Introduction
The development of malignant ascites is a common
complication of advanced or recurrent malignant tumors (1). Primary diseases that lead to
malignant ascites primarily include abdominal and pelvic malignant
tumors, such as malignant gastrointestinal tumors, ovarian tumors
and liver tumors (2). Recurrent
malignant ascites increases intra-abdominal pressure, abdominal
distention, dyspnea and severe loss of body weight, which
accelerates disease progression, affects anti-tumor treatment and
the patients' quality of life. In addition, patient prognosis is
poor once malignant ascites develop. It is reported that 18.3%
malignant ascites occur as a result of gastric cancer (3). The one-year survival rate of patients
with gastric cancer is ≤10%, and the average survival time is ≤6
months (4). The treatment options
for malignant ascites are limited. In the clinic, the primary
treatments include dieresis, puncture and aspiration, a peritoneal
shunt and systemic or intraperitoneal chemotherapy. Of these,
intraperitoneal chemotherapy has become an important treatment for
malignant ascites. Chemotherapy drugs are administered into the
peritoneal cavity, which may directly kill cancer cells. However,
chemotherapy cannot fully and effectively enhance patient survival
and control the metastasis of gastric tumors in the peritoneum
(5).
Studies have demonstrated that particular
antibacterial peptides are cytotoxic to transformed cells, whereas
they are less cytotoxic to non-transformed cells (6,7). The
antibacterial peptide, cecropin exhibits antitumor activity when
injected locally into solid tumors (8).
CecropinXJ is isolated from the larvae of Bombyx
mori (B. mori), which has a 37-amino acid cationic
antimicrobial peptide sequence with specific amphipathic α-helices
(9). CecropinXJ demonstrates a
broad activity against bacteria and fungi (10,11).
Previous studies have revealed that cecropinXJ may inhibit the
proliferation of human BGC823 gastric cancer cells in vitro
(12), whereas, it demonstrates no
hemolytic effects against human erythrocytes and no toxicity to
healthy mammalian cells (13,14).
However, to the best of the author's knowledge, no relevant studies
have been performed to date that explore the effects of cecropinXJ
on the growth of tumor-associated ascites in human gastric cancer.
In the present study, the therapeutic effects of cecropinXJ were
investigated in mice bearing malignant ascites. The results
revealed that cecropinXJ may effectively inhibit the formation and
growth of malignant ascites and improve the survival of
tumor-bearing mice, which was reflected by the normal blood and
biochemical indexes, and the lack of toxic effects on the liver,
kidney and spleen. These results suggested that cecropinXJ might be
utilized as a potential therapeutic drug for the treatment of
malignant ascites in patients with gastric cancer.
Materials and methods
Preparation of antimicrobial peptide
cecropinXJ
The cecropinXJ sequence of B. mori was
obtained via the Saccharomyces cerevisiae eukaryotic
expression system, and purified using a nickel-chelating Sepharose
column, as previously described (11). The concentration of purified
recombinant cecropinXJ protein was detected using a Bradford
protein assay kit (BioTeke Corporation, Beijing, China). The amino
acid sequence of cecropinXJ is as follows:
WKIFKKIEKMGRNIRDGIVKAGPAIEVLGSAKAIGK. Prior to use, the peptide was
dissolved in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) to a concentration of 1
mg/ml, and sterilized by filtration through a 0.22 µm filter.
Cell culture
The human gastric cancer cell line, BGC823, was
kindly provided by Professor Youyong Lv (Beijing Cancer Hospital,
Beijing, China). BGC823 cells were cultured in DMEM medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 µg/ml streptomycin and 100
U/ml penicillin (HyClone; GE Healthcare Life Sciences), in a
humidified atmosphere of 95% air with 5% CO2 at 37°C.
Cells growing in the mid-logarithmic growth phase were utilized in
all experiments.
Animals
BALB/C mice (weight, 17–22 g; age, 5–6 weeks) were
purchased from the Center for Disease Control and Prevention
(Xinjiang, China). The animals were maintained at a temperature of
23±2°C and a relative humidity of 50±10%, with 12 h light/dark
cycles. All experiments were conducted and approved by the Chinese
Animal Care for Laboratory Animals (Beijing, China).
