,000 × g for 10 min at 4°C and protein
concentration was measured using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology). Equal amounts of
extracted protein samples (50 µg) were separated by 10% SDS-PAGE
and transferred onto polyvinylidene difluoride membranes. Membranes
were blocked with 5% milk in TBST at room temperature for 1 h and
incubated overnight at 4°C with the following primary antibodies:
Anti-nuclear factor erythroid 2-related factor 2 (Nrf2; 1:500; cat.
no. sc-722; Santa Cruz Biotechnology, Inc.), anti-apoptosis
regulator Bcl-2 (Bcl-2; 1:500; cat. no. sc-783; Santa Cruz
Biotechnology, Inc.), anti-apoptosis regulator BAX (Bax; 1:500;
cat. no. sc-6236; Santa Cruz Biotechnology, Inc.),
anti-phosphorylated (p)-p38 MAPK (1:500; cat. no. sc-7975-R; Santa
Cruz Biotechnology, Inc.) and anti-GAPDH (1:1,000; cat. no.
sc-367714; Santa Cruz Biotechnology, Inc.). Membranes were washed
with TBS containing Tween-20 (0.1%) and then incubated with
horseradish peroxidase-conjugated anti-rabbit IgG (H+L),
Biotinylated Antibody (1:5,000; cat. no. 14708; Cell Signaling
Technology, Inc.) at 37°C for 2 h. Protein bands were visualized
using an enhanced chemiluminescence system (Beyotime Institute of
Biotechnology) and blots were semi-quantified using ImageJ software
version 3.1 (National Institutes of Health).
Statistical analysis
All data are expressed as the mean ± standard
deviation (n=3). Statistical analysis was performed with SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The statistical
significance of the differences between groups was assessed using
one-way analysis of variance with Tukey post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Treatment with artesunate reduces
