Introduction
Acute pancreatitis in pregnancy (APIP) is a rare
event, attacking approximately 1/10,000 to 1/1,000 pregnancies
(1,2), thereby the information on maternal
and fetal complications is limited. Although less frequent in
clinical practice, it was associated with up to 5% of maternal
deaths and fetal loss (3). APIP
usually occurs in the third trimester of pregnancy (4), and gallstones are the most common
cause and responsible for more than 60% of cases (1,2). As
in any other disease associated with pregnancy, APIP is associated
with greater concerns as it deals with two lives.
It has been widely accepted that the activation of
trypsinogen leads to self-digestion of pancreatic acinar cells and
then results in acute pancreatitis. Acute pancreatitis is
frequently complicated by an intensive systemic inflammatory
response, in which increased infiltration of inflammatory cells is
observed in multiple organs, such as the liver, kidney, and lung,
further leading to multiple organ dysfunction syndrome (5). Among these organs, the lung is the
most vulnerable one (6). Acute
lung injury is reported to occur in 10–25% of acute pancreatitis
cases, and it is responsible for up to 60% of acute
pancreatitis-associated deaths (7). Additionally, accumulated studies have
demonstrated that acute pancreatitis-triggered systemic
inflammatory response causes acute lung injury (8). Moreover, in acute
pancreatitis-induced lung injury, inflammatory cascade involving
the activation and release of various inflammatory cytokines, such
as nuclear factor-κB (NF-κB), tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6, was significantly induced (9). Therefore, reducing proinflammatory
mediators might be a good therapeutic strategy to attenuate acute
lung injury associated with APIP.
Macrophage migration inhibitory factor (MIF) is a
structurally unique pleiotropic cytokine that plays an important
role as an upstream regulator of innate and acquired immunity as
well as in cellular redox signaling (10). It regulates inflammatory response
through extra- and intracellular processes, such as binding to a
receptor complex made of CD74 with or without CD44, CXCR2, and
CXCR4 to initiate intracellular signaling (11,12).
Through these interactions, MIF negatively or positively regulates
MAPKs (13). For example, MIF
induced the phosphorylation of P38MAPK (14), which may ascribe to reduce the
expression of MKP-1, a critical phosphatase in physiological
counter-regulatory MIF-glucocorticoids (GCs) dyad (15). In most cases, MIF is recognized as
a pro-inflammatory cytokine whose neutralizing antibody (16) or small-molecule inhibitor (17) is used for suppressing inflammation
with high levels of MIF in blood circulation or local tissue in
various animal models, such as severe sepsis (18), rheumatoid arthritis (19), allergic airway inflammation
(20), colitis (21) and chronic obstructive pulmonary
disease (22). Therefore, a
promising therapeutic approach to diminish pathological
inflammation is to inhibit the production and/or biological
activity of MIF.
(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole
acetic acid methyl ester (ISO-1), a small molecule antagonist of
MIF, is from the isoxazole series, whose active compounds were
identified by virtue of their ability to inhibit the tautomerase
activity of MIF (17). ISO-1
inhibits Toll-like receptor-4 (TLR-4)-induced proinflammatory
cytokine production from monocytes (23), the macrophage release of TNF-α from
lipopolysaccharide (LPS)-stimulated mice and is moderately
protective in a clinically relevant model of sepsis when
administered intraperitoneally (17).
However, whether MIF inhibition by ISO-1 is
effective in protecting against acute lung injury induced by APIP
has not yet been elucidated. We hypothesized that MIF is involved
in the pathogenesis of acute lung injury induced by APIP, and MIF
antagonist ISO-1 can protect against the lung injury. Therefore, in
the present study, we attempted to investigate the effect and
potential mechanism of MIF antagonist ISO-1 in the development of
acute lung injury in rats with APIP. The results may provide a
theoretical basis for the treatment of acute lung injury associated
with APIP.
Materials and methods
Antibodies and reagents
ISO-1 and sodium taurocholate were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The primary
antibodies against P38, phosphorylated-P38, NF-κB/p65 and TNF-α
were purchased from Cell Signaling Technology Inc. (Danvers, MA,
USA). MIF primary antibody was from Abcam (Cambridge, CA). Rat
anti-MPO antibody was from Wuhan Goodbio Technology Co., Ltd.
(Wuhan, China). Rat TNF-α, IL-1β, IL-6 enzyme-linked immunosorbent
assay (ELISA) kits were purchased from Cusabio Corp (Wuhan,
China).
Animals
Eighteen pregnant Sprague-Dawley (SD) rats (17–18
days of the first gestation, weighing 390–450 g) were obtained from
the Experimental Animals Center of Huazhong University of Science
and Technology (Wuhan, China). The animals were kept under
standardized conditions with an ambient temperature of 23±2°C and a
12 h light and dark cycle. Before the induction of pancreatitis,
the animals were fed standard laboratory rodent chow, allowed free
access to sterile water. All rats were fasted for 12 h prior to the
modeling while given water ad libitum. All animal
experiments in this study were reviewed and approved by the Ethics
Committee of Wuhan University and performed in compliance with the
ARRIVE guidelines.
Experimental model and groups
Rats we re anesthetized with isoflurane (induced
with 5% isoflurane and maintaining with 3% in 2 l/min oxygen flow
in a sealed container) and underwent standardized surgical
procedures as described previously (24) and minor steps were revised.
Briefly, the APIP rat model was induced by retrograde infusion of
5% sodium taurocholate solution (1 ml/kg) into the
biliary-pancreatic duct at a constant speed of 0.10 ml/min. The
pancreas appeared to be hemorrhaged and necrotic after 5 mins,
indicating the APIP model was induced successfully. After closure,
20 ml/kg body weight of saline solution was compensated back
subcutaneously for fluid loss.
The rats were randomly assigned into three
experimental groups: i) Sham operation group (SO group); ii) APIP
group; and iii) ISO-1 + APIP group (ISO-1 group), including 6 rats
in each group. All SO group underwent the same procedures but were
retrogradely infused with equivalent saline water instead. The rats
of the ISO-1 group were intraperitoneally administered with 3.5
mg/kg ISO-1 (dissolved in 5% DMSO diluted in saline) 30 min before
the modeling. The dosage and time for ISO-1 were based on our
previous study (25), which was
non-toxic and effective. The rats in SO and APIP groups received an
equivalent volume of vehicle (5% DMSO diluted in saline) instead of
ISO-1 before the operation.
Collection of blood and tissue
samples
All the rats were sacrificed at 6 h after modeling,
which was based on our earlier study (2). Blood samples were collected by
inferior vena cava puncture and the serum was stored at −80°C for
further analysis. Subsequently, pancreas and lung tissues were
excised and fixed in 4% polyoxymethylene for histological detection
or were frozen immediately in liquid nitrogen and stored at −80°C
for the following assay.
Serum enzyme activity assay
Serum amylase (AMY) and lipase (LIPA) levels were
measured by a full automatic biochemical analyzer (Olympus AU680;
Olympus, Tokyo, Japan) using standard techniques.
Histopathology analysis
The pancreatic and lung specimens were fixed in 4%
polyformaldehyde, embedded with paraffin, sectioned at 4 µm thick,
and sequentially stained with hematoxylin and eosin (H&E). All
slides were assessed under the optical microscope (Olympus Optical
Ltd., Tokyo, Japan) by 3 experienced pathologists who are blind to
the research. The Pancreatic histological assessment was determined
by edema, hemorrhage, vacuolization, inflammatory cell
infiltration, and acinar necrosis according to the standard scale
system described by Schmidt et al (26). Similarly, lung injury was assessed
using a scale for interalveolar septal thickening, alveolar
hemorrhage and inflammatory cell infiltration and fibrosis, as
described by Werner et al (27).
