Introduction
Diabetes mellitus (DM) is a chronic metabolic
disease, affecting many individuals worldwide. The increase in
prevalence is followed by a global pandemic of diabetes-related
complications. Diabetic cardiomyopathy (DCM) is one of the major
complications of DM (1). However,
the exact molecular mechanisms underlying DCM remain unclear.
Matrix metalloproteinases (MMPs) are responsible for
cleaving extracellular matrix (ECM) proteins. Among the MMPs, MMP-2
and MMP-9 are the major gelatinases that play an important role in
the development of DCM by degrading the ECM (2). MMP-2 expression is higher in a
diabetic heart (3,4). Moreover, dysregulation of MMP
proteins and their endogenous inhibitor, namely, tissue inhibitor
of metalloproteinase (TIMP), has been observed in the diabetic
heart, suggesting that MMPs/TIMPs are involved in DCM. However,
whether high glucose levels affect the expression of MMPs/TIMPs in
human cardiac fibroblasts (HCF) is unclear.
Sodium-glucose cotransporters (SGLTs) are encoded by
a subfamily of solute carrier genes, which are members of the
sodium substrate symporter family. SGLT transport glucose by
following the sodium concentration gradient which is established by
the Na+/K+-ATPase pump. The primary SGLTs include SGLT1, which
accounts for glucose absorption from the small intestine, and
SGLT2, which is responsible for reabsorption of the glucose in the
proximal renal tubule. SGLT inhibitor has been developed as a novel
strategy for the treatment of type 2 DM patients. Recently, the
result of EMPA-REG OUTCOME trail demonstrated that, Empagliflozin,
a new member of the SGLT2 class, could significantly decreased the
cardiovascular morbidity and mortality in DM patients (5). In addition, Cefalu et al
(6), showed that dapagliflozin
plays a significant role in the reduction of HbA1c, BW, and SBP.
However, it had no adverse effect on cardiovascular safety,
compared to placebo treatment. In short-term studies, SGLT1
inhibition and combined SGLT1/SGLT2 inhibition were found to be
safe (7). SGLT inhibitors play a
cardiovascular protective role, possibly by inhibiting renal
reabsorption of glucose, thereby lowering blood glucose levels.
Recently, SGLT1 was found to be highly expressed in the human and
rodent heart, and to actually contribute to the pathogenesis of
PRKAG2 cardiomyopathy (8,9). Knockdown of SGLT1 could attenuate the
disease phenotype (10). SGLT1 was
also found to be expressed in cardiomyocytes (8). However, SGLT expression in the HCF
has not been previously tested, and the cardioprotective mechanism
of SLGT inhibitors involving direct inhibition of the SGLT in HCF,
in addition to lowering blood glucose levels, is unclear.
In the present study, we investigated whether high
glucose levels regulate the expression of MMPs and TIMPs in HCF. We
studied the effect of two SGLT inhibitors (phlorizin and
dapagliflozin) on glucose-induced MMP-2 expression in the
HCF as well as investigated the role of SGLT1 in this effect.
Materials and methods
Materials
The CS4Z055R (containing serum) and CS4Z3500R (not
containing serum) media were purchased from Cell Systems
Corporation, (Kirkland, WA, USA). D-(+)-Glucose, D-(+)-mannitol,
and diamidino-2-phenylindole (DAPI) were purchased from Nacalai
Tesque Inc., (Kyoto, Japan). Phlorizin and dapagliflozin were
purchased from Cayman Chemical Company, (Ann Arbor, MI, USA). All
other chemicals were of reagent grade and commercially
available.
Cell culture
Primary human cardiac fibroblast cells (ACBRI 5118)
at passage 2 were purchased from Cell Systems Corporation. All
studies were performed with HCFs at passage 4–10. Passage Reagent
Group (Cell Systems Corporation) were used for cell passaging. The
cells were seeded on 6-well tissue culture plates at a density of
1×105 cells/well, maintained in the CS4Z055R medium, and
grown in the cell incubator at 37°C, containing 95% O2
and 5% CO2. After sub-confluence, CS4Z3500R (without
serum) medium was used to synchronize the cells for 24 h. The cells
were washed twice with phosphate-buffered saline and cultured in
the medium to be further subjected to different treatments. Cells
were passaged in 0.05% trypsin-EDTA.
Groups and interventions
To test the effect of glucose on HCF, the cells were
divided into 7 groups: Control group, Glu 5.5 mM group (cultured
with 5.5 mM glucose), Glu 30 mM group (cultured with 30 mM
glucose), Glu 100 mM group (cultured with 100 mM glucose), osmotic
control (OC) 5.5 mM group (cultured with 5.5 mM mannitol), OC 30 mM
group (cultured with 30 mM mannitol), and OC 100 mM group (cultured
with 100 mM mannitol). Different times of incubation, including 1,
2, 4, 6, 12, 24, and 48 h, were adopted to evaluate the effects of
glucose on HCF. Then, to test the effect of phlorizin and
dapagliflozin on glucose-induced MMP-2 expression in HCF,
the cells were divided into 6 groups: Control group, Glu 30 mM
group (cultured with 30 mM glucose), Phlorizin 10 µM group
(cultured with 30 mM glucose and 10 µM phlorizin), Phlorizin 100 µM
group (cultured with 30 mM glucose and 100 µM phlorizin),
Dapagliflozin 10 µM group (cultured with 30 mM glucose and 10 µM
dapagliflozin), and Dapagliflozin 100 µM group (cultured with 30 mM
glucose and 100 µM dapagliflozin).