cerebral infarct volume and protects neurological function in CIRI
mice
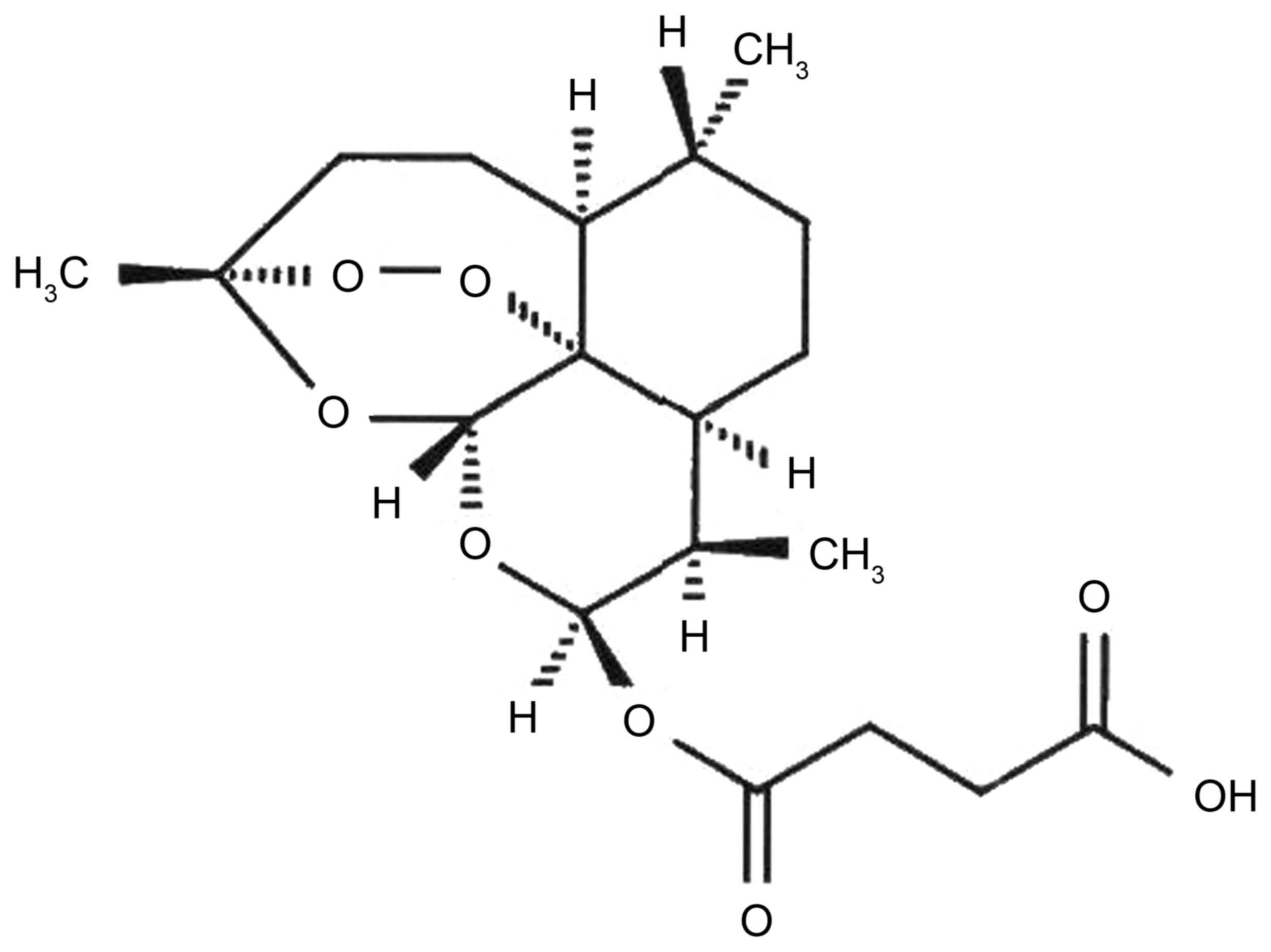
The chemical structure of artesunate is presented in
Fig. 1. The effects of artesunate
on cerebral infarct volume and its putative protective effects
against neurological function impairments were investigated in mice
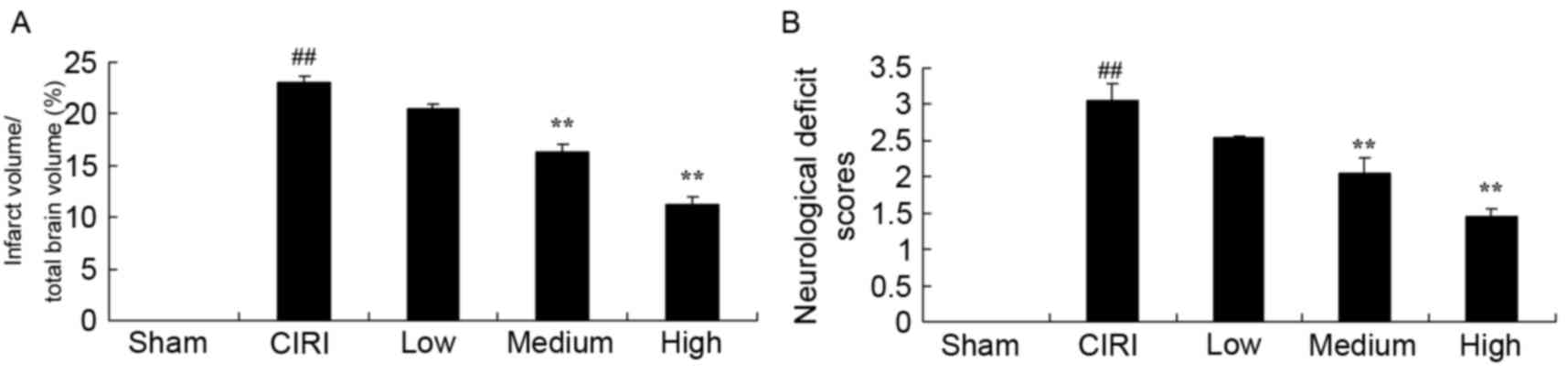
following CIRI. As demonstrated in Fig. 2A, cerebral infarct volumes in mice
from the CIRI model group were significantly larger compared with
mice from the sham group, and their neurological function was
significantly impaired (Fig. 2B).
However, following treatment with 20 or 40 mg/kg artesunate, the
cerebral infarct volume in CIRI mice was significantly reduced
compared with untreated mice from the CIRI model group (Fig. 2A). In addition, treatment with
medium and high doses of artesunate was revealed to significantly
protect mice from CIRI-associated neurological deficits (Fig. 2B).
Treatment with artesunate alleviates
cerebral histopathological alterations in CIRI mice
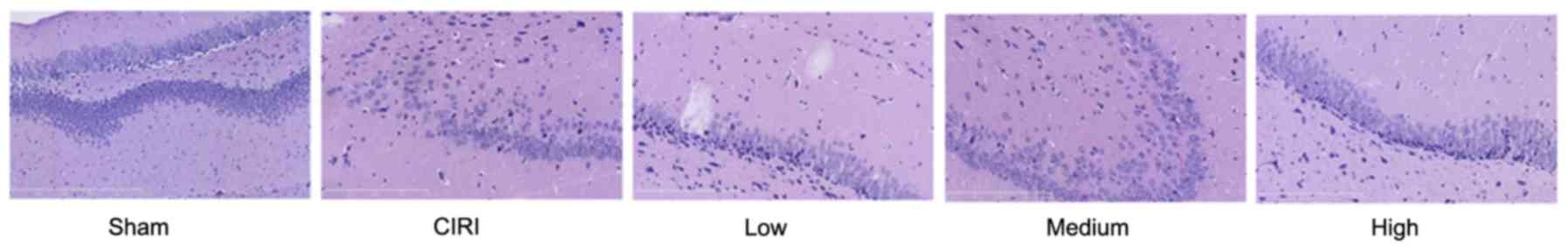
In order to investigate the putative protective
effects of artesunate against CIRI-induced histopathological
alterations in cerebral tissue, hippocampi were isolated and
stained with H&E. As presented in Fig. 3, mice from the CIRI model group
exhibited severe histological damage compared with mice from the
sham group. Treatment with 20 or 40 mg/kg artesunate was
demonstrated to markedly attenuate the CIRI-associated
histopathological alterations in hippocampal tissue compared with
tissue samples from untreated CIRI mice (Fig. 3).