ELISA
The serum concentrations of TNF-α, IL-1β, and IL-6
were detected by enzyme-linked immunosorbent assay (ELISA) using
corresponding ELISA kits according to the manufacturer's protocols.
The absorbance was read using an automated microplate reader at 450
nm and the concentrations were calculated according to the standard
curve run on each assay plate. All samples were duplicated 3
times.
Immunofluorescence assay
Myeloperoxidase (MPO), the marker of neutrophil
infiltration, was detected in the lung by immunofluorescence
analyses. Briefly, following xylene deparaffinization and hydration
using a graded series of ethanol solutions, the slides were boiled
for 10 min at 121°C in a pressure cooker containing 10 mM citrate
buffer (pH 9.0) for epitope retrieval. Subsequently, the slides
were cooled to room temperature and rinsed in phosphate-buffered
saline (PBS). After permeabilization with 0.2% Triton X-100 for 45
min, the slides were washed with PBS and then blocked with 10%
normal donkey serum to eliminate the nonspecific fluorescence. The
sections were incubated with the primary antibody against MPO
(1:200) at 4°C overnight in a humidity box. And followed by the
fluorescence-labeled secondary antibodies at room temperature for 1
h. Nuclei were counter-stained with DAPI. The negative control
experiments were performed in which PBS was substituted for the
primary antibody. All sections were examined and photographed using
an automatic fluorescence microscope (Olympus Optical Ltd.) under
blind conditions. And the staining was analyzed by Image Pro-Plus
6.0 system (Media Cybernetics Inc., Rockville, MD, USA).
Western blot analysis
The expression of MIF, phosphorylated-P38, P38,
TNF-α and NF-κB in the lung were determined by western blot
analysis. Lung tissues were homogenized and lysed on ice with lysis
buffer (nuclear-cytosol extraction kit; Applygen Technologies Inc.,
Beijing, China) in the presence of protease and phosphorylase
inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Lysates
were collected, and the concentrations of protein were detected
with BCA protein assay. In brief, equal amounts of protein samples
were electrophoresed on 10 or 12% sodium dodecyl
sulfate-polyacrylamide gels (SDS-PAGE) and then transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore). After
blocking with 5% fat-free milk dissolving in Tris-buffered saline
containing 0.1% Tween-20 (TBST) at room temperature for 2 h, the
membranes were subsequently incubated with the primary antibodies
(all of them were diluted as recommended 1:1,000) overnight at 4°C.
Following washing with TBST (5 min × 3), the membranes were
incubated with fluorescently-labeled secondary antibody at room
temperature for 1–2 h. Then the specific protein bands were scanned
by Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln,
NE, USA) according to the manufacturer's instructions. The relative
band intensity was quantified by Quantity One 4.6.2 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were expressed as mean ± SEM and analyzed
by the Graphpad Prism 7.0 software using one-way analysis of
variance (ANOVA) followed by Tukey's test. P<0.05 was considered
to indicate a statistically significant difference.
Results
ISO-1 reduced the serum pancreatic
enzymes and pancreatic histology
Since elevated activities of serum AMY and lipase
(LIPA) are considered the most sensitive and specific markers of AP
(28), firstly, we assessed the
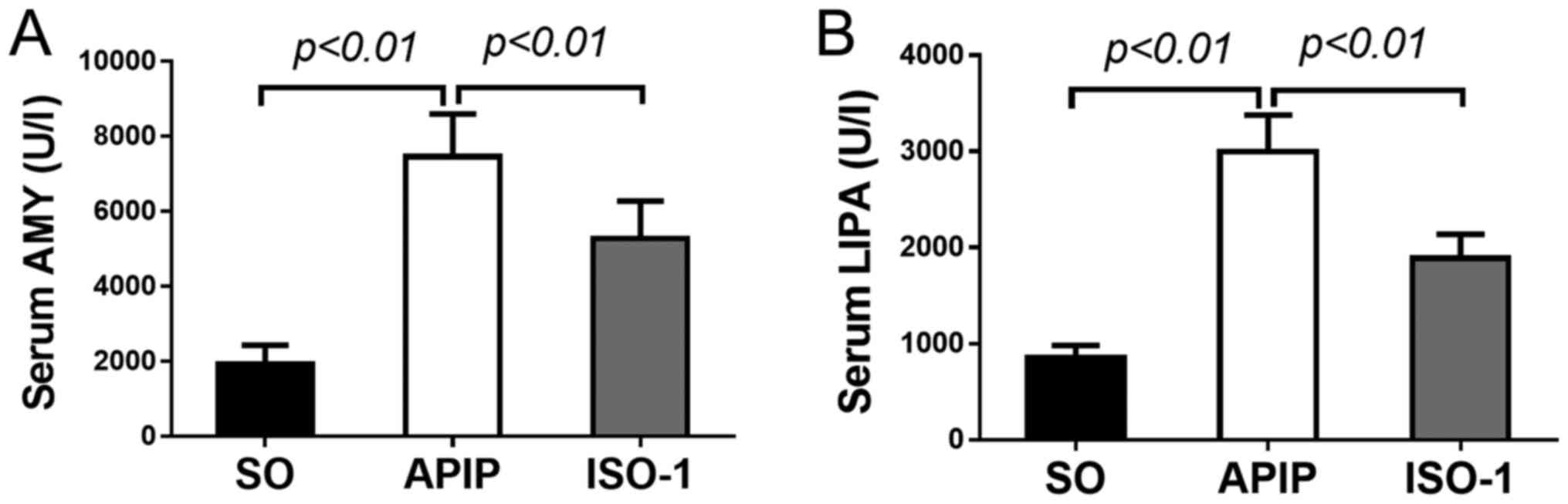
activities of these markers. As shown in Fig. 1A-B, compared with SO group, serum
AMY and LIPA levels were dramatically increased in the rats of APIP
group (P<0.01). However, ISO-1 pretreatment reversed the
increases compared with the APIP group (P<0.01).
Then pancreatic injury was estimated, based on
edema, inflammatory cell infiltration, hemorrhage, and necrosis. As
demonstrated in Fig. 2A, there was
a little morphological evidence of pancreatic injury in SO group.
While, conspicuous pancreatic edema, interstitial leukocyte
infiltration, intrapancreatic hemorrhage, and necrosis were
observed in the APIP group (Fig.
2B). Compared with APIP group, the extent and severity of the
pancreatic histological injury were significantly alleviated in the
ISO-1 group (Fig. 2C). As shown in
Fig. 2D, there was a significant
reduction of the pancreatic histological score in rats pretreated
with ISO-1 in comparison with APIP group (P<0.01).