Isolation of total mRNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
After group-specific treatment, RNeasy mini-kit
(Qiagen GmbH, Hilden, Germany) was used for extracting total RNA
from the cells. Then, the mRNA was used as the template to
synthesize complementary DNA (cDNA) with Thermo Script RT-PCR kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. Approximately 2 µl of
the cDNA was used for RT-PCR. The forward and reverse primer
sequences are shown in Table I.
The reaction conditions included the following: step 1: 95°C for 30
sec; step 2: 40 cycles at 95°C for 5 sec and 60°C for 34 sec; step
3: 95°C for 15 sec, 60°C for 60 sec, and 95°C for 15 sec. The final
concentration of MMP-2, TIMP-1, TIMP-2, and SGLT-1
was expressed relative to that of GAPDH from the same RNA
sample.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Primer name | Primer sequence
(5′-3′) |
|---|
| MMP-2 (Forward) |
CACATCGCAGATGCCTGGAA |
| MMP-2 (Reverse) |
TTCAGGTAATAGGCACCCTTGAAGA |
| MMP-9 (Forward) |
CAAGCTGGACTCGGTCTTTGA |
| MMP-9 (Reverse) |
GCCTGTGTACACCCACACCT |
| TIMP-1
(Forward) |
AAGAACTACACTGTTGGCTGTGAG |
| TIMP-1
(Reverse) |
GTCCGTCCACAAGCAATGAG |
| TIMP-2
(Forward) |
GGAGCACTGTGTTTATGCTGGA |
| TIMP-2
(Reverse) |
ACATGCGCAGTCTGCTTGTC |
| SGLT-1
(Forward) |
GCCCAACACTCTGATTTGCATTTA |
| SGLT-1
(Reverse) |
CTGGTTCTACTTCACCCTGAGCAC |
| GAPDH
(Forward) |
GCACCGTCAAGGCTGAGAAC |
| GAPDH
(Reverse) |
ATGGTGGTGAAGACGCCAGT |
Western blot analysis
After 24 h incubation with the corresponding
intervention factors, the cellular protein was extracted using the
radioimmunoprecipitation assay (RIPA) lysis buffer for use in
western blotting. This was followed by determination of protein
content in the supernatant. The supernatant was then separated by
SDS-PAGE (10%) and transferred to a polyvinylidene fluoride
membrane. The membrane was blocked with the blocking buffer for 30
min at room temperature, and then incubated overnight with the
rabbit anti-SGLT1 monoclonal antibody (1:1,000 diluted) and rabbit
anti-β-actin monoclonal antibody (1:10,000 diluted) at 4°C. After
wash with TBS-T for 3 times, the membranes were then incubated with
the second antibody (1:10,000 diluted) for 1 h at room temperature.
The antigen was detected by using the standard chemical
luminescence method. The bands on the membranes were scanned on a
gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and analyzed by Quantity One v4.4.
