Introduction
Intrauterine adhesion (IUA), also known as Asherman
syndrome, was defined by Joseph Asherman in 1948 (1). IUA comprises of a filmy or dense
fibrous adhesive band within the uterine cavity resulting in the
adherence of opposing endometrium (1). The prevalence of IUA has increased
over the last 20 years due to the increased use of hysteroscopic
surgeries, including dilation or curettage (2). The main clinical manifestations of
IUA appear as menstrual abnormalities, hypomenorrhea, secondary
infertility, recurrent miscarriage and pelvic pain (3). Among the symptoms, secondary
infertility may affect a patients' life most severely. Therefore,
treatment is necessary in cases ofmoderate or severe IUA only for
patients with reproductive requirements (3). In clinical practice, transcervical
resection of adhesion (TCRA) is widely regarded as the standard
strategy for the treatment of moderate to severe IUA (4); however, IUA has a high rate of
recurrencefollowing TCRA, which may be ≥62% in severe IUA (5). Due to its high recurrence rate, IUA
management requires adjuvant treatment following surgery and
pharmacological therapies, and physical barriers are applied to
prevent postoperative re-adhesion (6,7).
Postoperative estrogen therapy is widely utilized to prevent
recurrent adhesion; however, the effects of estrogen therapy alone
for moderate to severe IUA remains less than impressive and
reproductive prognosis is poor (8). Thus, more effective strategies to
improve IUA patient fertility rates are necessary.
Endometrial receptivity (ER) is a transitory period
when the endometrium is acceptable to embryo implantation, which is
closely associated with embryo implantation rate and pregnancy rate
(9). Almost two-thirds of
implantation failures correlate with poor ER (10,11);
as such, ER is one of the most important prognostic factors of
fertility (9). In the field of
artificial assisted reproduction technology (ART), ER, as well as
the number of embryos and embryo quality, are decisive factors for
fertility outcomes. In addition, in the evaluation of IUA therapy,
ER also serves as a crucial prognostic factor of fertility.
Integrin αvβ٣ and laminin (LAM) are acknowledged as valuable
indicators of ER. Integrin αvβ٣ is a member of the integrin family,
whichis a group of cell membrane bound proteins that connect the
cytoskeleton and extracellular matrix (ECM) (12). αvβ٣, also known as the vitronectin
(VN) receptor, contains 2 subunits, α and β (13). It is reported to bind to a variety
of ECM proteins, including VN, fibronectin, fibrinogen, osteopontin
and bon sialic 1 (14). It has
been reported that αvβ3 and LAM are ER markers (15).
Aspirin is a non-steroidal anti-inflammatory drug
(NSAID), the main component of which is acetylsalicylic acid
(16). It is widely used in
numerous different types of diseases and conditions, including
alleviating fever and pain, inflammation, myocardial infarction,
cardiovascular morbidity and certain types of cancer (16–18).
Aspirin has been reported to increase pregnancy rates in patients
undergoing in vitro fertilization (IVF) by enhancing ER
(19). Aspirin has been scarcely
reported for use in IUA treatment; however, oral estrogen therapy
alone has deficits. The first-pass elimination of oral estrogen by
the liver or gut wall limits blood concentration (20). In addition, the side effects of
orally taken estrogen on organs (21,22)
restrict the use of estrogen in IUA postoperative treatment. Most
importantly, estrogen therapy alone contributes little to
increasing pregnancy rates in IUA patients (23). Thus, the present study investigated
whether a therapeutic combination of transdermal estrogen gel and
oral aspirin may enhance ER and fertility prognosis.
Previous research has demonstrated that patients
with transdermal estrogen gel therapy exhibit better outcomes
(24) and lower thrombotic risk
(25) compared with oral estrogen
therapy. In addition, to avoid oral drug interaction, the present
study used transdermal estrogen therapy instead of oral estrogen
therapy. The results indicated that the combination of transdermal
estrogen gel and oral aspirin may significantly enhance ER by
increasing the resistant (RI) and pulsatility indices (PI) and by
promoting angiogenesis. Combination therapy revealed a higher
efficacy in preventing fibrosis than estrogen therapy alone. The
advantage in enhancing ER makes the combination of transdermal
estrogen gel and oral aspirin a more promising treatment for IUA
postoperative management. The findings of the present study may
provide novel ideas for the clinical treatment of IUA.
Materials and methods
Patient samples
The patients selected in the present study were
admitted to the Chongqing Health Center for Women and Children
(Chongqing, China) between September 2016 and February 2017, and
were diagnosed with IUA by hysteroscopy. A total of 40 female
patients were randomly selected; however, only 38 cases completed
the study (1 case in the control group was removed in the middle of
the study due to an allergy to alcohol, which was a solvent
component in the control group; 1 case in the control group failed
to appear for a scheduled follow-up). The present study was
approved by the Ethics Committee of Chongqing Health Center for
Women and Children, and written informed consent was obtained from
all patients prior to their inclusion in the present study. The
patients were aged 21 to 45 years, with a mean age of 29 years. All
of the pathologies were confirmed by pathological examination.
Patients, who had additional endometrial complications, including
dysfunctional uterine bleeding, adenomyosis, polycystic ovary
syndrome and other hormone dependent diseases, were excluded. All
of the patients included in the present study had regular menstrual
cycles, had not received hormone therapy during the 3 months prior
to surgeryand were not pregnant or lactating during the study
period.
Postoperative artificial menstrual
cycle therapy and follow up
Patients with moderate or severe IUA were treated by
TCRA combined with intrauterine balloon placement following
American Fertility Society (AFS)-score determination (26). The patients were randomly divided
into 2 groups: Group A and group B. Patients (n=18) in group A
(control group) were postoperatively administered 5 g/day
transdermal estrogen gel (EstradiolGel; Besins Manufacturing
Belgium Co., Ltd., Drogenbos, Belgium) while patients in group B
(treatment group; n=20) were postoperatively given 5 g/day
transdermal estrogen and 100 mg/day oral aspirin. Patients in the 2
groups were treated for 2 consecutive months. During the second
month, urinary luteinizing hormone (LH) test strips were used to
monitor ovulation in all patients. Once ovulation was detected, a
reasonable size (10–20 mm3) of endometrium was excised
and processed based on the subsequent experimental requirements.
Following the 2 months of therapy, follow-up examinations were
performed on all patients once their first menstrual period ended.