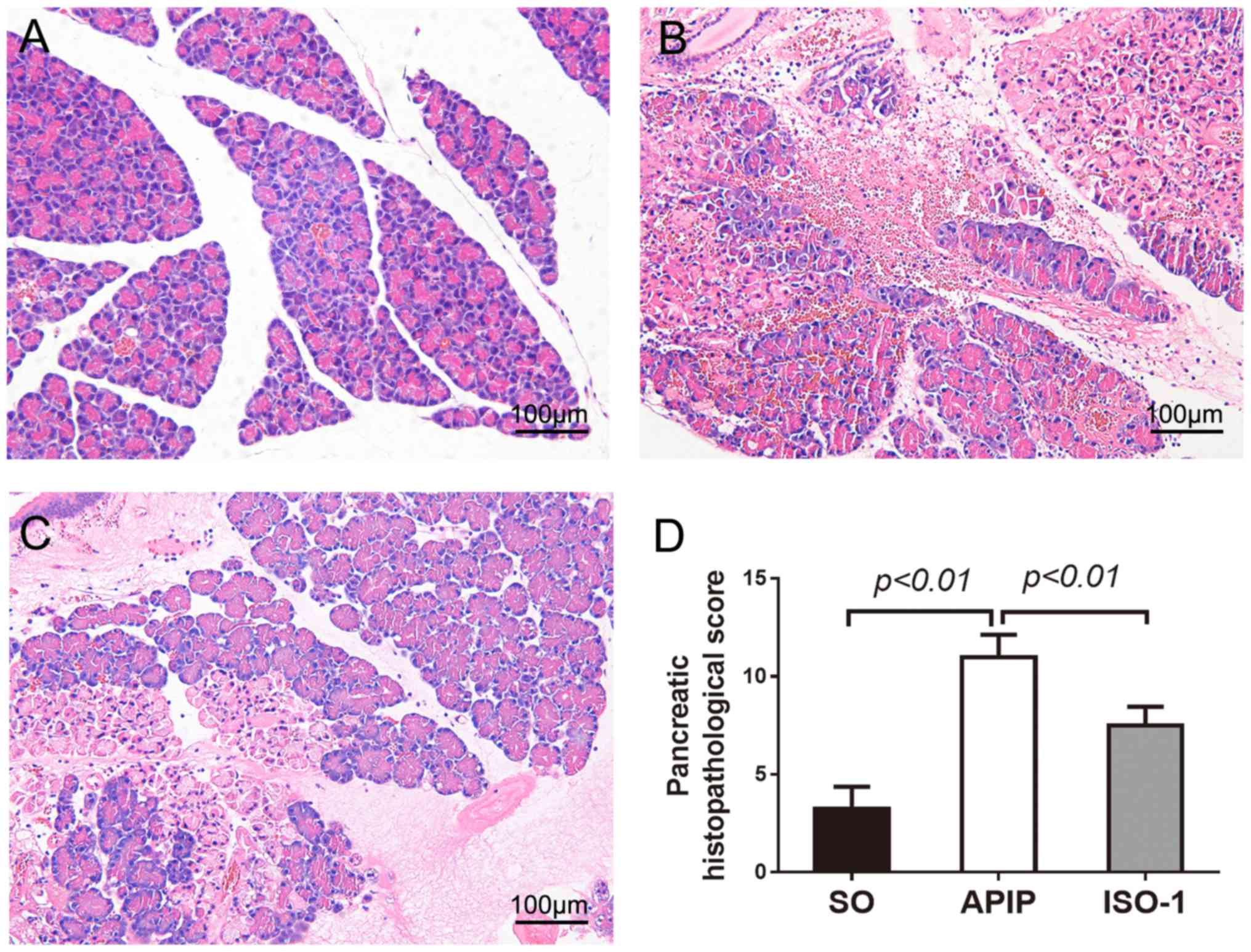 | Figure 2.Morphologic changes and
histopathological score of pancreas in all groups. H&E sections
were examined by light microscopy (original magnification, ×200).
(A) SO group, (B) APIP group, (C) ISO-1 group, (D) Comparison of
the total pathological scores of pancreas in all groups. P<0.05
indicates a significant difference between the marked groups. SO,
sham operation group; APIP, acute pancreatitis in pregnancy group;
ISO-1, ISO-1+APIP group. ISO-1,
(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl
ester. |
ISO-1 decreased the proinflammatory
cytokines following APIP
Serum concentrations of proinflammatory cytokines
such as TNF-α, IL-1β, and IL-6 greatly increase in AP (29,30),
so we measured their levels to obtain insight into the effect of
MIF antagonist ISO-1 on the inflammatory process in APIP. As
illustrated in Fig. 3A-C,
concomitant with the taurocholate administration, marked increases
in serum levels of TNF-α, IL-1β and IL-6 formation were observed in
the APIP rats compared with that in SO group (P<0.01). By
contrast, the increases of these cytokines were obviously decreased
by the pharmacological blockade of MIF antagonist ISO-1
(P<0.01).
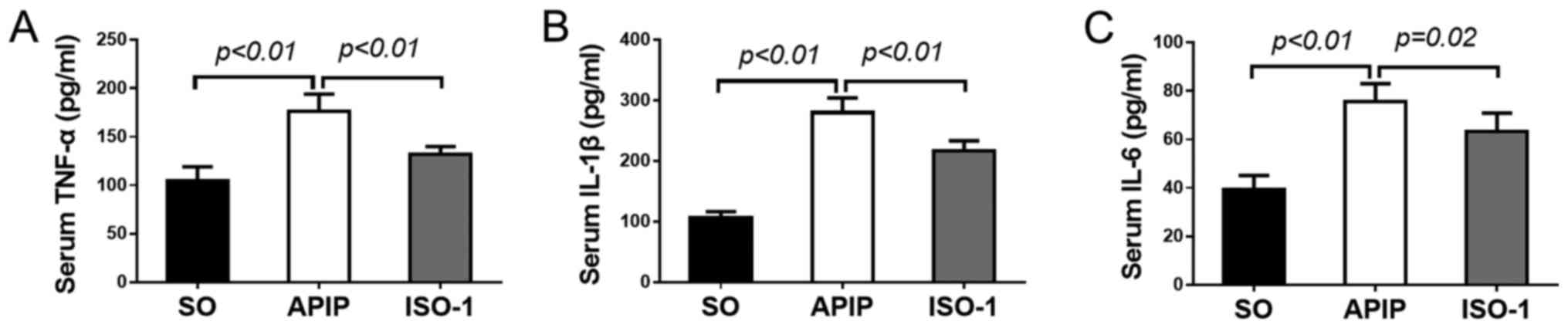 | Figure 3.Effects of ISO-1 on the activities of
serum proinflammatory cytokines in all groups. (A) TNF-α, (B)
IL-1β, and (C) IL-6 concentrations were quantified by ELISA assay.
(mean ± SD, n=6). P<0.05 indicates a significant difference
between the marked groups. SO, sham operation group; APIP, acute
pancreatitis in pregnancy group; ISO-1, ISO-1+APIP group. ISO-1,
(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl
ester; TNF-α, tumor necrosis factor-α; IL, interleukin. |
ISO-1 alleviated the lung
histopathology and the inflammatory cell infiltration
Next, we investigated the effect of ISO-1 on the
lung injury. As shown in Fig. 4A,
the lung sections of SO rats showed a normal alveolar morphology.
However, rats that underwent pancreatitis demonstrated the
recognized features of lung injury including alveolar wall
thickening, and increased exudates as well as inflammatory cell
infiltration in the alveolar spaces (Fig. 4B). The intervention of MIF
antagonist ISO-1 significantly amended the APIP-induced
histopathologic changes of the lung (Fig. 4C). In addition, the
histopathological score of lung injury in the ISO-1 group was
significantly reduced than the score of the APIP group (P<0.01;
Fig. 4D).