Statistical analysis
Statistical analysis was performed using the
GraphPad Prism (v6.0; GraphPad Software, Inc., La Jolla, CA, USA)
software. One-way analysis of variance (ANOVA), followed by Tukey's
post-hoc analysis, was performed to compare between multiple
experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
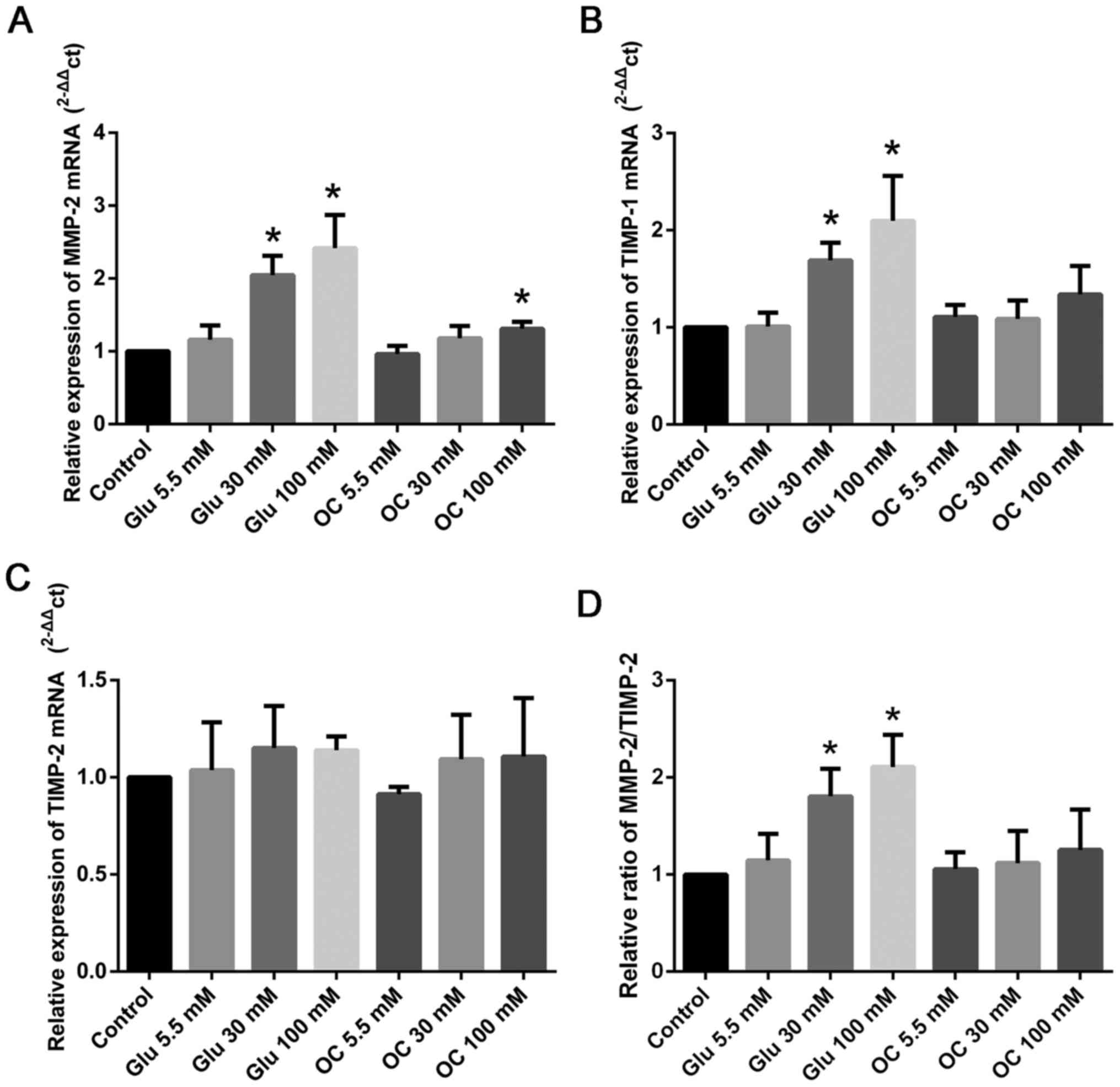
High glucose levels induce MMP-2 and
TIMP-1 expression in HCF
We performed RT-qPCR assay to test the effect of
glucose on HCF. As shown in Fig.
1, we found that the relative expression of MMP-2 and
TIMP-1 was up-regulated by glucose at 30 and 100 mM
(P<0.05; Fig. 1A-B). However,
high glucose levels did not have any effect on the expression of
TIMP-2 (Fig. 1C). As the
ratio of MMP-2 to TIMP-2 is usually considered
representative of ECM balance (3,11,12),
we tested the effect of high glucose levels on it; we found that
high glucose levels could increase the MMP-2/TIMP-2
ratio (P<0.05; Fig. 1D).
Moreover, MMP-9 mRNA expression was very low, and therefore,
we did not test the expression of MMP-9 in the subsequent
experiments. We found that mannitol at 30 and 100 mM had no effect
on the expression of MMP-2 and TIMP-1/2; these
results indicated that high glucose levels, and not osmotic effect,
were responsible.