Treatment with artesunate protects
endogenous antioxidant activity in CIRI mice
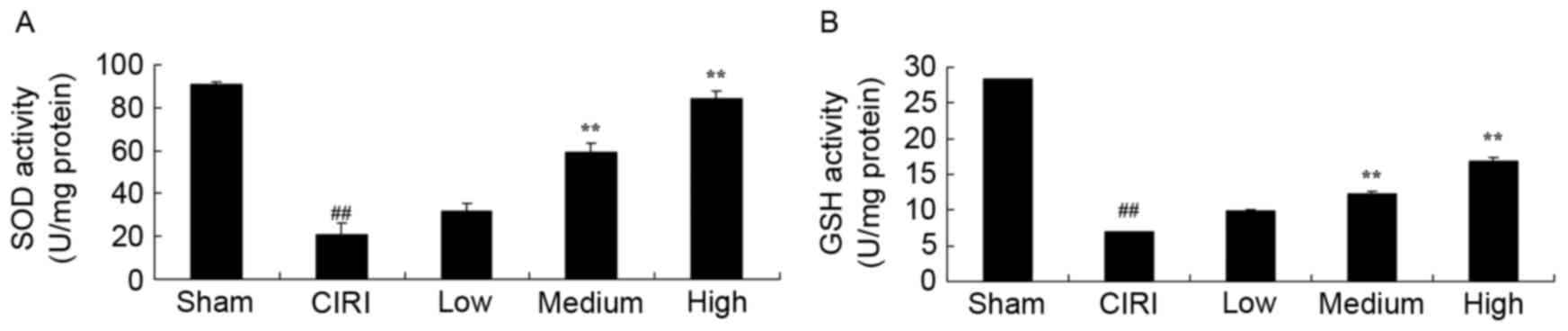
In order to investigate the antioxidative effects of
artesunate during CIRI, the activity of the endogenous antioxidants
SOD and GSH were assessed using ELISA kits. Mice from the CIRI
model group exhibited significantly reduced SOD and GSH activity
compared with mice from the sham group (Fig. 4A and B). Notably, treatment with 20
or 40 mg/kg artesunate was revealed to restore the activity of SOD
and GSH compared with untreated CIRI mice (Fig. 4A and B).
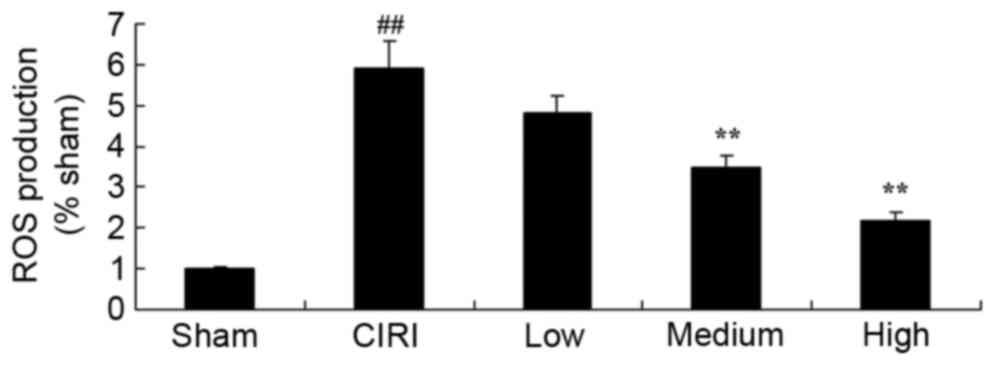
Treatment with artesunate suppresses
ROS production in CIRI mice
In order to further investigate the effects of
artesunate on oxidative stress during CIRI, ROS production was
assessed. The present results demonstrated that ROS production was
significantly potentiated in CIRI mice (Fig. 5). However, following treatment with
20 or 40 mg/kg artesunate, ROS production was significantly
suppressed compared with untreated mice from the CIRI model group
(Fig. 5).
Treatment with artesunate suppresses
inflammatory responses in CIRI mice
In order to evaluate the putative anti-inflammatory
properties of artesunate during CIRI, ELISA kits were used to
assess the activity of the proinflammatory cytokines IL-1β, IL-6
and TNF-α, and of the anti-inflammatory cytokine IL-10. In CIRI
mice, the activity of TNF-α, IL-1β and IL-6 was significantly
enhanced, whereas the activity of IL-10 was significantly
suppressed compared with in sham-operated mice (Fig. 6A-D). Following treatment with 20 or
40 mg/kg artesunate, TNF-α, IL-1β and IL-6 activity was
significantly reduced, whereas the activity of IL-10 was
significantly enhanced compared with untreated mice from the CIRI
model group (Fig. 6A-D).