For patients that failed to recover their menstrual cycle, the
follow-up was arranged immediately following 2 months of therapy.
During the follow-up, details of theirmenstrual cycle, ultrasonic
examination and hysteroscopy were used to assess the efficacy of
the therapies, by measuring menstrual flow, uterine length,
endometrium thickness, endometrial volume, average AFS-score, and
artery RI and PI during the follow-up examinations. TCRA was
performed again if necessary.
Estrogen has been well established as the most
important drug for IUA patients following TCRA (7), thus, the present study did not
includea group of patients treated with aspirin only; however,
comparisons between the estrogen only treatment group and the
combination therapy of estrogen and aspirin groups may provide
equivalent evidence to demonstrate the effects of aspirin in IUA
patients.
Histological staining, H&E
staining and immunohistochemistry (IHC)
Endometrial tissues were fixed in 4%
paraformaldehyde solution at room temperature for 24 h, embedded in
paraffin and sliced into 4 µm sections. Deparaffinized sections
were stained according to Masson's Trichrome staining protocol (BJS
Biological Technology Co., Ltd., Nanjing, China). The percentage of
blue staining indicated the extent of endometrial fibrosis. For
H&E staining, deparaffinized sections were stained using a
Solarbio H&E staining kit according to its manufacturer's
protocols (G1120; Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). For IHC, tissue paraffin sections were
deparaffinized in xylene and rehydrated using an alcohol gradient.
Antigen retrieval was performed by heating in a microwave
(>95°C) and endogenous peroxidase activity was quenched by
immersing the samples in 3% H2O2. The samples
were blocked with normal goat serum (ZSGB-Bio, Beijing, China) at
37°C for 10 min. Then samples were incubated overnight at 4°C with
anti-rabbit transforming growth factor-β (TGF-β; 1:100; bs-0086R;
BIOSS, Beijing, China), anti-rabbit CCCTC-binding factor (CTGF;
1:100; bs-0743R; BIOSS), anti-rabbit vascular endothelial growth
factor (VEGF; 1:100; bs-1313R; BIOSS), anti-rabbit αvβ٣ antibody
(1:100; bs-0342R; BIOSS), anti-rabbit collagen I (COI; 1:100;
bs-0578R; BIOSS), anti-rabbit collagen III (COIII; 1:100; bs-0948R;
BIOSS) or anti-rabbit cluster of differentiation (CD31; 1:100;
bs-20320R; BIOSS) primary antibodies. The slides were washed with
PBS and incubated with secondary antibody (1:1, Rabbit
Streptavidin-Biotin Detection system, SP9001; OriGene Technologies,
Inc.) at 37°C for 15 min. The slides were then stained with
3,3-diaminobenzidine (DAB) at room temperature for 3 min. Cell
nuclei were stained with hematoxylin at room temperature for 3 min.
All images were observed under an optical microscope
(magnification, ×200; Eclipse 50i; Nikon Corporation, Tokyo, Japan)
with NIS-Element DS-Ri1-U3 software (Nikon Corporation). Each
imageof H&E staining, Masson's staining or IHC staining was
captured at ×200 magnification.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of endometrium tissues was isolated using
a High PureRNA Isolation kit (BioTeke Corporation, Beijing, China)
according to the manufacturer's protocols. cDNA was synthesized
using aniSCRIPT cDNA Synthesis kit (GeneCopoeia, Inc., Rockville,
MD, USA) with following procedure: adding 1 µg oligo dT primer into
RNA, 65°C for 10 min; adding the remaining agents, 37°C for 1 h,
85°C for 5 min. The primers were synthesized by GeneCopoeia, Inc.
The primers sequences were as follows: TGF-β forward,
5′-TGGTGGAAACCCACAACGAA-3′ and reverse, 5′-GAGCAACACGGGTTCAGGTA-3′;
CTGF forward, 5′-TGACCCCTGCGACCCACA-3′ and reverse,
5′-TACACCGACCCACCGAAGACACAG-3′; VEGF forward,
5′-ATGAACTTTCTGCTGTCTTGG-3′ and reverse,
5′-TCACCGCCTCGGCTTGTCACA-3′; CD31 forward,
5′-TGTTGACATGAAGAGCCTGC-3′ and reverse, 5′-ACAGTTGACCCTCACGATCC-3′;
αv forward, 5′-TCCGATTCCAAACTGGGAGC-3′ and reverse,
5′-AAGGCCACTGAAGATGGAGC-3′; β٣ forward, 5′-CGAGTGCCTCTGTGGTCAAT-3′
and reverse, 5′-AGAAGTCGTCACACTCGCAG-3′; LAM forward,
5′-AAGTGGCACACGGTCAAGAC-3′ and reverse, 5′-GACAAGAGCTGCATATCCGC-3′;
COI forward, 5′-AGGCTTCAGTGGTTTGGATG-3′ and reverse,
5′-CACCAACAGCACCATCGTTA-3′; COIII forward,
5′-CCCAACCCAGAGATCCCATT-3′ and reverse,
5′-GAAGCACAGGAGCAGGTGTAGA-3′; and GAPDH forward,
5′-ACTCCACTCACGGCAAATTC-3′ and reverse, 5′-TCTCCATGGTGGTGAAGACA-3′.
The SYBR-Green RT-qPCR kit (2X All-in-One qPCR Mix) was purchased
from GeneCopoeiaInc. Relative quantification of mRNA was performed
using the comparative quantification cycles (Cq) method; GAPDH was
used as the reference gene. This value was used to plot the gene
expression employing the formula 2−ΔΔCq (27). qPCR cycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec,
60°C for 20 sec and 72°C for 10 sec. The experiments were performed
in triplicate for each sample.
Western blotting
Tissues were lysed with a radio immune precipitation
assay buffer containing protease inhibitors. Proteins were
quantified using a Bicinchoninc Acid protein assay kit (Beyotime
Institute of Biotechnology, Beijing, China) according to the
manufacturer's protocols. Aliquots of the lysates containing 40 µg
total protein were separated via 8 or 10% SDS-PAGE. The proteins
were transferred to a polyvinylidene fluoride membrane and blocked
via incubation with TBST (20 mM TrisHCl, pH 7.4, 150 mM NaCl, 0.05%
Tween-20) containing 5% non-fat milk powder for 1 h at room
temperature. The membrane was incubated with anti-rabbit TGF-β
antibody (1:100; BIOSS), anti-rabbit CTGF antibody (1:100; BIOSS),
anti-rabbit VEGF antibody (1:100; BIOSS), anti-rabbit αvβ٣ antibody
(١:١٠٠; BIOSS), anti-rabbit COI antibody (1:100; BIOSS),
anti-rabbit COIII antibody (1:100; BIOSS) or anti-rabbit CD31
antibody (1:100; BIOSS) overnight at 4°C. The membrane was then
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000, ab6721; Abcam, Cambridge, UK) for 1 h at 37°C.
