Introduction
Osteoporosis and fractures are the most common
orthopedic diseases in the elderly (1). have been plagued by the delayed
healing and nonunion of fractures, bone defects and osteoporosis
have become a growing concern for orthopedic clinicians, and the
outcome of current clinical treatments are not very satisfactory.
Therefore, in order to prevent and reduce the occurrence of
osteoporosis and fracture, understanding the mechanisms underlying
osteoporosis and fracture healing has become important in research
associated with bone injury.
The maintenance of the normal functional state of
the body is dependent on an appropriate supply of oxygen. In
addition, maintaining homeostasis in regard to oxygen levels is a
prerequisite for cell life activity (2), and tissue oxygen concentrations must
be precisely controlled to fluctuate only within a very small range
(2,3). Due to a reduction in blood supply
following bone or soft tissue injury, the microenvironment
surrounding lesions enters a hypoxia state (4). Thus, angiogenesis serves an important
role in the process of fracture healing (5,6).
Osteogenesis is closely associated with angiogenesis in the
formation and repair of bone (5–7).
Vessels carry oxygen and nutrients; however, they also serve a
pivotal role in the formation, reshaping and alteration of bone
through interactions between osteoblasts, osteocytes or osteoclasts
and cytokines in the blood vessels (5–7).
Hypoxia is considered to be an important stimulus in angiogenesis
(8); this stimulus is now thought
to be achieved by hypoxia inducible factor-1α (HIF-1α) (9). HIF-1α is a key regulator of
vertebrate adaptation to hypoxia (9). The study of HIF-1α expression levels,
its function and hypoxia status under physiological and
pathological conditions in the skeletal system has become an area
of growing interest (10,11). There are two main aspects
associated with the regulatory function of HIF-1α in fracture
healing. The first being that HIF-1α can induce the formation of
blood vessels during the healing process of the fracture by
stimulating the expression of vascular endothelial growth factor
(VEGF) (10,11). Secondly, HIF-1α is directly
involved with the regulation of cell functioning, including in
osteoblasts, osteoclasts and chondrocytes (10,11).
However, the detailed mechanisms of HIF-1α in the proliferation,
apoptosis and differentiation of osteoblasts have not been fully
elucidated. Previous studies have revealed that blocking expression
of runt-related transcription factor 2 (Runx2) and HIF-1α inhibited
the formation of heterotopic ossification (11,12).
Runx2 can stabilize HIF-1α structure via the inhibition of HIF-1α
ubiquitination in order to promote angiogenesis in growth plate
hypertrophic chondrocytes (12).
Forkhead box class O1 (Foxo1) is one of the earliest
members identified in the Foxo family, and is also the most
representative of the Foxo family. Previous studies had
demonstrated that they serve an important role in a number of
physiological and pathological processes, including proliferation,
apoptosis, phagocytosis, metabolism, inflammation, differentiation
and oxidative stress (13,14). Previous studies revealed that Foxo1
mediates dendritic cells and macrophages in order to regulate
associated target genes in inflammatory responses (15); osteoclasts, dendritic cells and
macrophages share a common precursor cell line. However, only Foxo1
is the transcription factor required for osteoblast proliferation
and the maintenance of the body's redox balance (16). In addition, previous studies have
demonstrated that interactions and cooperation between Foxo1and
Runx2 serve a key role in the transcriptional regulation of
osteoblast markers, including alkaline phosphatase (ALP), and
osteocalcin (17,18). Runx2, ALP and osteocalcin are
closely associated with the development of osteoblasts.
In addition, orthopedic diseases in children and
adolescents, such as osteoporosis, and children with avascular
necrosis and non-traumatic avascular necrosis of the femoral head,
have received little attention when compared with orthopedic
diseases observed in the elderly (19–21).
A previous study revealed that the negative associations between
HIF-1α and the rate of bone cell apoptosis was involved in the
non-traumatic avascular necrosis of the femoral head (22). Furthermore, there has been no
report regarding the associations between HIF-1α and Foxo1. Thus,
in the present study, children's iliac cancellous bone was used to
determine whether HIF-1α regulates the proliferation,
differentiation and apoptosis of osteoblasts through the regulation
of Foxo1 expression.
Materials and methods
Cell culture
Bone tissues were obtained between February 2015 and
March 2017 from children with congenital dislocation of the hip
when they underwent surgery for extra iliac bone at Department of
Orthopedics, the Children's Hospital, Zhejiang University School of
Medicine. The present study was approved by the institutional
review board of The Children's Hospital (Zhejiang, China) and
written informed consent was obtained from the parents of each
participant. Only children who were not taking hormones or other
drugs, and had no metabolic bone disease were enrolled, comprising
2 males and 2 females, aged 3–5 years old. The obtained bone
tissues were maintained aseptically, and placed in DMEM/F-12
serum-free medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) for
storage at 4°C. Bone tissues were repeatedly rinsed with 0.9%
sterile saline until the rinse solution was clear without any
precipitates, and were washed twice with Dulbecco's PBS. The bone
tissue was cut to a size with ~1 mm3 volume and digested
with 0.25% trypsin at 37°C for 30 min.
Bone particles were then digested 4 times using 0.1%
collagenase II at 37°C for 30 min. The cells were collected by
centrifugation at 500 × g for 10 min at 4°C. They were inoculated
into four 100 ml flasks, DMEM/F-12 serum-free medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added and the
cells were incubated at 37°C in a humidified atmosphere containing
5% CO2. According to levels of the growth, culture
medium was replaced in the first 3–5 days, then it was subsequently
replaced every 2–3 days. When the primary cells were grown into
monolayers, the cells were digested with 0.25% trypsin for 3–5 min
at 37°C to continue subculture. In addition to natural
purification, enzymatic digestion and repeated adherence methods
were used to purify cells (23).
Identification of osteoblasts
The isolated cells were cultured in primary culture,
and morphological observation and imaging were performed under an
inverted phase contrast microscope when the cells were subcultured
to 80% confluence.
Cell osteocalcin immunofluorescence staining was
also performed to identify osteoblasts. Osteoblasts were inoculated
on coverslips and the medium was discarded when the cells reached
80% confluency; cells were then fixed with 95% ethanol for 10 min.
Cell climbing slices were washed with PBS three times for 5 min
each, incubated at room temperature with 0.5% Triton X-100 for 10
min, and then washed 3 times with PBS for 5 min each. Subsequently,
once the slices were incubated with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 20 min at room temperature, the
anti-osteocalcin antibody (ab13418; 31:100; Abcam, Cambridge, UK)
was added for incubation overnight at 4°C; this was followed by 3
washes with PBS for 5 min. The slices were then incubated with a
TRITC-labeled secondary antibody (YB1130; 1:50; Dako; Agilent
Technologies GmbH, Waldbronn, Germany) for 45 min at room
temperature. Subsequently, DAPI staining was performed to stain the
nuclei for 15 min at room temperature, which was followed by 3
washes with PBS for 5 min each. Then five fields were randomly
selected from each section and observed and imaged under a laser
confocal microscope.
Cell transfection
HIF-1α small interfering (si)RNA
(5′CCAACCTCAGTGTGGGT-AT3′) and negative siRNA control
(5′CCATGTAG-GCGCAGTCTAT3′) were synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China) and recombinant plasmid containing
HIF-1α (Addgene, Inc., Cambridge, MA, USA) were transfected into
cells with Lipofectamine 2000® (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Briefly, prior
treatment with siRNA, cells were seeded in 6-well plates and grown
to 50% confluence. Transfection of 50 nM siRNA in cells was carried
out using Lipofectamine 2000® following the
manufacturer's protocols. Cells were then incubated for 5 h at 37°C
and the medium was replaced with complete DMEM medium
(Sigma-Aldrich; Merck KGaA). Cells were harvested at least 24 h
following transfection for use in the following experiments.
Cell viability assay
Cell viability was determined using a Cell Counting
kit (CCK)-8 assay. Cells collected at 24, 48, 72 and 96 h following
transfection were inoculated in 96-well plates (2×105
cells/well) and 20 µl of CCK-8 (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was added to each well. Following incubation
for 4 h at 37°C, the absorption was read at 450 nm on an ELISA
reader (ELx800™; BioTek Instruments, Minneapolis, MN, USA).