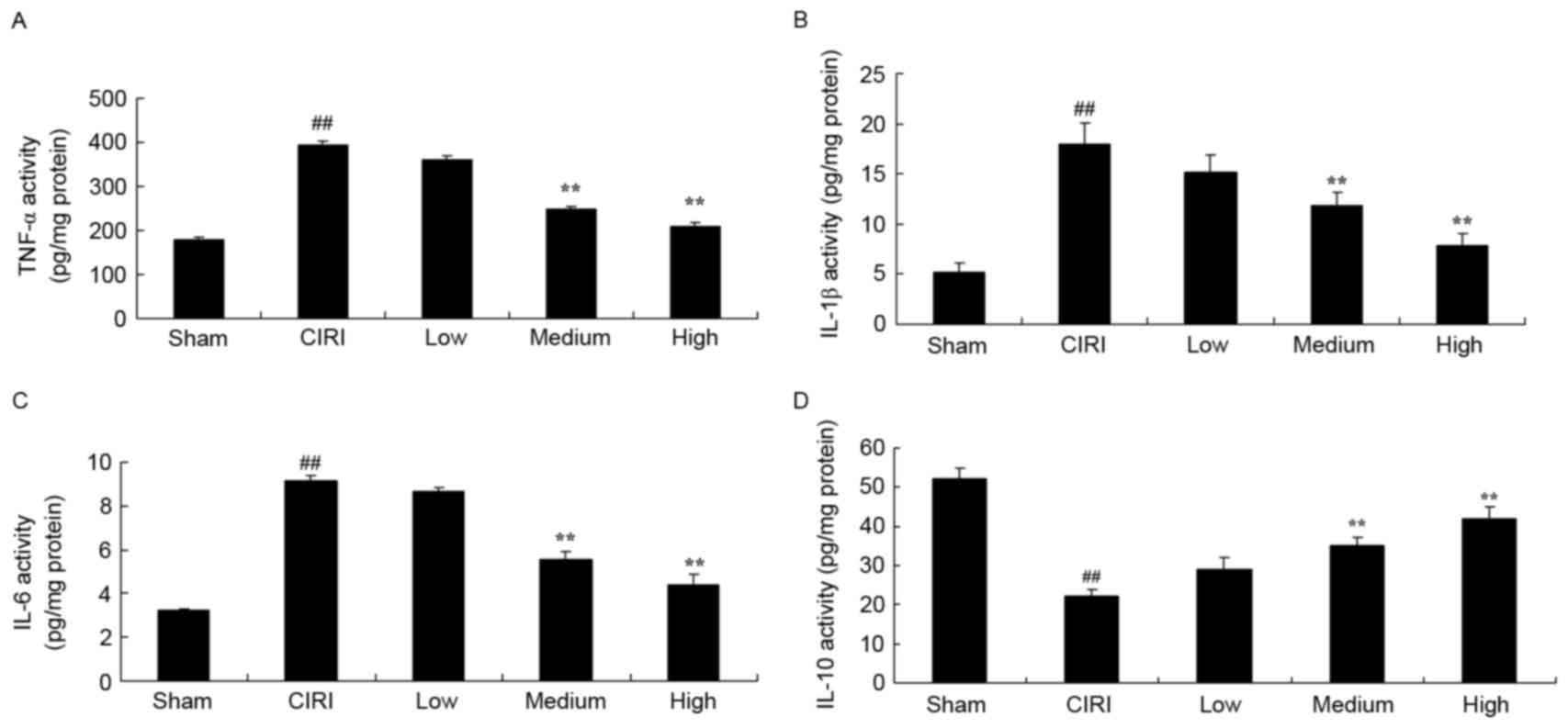 | Figure 6.Treatment with artesunate suppresses
inflammatory responses in CIRI mice. Following treatment with
artesunate the activity of (A) TNF-α, (B) IL-1β and (C) IL-6 was
inhibited, whereas the activity of (D) IL-10 was enhanced. Sham,
sham-operated group; CIRI, CIRI model group; Low, CIRI + 10 mg/kg
artesunate for 7 days group; Medium, CIRI + 20 mg/kg artesunate for
7 days group; High, CIRI + 40 mg/kg artesunate for 7 days group.
##P<0.01 vs. Sham; **P<0.01 vs. CIRI. CIRI,
cerebral ischemia/reperfusion injury; TNF, tumor necrosis factor;
IL, interleukin. |
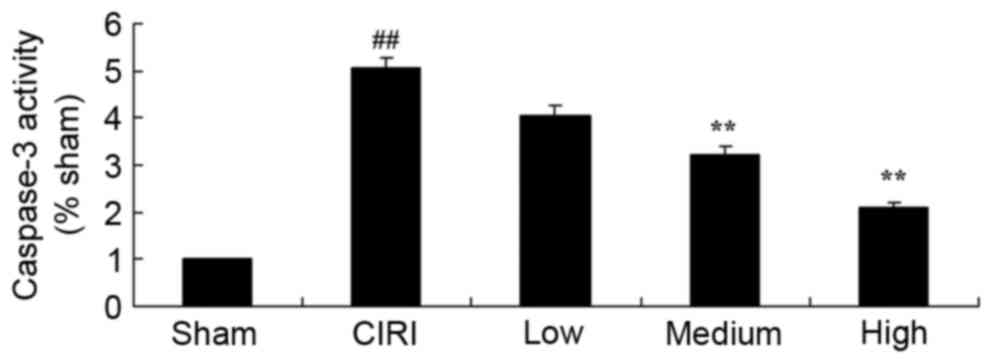
Treatment with artesunate prevents
caspase-3 activation in CIRI mice
In order to investigate the molecular mechanisms
underlying the protective effects of artesunate during CIRI, the
activity of the proapoptotic caspase-3 was assessed. As revealed in
Fig. 7, caspase-3 activity was
significantly increased in mice from the CIRI model group compared
with the sham-operated mice. Notably, following treatment with
artesunate, the activation of caspase-3 was significantly
suppressed compared with untreated CIRI mice (Fig. 7).