The western blotting bands were visualized via enhanced
chemiluminescence (Nanjing Keygen Biotech Co., Ltd., Nanjing,
China). The bands were captured by a gel imaging system (ChemiDoc
MP; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and analyzed
with Quantity One software, version 4.6.2 (Bio-Rad Laboratories,
Inc.) and calculated by comparison against the internal control
(anti-rabbit GAPDH antibody; 1:1,000; bs-10900R; BIOSS).
Statistical analysis
Statistical analyses were performed using SPSS
version 17 software (SPSS, Inc., Chicago, IL, USA). Each experiment
was performed in triplicate. Data were presented as the mean ±
standard deviation. Multiple comparisons were performed using
Dunnett's analysis of variance, and differences between two groups
were calculated using a t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic data in study groups
The baseline demographics and clinical
characteristics, including age, parity and most probable etiology
of IUA, of the 38 patients included in the present studyrevealed no
statistically significant effectsin recruiting the cases (Table I).
 | Table I.Demographic data in study groups. |
Table I.
Demographic data in study groups.
| Variable | Group A (n=18) | Group B (n=20) |
|---|
| Average age
(years) | 30±4.80 | 29±5.81 |
| Parity (average
times) | 1±0.45 | 1±0.63 |
| IUA grade (n) |
|
|
|
Moderate | 11 (61.11%) | 12 (60.00%) |
|
Severe | 7
(38.89%) | 8
(40.00%) |
| Most probable
etiology of IUA |
|
|
|
Caesarean section history
(n) | 8 (44.44%) | 9
(45.00%) |
|
Dilatation section and
curettage (average times) | 3±0.45 | 3±0.67 |
| Pelvic
inflammatory disease | No | No |
|
Excision of uterine
septum | No | No |
| Genital
tuberculosis | No | No |
IUA patients with a combination of
transdermal estrogen gel and aspirin therapy exhibit better effects
than simple transdermal therapy
All patients from both groups received TCRA and the
placement of an intrauterine balloon in the uterus. The typical
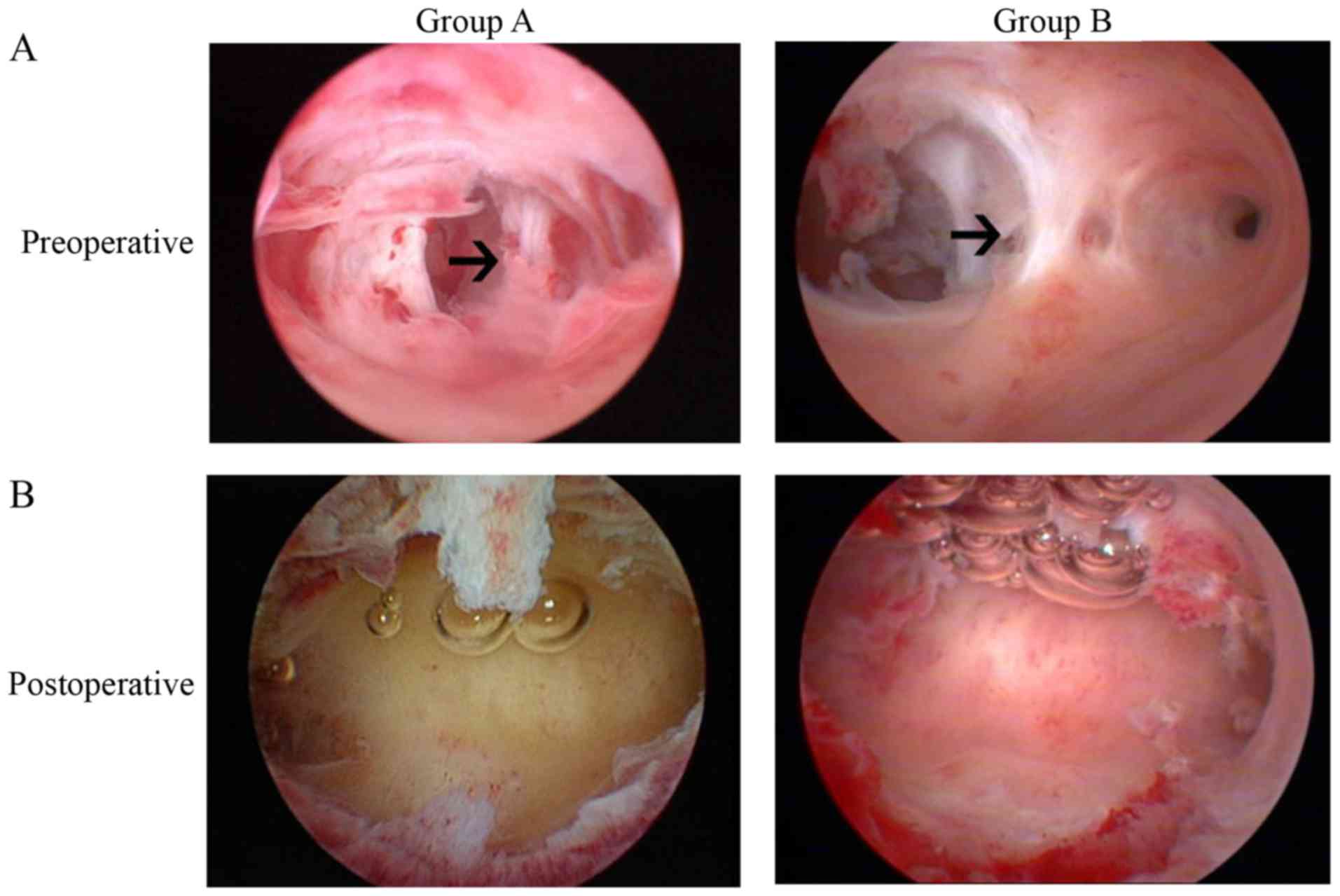
hysteroscopic views of patients prior to and following TCRA are
presented in Fig. 1. During the
postoperative follow-up examinations, there was no clinical
evidence of postoperative infection or abdominal pain reported in
any of the cases. The outcomes of the two different therapies were
assessed by the following indicators: Uterine length, menstrual
flow, postoperative adhesion score according to the AFS standard
(26), endometrium thickness and
pregnancy condition. Short-term follow-ups were scheduled ~1 year
(1±0.2 years) and the number of pregnancy caseswas recorded. The
results revealed that patients with a combined therapy of
transdermal estrogen gel and oral aspirin had significant
improvements in average AFS-score, with an absence of adhesions
recorded in 8 out of 18 cases (44.44%) in group A and 15 out of 20
cases (75.00%) in group B (Table
II). The two groups demonstrated significant differences in
uterine length and menstrual flow when comparing the preoperative
and postoperative values within the same group. In addition, group
B exhibited greater improvements (Table II). From the pregnancy data,
patients with combination therapy had a relatively higher rate of
pregnancy compared with patients with estrogen-alone therapy
(Table II). However, long-term
follow-upsare required to obtain data that are more specific. These
data indicated that, compared with simple transdermal estrogen
therapy, combination therapy with transdermal estrogen gel and oral
aspirin may improve the postoperative prognosis and increase the
rate of pregnancy in patients with IUA.