Experimental animal grouping and
administration
BGC823 cells were suspended in PBS at a
concentration of 2×108 cells/ml, and 0.5 ml of the
suspension was injected into the peritoneal cavity of each animal.
In total, we selected 30 mice with abdominal bulging. Mice were
divided into the following 3 groups at random (n=10): The negative
control group, where mice were treated with bovine serum albumin
(BSA, 5 mg/kg, d1~d10; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany); the cecropinXJ treatment group (5 mg/kg,
d1~d10); the positive control group, where
mice were treated with doxorubicin (Dox, 5 mg/kg,
d1~d10). In addition, we set the healthy
group (n=10), where mice were not injected with tumor cells. The
concentration of BSA, cecropinXJ and Dox was adjusted with saline,
and mice were administered with treatments once every day, for 10
days by intraperitoneal injection. The body weight and abdominal
circumference of mice was measured every day. Blood was collected
from the posterior orbital venous plexus at 24 h following the
final treatment, in order to measure blood physiological and serum
biochemical indexes. In addition, the volume of ascites and rate of
apoptosis were determined. Three mice in each group were selected
at random and sacrificed by cervical spine dislocation. Ascites
supernatant and abdominal viscera samples were collected and stored
at −80°C. The remaining mice in each group were used for survival
analysis.
Body weight analysis
Alterations in appetite, mental state and abdominal
circumference of mice in each group were observed. In addition, the
body weight of the mice in each group was monitored for a period
over 18 days, recorded and used to generate a body weight
alteration curve.
Abdominal circumference analysis
Abdominal bulging was observed in all groups, and
the abdominal circumference of the mice in each group was measured
and recorded daily for 18 days. The values were used to generate a
curve of abdominal circumference alterations.
Physiological and biochemical
analysis
Blood biochemical factors may alter during the
development of a tumor (15). A
total of 0.5 ml blood from each mouse was obtained at 24 h
following the final treatment, and blood factors (Hemoglobin, Red
blood cells count, White blood cells count and differential
leukocyte count) were measured with an automatic blood cell
analyzer (Blood Cell Counter 3-Part/Diff Hematology Analyzer,
Poweam Medical Co., ltd., Nanjing) according to the manufacturer's
protocol. For hepatic and renal function tests, blood was
centrifuged at 2,000 × g at 4°C for 10 min to prepare the serum.
The levels of albumin, globulin, alanine aminotransferase (ALT),
aspartate aminotransferase (AST), urea, creatinine and uric acid
were analyzed using the automatic blood cell analyzer according to
the manufacturer's protocol.
Volume of ascites in tumor-bearing
mice
Three mice in each group were sacrificed by cervical
spine dislocation. A laparotomy was performed under aseptic
conditions. Ascites fluid was collected and placed in a centrifuge
tube, and the volume was measured.
Tumor cell apoptosis in ascites fluid
of tumor-bearing mice
Annexin V and propidium iodide (PI) staining was
employed to quantify the effect of antimicrobial peptide on
apoptosis. Three mice in each group were sacrificed by cervical
spine dislocation. A laparotomy was performed under aseptic
conditions. Ascites fluid was collected and placed in a centrifuge
tube, and used for tumor cell apoptosis analysis. An Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(BestBio Biotechnologies, cat. no. 401003, Shanghai, China) was
used according to the manufacturer's protocol, and the results were
quantified by flow cytometric analysis. Briefly, cells of ascites
fluid were collected and washed with ice-cold phosphate-buffered
saline (PBS) prior to detaching cells with trypsin. Cells were
centrifuged at 2,000 × g for 5 min at 4°C and resuspended in 400 µl
PBS. Cells were centrifuged again for 5 min at 4°C, resuspended in
200 µl Annexin V binding buffer, and incubated with Annexin V-FITC
(5 µl) and PI (10 µl) at 4°C for 15 min in the dark. Cells were
analyzed using a FACScan flow cytometer with CellQuest software
version 3.0 (BD Biosciences, Franklin Lakes, NJ, USA).