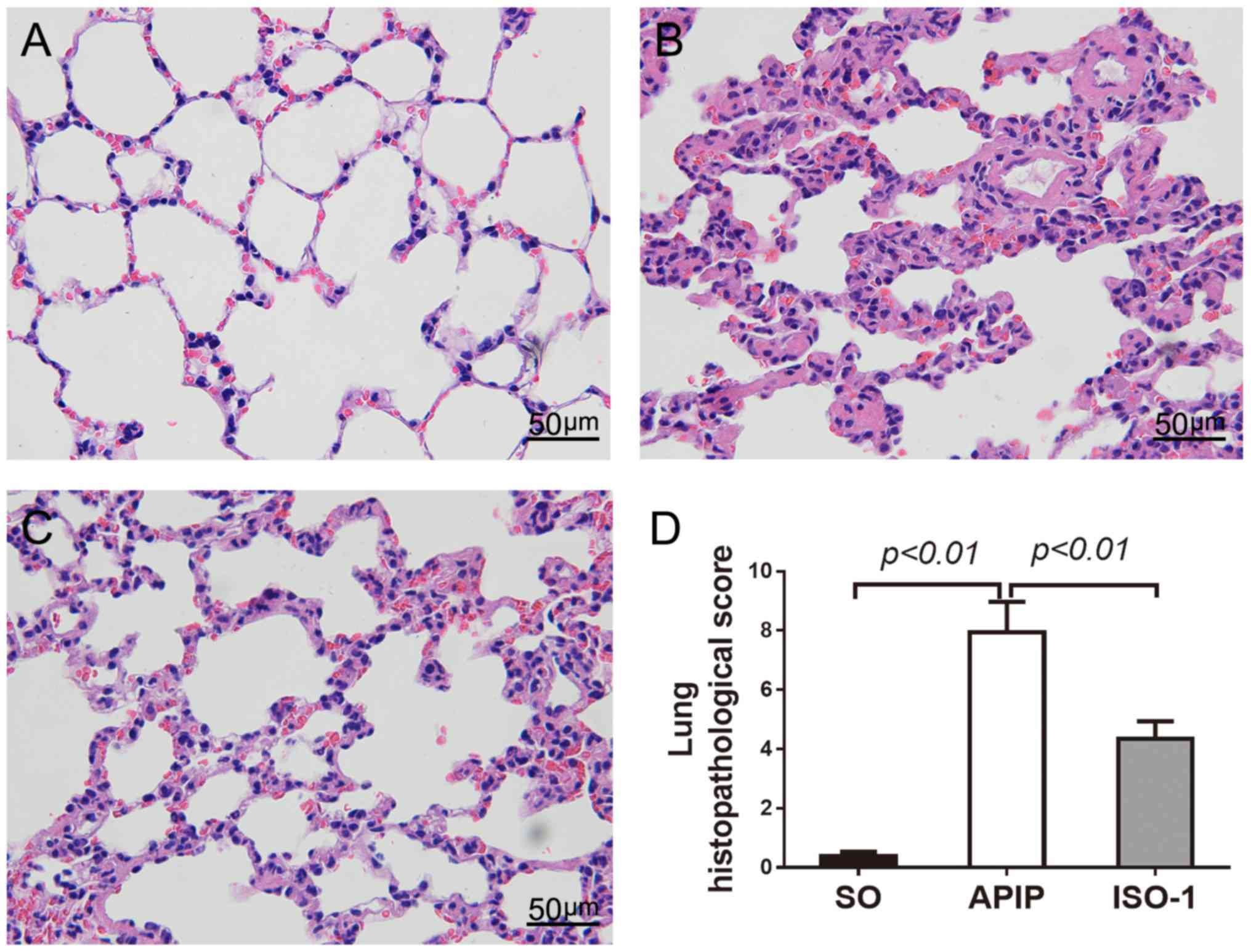 | Figure 4.Representative histopathological
changes of lung injury in each group (original magnification,
×400). (A) SO group, (B) APIP group, (C) ISO-1 group, (D)
Comparison of the total pathological scores of lung injury.
P<0.05 indicates a significant difference between the marked
groups. SO, sham operation group; APIP, acute pancreatitis in
pregnancy group; ISO-1, ISO-1+APIP group. ISO-1,
(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl
ester. |
Acute lung injury is usually accompanied by
increased inflammatory cells accumulation, mainly including
neutrophil, which can be marked by MPO (31). So, we explored whether ISO-1 could
alleviate the inflammatory cell infiltration in the lung by
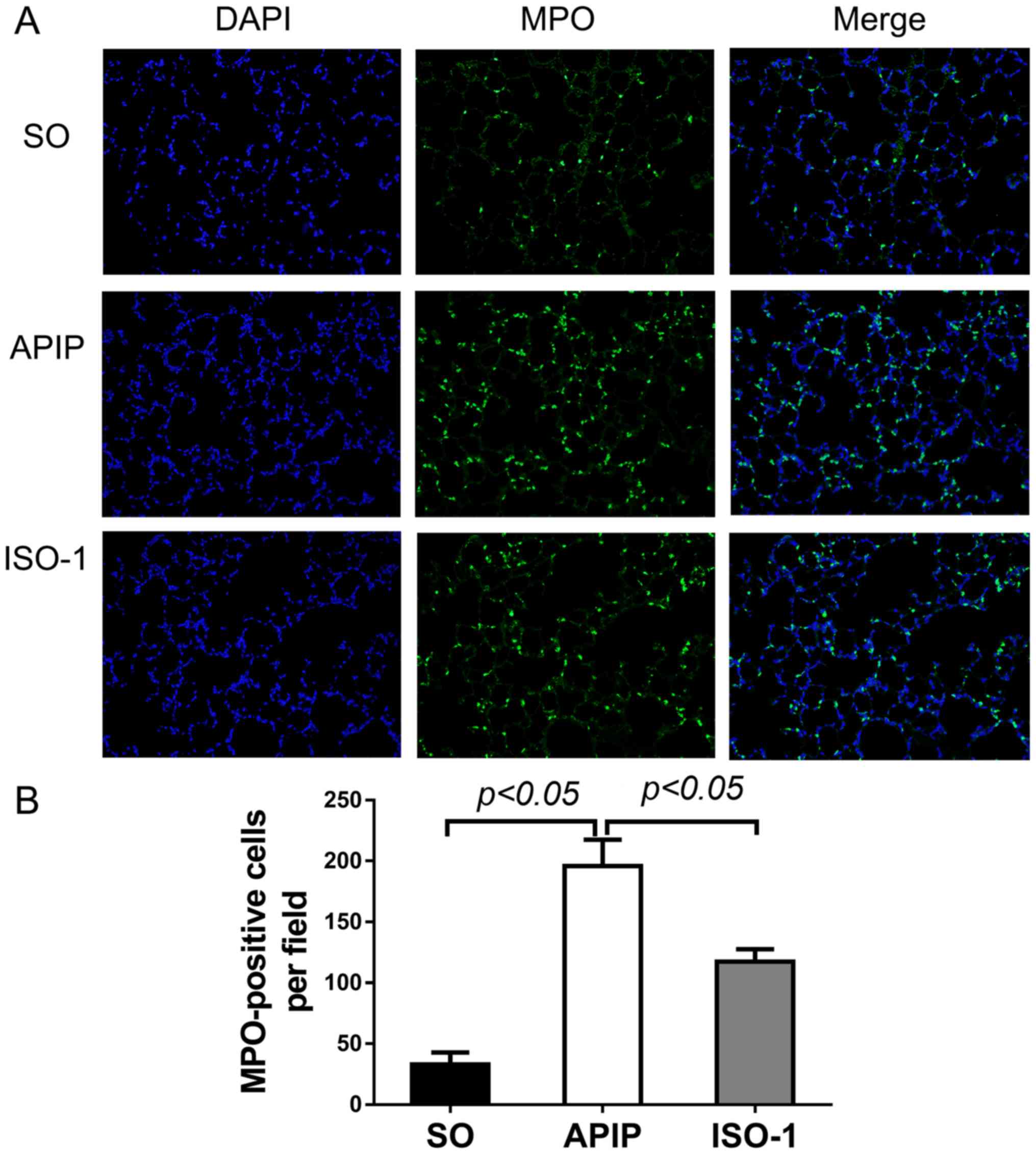
performing immunofluorescent assay of MPO. As shown in Fig. 5A, green-colored MPO-positive cells
in the lung were indicative of neutrophil cells. The number of
MPO-positive cells was significantly elevated after taurocholate
induction compared to the SO group (P<0.01). In contrast, when
pretreated with ISO-1, the number was apparently reduced
(P<0.01; Fig. 5B).