 | Table II.Outcome measures in study groups. |
Table II.
Outcome measures in study groups.
| Variable | Group A (n=18) | Group B (n=20) |
|---|
| Uterine length
(cm) |
|
|
|
Preoperative | 5.92±1.34 | 6.11±1.27 |
|
Postoperative | 6.97±1.46 | 7.72±1.41 |
| Menstrual flow
(days) |
|
|
|
Preoperative | 3±0.73 | 3±0.81 |
|
Postoperative | 5±1.36b | 5±1.71a,b |
| Endometrium
thickness (mm) |
|
|
|
Preoperative | 4.25±0.72 | 4.18±0.91 |
|
Postoperative |
7.64±1.54b |
9.12±1.78a,b |
| Menstrual volume
(ml) |
|
|
|
Preoperative | 38.77±7.46 | 40.76±8.92 |
|
Postoperative |
64.78±6.33b |
69.72±8.01a,b |
| Postoperative
adhesion cases (n) |
|
|
| No | 8 (44.44%) | 15
(75.00%)a |
|
Mild | 4 (22.22%) | 3 (15.00%) |
|
Moderate | 3 (16.67%) | 1 (5.00%) |
|
Severe | 3 (16.67%) | 1 (5.00%) |
| Postoperative
average AFS-score | 6±0.45 | 3±0.73a |
| Postoperative
pregnant cases (n) | 3 (16.67%) | 6
(30.00%)a |
Combination therapy with transdermal
estrogen gel and oral aspirin enhances ER during endometrium
rehabilitation in patients with IUA
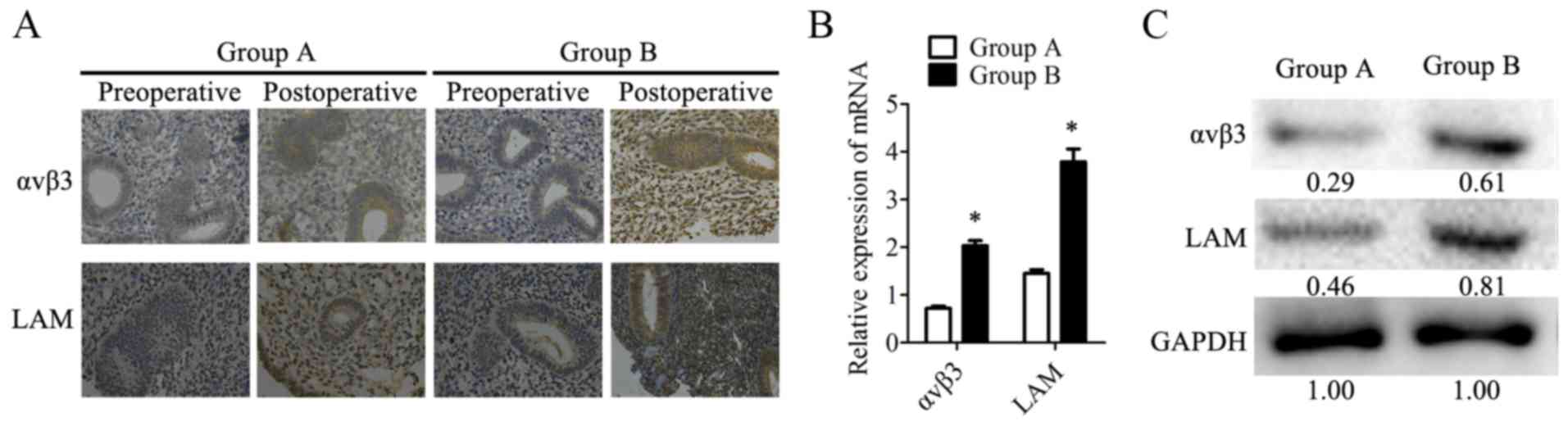
In the present study, αvβ٣ and LAM, two ER markers,
were analyzed to evaluate ER in patients. IHC was used to assess
the expression of αvβ3 or LAM in tissues from the two groups of
patients. Tissues were collected on the 20–24th day of the second
postoperative menstrual cycle when patients detected ovulation with
a LH rapid test strip. The IHC results demonstrated that αvβ3 and
LAM were mildly expressed or not detected in preoperative samples.
Postoperatively, αvβ٣ and LAM stained strongly in group B, while
revealing only moderate expression in group A (Fig. 2A). Furthermore, the mRNA expression
levels of αvβ3 and LAM were investigated with RT-qPCR and the
protein expression levels of these two markers were analyzed by
western blotting in postoperative tissues from the two groups.
Consistent with the IHC results, αvβ٣ and LAM were highly expressed
in group B at the mRNA (Fig. 2B)
and protein levels (Fig. 2C) in
postoperative tissues when compared with group A. These data
indicated that acombination therapy of transdermal estrogen and
oral aspirin improved ER during endometrium rehabilitation in
patients with IUA.
Combination therapy improves
neovascularization during endometrium rehabilitation in patients
with IUA
In the present study, based on ultrasonography, the
RI and PI were analyzed (Fig. 3A).