Determination of the liver-body weight
ratio, and the thymus and spleen index
The spleen, thymus and liver of sacrificed mice as
described earlier were removed and weighed. The hepatosomatic
index, kidney somatic index, thymus index and spleen index were
calculated according to the following formulae: Hepatosomatic Index
(HSI)=liver weight (g)/body weight (g); kidney somatic index
(KSI)=kidney weight (mg)/body weight (g); thymus index=thymus
weight (mg)/body weight (g) ×10; spleen index=spleen weight
(mg)/body weight (g) ×10.
Metastasis to abdominal viscera of
mice
The spleen, thymus and liver that were removed from
mice were weighed, fixed in 10% formalin solution at room
temperature for 24 h and embedded in a paraffin block. The paraffin
block was cut into 5 µm sections and stained with hematoxylin and
eosin at room temperature for 5 min. The pathological alterations,
including inflammation, necrosis, mitotic index and apoptosis index
from 5 random fields were observed under an optical microscope
(data not shown).
Survival time of tumor-bearing
mice
The survival of the seven remaining mice in each
group was recorded for 30 days, and the increase in life span was
calculated according to the following formula: Increase in life
span (%)=(T/C-1) ×100, where T represented the average survival
(days) of mice in each treatment group, and C represented the
average survival (days) of mice in the negative control group.
Statistical analysis
Experiments were repeated at least three times. Data
are expressed as the mean ± standard deviation. A Student's t-test
was used to analyze differences between two groups, whereas
differences among ≥3 groups were analyzed using one-way analysis of
variance followed by a Newman-Keuls test. SPSS software (version,
13.0; SPSS, Inc., Chicago, IL, USA) was used to analyze the data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CecropinXJ inhibits ascites
development in mice
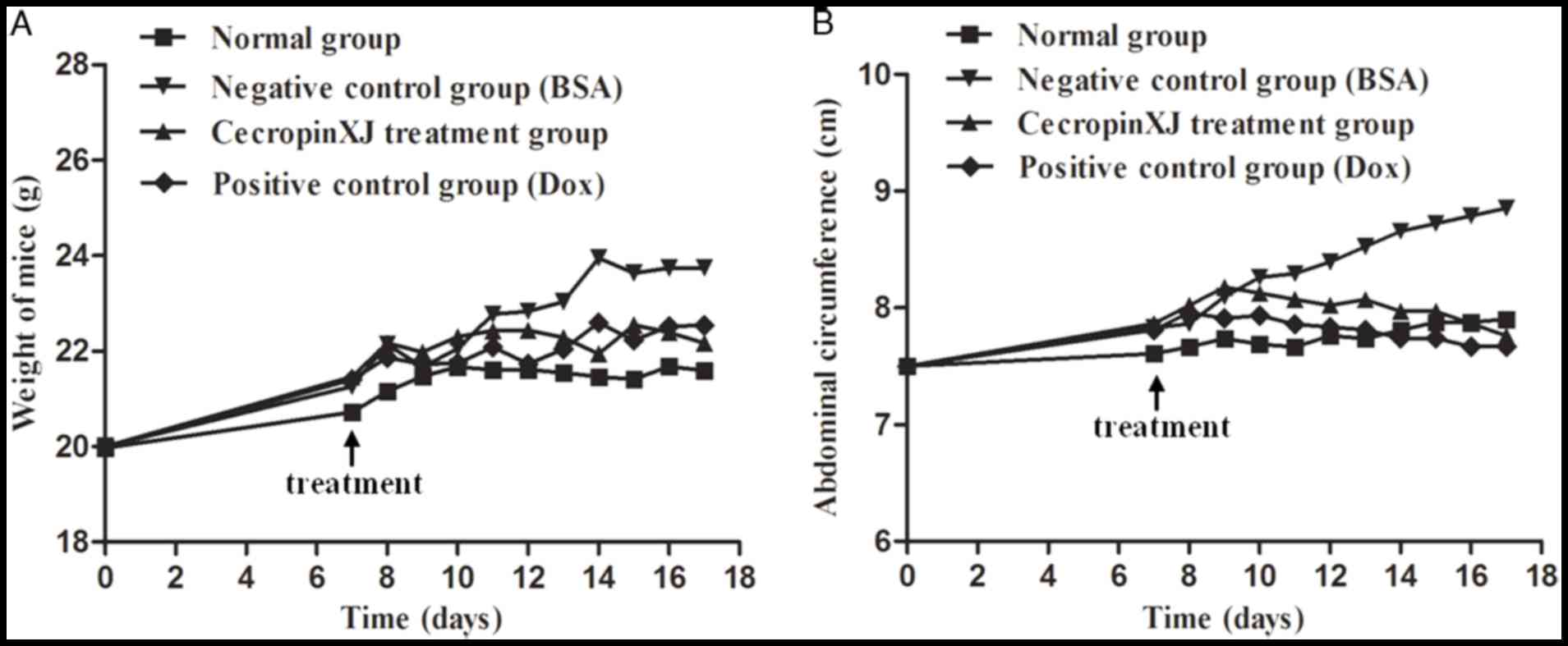
Body weight and abdominal circumference measurements
are direct indexes for determining the development of abdominal
ascites tumors in mice (16,17).