Reactive oxygen species (ROS)
assay
Cells were harvested from all groups [control,
negative control (NC), HIF1a, Mock and siHIF1a] and washed with PBS
following transfection for 24 h. Cells were then incubated in 20 µM
2′,7′-Dichorofluorescin diacetate (Sigma-Aldrich; Merck KGaA) at
37°C for 1 h following the manufacturer's protocols. Following
washing with PBS, ROS levels in cells were determined using FACS
Aria II flow cytometer (BD Biosciences, San Jose, CA, USA) and data
analysis was performed using FlowJo version 7.6 (FlowJo LLC,
Ashland, OR, USA).
Apoptosis determination by flow
cytometry assay
Cells collected from all groups (control, NC, HIF1a,
Mock and siHIF1a) were digested using 0.25% trypsin-EDTA for 3–5
min at 37°C. Subsequently, cells were harvested at a density of
1×106 by centrifugation at 500 × g for 4 min at 4°C.
Cells were washed with PBS and then placed in binding buffer (140
mM NaCl and 2·5 mM CaCl2 in 10 mM HEPES/NaOH; pH 7·4). A total of 5
µl propidium iodide (PI) and fluorescein isothiocyanate
(FITC)-labeled Annexin V (Biodesign International; Meridian Life
Science, Inc., Memphis, TN, USA) were added to cells for incubation
at room temperature for 10 min. Samples were analyzed by FACS Aria
II flow cytometer (BD Biosciences) and data analysis was performed
using FlowJo version 7.6 (FlowJo LLC). Those that were Annexin
V-FITC positive and PI negative were considered early apoptotic
cells, and late apoptotic cells were indicated by Annexin V-FITC
positive and PI positive.
Western blot assay
Cells were collected from all groups and washed
twice with ice-cold PBS. Cells were then lysed in
radioimmunoprecipitation assay lysis buffer (50 mM Tris-HCl, 200 mM
NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.25% deoxycholate,
and protease and phosphatase inhibitors) at 37°C for 30 min and
centrifuged for 20 min at 6,000 × g at 4°C. The supernatants were
collected and 50 µg of cell lysate was used to separate proteins by
10% SDS-PAGE, which were then transferred onto a nitrocellulose
membrane. The membranes were blocked with 5% non-fat dry milk for 1
h at room temperature. Subsequently, the blots were incubated with
primary antibodies against apoptosis-inducing factor (AIF; ab32516;
1:1,000; Abcam), B-cell lymphoma 2 (Bcl-2; ab32124; 1:1,000;
Abcam), Bcl-2-associated X protein (Bax; ab32503; 1:2,000; Abcam),
caspase-3 (ab13585; 1:1,000; Abcam), Runx2 (ab76956; 1:1,000;
Abcam), ALP (ab224335; 1:1,000; Abcam), osteocalcin (ab13420;
1:1,000; Abcam), F-actin (ab205; 1:500; Abcam) and Foxo1 (ab207204;
1:1,000; Abcam), at the appropriate dilution at 4°C overnight. The
blots were then washed three times with TBS and incubated with
horseradish peroxidase-conjugated secondary antibodies (P0260;
1:2,000 dilution; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) for at room temperature for 1 h. The protein-antibody
complexes were detected using an enhanced chemiluminescence system
(GE Healthcare Life Sciences, Little Chalfont, UK). ImageJ software
(version 1.42; National Institutes of Health, Bethesda, MD, USA)
was used to determine densitometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Cells collected from all groups were washed twice
with ice-cold PBS. RNA was isolated using the RNeasy mini-kit
(Qiagen GmbH, Hilden, Germany) following the manufacturer's
protocols. Reverse transcription was carried out at with the
iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) according to the manufacturer's protocols. The temperature
protocol used for RT-PCR was: 30°C for 10 min, 42°C for 30 min,
99°C for 5 min and 4°C for 5 min. Subsequently, qPCR was performed
using the iQ SYBR-Green Supermix (Bio-Rad Laboratories, Inc.) and
iCycleriQ thermal cycler (Bio-Rad Laboratories, Inc.) following the
manufacturer's protocols. The thermocycling conditions for qPCR
were: 45°C for 10 min, 95°C for 10 min, 40 cycles of 95°C 15 sec
and 60°C for 45 sec. Each sample was performed in duplicate. Data
was calculated using the 2−∆∆Cq method (24) and relative expression was
normalized to housekeeping gene (GAPDH). The primer sequences used
were as follows: HIF-1α forward, 5′-TCCAAGAAGCCCTAACGTGT-3′ and
reverse, 5′-TGATCGTCTGGCTGCTGTAA-3′; AIF forward,
5′-TCTACCCTCTATGCCAGGACT-3′ and reverse,
5′-ACCCAGATGTTAGAGCGTGC-3′; Bax forward, 5′-TCATGGGCTGGACACTGGAC-3′
and reverse, 5′-CACAGTCCAAGGCAGTGGGA-3′; Bcl-2 forward,
5′-TGGGCCACAAGTGAAGTCAA-3′ and reverse, 5′-TGATGCGGAAGTCACCGAAA-3′;
caspase-3 forward, 5′-TCTGGTTTTCGGTGGGTGTG-3′ and reverse,
5′-GTCGGCCTCCACTGGTATTT-3′; Foxo1 forward,
5′-GCGCTTAGACTGTGACATGG-3′ and reverse, 5′-ACTAACCCTCAGCCTGACAC-3′;
Runx2 forward, 5′-CTGTGGTTACTGTCATGGCG-3′ and reverse,
5′-AGGTAGCTACTTGGGGAGGA-3′; ALP forward, 5′-GTCAGTGGGAGTGGTAACCA-3′
and reverse, 5′-ACATGTACTTTCGGCCTCCA-3′; Osteocalcin forward,
5′-AATCCGGACTGTGACGAGTT-3′ and reverse, 5′-TTATTTGGGAGCAGCTGGGA-3′;
and GAPDH forward, 5′-CGGGAAACTGTGGCGTGATG-3′, and reverse
5′-ATGACCTTGCCCACAGCCTT-3′.
Statistical analysis
All of the experimental data were expressed as the
mean ± standard deviation. A t-test was used for comparisons
between two groups and a one-way analysis of variance was performed
for multiple comparisons and Bonferroni post hoc test was used for
pairwise comparison. All experiments were repeated at least 3
times. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
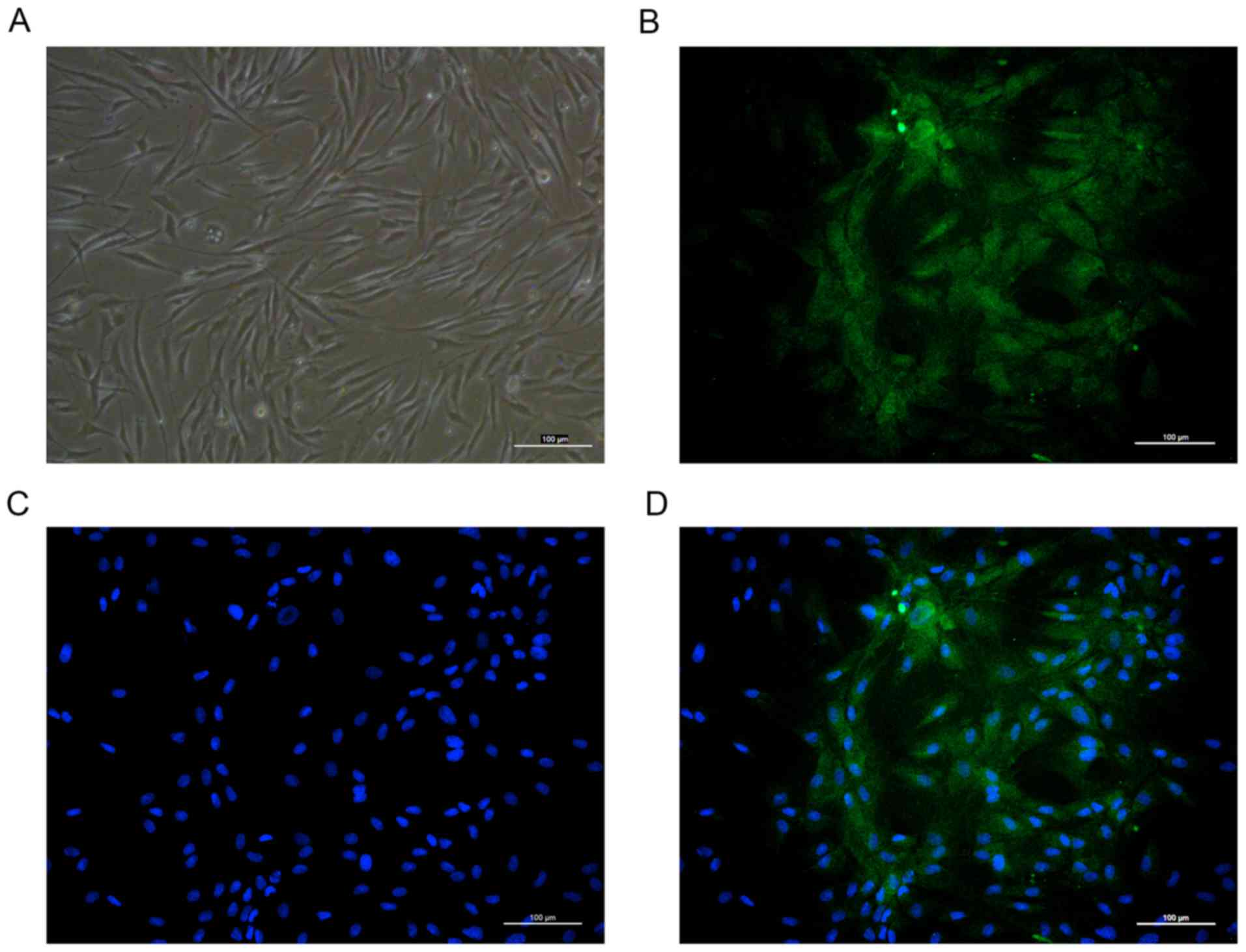
Identification of osteoblasts by
morphology and fluorescence immunity
The primary cultured human osteoblasts were observed
under an inverted phase contrast microscope and revealed spherical
morphology prior to adherent growth. Following incubation, the
cells adhered and distributed evenly on the wall of the bottle. The
cells were irregularly shaped, with long fusiform, star or
irregular polygons. The cytoplasm was homogeneous and the central
nucleus was round, oval centered or biased (Fig. 1A). To further confirm the
osteoblasts obtained, the present study performed
immunofluorescence staining of osteocalcin and osteoblasts were
observed to exhibit intense cytoplasmic staining for osteocalcin
(Fig. 1B-D).