The results revealed that patients from group B exhibited lower RI
and PI, indicating that the combination therapy had vasodilatory
effects on the uterus, potentially due to endometrial
neovascularization. Microvesseldensity (MVD) was determined based
on H&E staining in the postoperative endometrial patient
samples (Fig. 3B). It was observed
that samples from group B had a 1.8-fold increase in MVD when
compared with group A (Fig. 3B).
Also, the expression of the angiogenesis markers CD31 and VEGF
(28) were determined by IHC
(Fig. 3C), RT-qPCR and western
blotting in postoperative samples. Compared with group A, VEGF was
highly expressed at the mRNA (Fig.
3D) and protein levels in group B (Fig. 3E). These data suggested that
combination therapy with transdermal estrogen gel and oral aspirin
may have improved neovascularization during endometrium
rehabilitation in patients with IUA.
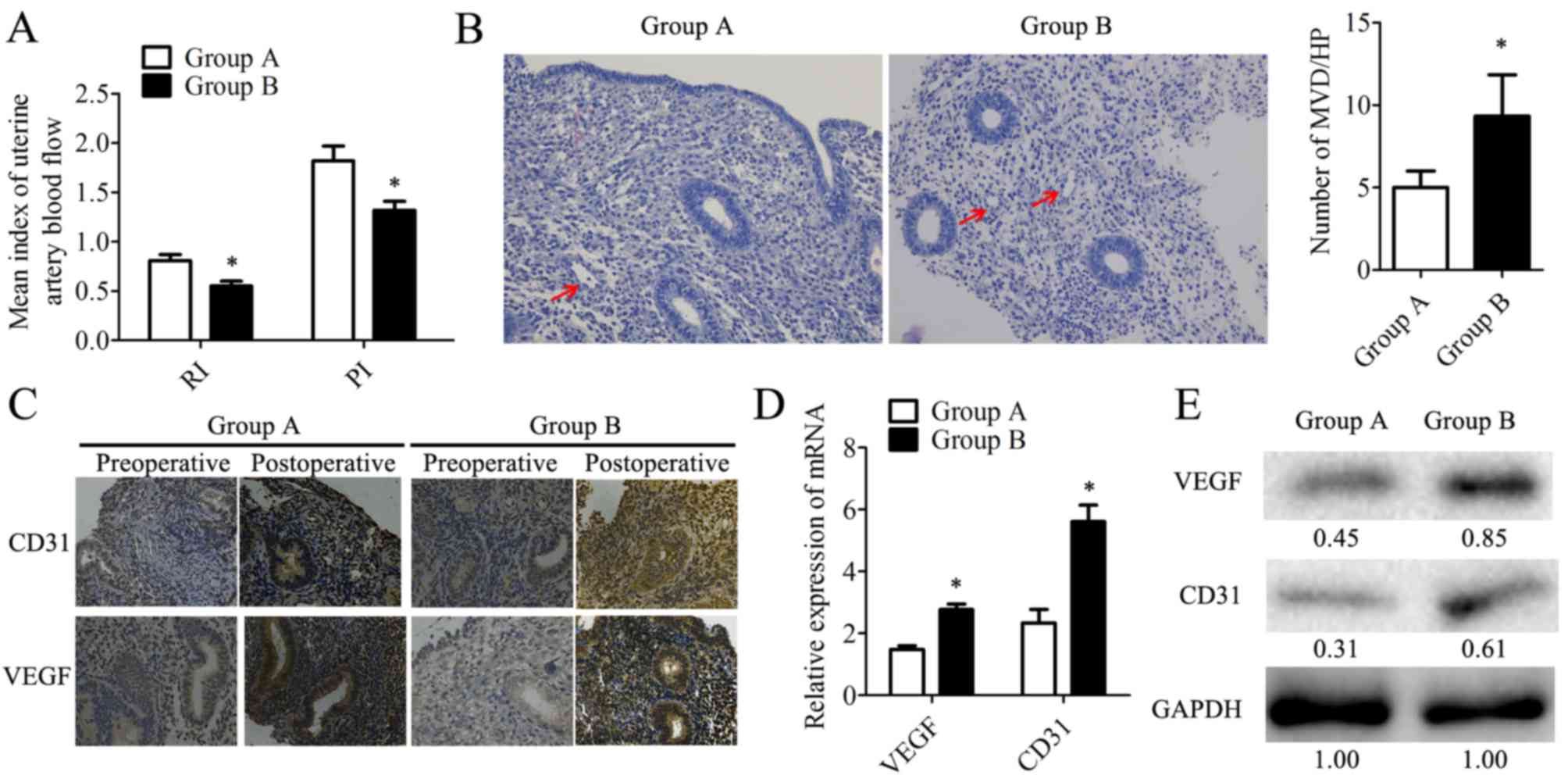 | Figure 3.Angiogenesis is significantly
increased in endometrium tissues within the combination therapy
group following treatment. (A) RI and PI were determined based on
the ultrasonic examination. (B) Microvessels (indicated by arrows)
were observed by hematoxylin staining. MVD was counted in 10
different HP fields. Magnification, ×200. (C) The expression of
CD31 and VEGF was detected by immunohistochemistry. Magnification,
×200. The expression of CD31 and VEGF in human endometrial tissues
was determined at the mRNA level by (D) reverse
transcription-quantitative polymerase chain reaction and at the
protein level by (E) western blotting. The relative quantity of
proteins was calculated by comparison against the internal control,
GAPDH, and noted below the bands. The postoperative tissues were
obtained during the follow-up at the 20–24th day of the second
menstrual cycle following transcervical resection of adhesion. Data
are presented as the mean ± standard deviation. *P<0.05 vs.