The body weight and abdominal circumference of normal healthy mice
increased at a steady rate, whereas BGC823 tumor-bearing mice
exhibited a sharp increase in body weight and abdominal
circumference (Fig. 1). This
suggested that the BGC823 ascites model was successfully
established. To determine the effect of cecropinXJ on malignant
ascites formation and tumor growth, mice bearing ascites tumors
were administered with cecropinXJ for 10 consecutive days. When
compared with the BSA control group, the body weight of mice in the
cecropinXJ and Dox treatment groups increased slowly and the
abdominal circumference started to decrease at 4 days following the
commencement of treatment (Fig.
1). The inhibitory effect of cecropinXJ on the body weight and
abdominal circumference of mice was similar to Dox treatment. At
the end of treatment, the body weight and abdominal circumference
of mice in the cecropinXJ treatment group was reduced by 6.61 and
12.36%, respectively, when compared with the BSA control group
(Fig. 1). The body weight and
abdominal circumference of mice in the Dox treatment group were
reduced by 6.05 and 12.69%, respectively, when compared with the
BSA control group (Fig. 1).
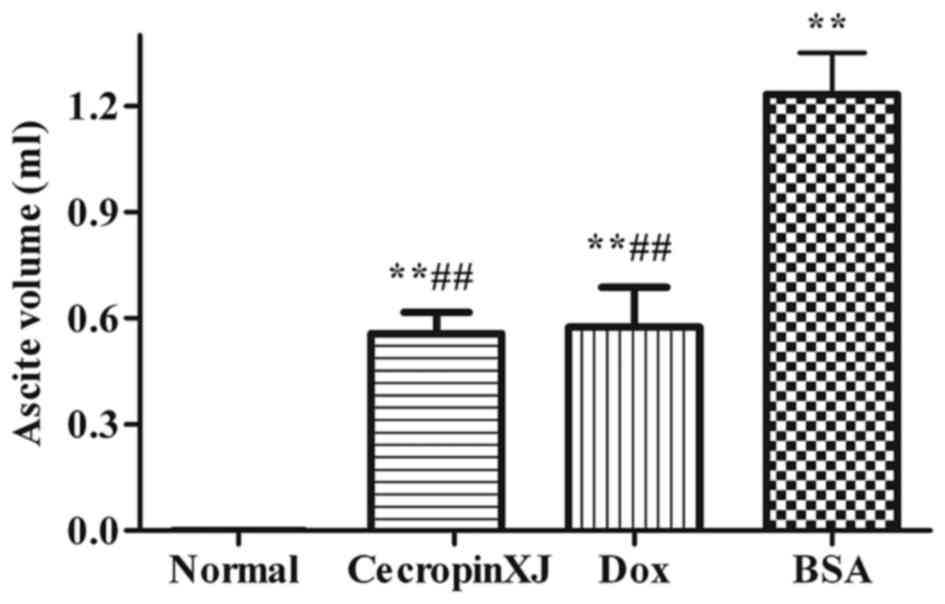
Ascites formation in mice
Ascites formation was significantly inhibited by
cecropinXJ (Fig. 2). The ascites
volume of tumor-bearing mice in the BSA control, cecropinXJ and Dox
treatment groups was 1.23, 0.57 and 0.55 ml, respectively. The
ascites volume in mice treated with cecropinXJ and Dox was
significantly reduced when compared with the BSA control group
(P<0.01); however, there was no significant difference between
the cecropinXJ and Dox treatment groups (Fig. 2).