Treatment with artesunate restores
Nrf2 protein expression in CIRI mice
To further investigate the protective mechanisms
implicated in the effects of artesunate during CIRI, western blot
analysis was used to assess the protein expression levels of the
transcription factor Nrf2 (Fig.
8A). As demonstrated in Fig.
8B, Nrf2 protein expression was significantly downregulated in
CIRI mice compared with mice from the sham group. Conversely,
treatment with 20 or 40 mg/kg artesunate was revealed to
significantly upregulate Nrf2 protein expression in CIRI mice
compared with untreated mice from the CIRI model group (Fig. 8B).
 | Figure 8.Treatment with artesunate restores
Nrf2 protein expression and the Bax/Bcl-2 ratio, and suppresses p38
MAPK phosphorylation in CIRI mice. (A) Protein expression was
detected using western blot analysis. (B) Blots were
semi-quantified and statistical analysis demonstrated that
treatment of CIRI mice with artesunate prevented the CIRI-induced
downregulation of Nrf2 protein expression. (C) Statistical analysis
demonstrated that treatment of CIRI mice with artesunate prevented
the CIRI-induced increase in the Bax/Bcl-2 protein expression
ratio. (D) Statistical analysis demonstrated that treatment of CIRI
mice with artesunate prevented the CIRI-induced upregulation of
p-p38 MAPK protein expression. Sham, sham-operated group; CIRI,
CIRI model group; Low, CIRI + 10 mg/kg artesunate for 7 days group;
Medium, CIRI + 20 mg/kg artesunate for 7 days group; High, CIRI +
40 mg/kg artesunate for 7 days group. ##P<0.01 vs.
Sham; **P<0.01 vs. CIRI. Nrf2, nuclear factor erythroid
2-related factor 2; CIRI, cerebral ischemia/reperfusion injury;
Bax, apoptosis regulator Bax; Bcl-2, apoptosis regulator Bcl-2; p,
phosphorylated; MAPK, mitogen-activated protein kinase. |
Treatment with artesunate restores the
Bax/Bcl-2 expression ratio in CIRI mice
To further explore the anti-apoptotic effects of
artesunate during CIRI, the protein expression levels of the
proapoptotic factor Bax and the antiapoptotic factor Bcl-2 were
assessed using western blot analysis (Fig. 8A). The present results demonstrated
that the Bax/Bcl-2 protein expression ratio was significantly
increased in CIRI mice compared with in sham-operated mice
(Fig. 8C). Following treatment
with artesunate (20 or 40 mg/kg), the Bax/Bcl-2 protein expression
ratio was significantly reduced compared with untreated mice from
the CIRI model group (Fig.
8C).
Treatment with artesunate suppresses
p38 MAPK phosphorylation in CIRI mice
In order to investigate the involvement of the MAPK
signaling pathway in the effects of artesunate during CIRI, the
protein expression levels of p-p38 MAPK were assessed using western
blot analysis (Fig. 8A). The
present results revealed that the protein expression levels of
p-p38 MAPK were significantly upregulated in CIRI mice compared
with in mice from the sham group (Fig.
8D). Following treatment with 20 or 40 mg/kg artesunate, p-p38
protein expression levels in CIRI mice were significantly reduced
compared with untreated mice from the CIRI model group (Fig. 8D).
Discussion
Cerebrovascular disease is also known as
cerebrovascular accident or stroke, and is a common disease of the
cerebral circulation with a high incidence rate (19). According to the third national
survey of mortality causes released by the Ministry of Health in
2012, 136.64 out of 100,000 patients with stroke succumbed in
China, raising the mortality of strokes to more than that of
malignant tumors (20). In China,
stroke has become the disease with the highest mortality rate
(21). Thrombolysis, angioplasty,
rehabilitation and other therapies, as well as early intervention,
can restore cerebral blood supply in patients with stroke and
alleviate neuronal damage, thus improving the survival rate of
stroke victims (22). However,
following the restoration of the blood supply to ischemic tissues,
the structure and function of cells undergo further damage caused
by reperfusion, thus resulting in the development of CIRI (19). The results of the present study
suggested that treatment with artesunate may prevent CIRI-induced
neurological impairments, reduce the cerebral infarct volume and
attenuate CIRI-associated histological damage in mice.