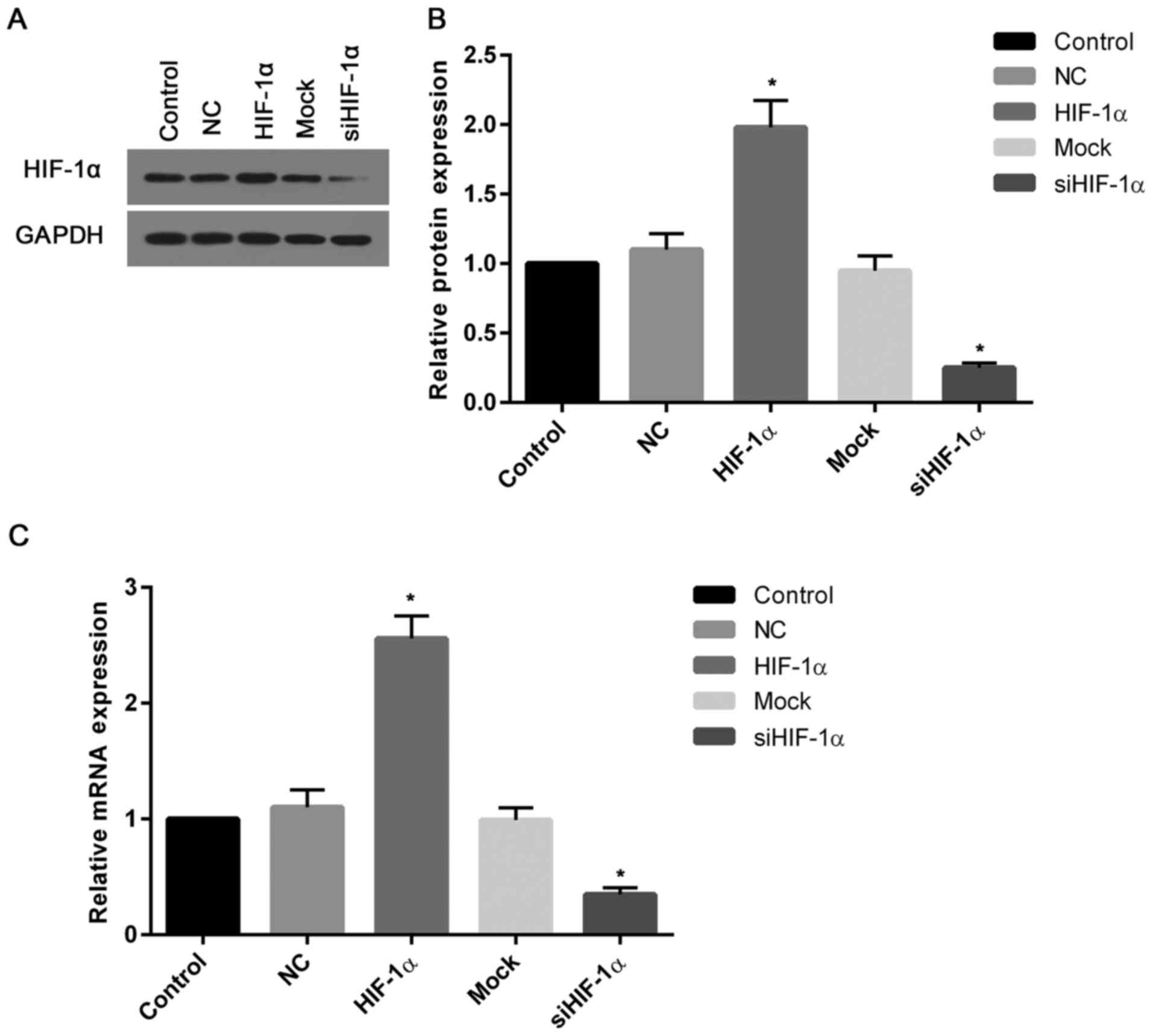
Expression of HIF-1α at the protein
and mRNA levels
To assess the transfection efficiency, the present
study determined the transcriptional and translational levels of
HIF-1α in cells. The western blotting and mRNA assays demonstrated
that the levels of HIF-1α protein and mRNA in cells transfected
with recombinant HIF-1α were ~2 and 2.5-fold greater than that of
the control (Fig. 2A and B).
However, the expression of HIF-1α protein and mRNA in cells treated
with HIF-1α siRNA were markedly suppressed when compared with the
control (Fig. 2C).