MIF was activated in the lung of APIP
rats
Abnormal MIF expression is regarded as an important
process in inflammatory reaction (32,33).
Here, results from western blot suggested that compared with the SO
group, the expression of MIF in lung tissues of APIP rats was
significantly increased (P<0.01). ISO-1 pretreatment slightly
decreased the MIF expression, but there was no statistical
difference (P>0.05; Fig.
6A).
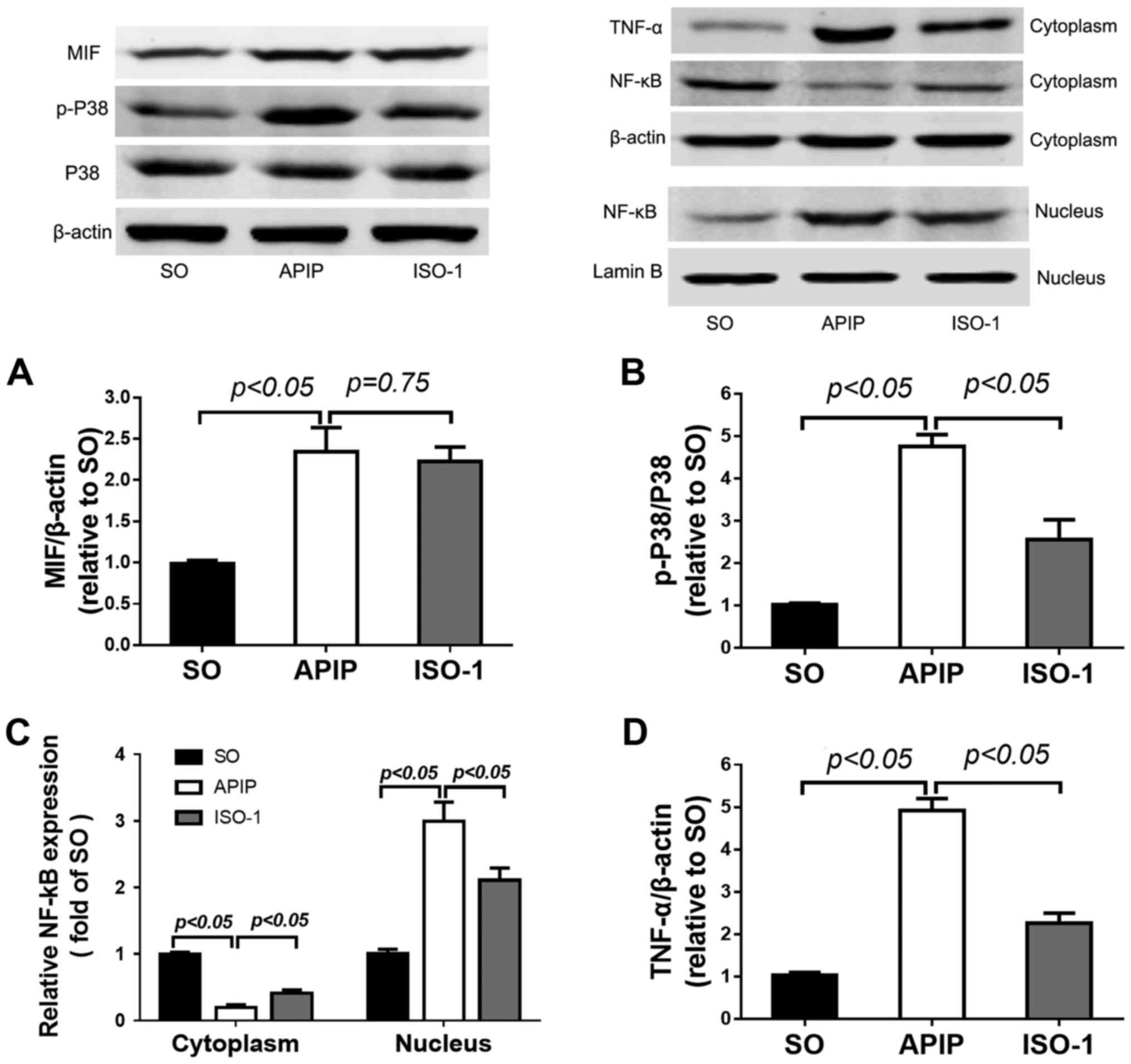 | Figure 6.Effects of MIF antagonist ISO-1 on
the P38MAPK and NF-κB signaling pathway in the lung injury induced
by APIP. Lung samples were obtained at 6 h after modeling. MIF,
total and phosphorylated P38 and TNF-α in the cytoplasm, as well as
NF-κB in the cytoplasm and nucleus were measured by western blot
assay. β-actin was used as internal control of cytoplasm, and
LaminB was used as nucleus internal control. Densitometry
quantification of (A) MIF, (B) p-P38, (C) NF-κB, and (D) TNF-α was
evaluated by the Quantity One software. Data are expressed as mean
± SD (n=6). P<0.05 indicates a significant difference between
the marked groups. SO, sham operation group; APIP, acute
pancreatitis in pregnancy group; ISO-1, ISO-1+APIP group. MIF,
macrophage migration inhibitory factor; ISO-1,
(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl
ester; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor-κB |
ISO-1 inhibited the P38MAPK and NF-κB
activation in the lung
Taken together, all of the above results indicated
that MIF antagonist ISO-1 mitigated the degree of pancreatitis and
associated lung injury, but the underlying mechanism was unknown.
Since the P38MAPK and NF-κB signaling pathways play key roles in
the induction of several inflammatory diseases (34,35),
next we investigated if ISO-1 exerts its anti-inflammatory
activities by affecting the two pathways in lung injury induced by
APIP. As shown in Fig. 6B, APIP
induced a marked increase in the phosphorylated level of P38MAPK
compared with SO group (P<0.01). In addition, pretreated with
the MIF antagonist ISO-1 markedly reduced the phosphorylation of
P38MAPK in the lung following APIP.
The translocation of NF-κB indicated the activation
of NF-κB signaling pathway. As the western blot showed in Fig. 6C, the expression of NF-κB increased
significantly in the nucleus following APIP, accompanied by the
reduction in the cytoplasm. However, pretreatment with ISO-1
inhibited this translocation process. We also found that the
expression of TNF-α in lung tissues was greatly upregulated after
APIP, but ISO-1 treatment reversed the increase (P<0.01;
Fig. 6D).