group A. CD31, cluster of differentiation 31; group A, transdermal
estrogen gel therapy; group B, combination therapy of transdermal
estrogen gel and oral aspirin therapy; HP, high power; MVD,
microvessel density; PI, pulsatility indices; RI, resistant
indices; VEGF, vascular endothelial growth factor. |
Transdermal estrogen gel and oral
aspirin combination therapy inhibits endometrial fibrosis during
endometrium rehabilitation in patients with IUA
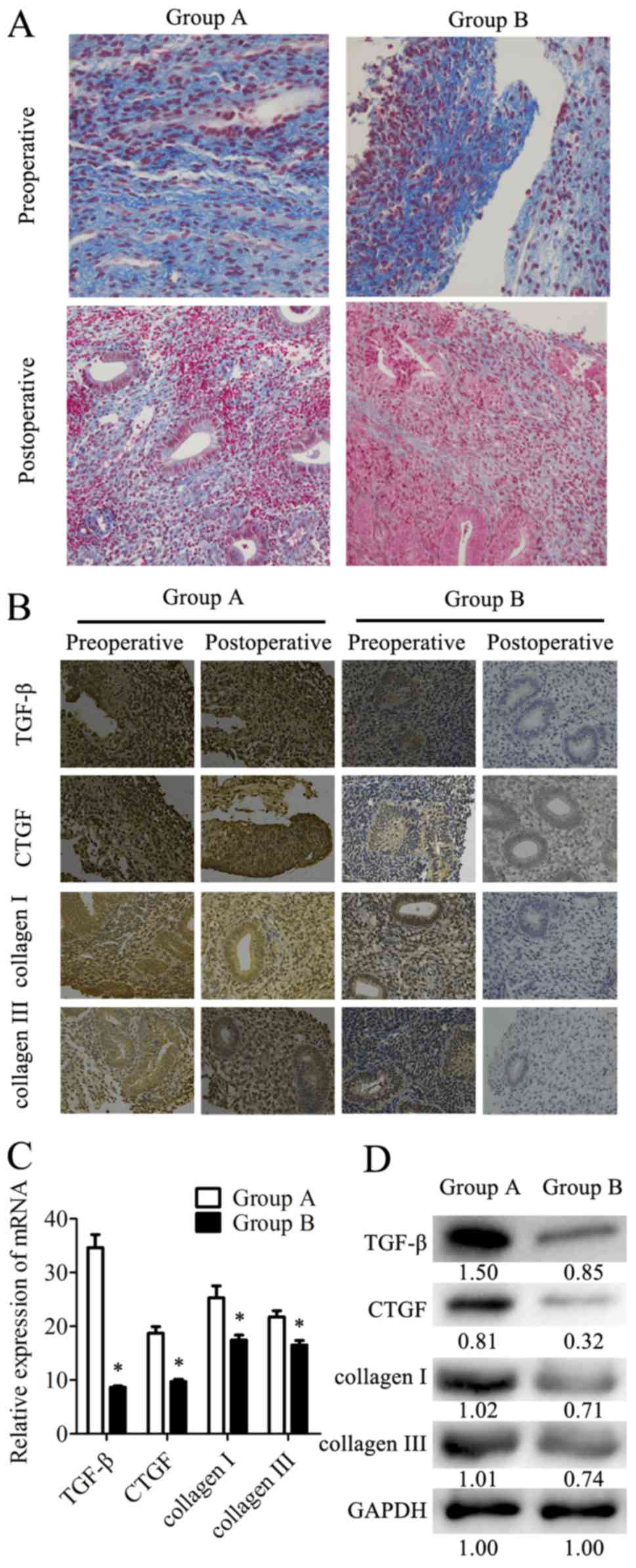
Endometrial fibrosis is accepted as an important
factor for IUA formation. Fibrosis was determined in the tissues by
Masson's Trichrome staining. The results revealed that the
endometrial tissues from the combination therapy group had less
fibroses compared with the tissues from the estrogen therapy group
(Fig. 4A). Additionally, the
expression levels of the fibrotic markers (TGF-β, CTGF, COI and
COIII) in tissues were analyzed by IHC staining (Fig. 4B). TGF-β, CTGF, COI and COIII were
expressed in the cytoplasm and nucleus of the epithelial and
stromal cells. There was also strong staining in the preoperative
endometrial tissues from the two groups. However, these four
proteins were mildly expressed or were undetected in the
postoperative tissues from group B, while the postoperative tissues
from group A exhibited higher expression levels of these markers.
In postoperative tissues, the expression levels of fibrotic markers
were examined at the mRNA level by RT-qPCR and at the protein level
by western blotting. In samples from group B, the TGF-β mRNA
expression levels were 75% lower than in group A. Similarly, the
mRNA expression levels of CTGF, COI and COIII were 48, 31 and 24%
lower, respectively, in group B than in group A (Fig. 4C). The relative protein expression
levels determined by western blotting (Fig. 4D) were consistent with the results
of RT-qPCR. These data indicated that combination therapy with
transdermal estrogen gel and oral aspirin may have inhibited
postoperative endometrial fibrosis during endometrium
rehabilitation in patients with IUA.
Discussion
IUA is one of the most common reproductive system
diseases in women of childbearing age (23). The incidence is rising due to the
increasing use of intrauterine operations, including curettage,
dilation and hysteromyomectomy (29). A total of ~40–50% of severe IUA
patients have secondary infertility, which is the most severe
symptom and requires treatment (30,31).
Transcervical resection of adhesion is the mainstay treatment for
moderate and severe IUA (5).
However, high rates of postoperative recurrence of IUA and low
pregnancy rates pose great challenges to clinical management
(3). A wide range of treatment
techniques are applied to prevent re-adhesion (5); however, these treatments are seldom
successful in improving pregnancy rates for patients. Oral estrogen
is widely used in IUA patients following TCRA and a previous study
has reported that estrogen may help with endometrium repair,
inhibition of fibrosis and angiogenesis (4). However, oral estrogen therapy
presents notable barriers. The first-pass elimination of oral
estrogen impairs its blood concentration in some patients. Thus,
transdermal estrogen gel was employed in the present study, which
has been proven to have equivalent effects in treating IUA
following surgery (24).
Additionally, transdermal estrogen causes less harm to the liver
and has lower thrombotic risk (25). The majority of patients with IUA
are of reproductive age; however, the effect of estrogen on
improving fertility is not ideal (4,24).
Aspirin is an NSAID that is widely used to relieve pain and reduce
the risk of cardiac diseases, such as myocardial infarction
(16,17). It has been reported that
low-dose-aspirin (LDA) may improve the prognosis of IVF (19); however, whether aspirin is
effective in the treatment of IUA requires further investigation.
In the present study, the effects of a combination therapy
constituting transdermal estrogen gel and oral aspirin in IUA
patients were analyzed. The results demonstrated that the
combination therapy exhibited better effects inimproving ER when
compared with transdermal estrogen gel therapy alone. These
effectsmay be the result of the decreased RI and PI of the uterine
artery, and increased MVD produced by the combination therapy,
which also exhibited greater antifibrotic effects that may reduce
the re-adhesion rate over time.