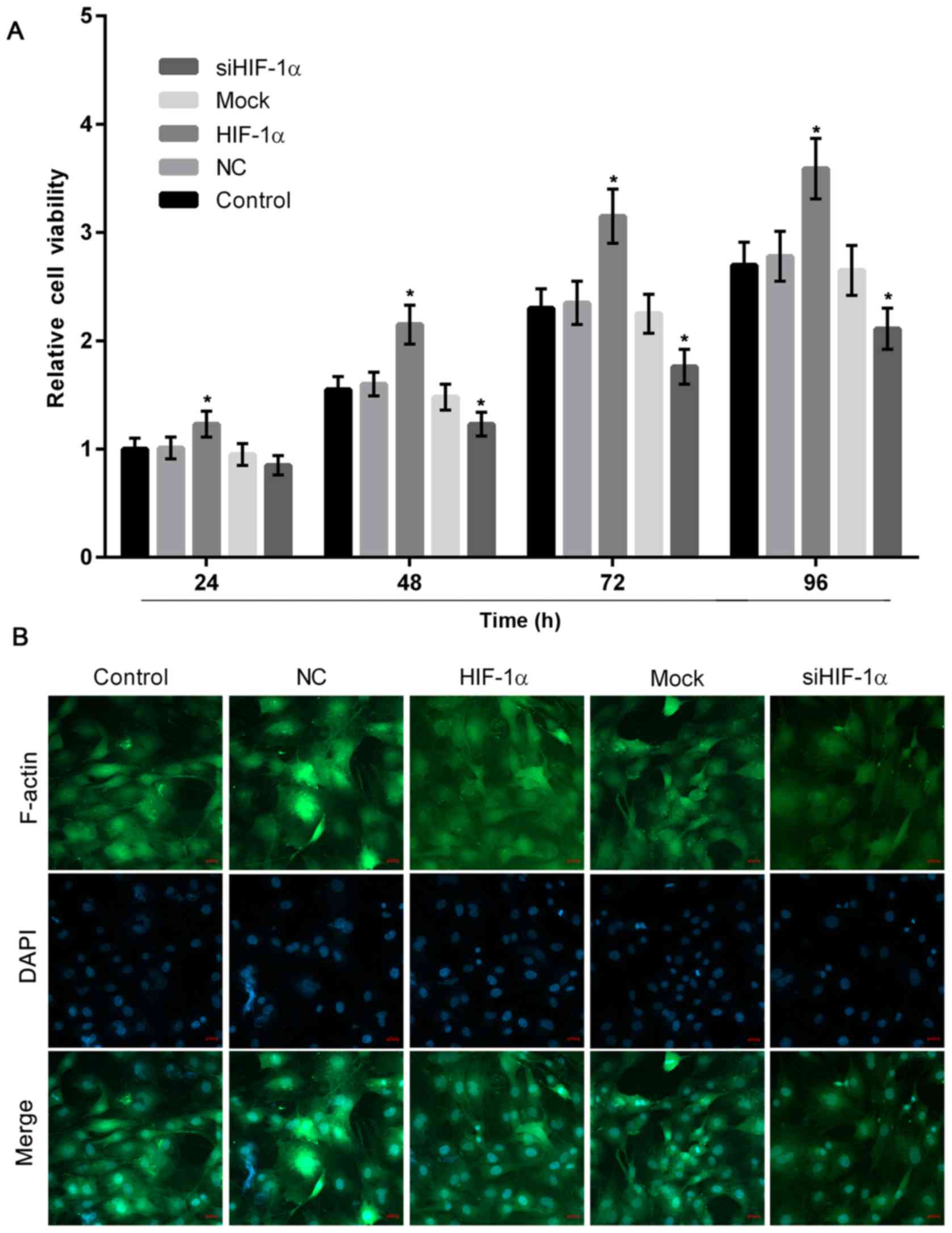
HIF-1α overexpression or knockdown
induces or suppresses the proliferation of osteoblasts
To evaluate the effect of differentiated HIF-1α
expression in osteoblasts, the present study performed a CCK-8
assay, which revealed that HIF-1α overexpression significantly
stimulated osteoblast proliferation, while downregulation of HIF-1α
significantly decreased the growth of osteoblasts when compared
with the control (Fig. 3A).
Furthermore, in the HIF-1α overexpression group, F-actin positive
immunofluorescence staining was greater and osteoblast
proliferation was higher, when compared with the group with HIF-1α
downregulation (Fig. 3B).
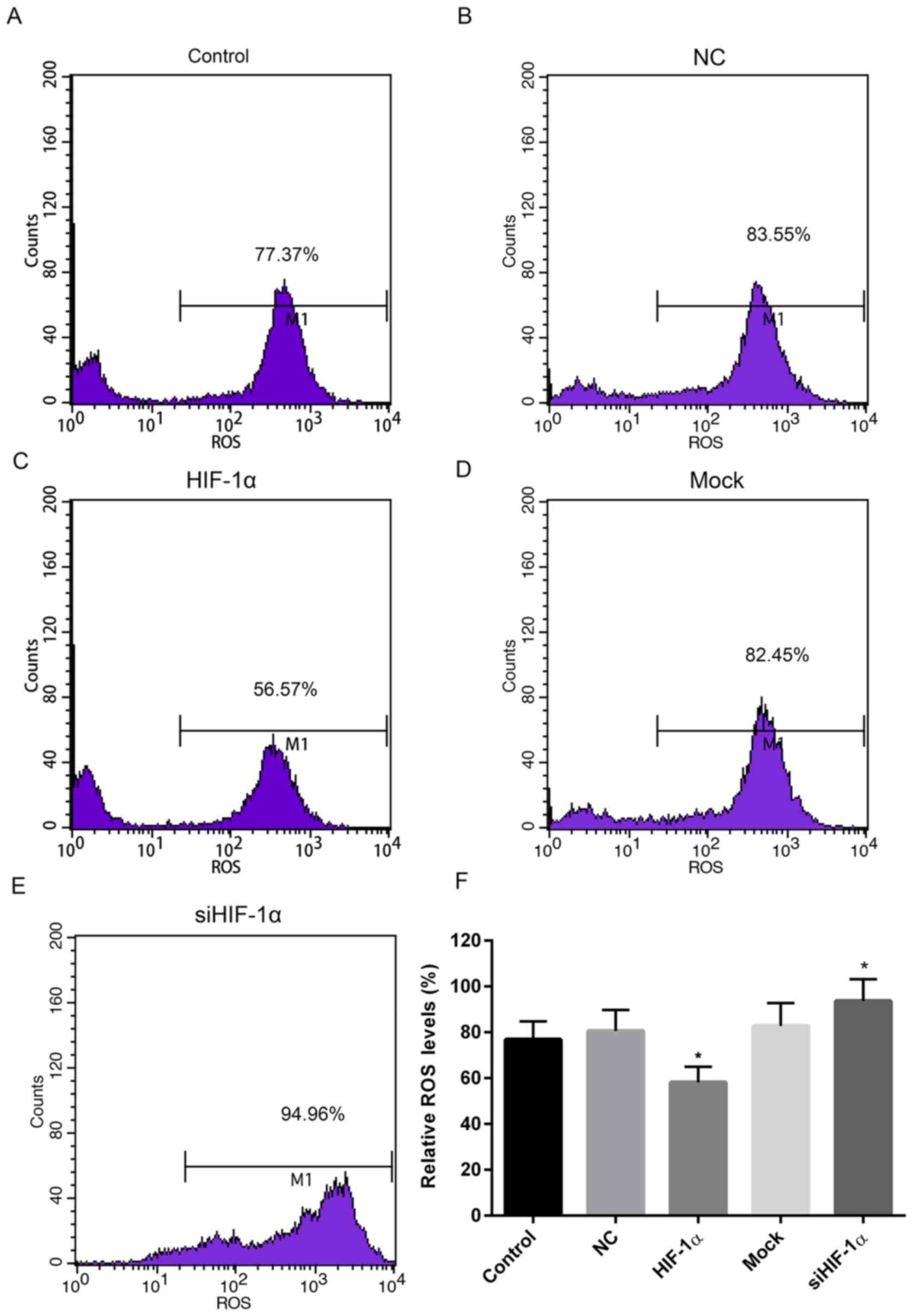
ROS levels increase in cells with
HIF-1α downregulation
To measure the levels of oxidative stress in cells
up- or downregulated HIF-1α, the ROS levels were determined. When
compared with the control and NC groups, the relative ROS level in
cells treated with recombinant HIF-1α was markedly reduced. By
contrast, the relative ROS level in cells treated with HIF-1α siRNA
was elevated when compared with the control and mock groups
(Fig. 4).