Discussion
In the present study, the effect of MIF antagonist
ISO-1 on APIP and associated lung injury as well as the underlying
mechanism was preliminarily explored. Our results indicated that
MIF expression was upregulated in the lung of APIP rats, and ISO-1
pretreatment could ameliorate pancreatitis and associated lung
injury. In addition, ISO-1 reduced serum AMY and LIPA
concentrations, pro-inflammatory mediators and inflammatory cell
infiltration in the lung. We also demonstrated that the inhibitory
effect of ISO-1 on APIP and associated lung injury may be through
deactivating P38MAPK and NF-κB signaling pathways. All of these
observations indicate that MIF antagonist ISO-1 exerts potent
anti-inflammatory effects and ameliorates the degree of APIP and
associated lung injury in rats.
AP is a challenging clinical problem characterized
by increased mortality depending on the severity. Although there
have been few clinical trials with pharmacological agents, no
effective treatment exists (36).
APIP is a severe complication of pregnancy, which easily results in
miscarriage, stillbirth and premature birth, and the fetal
mortality can be up to 10–20%, even 30% as reported (37,38).
The incidence of APIP increased during recent years with the change
of dietary habits. It mostly occurs in the third trimester. The
most frequent cause of APIP is gallstones, which is responsible for
more than 60% of cases (1,39). Gallstones are more likely to form
during pregnancy because of elevated progesterone level and it
induces biliary hypotonia and increase the pressure of Oddi
sphincter, which would lead to bile stasis and stone formation
(40). So retrograde infusion of
sodium taurocholate solution into the biliary-pancreatic duct to
induce APIP is in line with the pathophysiological changes of
APIP.
MIF, as an important multifunctional cytokine,
involves in various physiological and pathological processes
including inflammation, immunity, tumor, and pregnancy. Studies
have shown that MIF plays a certain role in the initiation and
development of acute necrotizing pancreatitis (41), liver injury (42), acute respiratory distress syndrome
(43) and sepsis (18). ISO-1 can selectively bind to the
MIF tautomerase site, inhibiting its enzyme activity, thereby
suppressing some of the biological function of MIF. Lung injury is
the most common organ damage in AP and is responsible for 43%
mortality, a main reason for the death of AP patients (5). However, whether inhibition of MIF
with ISO-1 could ameliorate lung injury induced by APIP has been
unknown. Therefore, we hypothesized that MIF may be related to
acute lung injury induced by APIP. In our study, it was
demonstrated that MIF was significantly upregulated in the lung of
APIP rats, suggesting it may be involved in the pathogenesis of
acute lung injury. While, ISO-1 pretreated did not downregulate the
MIF expression, which was consistent with the earlier study that
ISO-1 can ‘bind-onto’ the tautomerase site of MIF thereby blocking
its recognition whereas it can't inhibit MIF synthesis (17).
Generally, pancreatic digestive enzymes such as AMY
and LIPA are most commonly obtained as the biochemical marker of
pancreatic disease, particularly AP. It is contributed at an early
stage to the damage of acinar cells and, consequently, to
inflammatory processes and cytokine production into the pancreas.
In our experiments, conspicuous hyperamylasemia, hyperlipasemia,
and pathological evidences like pancreatic hemorrhage and necrosis
were observed in the sodium taurocholate induced APIP rats. These
results showed that the APIP model was successfully induced.
In AP, inflammatory response and pro-inflammatory
cytokines play pivotal roles and exert major influences on the
outcome of the disease, in particular by triggering the systemic
inflammatory response and multisystem organ failure (44,45).
Growing evidences have identified crucial contribution of
inflammation to AP-induced lung injury. In the study, elevated
levels of TNF-α, IL-1β, and IL-6 in the serum of APIP rats were
observed. The proinflammatory cytokine TNF-α is regarded as one of
the key cytokine initiators of the inflammatory cascade of AP and
the degree of pancreatic injury in AP correlates directly with the
level of TNF-α (46). IL-1β and
IL-6 are the principal mediators in the synthesis of acute-phase
proteins and in the regulation of immune responses and the
inflammatory process (47). In
acute lung injury, these inflammatory mediators have cytotoxic
effects, such as inducing apoptosis of multiple cells including
alveolar epithelial cells, increasing capillary permeability and
damaging intercellular tight junctions, further resulting in
increasing extravasation of vascular fluid, inflammatory cells and
more inflammatory mediators (48).
In our study, recognized features of lung injury including alveolar
wall thickening, and increased exudates as well as inflammatory
cell infiltration in the alveolar spaces of rats that underwent
pancreatitis were observed.
MPO has been used as a biochemical marker for
inflammatory cells infiltration in studies of multiple-organ injury
in AP (49). In addition, its
activity in the lung were correlated with the degree of lung injury
(31). In this study, the number
of MPO-positive cells were dramatically increased in the lung of
APIP rats. Together with aggravating morphological changes of the
lung and increased inflammatory cells infiltration demonstrated
obvious lung injury during the progression of pancreatitis.
However, ISO-1 pretreatment greatly inhibited the
elevation of serum AMY and LIPA, in addition, we also found that
ISO-1 significantly improved the pathological state of the pancreas
and lung, inhibited proinflammatory cytokines, and reduced the
number of MPO-positive cells. All of these observations indicate
that the MIF antagonist ISO-1 exerts potent anti-inflammatory
effects and ameliorates the degree of APIP and associated lung
injury in rats.
Published studies have showed P38MAPK and NF-κB are
both essential pathways involved in regulating the expression of
inflammatory mediators in the pathogenesis of SAP (50,51).
The expression of phosphorylated P38MAPK in the pancreatic and lung
tissue was increased rapidly in the SAP rat model (50). And inhibiting P38MAPK expression
ameliorates the severity of the disease (52). Therefore, P38MAPK activation may
represent a major regulatory mechanism during severe acute
pancreatitis. Our results revealed that taurocholate stimulation
could induce the phosphorylation of P38MAPK in the lung tissue. As
anticipated, ISO-1 pretreatment greatly inhibited the APIP-induced
P38MAPK phosphorylation. In addition, we found that the MIF
antagonist ISO-1 can simultaneously inhibit NF-κB signaling pathway
in the lung. Activation of NF-κB pathway needs NF-κB translocation
from cell plasma to the nucleus, binding to the promoter region of
various pro-inflammatory NF-κB responsive genes and activates
transcription (53). Our data
showed that NF-κB increased significantly in the nucleus following
APIP-induced lung injury, accompanied by the reduction in the
cytoplasm, implying the activation of NF-κB signaling pathway. We
found that pretreatment with ISO-1 effectively attenuated NF-κB
intranuclear translocation in the lung. Inhibition of NF-κB
activation reduces the severity of severe acute pancreatitis
(54). ISO-1 inhibits the NF-κB
activation and thus ameliorates severe acute pancreatitis-induced
lung injury. Previous studies have shown that NF-κB activating can
produce multiple proinflammatory cytokines (55). In this study, we detected the
expression of TNF-α in the lung tissues. The results showed that
the expression of TNF-α was greatly increased in the lung of APIP
rats, whereas ISO-1 pretreatment reduced the increase. These
results indicate that the protective effects of ISO-1 against lung
injury induced by APIP are correlated with the deactivation of
P38MAPK and NF-κB signaling pathways.