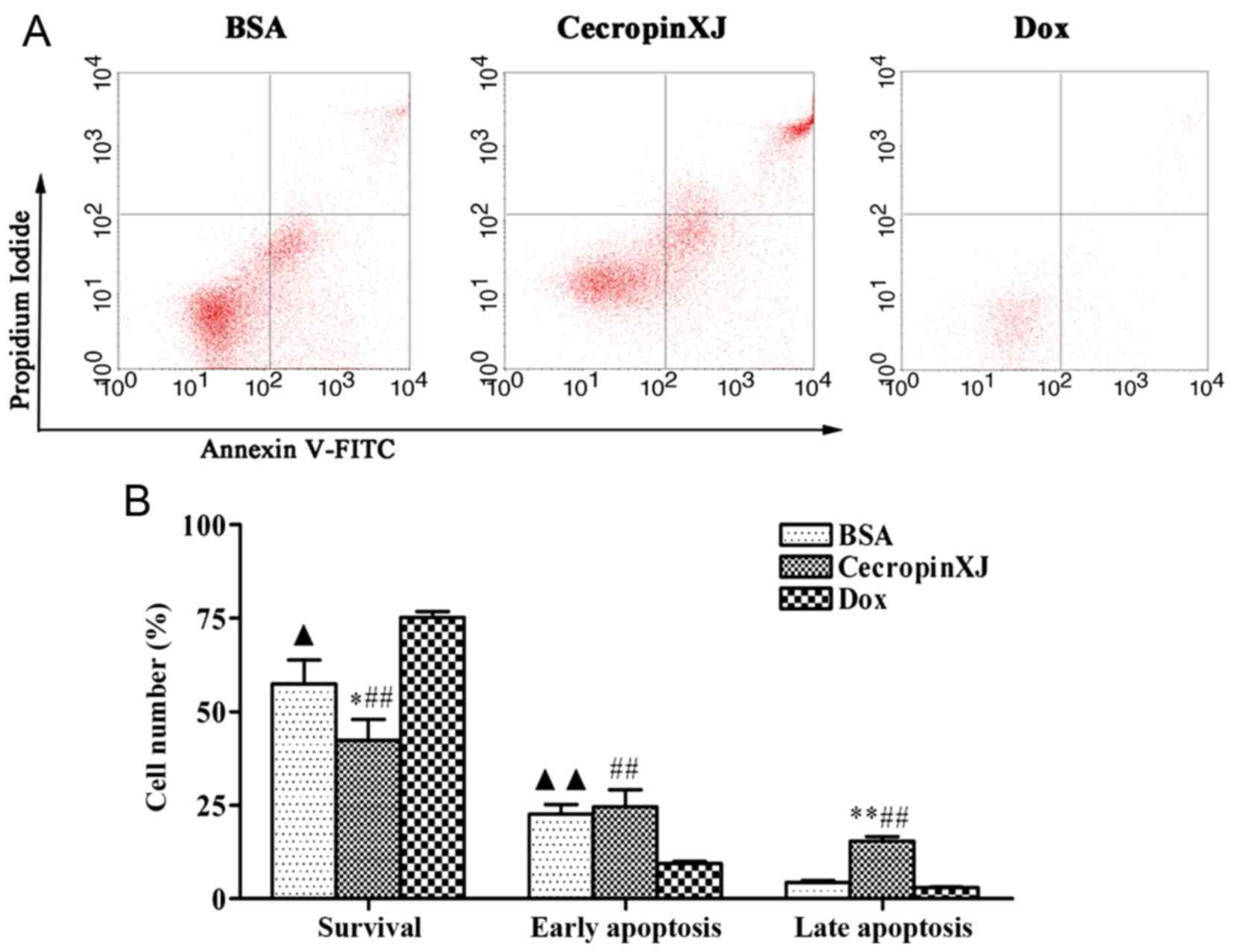
Apoptosis of tumor cells in ascites
fluid of tumor-bearing mice
Apoptosis of tumor cells in ascites fluid was
detected by flow cytometry analysis. As demonstrated in Fig. 3, cecropinXJ treatment increased the
level of apoptosis in ascites fluid-derived tumor cells. Apoptosis
increased from 21.25±2.13 (BSA) to 32.18±4.36 (cecropinXJ; Fig. 3). The results suggested that
intraperitoneal injection of cecropinXJ into tumors may inhibit
tumor growth, prevent the formation of ascites fluid and induce
apoptosis of tumor cells.