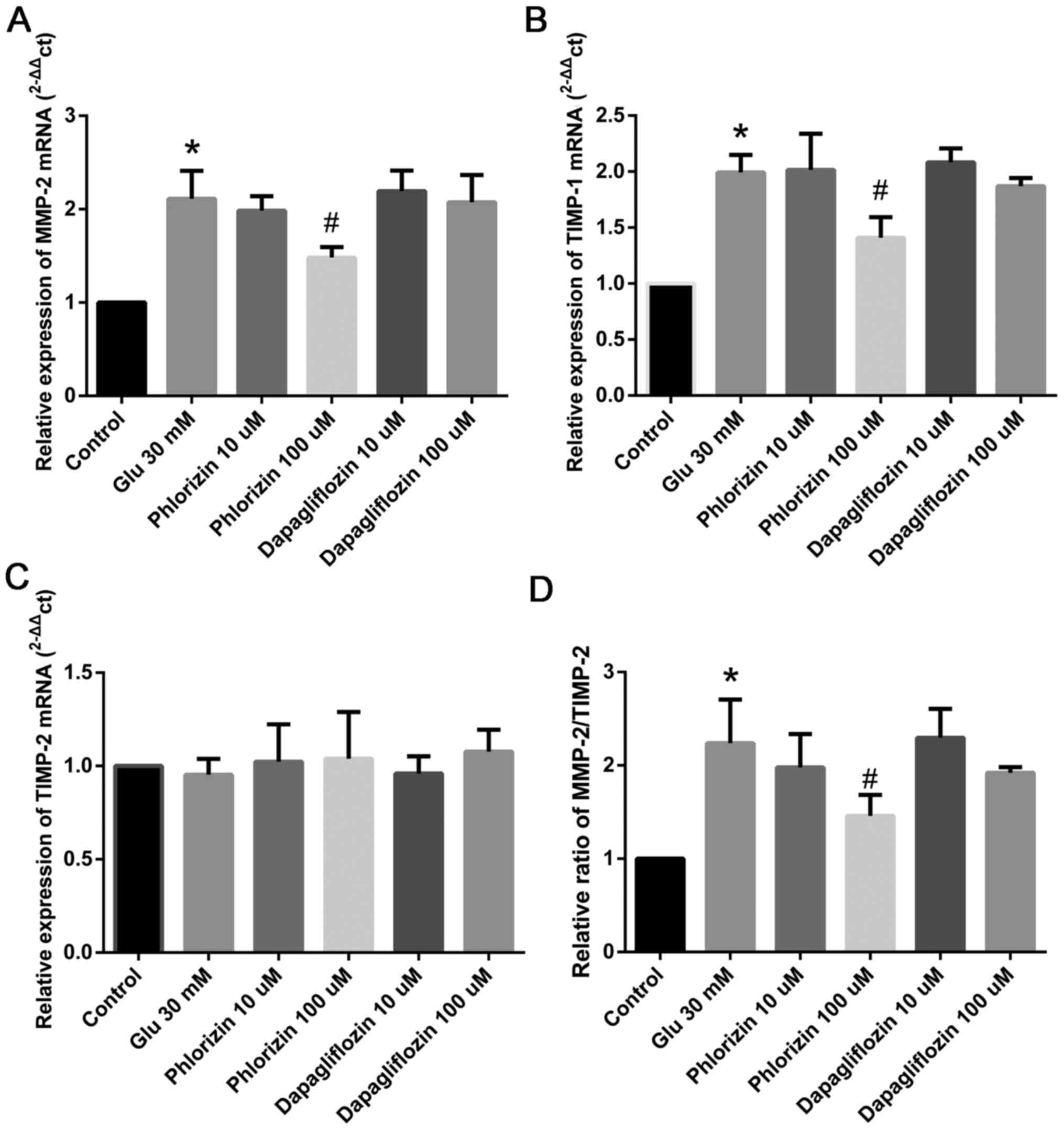
Phlorizin inhibits high glucose
level-induced MMP-2 and TIMP-1 expression in HCF
Based on the findings of previous studies (13) as well as the above-described
results, 30 mM glucose was used to induce the expression of
MMP-2 and TIMP-1. As shown in Fig. 2, 100 µM phlorizin inhibits
MMP-2 and TIMP-1 expression in HCF, which is induced
by high glucose (P<0.05); however, dapagliflozin had no effect
(Fig. 2A-B). Both phlorizin and
dapagliflozin had no effect on the expression of TIMP-2
(Fig. 2C). We also found that
phlorizin could decrease the ratio of MMP-2/TIMP-2
(P<0.05; Fig. 2D).
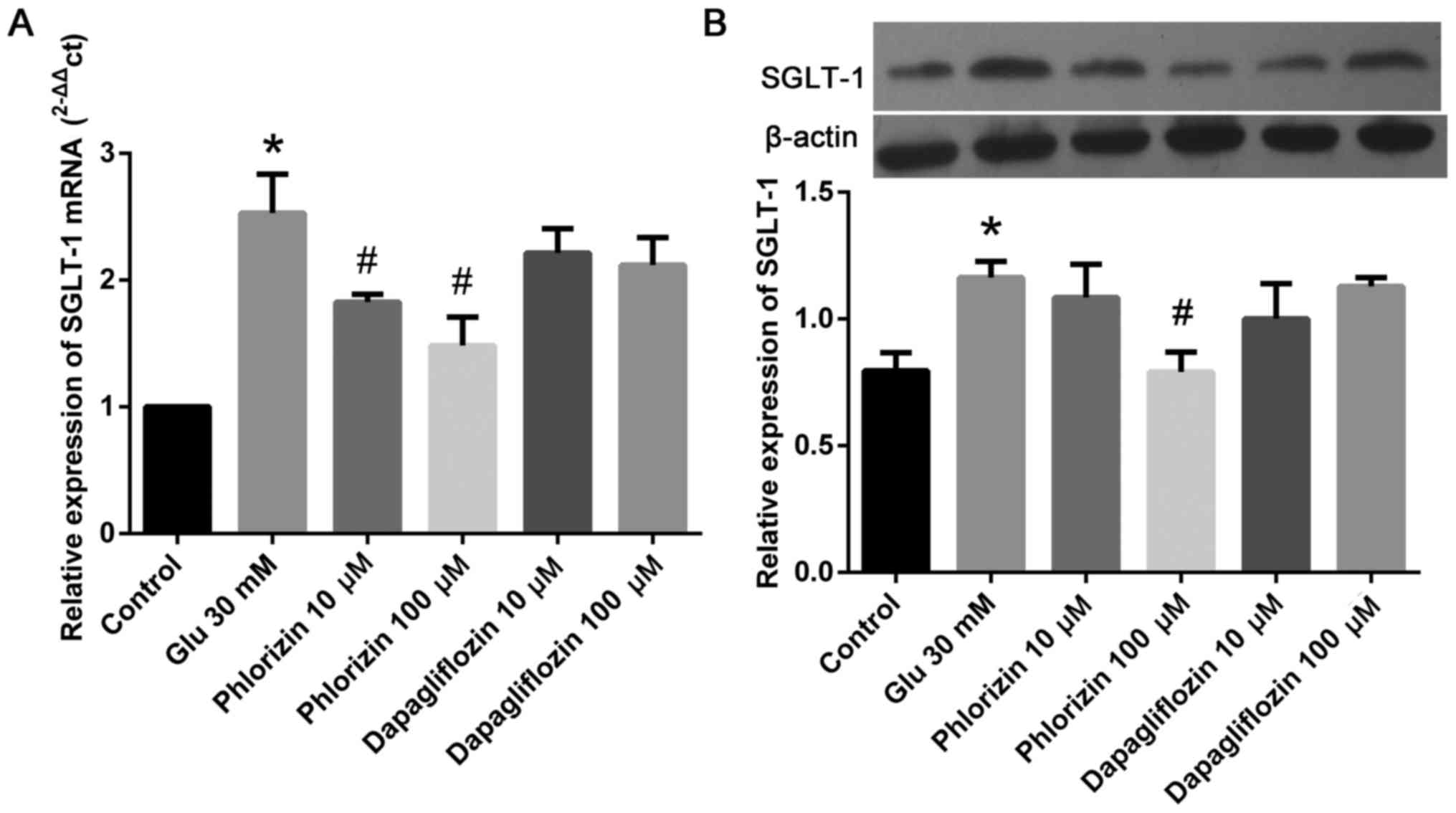
Phlorizin inhibits high glucose
level-induced SGLT-1 expression in HCF
To determine the involvement of SGLT-1, western
blotting was used to detect SGLT-1 expression in each group. As
shown in Fig. 3, we found that
high glucose levels increase the expression of SGLT-1 in HCF, and
phlorizin inhibits the expression of SGLT-1 (P<0.05). Meanwhile,
dapagliflozin did not have this effect. These results indicated
that high glucose levels might induce MMP-2 and
TIMP-1 expression by up-regulating SGLT-1.
Discussion
DCM, which was first described in 1972, is defined
as myocardial dysfunction in the DM patients without hypertension
and coronary artery disease; it could finally result in heart
failure (14). There is growing
evidence that myocardial fibrosis, cardiomyocyte apoptosis,
inflammation, oxidative stress, impaired calcium handling,
renin-angiotensin system activation, and mitochondrial dysfunction
are involved in the development of DCM (15). Among all these factors, myocardial
fibrosis is the most frequently proposed mechanism to explain
cardiac changes in DCM. Many studies have shown that inhibiting
myocardial fibrosis can attenuate the progress of DCM in animal
experiments (16,17).
Cardiac fibroblasts are the main cell type
constituting the heart, and are responsible for the basal
deposition and degradation of the ECM. As the primary structural
cells of the heart, cardiac fibroblasts are critically involved in
all cardiac fibrotic conditions. Liu et al (13), showed that high glucose levels
induced cardiac fibrosis in diabetic mice by increasing the
proliferation of and collagen synthesis by cardiac fibroblasts. In
addition, in the in vitro experiment, Wang et al