Overexpression or downregulation of
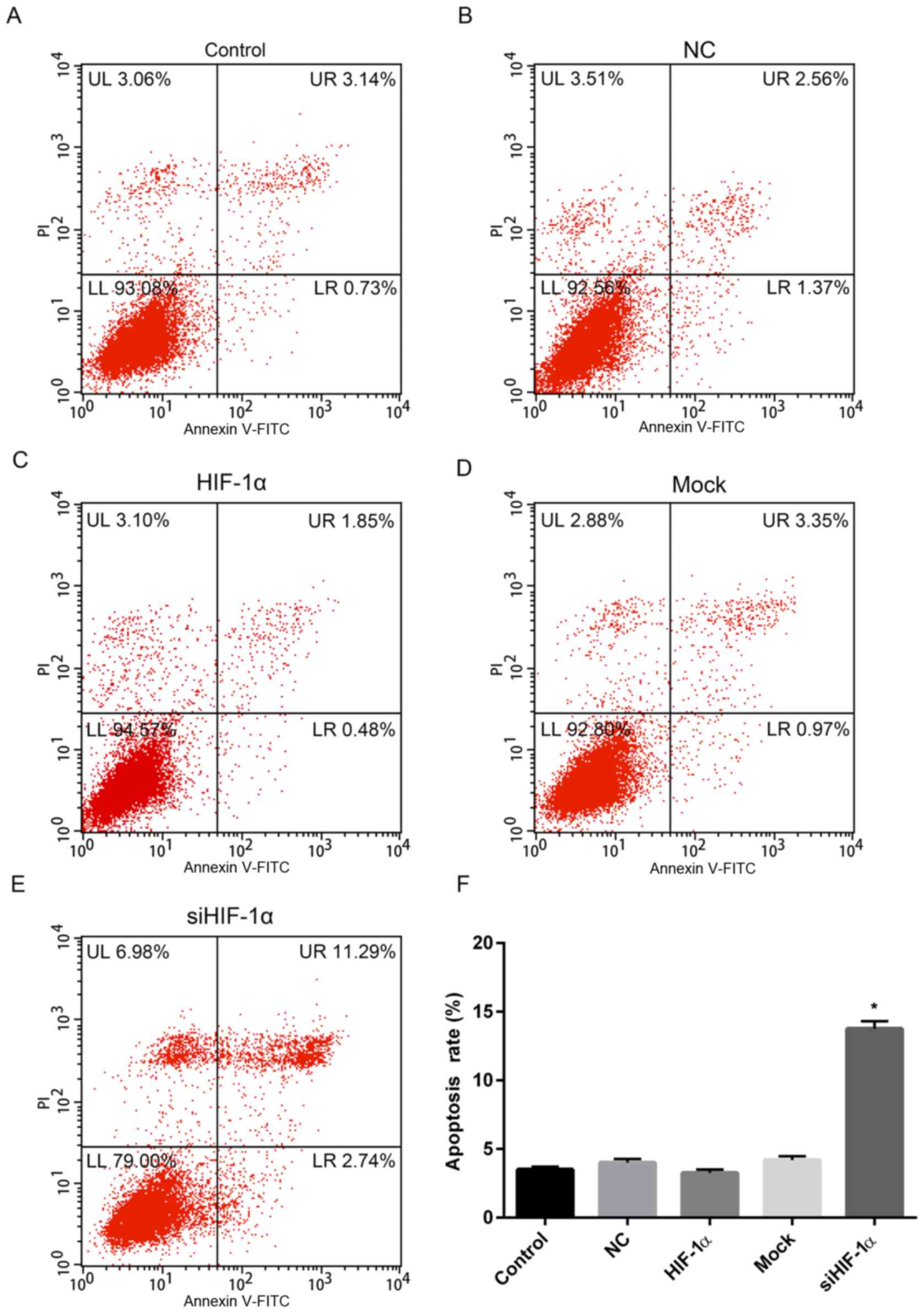
HIF-1α suppresses or stimulates apoptosis, respectively
The effect of overexpression or downregulation of
HIF-1α on apoptosis was investigated and the results revealed that
the apoptosis rate of cells with HIF-1α overexpression was 2.33%,
which was slightly lower than that of the control (3.87%) and NC
(3.93%) groups (Fig. 5A-C and F).
However, the rate of apoptosis for cells treated with HIF-1α siRNA
was 14.03%, which was significantly higher than that of control and
mock (4.32%) groups (Fig.
5D-F).
Up- or downregulated HIF-1α alters the
expression of apoptosis-associated genes
To investigate the effect of HIF-1α on the
expression of genes associated with apoptosis, the present study
further detected the expression of AIF, Bax, Bcl-2 and caspase-3.
The expression levels of AIF, Bax and caspase-3 mRNA and protein
decreased in cells with overexpressed HIF-1α, compared with the
control and NC groups (Fig. 6). By
contrast, they were increased in cells with downregulated HIF-1α,
when compared with the control and mock groups. However, unlike
Bax, the Bcl-2 mRNA and protein levels were increased in cells
treated with recombinant HIF-1α and decreased in cells treated with
HIF-1α siRNA when compared with the control (Fig. 6).
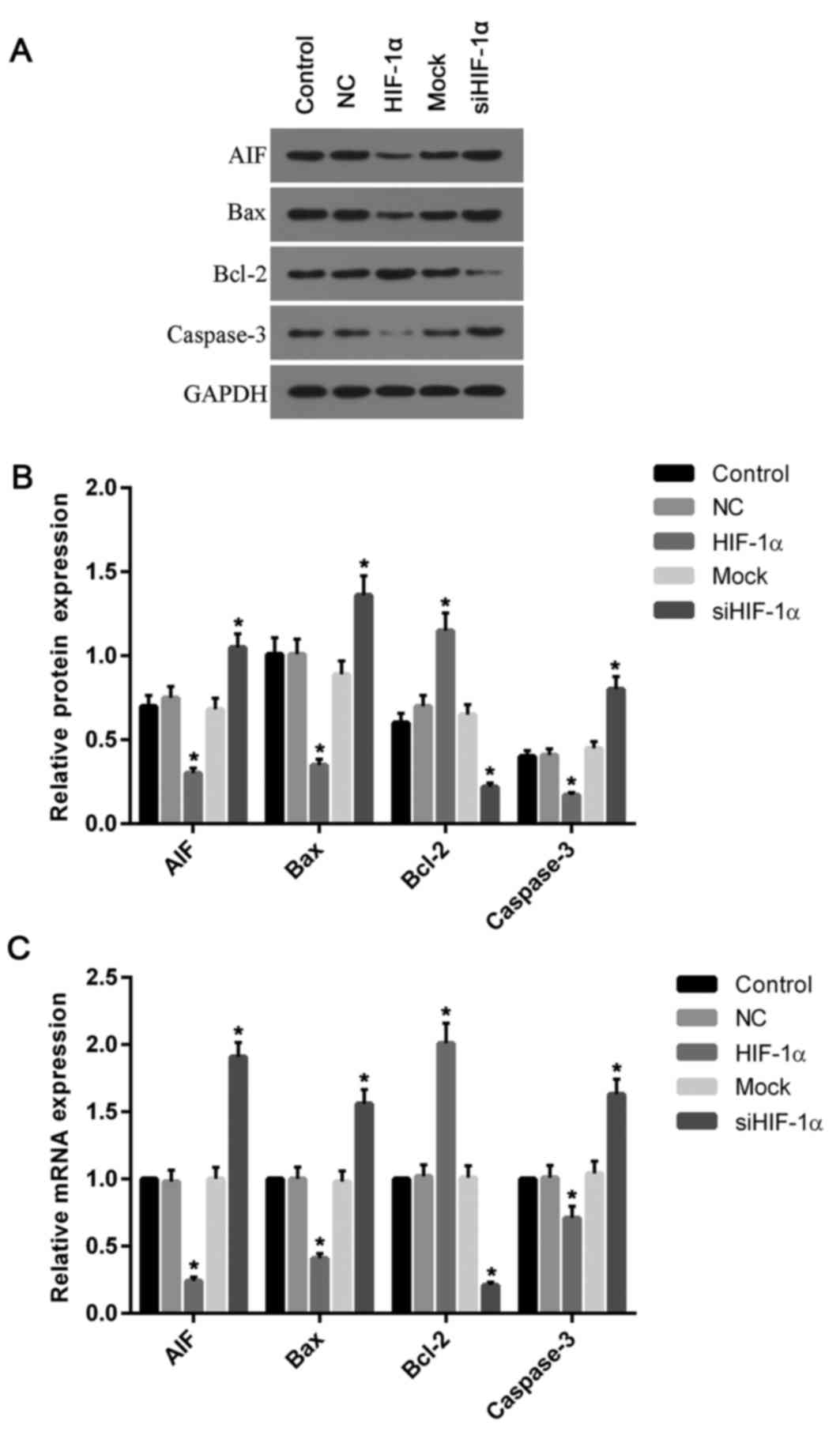 | Figure 6.Expression of apoptosis associated
genes was altered by the overexpression and silencing of HIF-1α.
(A) Western blotting was performed to analyze the proteins levels
of AIF, Bax, Bcl-2 and caspase-3. (B) The protein levels of AIF,
Bax and caspase-3 were decreased in cells overexpressing HIF-1α
compared with the control and NC groups, while the level of Bcl-2
was increased when compared with control. (C) Similarly, the
relative mRNA levels of AIF, Bax and caspase-3 were decreased in
cells with HIF-1α overexpression compared with the control and NC
groups; however, the levels of Bcl-2 mRNA were elevated when
compared with control. Silencing of HIF-1α had the opposite effects
on protein and mRNA levels, with Bcl-2 levels significantly
decreased and AIF, Bax and caspase-3 levels significantly
increased. *P<0.05 vs. control. HIF-1α, hypoxia inducible
factor-1α; NC, negative control; siRNA, small interfering RNA; AIF,
apoptosis-inducing factor; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein. |
Overexpression or down-regulation of
HIF-1α increases or decreases the expression of Foxo1 and
osteoblast markers
To explore the function of HIF-1α in the regulation
of the expression of Foxo1 and osteoblast markers including Runx2,
ALP and osteocalcin, the present study determined their expression
following the upregulation or silencing of HIF-1α. The results
demonstrated that the protein levels of Foxo1, Runx2, ALP and
osteocalcin were significantly elevated in cells treated with
recombinant HIF-1α when compared with those of the control and NC
groups (Fig. 7A and B). However,
in cells treated with HIF-1α siRNA, the protein levels of Foxo1,
Runx2, ALP and osteocalcin were markedly decreased compared with
the control and mock groups (Fig. 7A
and B). Similarly, the mRNA expression levels of Foxo1, Runx2,
ALP and osteocalcin in cells overexpressing HIF-1α were ~1.5, 2,
2.2 and 2.7-fold greater of that of the control and NC groups,
respectively. Furthermore, the expression levels of Foxo1, Runx2,
ALP and osteocalcin mRNA in cells treated with HIF-1α siRNA were
significantly suppressed when compared with the control and mock
groups (Fig. 7C). To further
confirm these altered osteoblast marker expressions, the expression
of the osteogenic marker Runx2 was evaluated by immunofluorescence
and the greatest Runx2 expression levels were observed in the
HIF-1α overexpression group, while the lowest levels were seen in
cells with HIF-1α silencing (Fig.