In summary, our study provides evidences that MIF
upregulation participated in the lung injury following APIP, and
ISO-1, an MIF antagonist, markedly ameliorated the severity of
pancreatitis and lung injury, which may through the deactivation of
P38MAPK and NF-κB signaling pathways. Targeting its upstream or
downstream substrates may attenuate lung injury and improve APIP
outcomes, but the specific mechanisms need further investigations.
Therefore, the findings presented in this study may stimulate
interest in the development of more potent and specific MIF
inhibitors for the prevention and treatment of APIP, and associated
lung injury.
Acknowledgements
The authors are grateful to the Central Laboratory,
Hubei Key Laboratory of Digestive System Disease of Renmin Hospital
of Wuhan University for providing relevant experimental facilities
and technical support.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 81370562).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed the study, performed the majority of
experiments and wrote this article. LZ and YD analyzed the data and
helped to write the paper. FM and YH performed the
immunofluorescence and western blot assay. HX performed the animal
experiments. TZ participated in the design of the study and
performed the ELISA assay. WW participated in the design of this
study and provided financial support for this work. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments in this study were reviewed
and approved by the Ethics Committee of Wuhan University and
performed in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MIF
|
macrophage migration inhibitory
factor
|
|
APIP
|
acute pancreatitis in pregnancy
|
|
ISO-1
|
(S,R)3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl
ester
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL
|
interleukin
|
|
MPO
|
myeloperoxidase
|
|
NF-κB
|
nuclear factor-κB
|
References
|
1
|
Mali P: Pancreatitis in pregnancy:
Etiology, diagnosis, treatment, and outcomes. Hepatobiliary
Pancreat Dis Int. 15:434–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuo T, Yu J, Wang WX, Zhao KL, Chen C,
Deng WH, He XB, Wang P, Shi Q and Guo WY: Mitogen-activated protein
kinases are activated in placental injury in rat model of acute
pancreatitis in pregnancy. Pancreas. 45:850–857. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akcakaya A, Ozkan OV, Okan I, Kocaman O
and Sahin M: Endoscopic retrograde cholangiopancreatography during
pregnancy without radiation. World J Gastroenterol. 15:3649–3652.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernandez A, Petrov MS, Brooks DC, Banks
PA, Ashley SW and Tavakkolizadeh A: Acute pancreatitis and
pregnancy: A 10-year single center experience. J Gastrointest Surg.
11:1623–1627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halonen KI, Pettilä V, Leppäniemi AK,
Kemppainen EA, Puolakkainen PA and Haapiainen RK: Multiple organ
dysfunction associated with severe acute pancreatitis. Crit Care
Med. 30:1274–1279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mole DJ, Webster SP, Uings I, Zheng X,
Binnie M, Wilson K, Hutchinson JP, Mirguet O, Walker A, Beaufils B,
et al: Kynurenine-3-monooxygenase inhibition prevents multiple
organ failure in rodent models of acute pancreatitis. Nat Med.
22:202–209. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elder AS, Saccone GT and Dixon DL: Lung
injury in acute pancreatitis: Mechanisms underlying augmented
secondary injury. Pancreatology. 12:49–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pastor CM, Matthay MA and Frossard JL:
Pancreatitis-associated acute lung injury: New insights. Chest.
124:2341–2351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaffray C, Yang J, Carter G, Mendez C and
Norman J: Pancreatic elastase activates pulmonary nuclear factor
kappa B and inhibitory kappa B, mimicking pancreatitis-associated
adult respiratory distress syndrome. Surgery. 128:225–231. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu H and Bai Y, Wu L, Hong W, Liang Y,
Chen B and Bai Y: Inhibition of macrophage migration inhibitory
factor protects against inflammation and matrix deposition in
kidney tissues after injury. Mediat Inflamm. 2016:21746822016.
View Article : Google Scholar
|
|
11
|
Leng L, Metz CN, Fang Y, Xu J, Donnelly S,
Baugh J, Delohery T, Chen Y, Mitchell RA and Bucala R: MIF signal
transduction initiated by binding to CD74. J Exp Med.
197:1467–1476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lue H, Thiele M, Franz J, Dahl E,
Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B and
Bernhagen J: Macrophage migration inhibitory factor (MIF) promotes
cell survival by activation of the Akt pathway and role for
CSN5/JAB1 in the control of autocrine MIF activity. Oncogene.
26:5046–5059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao Y, Hou R, Liu F, Liu H, Fei Q, Han Y,
Cai R, Peng C and Qi Y: Obacunone causes sustained expression of
MKP-1 thus inactivating p38 MAPK to suppress pro-inflammatory
mediators through intracellular MIF. J Cell Biochem. 119:837–849.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roger T, Schneider A, Weier M, Sweep FC,
Le Roy D, Bernhagen J, Calandra T and Giannoni E: High expression
levels of macrophage migration inhibitory factor sustain the innate
immune responses of neonates. Proc Natl Acad Sci USA. 113:pp.
E997–E1005. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aeberli D, Leech M and Morand EF:
Macrophage migration inhibitory factor and glucocorticoid
sensitivity. Rheumatology (Oxford). 45:937–943. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calandra T, Echtenacher B, Roy DL, Pugin
J, Metz CN, Hültner L, Heumann D, Männel D, Bucala R and Glauser
MP: Protection from septic shock by neutralization of macrophage
migration inhibitory factor. Nat Med. 6:164–170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Abed Y, Dabideen D, Aljabari B, Valster
A, Messmer D, Ochani M, Tanovic M, Ochani K, Bacher M, Nicoletti F,
et al: ISO-1 binding to the tautomerase active site of MIF inhibits
its pro-inflammatory activity and increases survival in severe
sepsis. J Biol Chem. 280:36541–36544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bozza FA, Gomes RN, Japiassú AM, Soares M,
Castro-Faria-Neto HC, Bozza PT and Bozza MT: Macrophage migration
inhibitory factor levels correlate with fatal outcome in sepsis.
Shock. 22:309–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mikulowska A, Metz CN, Bucala R and
Holmdahl R: Macrophage migration inhibitory factor is involved in
the pathogenesis of collagen type II-induced arthritis in mice. J
Immunol. 158:5514–5517. 1997.PubMed/NCBI
|
|
20
|
Amano T, Nishihira J and Miki I: Blockade
of macrophage migration inhibitory factor (MIF) prevents the
antigen-induced response in a murine model of allergic airway
inflammation. Inflamm Res. 56:24–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohkawara T, Nishihira J, Takeda H, Hige S,
Kato M, Sugiyama T, Iwanaga T, Nakamura H, Mizue Y and Asaka M:
Amelioration of dextran sulfate sodium-induced colitis by
anti-macrophage migration inhibitory factor antibody in mice.
Gastroenterology. 123:256–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russell KE, Chung KF, Clarke CJ, Durham
AL, Mallia P, Footitt J, Johnston SL, Barnes PJ, Hall SR, Simpson
KD, et al: The MIF antagonist ISO-1 attenuates
corticosteroid-insensitive inflammation and airways
hyperresponsiveness in an ozone-induced model of COPD. PLoS One.
11:e1461022016. View Article : Google Scholar
|
|
23
|
West PW, Parker LC, Ward JR and Sabroe I:
Differential and cell-type specific regulation of responses to
Toll-like receptor agonists by ISO-1. Immunology. 125:101–110.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Deng W, Wang W, Ding Y, Jin H, Chen
C, Chen X, Xiong X and Xu S: Inhibition of poly(ADP-ribose)
polymerase attenuates acute kidney injury in sodium
taurocholate-induced acute pancreatitis in rats. Pancreas.