(18), found that high glucose
levels induced collagen formation and cytoskeleton degradation in
cardiac fibroblasts. Li et al (19), also showed that inhibiting high
glucose level-induced proliferation and differentiation of and
collagen accumulation by cardiac fibroblasts could be a new
therapeutic strategy for diabetes. In this study, we found that
high levels of glucose can induce MMP-2 expression in the
HCF. MMP-2 is the primary kind of gelatinases that can degrade the
ECM. Synthesis and decomposition of ECM are ongoing processes. In
the normal heart, the decomposition and synthesis of ECM are in
dynamic equilibrium. The balance is maintained by MMPs and TIMPs
(20). As TIMP-2 is an important
member of the TIMP family, it can effectively inhibit the activity
of MMP-2. Therefore, we further determined the
MMP-2/TIMP-2 ratio and found that high glucose level
also increases this ratio. Thus, these results indicate that high
glucose levels can degrade the ECM and attenuate cardiac fibrosis
by up-regulating MMP-2. However, as we know, during cardiac
remodeling, ECM degradation and synthesis are activated
simultaneously. Derangement of MMP-2 expression and activity
alters the balance between ECM synthesis and degradation, resulting
in excessive collagen deposition and reduced structural integrity
in the myocardium. Increasing degradation of ECM supplies space for
the proliferation and migration of HCF and other macrophagocytes,
which secrete inflammatory and growth factors, further enhancing
the cardiac remodeling process (21). Siddesha et al (22), showed that sustained induction and
activation of MMPs and the destruction and deposition of ECM can
result in cardiac fibrosis. In addition to its canonical function
in ECM degradation, studies in the recent year have highlighted new
functions of MMP-2 to induce cardiac conditions such as proteolysis
of novel substrates other than ECM proteins such as troponin I
(23), localization to subcellular
organelles like the mitochondria (24), and proteolysis of susceptible
intracellular proteins in subcellular compartments, such as
monocyte chemoattractant protein-3 (25). All these functions subsequently
resulted in cardiac remodeling and heart failure. In accordance
with our results, animal studies also showed that MMP-2 expression
and activity was increased in the diabetic heart (3,4).