For the majority patients with IUA, successfully
becoming pregnant is the ultimate goal (3). The present study reported that the
expression of αvβ3 and LAM was higher in tissues from the
combination therapy group than in those from the estrogen-alone
therapy group. These results indicated that the combination therapy
of transdermal estrogen gel and oral aspirin may enhance ER; higher
ER may lead to an increased chance of success in achieving
pregnancy. Previously, it had been reported that LDA exhibited a
positive effect on ER and implantation in ART (32,33),
which is consistent with the results reported in the present study.
Ultrasound examinationsrevealed lower PI and RI in IUA patients
with combination therapy in the present study. Previously, LDA
increased uterine blood flow (34), which is also consistent with the
present study. Lower RI and PI are associated with lower impedance
to uterine artery blood flow, which leads to a greater blood flow
to the endometrium. The anticoagulant drug aspirin has a
vasodilatory effect (35) and
suppresses the formation of thromboxane A without affecting
prostaglandin I2 (36). This may
explain the reduction in PI and RI observed more significantly with
the combination therapy than with estrogen-only therapy. In
addition, the expression levels of CD31 and VEGF were higher in the
tissues from the combination therapy group. MVD was also higher in
the combination therapy group. These results indicated that
combination therapy promoted angiogenesis in IUA. It has been
previously reported that the use of estrogen may promote
angiogenesis in IUA following surgery (4), which provides suitable conditions for
endometrial repair. Aspirin has been reported to inhibit
angiogenesis (18); however, the
use of estrogen compensated the effect to a certain degree in the
combination therapy in IUA, though, further study is required to
investigate the effects of aspirin on angiogenesis, when combined
with estrogen, for the treatment of IUA.
In the present study, the expression levels of
fibrosis markers (TGF-β, CTGF, COI and COIII) were lower in tissues
from the combination group than from estrogen therapy. Accordingly,
Masson's Trichrome staining demonstrated fewer fibrotic tissues in
the combination therapy group. These data indicated that the
combination therapy of transdermal estragon gel and oral aspirin
was a more effective anti-fibrotic strategy for the management of
postoperative IUA. Inflammation is one of the causes of fibrosis
(37,38). TGF-β is overexpressed in inflamed
tissues and induces the TGF-β signaling pathway, which stimulates
an increase of COI and COIII secretion and deposition (39). Inflammation itself results in
tissue necrosis and edema, consequently leading to fibrosis during
the tissue repair process. As an anti-inflammatory agent, aspirin
has been reported to revert fibrosis in a chronic pulmonary murine
model (40). Estrogen has been
demonstrated to prevent fibrosis via suppression of the TGF-β
signaling pathway in IUA (39).
These findings may explain the synergy between the antifibrotic
effects of aspirin and estrogen. Collectively, the combination of
transdermal estrogen gel and oral aspirin is more effective in
enhancing ER and preventing re-adhesion in the postoperative
treatment of IUA.
However, the present study has some limitations: One
potential weakness is that the present study lacked a comparison of
differing methods of estrogen delivery. It was considered that
first-pass elimination and the individual differences of patients
may determine the uncertainty of treatment results, and provide a
suitable comparison between a combination therapy of transdermal
estrogen gel with oral aspirin group and a transdermal estrogen
therapy group, without including different estrogen delivery
schemes. Additionally, pregnancy data acquired from longer-period
follow-upsare necessary in future studies to confirm whether
combination therapy induces a greater prognosis in fertility. As
well asthe longer-term data, an IUA mice model may be constructedto
confirm the findings of the present study and investigate the
potential underlying mechanisms.
In conclusion, the combination of transdermal
estrogen gel and oral aspirin therapy may be a better strategy than
transdermal estrogen therapy alone in the treatment of IUA. For
postoperative management, combination therapy is more efficacious
in enhancing ER by increasing uterine blood and MVD. This may
contribute to improved fertility prognoses. The findings of the
present study may provide novel guidance into the clinical
treatment of IUA.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Foundation of
Health and Family Planning Commission of Chongqing (grant nos.
2016MSXM092 and 20142099).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contribution
YC, LL, YL, JH, YM and LH were involved in the
protocol and project development process, and the collection and/or
management of the data. PH also aided the data
collection/management process and performed the data analyses. YC
and LH produced the manuscript, which was critically revised for
important intellectual content by LH and YM.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing Health Center for Women and Children
(Chongqing Health Center), and written informed consent was
obtained from all patients prior to their inclusion in the
study.
Consent for publication
The patients or their guardians have provided
written informed consent for the publication of any associated data
and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asherman JG: Amenorrhoea traumatica
(atretica). J Obstet Gynaecol Br Emp. 55:23–30. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dalton VK, Saunders NA, Harris LH,
Williams JA and Lebovic DI: Intrauterine adhesions after manual
vacuum aspiration for early pregnancy failure. Fertil Steril.
85:18232006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deans R and Abbott J: Review of
intrauterine adhesions. J Minim Invasive Gynecol. 27:555–569. 2010.
View Article : Google Scholar
|
|
4
|
Johary J, Xue M, Zhu X, Xu D and Velu PP:
Efficacy of estrogen therapy in patients with intrauterine
adhesions: Systematic review. J Minim Invasive Gynecol. 21:44–54.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capella-Allouc S, Morsad F,
Rongieres-Bertrand C, Taylor S and Fernandez H: Hysteroscopic
treatment of severe Asherman's syndrome and subsequent fertility.