7D). In addition, to confirm the role of Foxo1 in
HIF-1α-induced osteoblast proliferation, further blotting
experiments were performed. The results revealed that Runx2 and ALP
expression induced by HIF-1α were markedly reversed by Foxo1 siRNA,
while osteocalcin was not affected by Foxo1 siRNA (Fig. 7E). Thus, it was proposed that
HIF-1α-induced expression of Runx2 and ALP may be completely
dependent on the expression levels of Foxo1, and in turn,
osteocalcin may be partially dependent on Foxo1, though to a much
lesser degree.
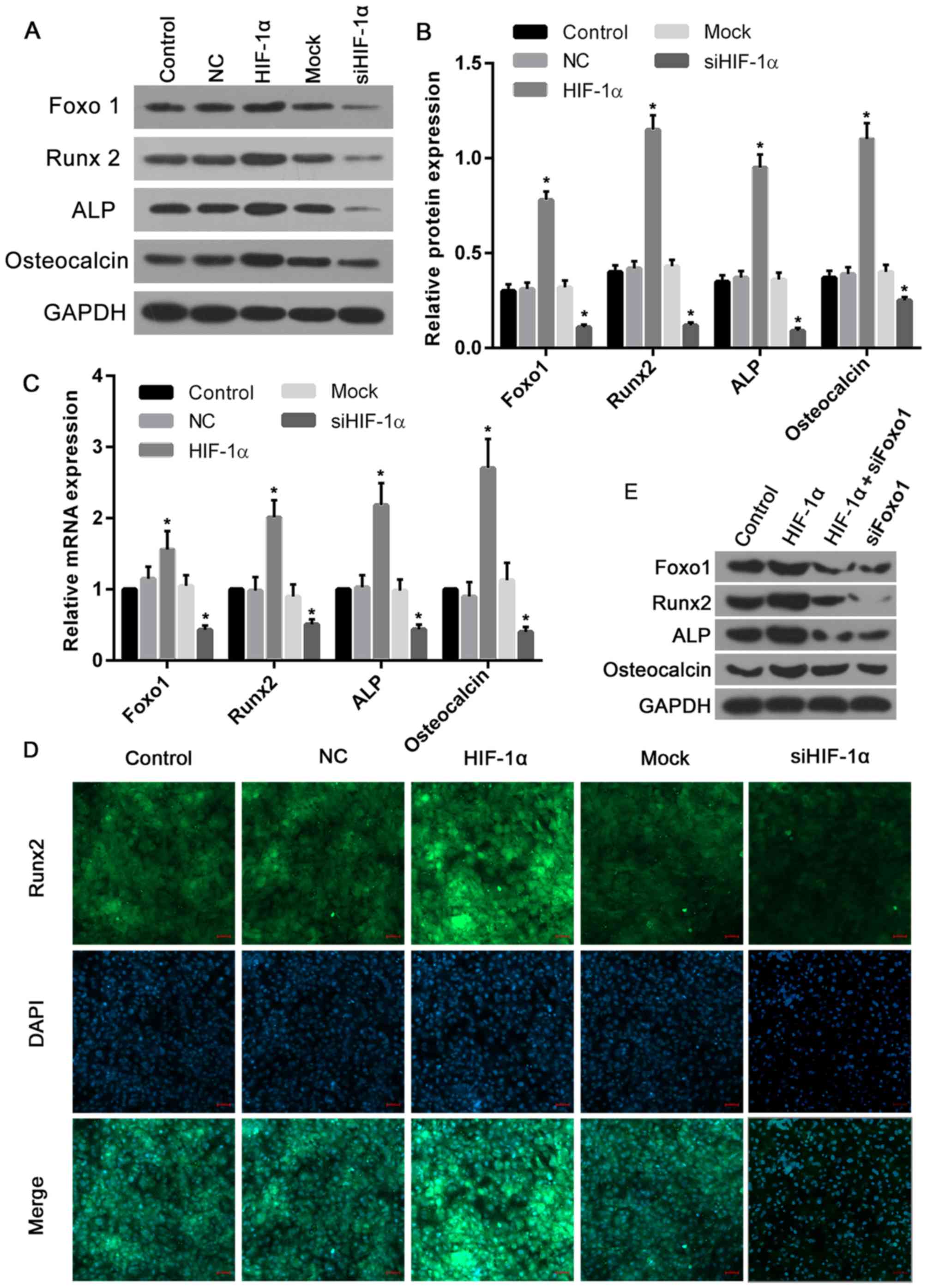 | Figure 7.HIF-1α overexpression increased, and
knockdown decreased, the expression levels of Foxo1 and osteoblast
markers. (A) Western blotting was performed to analyze the proteins
levels of Foxo1, Runx2, ALP and osteocalcin. (B) Their protein
expression levels significantly increased in cells overexpressing
HIF-1α when compared with the control and NC groups. However, the
protein levels significantly decreased in cells treated with HIF-1α
siRNA, compared with the control and mock groups. (C) Similarly,
the mRNA expression levels of Foxo, Runx2, ALP and osteocalcin in
cells with HIF-1α overexpression were elevated, while the mRNA
levels in cells treated with HIF-1α siRNA were significantly
inhibited. (D) Immunofluorescence analysis confirmed that the
highest expression of Runx2 was observed in cells overexpressing
HIF-1α, while the lowest expression of Runx2 was exhibited by cells
with HIF-1α silencing (scale bars, 50 µm). (E) Runx2 and ALP
protein expression induced by HIF1α were markedly decreased by
Foxo1 siRNA, while only a slight reduction in osteocalcin was
produced by Foxo1 siRNA. *P<0.05 vs. control. HIF-1α, hypoxia
inducible factor-1α; NC, negative control; si-/siRNA, small
interfering RNA; Runx2, runt-related transcription factor 2; ALP,
alkaline phosphatase; Foxo1, forkhead box class O1. |
Discussion
HIF-1α serves a pivotal role in the stimulation of
bone formation via the regulation of several key factors such as
Runx2 (12). The Foxo subfamily
regulates the expression of genes associated with a variety of
physiological and pathological processes (14); however, they also have a role in
the proliferation, differentiation and apoptosis of osteoblasts,
which to date has been quite well studied. Previous studies have
demonstrated that Foxo1 can stimulate the growth of osteoblasts by
increasing the expression of Runx2 (17). Therefore, the present study
investigated whether HIF-1α affects the expression of Runx2 by
regulating Foxo1. The results revealed that the interactions
between HIF-1α and Foxo1 serve a key role in the proliferation,
differentiation and apoptosis of osteoblasts.
HIF-1α is an important transcription factor involved
in cell metabolism, with roles such as promoting glycolysis and
inhibiting mitochondrial respiration (25). HIF-1α upregulates pyruvate
dehydrogenase kinase, inhibits pyruvate dehydrogenase activity and
blocks pyruvate entry into tricarboxylic acid (TCA) cycle, thereby
inhibiting mitochondrial oxidative phosphorylation (25–27).
As mitochondrial respiration is the primary source of ROS, it was
hypothesized that HIF-1α may reduce ROS production. The results
demonstrated that ROS levels were decreased in cells with
overexpressed HIF-1α, which is consistent with the authors'
hypothesis. In addition, ROS levels were markedly increased in
cells treated with HIF-1α siRNA compared with normal osteoblasts.
These results suggested that the underlying mechanism may involve
the suppression effect of HIF-1α on ROS in osteoblasts.