Therefore, we suggest that high glucose levels up-regulate the
expression of MMP-2, which further promotes ECM degradation,
increased HCF migration and proliferation, finally resulting in
fibrosis and DCM.
SGLT1 has been reported to exist in the small
intestine, skeletal muscle, heart, kidney, trachea, prostate,
cervix, and mesenteric adipose tissue (26). It is also expressed in the kidney
and intestine (26). In the heart,
SGLT1 has been shown to be expressed in the cardiomyocytes and
endothelial cells (27). In the
present study, we found that SGLT1 was expressed in HCF. This is,
to the best of our knowledge, the first report that SGLT1 is
present in HCF. In addition, we found that SGLT1 is up-regulated by
glucose levels. Previous studies had found that SGLT1 is
substantially expressed in the myocardium and actually contributes
to the pathogenesis of PRKAG2 cardiomyopathy (8,9), and
that knockdown of SGLT1 can attenuate the disease phenotype
(10). Thus, SGLT1 might be
involved in DCM. In order to explore the effect of SGLT1, we used
phlorizin (inhibits SGLT1 and SGLT2) and dapagliflozin (inhibits
SGLT2). Our results showed that phlorizin can inhibit SGLT-1
expression in HCF. In addition, phlorizin can inhibit high glucose
level-induced MMP-2 and TIMP-1 expression in HCF. Also,
dapagliflozin did not exert this effect. These results indicated
that the up-regulation of SGLT1 is necessary for the induction of
MMP-2 expression by high glucose levels, and that the inhibition of
SGLT1 can attenuate this effect. Balteau et al (28), showed that SGLT1 is linked with
NADPH oxidase activation. Later, Van Steenbergen et al
(29), found that SGLT1 mediated
the production of reactive oxygen species induced by hyperglycemia
in the heart. Both NADPH oxidase activation and production of
reactive oxygen species enhanced MMP-2 expression and activation.
Thus, this might be the mechanism involved in the down-regulation
of MMP-2 expression by inhibited SGLT1. In the subsequent
experiment, we will use siRNA to knock down SGLT1 and over express
SGLT1 to further verify the relationship between SGLT1 and
MMP-2.
In a recent study, myocardial ischemia and
hypertrophy were found to be associated with SGLT1 up-regulation,
while SGLT2 was not expressed (30). SGLT1 inhibition in the heart, which
was previously thought to inhibit SGLT1 expression in the
cardiomyocytes, could result in potential improvement of cardiac
function and reduction of arrhythmic risk. Our study suggests
another mechanism used by SGLT1 to protect the diabetic heart, that
is, by attenuating glucose-induced MMP-2 expression in HCF.
Although dapagliflozin has demonstrated anti-DCM effect in previous
studies (31,32), in the present study, it showed no
effect on glucose-induced MMP2 expression. The possible mechanism
can be down-regulation of serum glucose levels or direct influence
on the cardiac tissue through some unknown mechanism.
In summary, inhibition of MMP-2 expression is
suggested to be cardioprotective in diabetes. In this study, we
showed that MMP-2 expression increased in the HCF in
response to high glucose levels, which could be reversed by
phlorizin, but not by dapagliflozin. In addition, we found that
SGLT1 exists in the HCF and that high glucose levels increase the
expression of SGLT1 in HCF, which could also be attenuated by
phlorizin. Thus, we concluded that high glucose levels induce
MMP-2 expression in HCF, possibly by up-regulation of
SGLT1.