Oxidative stress results from an imbalance between
oxidative and antioxidative processes, and may cause severe cell
damage (8). An increase in the
production of reactive oxygen and nitrogen species, and other free
radicals, is the main cause of oxidative stress (23). ROS can interfere with gene
expression regulation, thus altering the expression of signaling
proteins, and affecting intracellular signaling cascades (24). Physiological ROS generation is
critical for the maintenance of normal cellular functions; however,
under pathological conditions, including cerebral ischemia,
aberrant ROS production can result in the oxidation of cellular
components, thus causing cell damage (25). In the present study, artesunate was
demonstrated to significantly attenuate CIRI-induced impairments in
the activity of the antioxidative enzymes SOD and GSH in mice.
Neurons are characterized by high metabolic activity
and increased oxygen consumption. In addition, their relatively low
endogenous antioxidant contents make neurons particularly sensitive
to oxidative stress (26).
Furthermore, the brain is rich in lipids, which can react with ROS
to generate hydrogen peroxide free radicals, resulting in membrane
lipid peroxidation and cell damage (27). Therefore, during CIRI, oxidative
stress is among the main causes of neuronal injury. In the present
study, artesunate was revealed to significantly inhibit ROS
production in mice with CIRI. Cheng et al (14) suggested that artesunate may induce
endothelial cell apoptosis through an ROS-dependent p38
MAPK/mitochondrial pathway. Conversely, the present findings
suggested that the regulation of ROS production may be implicated
in the protective effects of artesunate during CIRI.
Mitochondria are important organelles during the
execution of apoptotic signals in neurons. Cerebral ischemia and
reperfusion enhance the mitochondrial production of ROS, which,
through the activation of the proapoptotic protein Bax, trigger the
mitochondrial release of cytochrome c, which binds with
apoptotic protease activating factor 1 and caspase-9 to form the
apoptosome, resulting in the activation of caspase-3 and other
caspases, including caspase-2, −6, −8 and −10 (28). Activated caspase-3 may subsequently
cleave DNA repair enzymes, thus inhibiting the repair of
ischemia-induced DNA damage, which results in cellular apoptosis
(29). Oxidative stress and
increased ROS production have been implicated in the development of
CIRI (30). Under physiological
conditions, low levels of ROS serve as signaling molecules in
various cellular processes; however, in pathological states, when
the endogenous antioxidative mechanisms are unable to counteract
excessive ROS production, high levels of ROS can oxidize lipids,
proteins, nucleic acids and intracellular components, thus causing
cell damage (31). Direct or
indirect protein oxidation by ROS can alter their structure,
increase their hydrophobicity and interfere with protein-protein
interactions, leading to the formation of protein aggregates, and
causing cell damage (32). In the
present study, artesunate was revealed to significantly suppress
the activity of the proapoptotic caspase-3, reduce the Bax/Bcl-2
protein expression ratio and upregulate the expression of the
antiapoptotic transcription factor Nrf2. Cao et al (16) reported that artesunate protected
against sepsis-induced lung injury through the activation of Nrf2
and heme oxygenase 1. Therefore, it may be hypothesized that
increased Nrf2 expression participates in the suppression of
ROS-induced apoptosis that is implicated in the protective effects
of artesunate during CIRI.
CIRI-induced brain damage has been reported to
involve several cellular processes, including increased production
of oxygen free radicals and ROS-mediated injury, intracellular
Ca2+ overload, cytokine-mediated injury, neurotoxicity
caused by the aberrant release of excitatory amino acids, and
disorders in neuronal metabolism (33). Inflammatory responses are
implicated in the development of CIRI, and the MAPK signaling
pathway has been reported to mediate inflammatory processes during
CIRI (29). The inhibition of
p38/MAPK signaling has been reported to reduce neuronal apoptosis
in the rat hippocampal CA1 region during cerebral ischemia, thus
indicating the implication of p38 MAPK activation in neuronal
apoptosis (33). Following its
activation via phosphorylation, p38 MAPK can activate downstream
kinases and various transcription factors, and regulate the
expression of target genes, including inducible nitric oxide
synthase, TNF-α and IL-1β (34).
These genes serve important roles in neuronal apoptosis and
inflammatory responses during CIRI. The present results
demonstrated that artesunate significantly suppressed the
activation of the proinflammatory cytokines TNF-α, IL-1β and IL-6,
and enhanced the activity of anti-inflammatory IL-10 during CIRI,
possibly through the suppression of p38 MAPK activation.
In conclusion, the results of the present study
suggested that artesunate may protect against the development of
CIRI, through the inhibition of oxidative and inflammatory
processes, which may be regulated through the activation of Nrf2
and ROS-dependent p38 MAPK signaling pathways. Therefore,
artesunate may have potential for the development of alternative
therapeutic strategies aimed at the prevention and treatment of
CIRI.
References
|
1
|
Sorond FA, Tan CO, LaRose S, Monk AD,
Fichorova R, Ryan S and Lipsitz LA: Deferoxamine, cerebrovascular
hemodynamics, and vascular aging: Potential role for
hypoxia-inducible transcription factor-1-regulated pathways.