In the process of bone development and regeneration,
angiogenesis is closely associated with bone neoplasm (28). Previous studies had observed that
HIF-1α can promote the proliferation and migration of vascular
endothelial cells, and increase the permeability of vascular
endothelial cells (29,30), which provides nutrition for the
growth of cells and the establishment of capillaries, and also
promotes the development of bone marrow-derived endothelial
progenitor cells that transfer to the site of hypoxic injury
(30,31). Thus, the present study measured the
cell viabilities and rates of apoptosis in osteoblasts with HIF-1α
overexpression or knockdown. The results revealed that the cell
viabilities and proliferation were increased in cells with
overexpression, and decreased in cells with downregulated HIF-1α.
Furthermore, apoptosis was significantly increased in cells with
silenced HIF-1α; however, the apoptosis rate in cells with
overexpressed HIF-1α was marginally decreased compared with normal
cells. Consistent with these results, the expression levels of the
proapoptotic genes AIF, Bax and caspase-3 were increased, while the
anti-apoptotic gene Bcl-2 was decreased in cells treated with
HIF-1α siRNA. These results and those of previous reports indicate
that the inhibition of HIF-1α function suppresses osteoblast
proliferation (12).
The Foxo family mainly includes Foxo1, Foxo3 and
Foxo4, and is a group comprised of multifunctional transcription
factors involved in the cell cycle, apoptosis and ROS metabolism
(14). In addition, previous
investigations revealed that Foxo1 is closely associated with the
proliferation, differentiation and apoptosis of osteoblasts
(16,18,32,33).
Therefore, the present study detected the expression levels of
Foxo1 in order to determine the associations between HIF-1α and
Foxo1. The transcriptional and translational levels of Foxo1 were
increased in cells with HIF-1α overexpression. Previous studies
have demonstrated that Foxo1 can upregulate the ROS reducing agent
manganese peroxidase, Catalase and sestrin 3, which produce
superoxide oxidation, antioxidant protein (overoxidized
peroxiredoxins) degradation of ROS (34,35).
Therefore, it was assumed in the present study that the increased
ROS levels in cells with HIF-1α silencing were associated with the
downregulation of Foxo1 induced by knockdown HIF-1α. Notably, the
complete knockdown of Foxo1 in vivo has previously been
observed to induce the incomplete development of the embryonic
vascular system, which in turn leads to the apoptosis of embryonic
cells and thus, the termination of the pregnancy (36). Furthermore, Foxo1 serves a pivotal
protective role in endoplasmic reticulum stress-, hypoxia- and
tumor necrosis factor-induced apoptosis in a variety of cell lines
(37–39). Therefore, the interactions between
HIF-1α and Foxo1 may be an important factor for the regulation of
osteoblast apoptosis.
To date, HIF-1 has been associated with the
regulation of a variety of genes including VEGF, bone morphogenetic
protein and osteocalcin, which in turn are closely associated with
angiogenesis and bone formation (30,31).
Several studies have reported that the deletion of HIF-1α results
in the downregulation of osteoblast markers, including Runx2, ALP
and osteocalcin (12,40,41).
However, the mechanism by which HIF-1α regulates these genes
remains unclear. In addition, a number of investigations have
revealed that knockout of Foxo1 markedly reduced the expression of
Runx2, ALP and osteocalcin, resulting in the reduction of culture
calcification even with exposure to osteogenic stimulants (17,19,32).
Thus, it was hypothesized in the present study that there may be a
close association between HIF-1α and Foxo1 in the regulation of the
expression of these genes. Therefore, the expression of these genes
was further investigated. The results revealed that Runx2 and ALP
expression induced by HIF1α were markedly reduced by Foxo1 siRNA;
however, osteocalcin was not notably affected by Foxo1 siRNA. It is
therefore a possibility that the HIF1α-induced expression of Runx2
and ALP may be completely dependent upon the expression levels of
Foxo1, and osteocalcin may be partially dependent on Foxo1. The
results of the present study were consistent with the authors'
hypothesis and with the results of previous studies (17,18,32).
Notably, the mRNA and protein levels of Runx2, ALP and osteocalcin
had similar expression profiles as those of HIF-1α and Foxo1.
Silencing HIF-1α resulted in the decreased expression of Runx2, ALP
and osteocalcin, while overexpression of HIF-1α led to an increased
expression of Runx2, ALP and osteocalcin. The accumulation of
HIF-1α protein has been associated with Runx2 in ATDC5 chondrocytes
and HEK293 cells (42). In
addition, Runx2 can promote the nuclear translocation of HIF-1α in
HEK293 cells (42). Runx2 can also
stabilize the structure of HIF-1α by suppressing the ubiquitination
of HIF-1α (40,42). In fact, there are only two specific
transcripts in osteoblasts, one encoding Runx2 and the other
encoding osteocalcin, in which osteocalcin is an inhibitor of
osteoclast function and is expressed only when osteoblasts are
completely differentiated (43,44).
Furthermore, Runx2 is required for the expression of
osteoblast-specific proteins such as osteocalcin (44). Through the regulation of
osteocalcin expression, Runx2 can promote bone formation in
differentiated osteoblasts (45).
In conclusion, the results of the present study
indicated that the dependent activation of Foxo1 by HIF-1α may be
essential for osteoblast cell survival, differentiation and
proliferation. The increased viabilities of osteoblasts derived
from children's iliac cancellous bone with elevated HIF-1α and
Foxo1 levels provides evidence for novel approaches that stimulate
the development of osteoblasts by activating HIF-1α and Foxo1 in
combination.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GX performed the experiments and wrote and revised
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of The Children's Hospital (Zhejiang, China).
Consent for publication
Written informed consent was obtained from the
parents of each participant.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Moradi R and Atik OS: Are orthopedic
surgeons more aware of medical treatment of osteoporotic fractures
in the last decade? Eklem Hastalik Cerrahisi. 25:80–84. 2014.(In
Turkish). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng L, Kelly CJ and Colgan SP:
Physiologic hypoxia and oxygen homeostasis in the healthy
intestine. A review in the theme: Cellular responses to hypoxia. Am
J Physiol Cell Physiol. 309:C350–C360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iranon NN and Miller DL: Interactions
between oxygen homeostasis, food availability, and hydrogen sulfide
signaling. Front Genet. 3:2572012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Atkinson PJ, Cooper TG, Anseth S, Walter
NE, Kargus R and Haut RC: Association of knee bone bruise frequency
with time postinjury and type of soft tissue injury. Orthopedics.
31:4402008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murata K, Ito H, Yoshitomi H, Yamamoto K,
Fukuda A, Yoshikawa J, Furu M, Ishikawa M, Shibuya H and Matsuda S:
Inhibition of miR-92a enhances fracture healing via promoting
angiogenesis in a model of stabilized fracture in young mice. J
Bone Miner Res. 29:316–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fauzi A, Kamal AF, Kurniawan A and Kodrat
E: Role of sildenafil in acceleration of delayed union fracture
healing on Sprague-Dawley rats model. Br J Med Med Res. 8:419–428.
2015. View Article : Google Scholar
|
|
7
|
Fang TD, Salim A, Xia W, Nacamuli RP,
Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia
AJ and Longaker MT: Angiogenesis is required for successful bone
induction during distraction osteogenesis. J Bone Miner Res.
20:1114–1124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molica S, Vitelli G, Levato D, Gandolfo GM
and Liso V: Increased serum levels of vascular endothelial growth
factor predict risk of progression in early B-cell chronic
lymphocytic leukaemia. Br J Haematol. 107:605–610. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benita Y, Kikuchi H, Smith AD, Zhang MQ,
Chung DC and Xavier RJ: An integrative genomics approach identifies
Hypoxia inducible factor-1 (HIF-1)-target genes that form the core
response to hypoxia. Nucleic Acids Res. 37:4587–4602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen D, Tian W, Li Y, Tang W and Zhang C:
Osteoblast-specific transcription factor Osterix (Osx) and HIF-1α
cooperatively regulate gene expression of vascular endothelial
growth factor (VEGF). Biochem Biophys Res Commun. 424:176–181.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lechler P, Klein SM, Prantl L, Englert C,
Renkawitz T and Grifka J: Hypoxic downregulation of cellular
proliferation and loss of phenotype stability in human osteoblasts
is mediated by HIF-1α. Clin Hemorheol Microcirc. 49:279–286.