This study has some limitations. First, we only
tested the mRNA levels of MMP-2, MMP-9, TIMP-1, and
TIMP-2; we have not evaluated the expression of these
proteins by western blotting. Second, SGLT1 over-expression or
knock-down has not been used to further verify the relationship
between SGLT1 and MMP-2. Third, it is only an in vitro
experiment using one cell line; evaluation of other cell lines and
in vivo experiments should be conducted in future to verify
this conclusion.
Acknowledgements
The present study was supported by the Basic Public
Welfare Research Project of Zhejiang Province (LGF18H020009) and
the Youth Research Fund Project of Shaoxing People's Hospital
(2017A02).
References
|
1
|
Fang ZY, Prins JB and Marwick TH: Diabetic
cardiomyopathy: Evidence, mechanisms and therapeutic implications.
Endocr Rev. 25:543–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeCoux A, Lindsey ML, Villarreal F, Garcia
RA and Schulz R: Myocardial matrix metalloproteinase-2: Inside out
and upside down. J Mol Cell Cardiol. 77:64–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Sun SZ, Wang Y, Tian YJ and Liu MH:
The roles of MMP-2/TIMP-2 in extracellular matrix remodelling in
the hearts of STZ-induced diabetic rats. Acta Cardiol. 62:485–491.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen SL, Hu ZY, Zuo GF, Li MH and Li B:
I(f) current channel inhibitor (ivabradine) deserves
cardioprotective effect via down-regulating the expression of
matrix metalloproteinase (MMP)-2 and attenuating apoptosis in
diabetic mice. BMC Cardiovasc Disord. 14:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zinman B, Wanner C, Lachin JM, Fitchett D,
Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ,
et al: Empagliflozin, cardiovascular outcomes and mortality in type
2 diabetes. N Engl J Med. 373:2117–2128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cefalu WT, Leiter LA, de Bruin TW,
Gause-Nilsson I, Sugg J and Parikh SJ: Dapagliflozin's effects on
glycemia and cardiovascular risk factors in high-risk patients with
type 2 diabetes: A 24-week, multicenter, randomized, double-blind,
placebo-controlled study with a 28-week extension. Diabetes Care.
38:1218–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song P, Onishi A, Koepsell H and Vallon V:
Sodium glucose cotransporter SGLT1 as a therapeutic target in
diabetes mellitus. Exp Opin Ther Targets. 20:1109–1125. 2016.
View Article : Google Scholar
|
|
8
|
Kashiwagi Y, Nagoshi T, Yoshino T, Tanaka
TD, Ito K, Harada T, Takahashi H, Ikegami M, Anzawa R and Yoshimura
M: Expression of SGLT1 in human hearts and impairment of cardiac
glucose uptake by phlorizin during ischemia-reperfusion injury in
mice. PLoS One. 10:e01306052015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banerjee SK, Ramani R, Saba S, Rager J,
Tian R, Mathier MA and Ahmad F: A PRKAG2 mutation causes biphasic
changes in myocardial AMPK activity and does not protect against
ischemia. Biochem Biophys Res Commun. 360:381–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramratnam M, Sharma RK, D'Auria S, Lee SJ,
Wang D, Huang XY and Ahmad F: Transgenic knockdown of cardiac
sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2
cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1
causes pathologic hypertrophy and dysfunction in mice. J Am Heart
Assoc. 3:e0008992014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das S, Mandal M, Chakraborti T, Mandal A
and Chakraborti S: Isolation of MMP-2 from MMP-2/TIMP-2 complex:
Characterization of the complex and the free enzyme in pulmonary
vascular smooth muscle plasma membrane. Biochim Biophys Acta.
1674:158–174. 2004.PubMed/NCBI
|
|
12
|
Avolio C, Filippi M, Tortorella C, Rocca
MA, Ruggieri M, Agosta F, Tomassini V, Pozzilli C, Stecchi S,
Giaquinto P, et al: Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in
multiple sclerosis: Relationships with different magnetic resonance
imaging measures of disease activity during IFN-beta-1a treatment.
Mult Scler. 11:441–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, Song X, Lu J, Chen X, Liang E, Liu
X, Zhang M, Zhang Y, Du Z and Zhao Y: Neferine inhibits
proliferation and collagen synthesis induced by high glucose in
cardiac fibroblasts and reduces cardiac fibrosis in diabetic mice.
Oncotarget. 7:61703–61715. 2016.PubMed/NCBI
|
|
14
|
Rubler S, Dlugash J, Yuceoglu YZ, Kumral
T, Branwood AW and Grishman A: New type of cardiomyopathy
associated with diabetic glomerulosclerosis. Am J Cardiol.
30:595–602. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia G, Whaley-Connell A and Sowers JR:
Diabetic cardiomyopathy: A hyperglycaemia- and
insulin-resistance-induced heart disease. Diabetologia. 61:21–28.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou C, Liu X, Xie R, Bao Y, Jin Q, Jia X,
Li L and Liu R: Deferiprone attenuates inflammation and myocardial
fibrosis in diabetic cardiomyopathy rats. Biochem Biophys Res
Commun. 486:930–936. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT
and Chen ZC: Cryptotanshinone inhibits STAT3 signaling to alleviate
cardiac fibrosis in type 1-like diabetic rats. Phytother Res.