Hum Reprod. 14:1230–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
AOrhue AA, Aziken ME and Igbefoh JO: A
comparison of two adjunctive treatments for intrauterine adhesions
following lysis. Int J Gynaecol Obstet. 82:49–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pabuccu R, Onalan G, Kaya C, Selam B,
Ceyhan T, Ornek T and Kuzudisli E: Efficiency and pregnancy outcome
of serial intrauterine device-guided hysteroscopic adhesiolysis of
intrauterine synechiae: Fertil Steril. 90:1973–1977. 2008.
|
|
8
|
Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu
D, Yang X, Zhang P, Sheng W, Xu J, et al: Etiology, treatment, and
reproductive prognosis of women with moderate-to-severe
intrauterine adhesions. Int J Gynaecol Obstet. 125:121–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makker A and Singh MM: Endometrial
receptivity: Clinical assessment in relation to fertility,
infertility, and antifertility. Med Res Rev. 26:699–746. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simon C, Moreno C, Remohi J and Pellicer
A: Cytokines and embryo implantation. J Reprod Immunol. 39:117–131.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lédée-Bataille N, Laprée-Delage G, Taupin
JL, Dubanchet S, Frydman R and Chaouat G: Concentration of
leukaemia inhibitory factor (LIF) in uterine flushing fluid is
highly predictive of embryo implantation. Hum Reprod. 17:213–218.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krutzsch HC, Choe BJ, Sipes JM, Guo Nh and
Roberts DD: Identification of an alpha3beta1 integrin recognition
sequence in thrombospondin-1. J Biol Chem. 274:24080–24086. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lessey BA, Damjanovich L, Coutifaris C,
Castelbaum A, Albelda SM and Buck CA: Integrin adhesion molecules
in the human endometrium: Correlation with the normal and abnormal
menstrual cycle. J Clin Invest. 90:188–195. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lessey BA: Endometrial receptivity and the
window of implantation. Baillieres Best Pract Res Clin Obstet
Gynaecol. 14:775–788. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patrono C: Aspirin as an antiplatelet
drug. N Engl J Med. 330:1287–1294. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krasopoulos G, Brister SJ, Beattie WS and
Buchanan MR: Aspirin ‘resistance’ and risk of cardiovascular
morbidity: Systematic review and meta-analysis. BMJ. 336:195–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuzick J, Otto F, Baron JA, Brown PH, Burn
J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, et
al: Aspirin and non-steroidal anti-inflammatory drugs for cancer
prevention: An international consensus statement. Lancet Oncol.
10:501–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rubinstein M, Marazzi A and Polak de Fried
E: Low-dose aspirin treatment improves ovarian responsiveness,
uterine and ovarian blood flow velocity, implantation, and
pregnancy rates in patients undergoing in vitro fertilization: A
prospective, randomized, double blind placebo controlled assay.
Fertil Steril. 71:825–829. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kenigsberg L, Balachandar S, Prasad K and
Shah B: Exogenous pubertal induction by oral versus transdermal
estrogen therapy. J Pediatr Adolesc Gynecol. 26:71–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shukla A, Jamwal R and Bala K: Adverse
effect of combined oral contraceptive pills. Asian J Pharma &
Clin Res. 10:17–21. 2017. View Article : Google Scholar
|
|
22
|
Vigen C, Hodis HN, Chandler WL, Lobo RA
and Mack WJ: Postmenopausal oral estrogen therapy affects
hemostatic factors, but does not account for reduction in the
progression of subclinical atherosclerosis. J Thromb Haemost.
5:1201–1208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johary J, Xue M, Zhu X, Xu D and Velu PP:
Efficacy of estrogen therapy in patients with intrauterine
adhesions: Systematic review. J Minim Invasive Gynecol. 21:44–54.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chi Y, Yang X, He P, Lei L, Yi Lan Y and
Liu L: Efficiency of estradiol gel in treatment of moderate or
severe intrauterine adhesion. J Reprod Med. 25:691–695. 2016.(In
Chinese).
|
|
25
|
Bagot CN, Marsh MS, Whitehead M, Sherwood
R, Roberts L, Patel RK and Arya R: The effect of estrone on
thrombin generation may explain the different thrombotic risk
between oral and transdermal hormone replacement therapy. J Thromb
Haemost. 8:1736–1744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
American Fertility Society (1988) The
American Fertility Society classifications of adnexal adhesions,
distal tubal occlusion, tubal occlusion secondary to tubal
ligation, tubal pregnancies, Mullerian anomalies and intrauterine
adhesions. Fertil Steril. 49:944–955. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Inany H: Intrauterine adhesions. An
update. Acta Obstet Gynecol Scand. 80:986–993. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Socolov R, Anton E, Butureanu S and
Socolov D: The endoscopic management of uterine synechiae. A
clinical study of 78 cases. Chirurgia (Bucur). 105:515–518.
2010.PubMed/NCBI
|
|
31
|
Capella-Allouc S, Morsad F,
Rongières-Bertrand C, Taylor S and Fernandez H: Hysteroscopic
treatment of severe Asherman's syndrome and subsequent fertility.
Hum Reprod. 14:1230–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schisterman EF, Gaskins AJ and Whitcomb
BW: Effects of low-dose aspirin in in-vitro fertilization. Curr
Opin Obstet Gynecol. 21:275–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao M, Chang C, Liu Z, Chen LM and Chen
Q: Treatment with low-dose aspirin increased the level LIF and
integrin β3 expression in mice during the implantation window.
Placenta. 31:1101–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang X, Wang T, He L, Xu H, Liu Z and Zhao
A: Effect of low-dose aspirin on midluteal phase uterine artery
blood flow in patients with recurrent pregnancy loss. J Ultrasound
Med. 35:2583–2587. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lazzarin N, Vaquero E, Exacoustos C,
Bertonotti E, Romanini ME and Arduini D: Low-dose aspirin and
omega-3 fatty acids improve uterine artery blood flow velocity in
women with recurrent miscarriage due to impaired uterine perfusion.
Fertil Steril. 92:296–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burch JW, Stanford N and Majerus PW:
Inhibition of platelet prostaglandin synthetase by oral aspirin. J
Clin Invest. 61:314–319. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kershenobich Stalnikowitz D and Weissbrod
AB: Liver fibrosis and inflammation. A review. Ann Hepatol.
2:159–163. 2003.PubMed/NCBI
|
|
38
|
Stramer BM, Mori R and Martin P: The
inflammation-fibrosis link? A Jekyll and Hyde role for blood cells
during wound repair. J Invest Dermatol. 127:1009–1017. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y
and Dai YT: Estradiol attenuates the TGF-β1-induced conversion of
primary TAFs into myofibroblasts and inhibits collagen production
and myofibroblast contraction by modulating the Smad and Rho/ROCK
signaling pathways. Int J Mol Med. 36:801–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Benjamim CF, Guilherme RDF, Nogueira TDO,
Neves JS and Canetti CDA: Aspirin-triggered Resolvin D1, atrvd1,
reverts chronic pulmonary fibrosis in a murine model pulmonary
fibrosis. American J Resp Crit Care Med. 193:A49382016.
|