2011.PubMed/NCBI
|
|
12
|
Lin L, Shen Q, Leng H, Duan X, Fu X and Yu
C: Synergistic inhibition of endochondral bone formation by
silencing Hif1α and Runx2 in trauma-induced heterotopic
ossification. Mol Ther. 19:1426–1432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HS, Nam JS, Le SS, Kim LS, Ryu BY,
Kang HJ, Choi BS, Ganbold B and Ko YC: Abstract 564: Foxo3a
regulates cell cycle arrest through the regulation of p53, p21 and
GADD45 signaling activity in Quercetin-treated MDA-MB-231 breast
cancer cells. Cancer Res. 73:5642013. View Article : Google Scholar
|
|
14
|
Lu H and Huang H: FOXO1: A potential
target for human diseases. Curr Drug Targets. 12:1235–1244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown J, Wang H, Suttles J, Graves DT and
Martin M: Mammalian target of rapamycin complex 2 (mTORC2)
negatively regulates toll-like receptor 4-mediated inflammatory
response via FoxO1. J Biol Chem. 286:44295–44305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar
G, Ajita J, Rhee Y, Kim CH and Lim SK: miR-182 is a negative
regulator of osteoblast proliferation, differentiation, and
skeletogenesis through targeting FoxO1. J Bone Miner Res.
27:1669–1679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Xu H, Yu S, Cao H, Fan J, Ge C,
Fransceschi RT, Dong HH and Xiao G: Foxo1 mediates insulin-like
growth factor 1 (IGF1)/insulin regulation of osteocalcin expression
by antagonizing Runx2 in osteoblasts. J Biol Chem. 286:19149–19158.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teixeira CC, Liu Y, Thant LM, Pang J,
Palmer G and Alikhani M: Foxo1, a novel regulator of osteoblast
differentiation and skeletogenesis. J Biol Chem. 285:31055–31065.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubat O, Šmigovec I, Đapić T and Antičević
D: Cystic-like lesions of proximal femur associated with fractures
in children and adolescents-diagnostic and therapeutic dilemma. J.
2012.
|
|
20
|
Madadi F, Shamsian BS, Alavi S, Madadi F,
Eajazi A and Aslani A: Avascular necrosis of the femoral head in
children with acute lymphoblastic leukemia: A 4- to 9-year
follow-up study. Orthopedics. 34:e593–e597. 2011.PubMed/NCBI
|
|
21
|
Canale ST: Fracture of hip in children and
adolescents. Orthop Clin North Am. 21:341–352. 1990.PubMed/NCBI
|
|
22
|
Karatoprak O, Korkmaz MF, Kara AN, Göğüş A
and Işiklar ZU: Early results of autologous mononuclear bone marrow
cell implantation in nontraumatic avascular necrosis of the femoral
head. Acta Orthop Traumatol Turc. 42:178–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siggelkow H, Rebenstorff K, Kurre W,
Niedhart C, Engel I, Schulz H, Atkinson MJ and Hüfner M:
Development of the osteoblast phenotype in primary human
osteoblasts in culture: Comparison with rat calvarial cells in
osteoblast differentiation. J Cell Biochem. 75:22–35. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bel Aiba RS, Dimova EY, Görlach A and
Kietzmann T: The role of hypoxia inducible factor-1 in cell
metabolism-a possible target in cancer therapy. Expert Opin Ther
Targets. 10:583–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sudarshan S, Sourbier C, Kong HS, Block K,
Valera Romero VA, Yang Y, Galindo C, Mollapour M, Scroggins B,
Goode N, et al: Fumarate hydratase deficiency in renal cancer
induces glycolytic addiction and hypoxia-inducible transcription
factor 1alpha stabilization by glucose-dependent generation of
reactive oxygen species. Mol Cell Biol. 29:4080–4090. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu CW, Lin SC, Chen KF, Lai YY and Tsai
SJ: Induction of pyruvate dehydrogenase kinase-3 by
hypoxia-inducible factor-1 promotes metabolic switch and drug
resistance. J Biol Chem. 283:28106–28114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai J and Rabie AB: VEGF: An essential
mediator of both angiogenesis and endochondral ossification. J Dent
Res. 86:937–950. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim CH, Kin JK and Yoon JH: Dendritic
epidermal T cells promote wound healing by production of vascular
endothelial growth factor mediated by HIF-1a signaling. Am J Respir
Crit Care Med. 185:A42722012. View Article : Google Scholar
|
|
30
|
Kiani AA, Kazemi A, Halabian R,
Mohammadipour M, Jahanian-Najafabadi A and Roudkenar MH: HIF-1α
confers resistance to induced stress in bone marrow-derived
mesenchymal stem cells. Arch Med Res. 44:185–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang C, Sun J, Dai Y, Cao P, Zhang L,
Peng S, Zhou Y, Li G, Tang J and Xiang J: HIF-1A and C/EBPs
transcriptionally regulate adipogenic differentiation of bone
marrow-derived MSCs in hypoxia. Stem Cell Res Ther. 6:212015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siqueira MF, Flowers S, Bhattacharya R,
Faibish D, Behl Y, Kotton DN, Gerstenfeld L, Moran E and Graves DT:
FOXO1 modulates osteoblast differentiation. Bone. 48:1043–1051.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moriishi T, Kawai Y, Komori H, Rokutanda
S, Eguchi Y, Tsujimoto Y, Asahina I and Komori T: Bcl2 deficiency
activates FoxO through Akt inactivation and accelerates osteoblast
differentiation. PLoS One. 9:e866292014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alikhani M, Maclellan CM, Raptis M, Vora
S, Trackman PC and Graves DT: Advanced glycation end products
induce apoptosis in fibroblasts through activation of ROS, MAP
kinases, and the FOXO1 transcription factor. Am J Physiol Cell
Physiol. 292:C850–C856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen CC, Jeon SM, Bhaskar PT, Nogueira V,
Sundararajan D, Tonic I, Park Y and Hay N: FoxOs inhibit mTORC1 and
activate Akt by inducing the expression of sestrin3 and rictor. Dev
Cell. 18:592–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosaka T, Biggs WH III, Tieu D, Boyer AD,
Varki NM, Cavenee WK and Arden KC: Disruption of forkhead
transcription factor (FOXO) family members in mice reveals their
functional diversification. Proc Natl Acad Sci USA. 101:pp.
2975–2980. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen B, Chao L and Chao J: Pivotal role of
JNK-dependent FOXO1 activation in downregulation of kallistatin
expression by oxidative stress. Am J Physiol Heart Circ Physiol.
298:H1048–H1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martinez S, Tanabe KM, Cras-Méneur C,
Abumrad NA, Bernal-Mizrachi E and Permutt M: Inhibition of Foxo1
protects pancreatic islet beta-cells against fatty acid and
endoplasmic reticulum stress-induced apoptosis. Diabetes.
57:846–859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alikhani M, Alikhani Z and Graves DT:
FOXO1 functions as a master switch that regulates gene expression
necessary for tumor necrosis factor-induced fibroblast apoptosis. J
Biol Chem. 280:12096–12102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kwon TG, Zhao X, Yang Q, Li Y, Ge C, Zhao
G and Franceschi RT: Physical and functional interactions between
Runx2 and HIF-1α induce vascular endothelial growth factor gene
expression. J Cell Biochem. 112:3582–3593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hirata M, Kugimiya F, Fukai A, Saito T,
Yano F, Ikeda T, Mabuchi A, Sapkota BR, Akune T, Nishida N, et al:
C/EBPβ and RUNX2 cooperate to degrade cartilage with MMP-13 as the
target and HIF-2α as the inducer in chondrocytes. Hum Mol Genet.
21:1111–1123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee SH, Che X, Jeong JH, Choi JY, Lee YJ,
Lee YH, Bae SC and Lee YM: Runx2 protein stabilizes
hypoxia-inducible factor-1α through competition with von
Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth
plate hypertrophic chondrocytes. J Biol Chem. 287:14760–14771.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sierra J, Villagra A, Paredes R, Cruzat F,
Gutierrez S, Javed A, Arriagada G, Olate J, Imschenetzky M, Van
Wijnen AJ, et al: Regulation of the bone-specific osteocalcin gene
by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not
p300 intrinsic histone acetyltransferase activity. Mol Cell Biol.
23:3339–3351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao G, Jiang D, Ge C, Zhao Z, Lai Y,
Boules H, Phimphilai M, Yang X, Karsenty G and Franceschi RT:
Cooperative interactions between activating transcription factor 4
and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene
expression. J Biol Chem. 280:30689–30696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Komori T: Regulation of osteoblast
differentiation by Runx2. Adv Exp Med Biol. 658:43–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|