41:1299–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mei F, Shi Q, Zuo T, Chen C, Wang P, Li C,
He B, Yang X, Hu P and Wang W: Dose-effect relationship and
protective effect of MIF inhibitor on pancreas and placenta
injuries in rats with acute necrotizing pancreatitis in late
pregnancy. Chin J Emerg Med. 25:45–49. 2016.
|
|
26
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Werner J, Z'Graggen K, Fernández-del
Castillo C, Lewandrowski KB, Compton CC and Warshaw AL: Specific
therapy for local and systemic complications of acute pancreatitis
with monoclonal antibodies against ICAM-1. Ann Surg. 229:834–842.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sha H, Ma Q, Jha RK and Wang Z:
Resveratrol ameliorates lung injury via inhibition of apoptosis in
rats with severe acute pancreatitis. Exp Lung Res. 35:344–358.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishiki Y, Adewola A, Hatanaka M, Templin
AT, Maier B and Mirmira RG: Translational control of inducible
nitric oxide synthase by p38 MAPK in islet β-cells. Mol Endocrinol.
27:336–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Que RS, Cao LP, Ding GP, Hu JA, Mao KJ and
Wang GF: Correlation of nitric oxide and other free radicals with
the severity of acute pancreatitis and complicated systemic
inflammatory response syndrome. Pancreas. 39:536–540. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim K, Li Y, Jin G, Chong W, Liu B, Lu J,
Lee K, Demoya M, Velmahos GC and Alam HB: Effect of valproic acid
on acute lung injury in a rodent model of intestinal ischemia
reperfusion. Resuscitation. 83:243–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bargagli E, Olivieri C, Nikiforakis N,
Cintorino M, Magi B, Perari MG, Vagaggini C, Spina D, Prasse A and
Rottoli P: Analysis of macrophage migration inhibitory factor (MIF)
in patients with idiopathic pulmonary fibrosis. Respir Physiol
Neurobiol. 167:261–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heinrichs D, Knauel M, Offermanns C,
Berres ML, Nellen A, Leng L, Schmitz P, Bucala R, Trautwein C,
Weber C, et al: Macrophage migration inhibitory factor (MIF) exerts
antifibrotic effects in experimental liver fibrosis via CD74. Proc
Natl Acad Sci USA. 108:pp. 17444–17449. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jaworek J, Jachimczak B, Tomaszewska R,
Konturek PC, Pawlik WW, Sendur R, Hahn EG, Stachura J and Konturek
SJ: Protective action of lipopolysaccharidesin rat
caerulein-induced pancreatitis: Role of nitric oxide. Digestion.
62:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao Y, Liu F, Fang L, Cai R, Zong C and Qi
Y: Genkwanin inhibits proinflammatory mediators mainly through the
regulation of miR-101/MKP-1/MAPK pathway in LPS-activated
macrophages. PLoS One. 9:e967412014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi SB, Bae GS, Jo IJ, Wang S, Song HJ
and Park SJ: Berberine inhibits inflammatory mediators and
attenuates acute pancreatitis through deactivation of JNK signaling
pathways. Mol Immunol. 74:27–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Geng Y, Li W, Sun L, Tong Z, Li N and Li
J: Severe acute pancreatitis during pregnancy: Eleven years
experience from a surgical intensive care unit. Dig Dis Sci.
56:3672–3677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stimac D and Stimac T: Acute pancreatitis
during pregnancy. Eur J Gastroenterol Hepatol. 23:839–844. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hacker FM, Whalen PS, Lee VR and Caughey
AB: Maternal and fetal outcomes of pancreatitis in pregnancy. Am J
Obstet Gynecol. 213:568.e1–5. 2015. View Article : Google Scholar
|
|
40
|
Robertson KW, Stewart IS and Imrie CW:
Severe acute pancreatitis and pregnancy. Pancreatology. 6:309–315.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahman SH, Menon KV, Holmfield JH, McMahon
MJ and Guillou JP: Serum macrophage migration inhibitory factor is
an early marker of pancreatic necrosis in acute pancreatitis. Ann
Surg. 245:282–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barnes MA, McMullen MR, Roychowdhury S,
Pisano SG, Liu X, Stavitsky AB, Bucala R and Nagy LE: Macrophage
migration inhibitory factor contributes to ethanol-induced liver
injury by mediating cell injury, steatohepatitis, and steatosis.
Hepatology. 57:1980–1991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lai KN, Leung JC, Metz CN, Lai FM, Bucala
R and Lan HY: Role for macrophage migration inhibitory factor in
acute respiratory distress syndrome. J Pathol. 199:496–508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Norman J, Franz M, Messina J, Riker A,
Fabri PJ, Rosemurgy AS and Gower WR Jr: Interleukin-1 receptor
antagonist decreases severity of experimental acute pancreatitis.
Surgery. 117:648–655. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hughes CB, Grewal HP, Gaber LW, Kotb M,
El-din AB, Mann L and Gaber AO: Anti-TNFalpha therapy improves
survival and ameliorates the pathophysiologic sequelae in acute
pancreatitis in the rat. Am J Surg. 171:274–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baker SJ and Reddy EP: Transducers of life
and death: TNF receptor superfamily and associated proteins.
Oncogene. 12:1–9. 1996.PubMed/NCBI
|
|
47
|
Papachristou GI: Prediction of severe
acute pancreatitis: Current knowledge and novel insights. World J
Gastroenterol. 14:6273–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lukacs NW and Ward PA: Inflammatory
mediators, cytokines, and adhesion molecules in pulmonary
inflammation and injury. Adv Immunol. 62:257–304. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen C, Xu S, Wang WX, Ding YM, Yu KH,
Wang B and Chen XY: Rosiglitazone attenuates the severity of sodium
taurocholate-induced acute pancreatitis and pancreatitis-associated
lung injury. Arch Med Res. 40:79–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Twait E, Williard DE and Samuel I:
Dominant negative p38 mitogen-activated protein kinase expression
inhibits NF-kappaB activation in AR42J cells. Pancreatology.
10:119–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lv W, Lv C, Yu S, Yang Y, Kong H, Xie J,
Sun H, Andersson R, Xu D, Chen B and Zhou M: Lipoxin A4 attenuation
of endothelial inflammation response mimicking pancreatitis-induced
lung injury. Exp Biol Med (Maywood). 238:1388–1395. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen P, Zhang Y, Qiao M and Yuan Y:
Activated protein C, an anticoagulant polypeptide, ameliorates
severe acute pancreatitis via regulation of mitogen-activated
protein kinases. J Gastroenterol. 42:887–896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong H, SuYang H, Erdjument-Bromage H,
Tempst P and Ghosh S: The transcriptional activity of NF-kappaB is
regulated by the IkappaB-associated PKAc subunit through a cyclic
AMP-independent mechanism. Cell. 89:413–424. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yubero S, Ramudo L, Manso MA and De Dios
I: Mechanisms of dexamethasone-mediated chemokine down-regulation
in mild and severe acute pancreatitis. Biochim Biophys Acta.
1792:1205–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim C, Sano Y, Todorova K, Carlson BA,
Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS and
Park JM: The kinase p38 alpha serves cell type-specific
inflammatory functions in skin injury and coordinates pro- and
anti-inflammatory gene expression. Nat Immunol. 9:1019–1027. 2008.
View Article : Google Scholar : PubMed/NCBI
|