31:638–646. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XW, Zhang FX, Yang F, Ding ZF,
Agarwal N, Guo ZK and Mehta JL: Effects of linagliptin and
liraglutide on glucose- and angiotensin II-induced collagen
formation and cytoskeleton degradation in cardiac fibroblasts in
vitro. Acta Pharmacol Sin. 37:1349–1358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Dai Y, Su Z and Wei G: MicroRNA-9
inhibits high glucose-induced proliferation, differentiation and
collagen accumulation of cardiac fibroblasts by down-regulation of
TGFBR2. Biosci Rep. 36:e004172016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng L, Liu L, Zhou C, Pan S, Zhai X,
Jiang C, Guo Y, Ji Z, Chi J, Peng F and Guo H: Polyphenols and
polypeptides in chinese rice wine inhibit homocysteine-induced
proliferation and migration of vascular smooth muscle cells. J
Cardiovasc Pharmacol. 67:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi YF, Chi JF, Tang WL, Xu FK, Liu LB, Ji
Z, Lv HT and Guo HY: Effects of rosuvastatin on the production and
activation of matrix metalloproteinase-2 and migration of cultured
rat vascular smooth muscle cells induced by homocysteine. J
Zhejiang Univ Sci B. 14:696–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siddesha JM, Valente AJ, Sakamuri SS,
Yoshida T, Gardner JD, Somanna N, Takahashi C, Noda M and
Chandrasekar B: Angiotensin II stimulates cardiac fibroblast
migration via the differential regulation of matrixins and RECK. J
Mol Cell Cardiol. 65:9–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cauwe B and Opdenakker G: Intracellular
substrate cleavage: A novel dimension in the biochemistry, biology
and pathology of matrix metalloproteinases. Crit Rev Biochem Mol
Biol. 45:351–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hughes BG, Fan X, Cho WJ and Schulz R:
MMP-2 is localized to the mitochondria-associated membrane of the
heart. Am J Physiol Heart Circ Physiol. 306:H764–H770. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westermann D, Savvatis K, Lindner D,
Zietsch C, Becher PM, Hammer E, Heimesaat MM, Bereswill S, Volker
U, Escher F, et al: Reduced degradation of the chemokine MCP-3 by
matrix metalloproteinase-2 exacerbates myocardial inflammation in
experimental viral cardiomyopathy. Circulation. 124:2082–2093.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Williams S, Ho S, Loraine H, Hagan
D, Whaley JM and Feder JN: Quantitative PCR tissue expression
profiling of the human SGLT2 gene and related family members.
Diabetes Ther. 1:57–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin X, Yi L, Chen ML, Chen CY, Chang H,
Zhang T, Wang L, Zhu JD, Zhang QY and Mi MT:
Delphinidin-3-glucoside protects against oxidized low-density
lipoprotein-induced mitochondrial dysfunction in vascular
endothelial cells via the sodium-dependent glucose transporter
SGLT1. PLoS One. 8:e686172013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balteau M, Tajeddine N, de Meester C,
Ginion A, Des Rosiers C, Brady NR, Sommereyns C, Horman S,
Vanoverschelde JL, Gailly P, et al: NADPH oxidase activation by
hyperglycaemia in cardiomyocytes is independent of glucose
metabolism but requires SGLT1. Cardiovasc Res. 92:237–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Steenbergen A, Balteau M, Ginion A,
Ferte L, Battault S, Ravenstein CM, Balligand JL, Daskalopoulos EP,
Gilon P, Despa F, et al: Sodium-myoinositol cotransporter-1, SMIT1,
mediates the production of reactive oxygen species induced by
hyperglycemia in the heart. Sci Rep. 7:411662017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Franco A, Cantini G, Tani A, Coppini R,
Zecchi-Orlandini S, Raimondi L, Luconi M and Mannucci E:
Sodium-dependent glucose transporters (SGLT) in human ischemic
heart: A new potential pharmacological target. Int J Cardiol.
243:86–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye Y, Bajaj M, Yang HC, Perez-Polo JR and
Birnbaum Y: SGLT-2 inhibition with dapagliflozin reduces the
activation of the Nlrp3/ASC inflammasome and attenuates the
development of diabetic cardiomyopathy in mice with type 2
diabetes. Further augmentation of the effects with saxagliptin, a
DPP4 inhibitor. Cardiovasc Drugs Ther. 31:119–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joubert M, Jagu B, Montaigne D, Marechal
X, Tesse A, Ayer A, Dollet L, Le May C, Toumaniantz G, Manrique A,
et al: The sodium-glucose cotransporter 2 inhibitor dapagliflozin
prevents cardiomyopathy in a diabetic lipodystrophic mouse model.
Diabetes. 66:1030–1040. 2017. View Article : Google Scholar : PubMed/NCBI
|