Stroke. 46:2576–2583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kernan WN, Viscoli CM, Furie KL, Young LH,
Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit
R, et al: Pioglitazone after ischemic stroke or transient ischemic
attack. N Engl J Med. 374:1321–1331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang X, Cheripelli BK, Lloyd SM, Kalladka
D, Moreton FC, Siddiqui A, Ford I and Muir KW: Alteplase versus
tenecteplase for thrombolysis after ischaemic stroke (ATTEST): A
phase 2, randomised, open-label, blinded endpoint study. Lancet
Neurol. 14:368–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liebeskind DS, Tomsick TA, Foster LD,
Yeatts SD, Carrozzella J, Demchuk AM, Jovin TG, Khatri P, von
Kummer R, Sugg RM, et al: Collaterals at angiography and outcomes
in the Interventional Management of Stroke (IMS) III trial. Stroke.
45:759–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shan J, Sun L, Wang D and Li X: Comparison
of the neuroprotective effects and recovery profiles of isoflurane,
sevoflurane and desflurane as neurosurgical pre-conditioning on
ischemia/reperfusion cerebral injury. Int J Clin Exp Pathol.
8:2001–2009. 2015.PubMed/NCBI
|
|
6
|
Jiang F, Yang J, Zhang L, Li R, Zhuo L,
Sun L and Zhao Q: Rosuvastatin reduces ischemia-reperfusion injury
in patients with acute coronary syndrome treated with percutaneous
coronary intervention. Clin Cardiol. 37:530–535. 2014.PubMed/NCBI
|
|
7
|
Yu Q, Lu Z, Tao L, Yang L, Guo Y, Yang Y,
Sun X and Ding Q: ROS-dependent neuroprotective effects of NaHS in
ischemia brain injury involves the PARP/AIF pathway. Cell Physiol
Biochem. 36:1539–1551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao B, Chen Y, Sun X, Zhou M, Ding J,
Zhan JJ and Guo LJ: Phenolic alkaloids from Menispermum dauricum
rhizome protect against brain ischemia injury via regulation of
GLT-1, EAAC1 and ROS generation. Molecules. 17:2725–2737. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang PR, Wang JS, Zhang C, Song XF, Tian N
and Kong LY: Huang-Lian-Jie-Du-Decotion induced protective
autophagy against the injury of cerebral ischemia/reperfusion via
MAPK-mTOR signaling pathway. J Ethnopharmacol. 149:270–280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng CY, Lin JG, Tang NY, Kao ST and
Hsieh CL: Electroacupuncture at different frequencies (5 Hz and 25
Hz) ameliorates cerebral ischemia-reperfusion injury in rats:
Possible involvement of p38 MAPK-mediated anti-apoptotic signaling
pathways. BMC Complement Altern Med. 15:2412015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Liu K, Seneviratne CJ, Li X,
Cheung GS, Jin L, Chu CH and Zhang C: Lipoteichoic acid from an
Enterococcus faecalis clinical strain promotes TNF-α expression
through the NF-κB and p38 MAPK signaling pathways in differentiated
THP-1 macrophages. Biomed Rep. 3:697–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nito C, Kamada H, Endo H, Niizuma K, Myer
DJ and Chan PH: Role of the p38 mitogen-activated protein
kinase/cytosolic phospholipase A2 signaling pathway in blood-brain
barrier disruption after focal cerebral ischemia and reperfusion. J
Cereb Blood Flow Metab. 28:1686–1696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jie P, Hong Z, Tian Y, Li Y, Lin L, Zhou
L, Du Y and Chen L and Chen L: Activation of transient receptor
potential vanilloid 4 induces apoptosis in hippocampus through
downregulating PI3K/Akt and upregulating p38 MAPK signaling
pathways. Cell Death Dis. 6:e17752015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng R, Li C, Li C, Wei L, Li L, Zhang Y,
Yao Y, Gu X, Cai W, Yang Z, et al: The artemisinin derivative
artesunate inhibits corneal neovascularization by inducing
ROS-dependent apoptosis in vascular endothelial cells. Invest
Ophthalmol Vis Sci. 54:3400–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thanaketpaisarn O, Waiwut P, Sakurai H and
Saiki I: Artesunate enhances TRAIL-induced apoptosis in human
cervical carcinoma cells through inhibition of the NF-κB and
PI3K/Akt signaling pathways. Int J Oncol. 39:279–285.
2011.PubMed/NCBI
|
|
16
|
Cao TH, Jin SG, Fei DS, Kang K, Jiang L,
Lian ZY, Pan SH, Zhao MR and Zhao MY: Artesunate protects against
sepsis-induced lung injury via heme oxygenase-1 modulation.
Inflammation. 39:651–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng DS, Liao W, Tan WS, Chan TK, Loh XY and
Wong WS: Anti-malarial drug artesunate protects against cigarette
smoke-induced lung injury in mice. Phytomedicine. 21:1638–1644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu F, Li J, Guo N, Wang XH and Liao YQ:
MiRNA-27a promotes the proliferation and invasion of human gastric
cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling
pathway. Am J Cancer Res. 7:405–416. 2017.PubMed/NCBI
|
|
19
|
Svensson LG, Blackstone EH,
Apperson-Hansen C, Ruggieri PM, Ainkaran P, Naugle RI, Lima B,
Roselli EE, Cooper M, Somogyi D, et al: Implications from
neurologic assessment of brain protection for total arch
replacement from a randomized trial. J Thorac Cardiovasc Surg.
150:1140–1147.e11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan Y, Yao YF, Hu SN, Gao J and Zhang LL:
MiR-133a is functionally involved in doxorubicin-resistance in
breast cancer cells MCF-7 via its regulation of the expression of
uncoupling protein 2. PLoS One. 10:e01298432015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernandez-Twinn DS, Blackmore HL, Siggens
L, Giussani DA, Cross CM, Foo R and Ozanne SE: The programming of
cardiac hypertrophy in the offspring by maternal obesity is
associated with hyperinsulinemia, AKT, ERK, and mTOR activation.
Endocrinology. 153:5961–5971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jones KM, Bhattacharjee R, Krishnamurthi
R, Blanton S, Theadom A, Barker-Collo S, Thrift A, Parmar P,
Maujean A, Ranta A, et al: Methodology of the stroke
self-management rehabilitation trial: An international, multisite
pilot trial. J Stroke Cerebrovasc Dis. 24:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saad MA, Abdelsalam RM, Kenawy SA and
Attia AS: Ischemic preconditioning and postconditioning alleviates
hippocampal tissue damage through abrogation of apoptosis modulated
by oxidative stress and inflammation during transient global
cerebral ischemia-reperfusion in rats. Chem Biol Interact.
232:21–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen MH, Zhang CB, Zhang JH and Li PF:
Electroacupuncture attenuates cerebral ischemia and reperfusion
injury in middle cerebral artery occlusion of rat via modulation of
apoptosis, inflammation, oxidative stress, and excitotoxicity. Evid
Based Complement Alternat Med. 2016:94386502016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sosunov SA, Ameer X, Niatsetskaya ZV,
Utkina-Sosunova I, Ratner VI and Ten VS: Isoflurane anesthesia
initiated at the onset of reperfusion attenuates oxidative and
hypoxic-ischemic brain injury. PLoS One. 10:e01204562015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buch P, Patel V, Ranpariya V, Sheth N and
Parmar S: Neuroprotective activityof Cymbopogon martinii against
cerebral ischemia/reperfusion-induced oxidative stress in rats. J
Ethnopharmacol. 142:35–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palencia G, Medrano JÁ, Ortiz-Plata A,
Farfán DJ, Sotelo J, Sánchez A and Trejo-Solís C: Anti-apoptotic,
anti-oxidant, and anti-inflammatory effects of thalidomide on
cerebral ischemia/reperfusion injury in rats. J Neurol Sci.
351:78–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Su L, Xiao Z and Liu X and Liu X:
Methyl jasmonate induces apoptosis and pro-apoptotic autophagy via
the ROS pathway in human non-small cell lung cancer. Am J Cancer
Res. 6:187–199. 2016.PubMed/NCBI
|
|
29
|
Zhou L, Chen L, Wang J and Deng Y:
Astragalus polysaccharide improves cardiac function in
doxorubicin-induced cardiomyopathy through ROS-p38 signaling. Int J
Clin Exp Med. 8:21839–21848. 2015.PubMed/NCBI
|
|
30
|
Yong Y, Matthew S, Wittwer J, Medrano JÁ,
Ortiz-Plata A, Farfán DJ, Sotelo J, Sánchez A and Trejo-Solís C:
Dichamanetin inhibits cancer cell growth by affecting ROS-related
signaling components through mitochondrial-mediated apoptosis.
Anticancer Res. 33:5349–5355. 2013.PubMed/NCBI
|
|
31
|
Lin H, Gao X, Chen G, Sun J, Chu J, Jing
K, Li P, Zeng R and Wei B: Indole-3-carbinol as inhibitors of
glucocorticoid-induced apoptosis in osteoblastic cells through
blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun.
460:422–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lucas IK and Kolodziej H:
Trans-resveratrol induces apoptosis through ROS-triggered
mitochondria-dependent pathways in A549 human lung adenocarcinoma
epithelial cells. Planta Med. 81:1038–1044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang M, Li J, Peng Q, Sun J, Chu J, Jing
K, Li P, Zeng R and Wei B: Neuroprotective effects of bilobalide on
cerebral ischemia and reperfusion injury are associated with
inhibition of pro-inflammatory mediator production and
down-regulation of JNK1/2 and p38 MAPK activation. J
Neuroinflammation. 11:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qi SH, Hao LY, Yue J, Zong YY and Zhang
GY: Exogenous nitric oxide negatively regulates the S-nitrosylation
p38 mitogen-activated protein kinase activation during cerebral
ischaemia and reperfusion. Neuropathol Appl Neurobiol. 39:284–297.
2013. View Article : Google Scholar : PubMed/NCBI
|