Prolongation of cecropinXJ on survival
time of tumor-bearing mice
Effective anti-tumor chemotherapy drugs may
significantly prolong the survival of tumor-bearing mice. In the
present study, tumor-bearing mice were treated with BSA, cecropinXJ
or Dox for 10 consecutive days and the survival time was recorded.
The increase in life-span of tumor-bearing mice in the cecropinXJ
treatment group was 66%, whereas the increase in survival of mice
treated with Dox was 63% when compared with the BSA control group
(Fig. 4).
Effect of cecropinXJ on blood
parameters
The level of routine blood factors in mice from each
group was analyzed, and included red and white blood cell counts
and hemoglobin levels. During the development of a tumor, the
levels of hemoglobin and red blood cells are frequently reduced,
whereas the white blood cell count is increased (18). As demonstrated in Table I, treatment with cecropinXJ and Dox
resulted in enhanced levels of hemoglobin (P<0.01) and red blood
cells (P<0.05), and significantly reduced the white blood cell
count (P<0.01) when compared with mice treated with BSA.
 | Table I.Blood hematological index in mice. |
Table I.
Blood hematological index in mice.
| Parameter | Healthy mice
group | Negative control
group (BSA) | CecropinXJ treatment
group | Positive control
group (Dox) |
|---|
| Hemoglobin (g/l) |
153.67±0.58 |
100.50±0.71b |
123.5±0.71d |
131.33±2.89d |
| RBC (109
cells/ml) |
10.05±0.19 |
8.37±0.51b |
9.15±0.01c |
9.56±0.25c |
| WBC (106
cells/ml) |
2.73±0.18 |
7.19±0.46b |
4.52±0.27d |
5.19±0.25a,d |
| Platelet (106
cells/ml) |
563.00±32.70 |
996.5±416.49b |
878.00±410.12a |
1,139.33±150.19b |
| Neutrophils (%) |
22.43±0.86 |
24.70±0.29 |
27.20±0.71 |
25.77±0.93 |
| Lymphocytes (%) |
77.23±1.36 |
93.95±1.06a |
92.25±1.48 |
92.27±0.75 |
| Monocytes (%) |
0.33±0.56 |
1.75±0.21 |
0.55±0.78 |
1.97±0.42a |
Effect of cecropinXJ on hepatic and
renal function
Blood biochemical indexes of mice in each group were
measured, including indicators of liver function, such as albumin,
globulin, ALT, AST and indicators of renal function, such as urea,
creatinine and uric acid levels. As demonstrated in Table II, almost all parameters in mice
from the BSA control group were significantly altered when compared
with the normal healthy control group, indicating that the liver
and kidney may have been damaged. However, treatment with
cecropinXJ reversed these alterations in hepatic and renal function
in mice. Compared with BSA-treated mice, ALT, BUN (P<0.01) and
AST (P<0.05) were reduced, and Uric acid (P<0.05) were
enhanced in cecropinXJ-treated mice.
 | Table II.Blood physiochemical indexes in
mice. |
Table II.
Blood physiochemical indexes in
mice.
| Parameters | Healthy mice
group | Negative control
group (BSA) | CecropinXJ
treatment group | Positive control
group (Dox) |
|---|
| Albumin (g/l) |
30.37±0.60 |
26.87±0.47a |
26.60±0.71 |
27.25±0.64 |
| Globulin (g/l) |
21.27±1.55 |
27.27±0.93a |
30.60±0.71a |
28.25±2.76a |
| ALT (U/l) |
41.33±2.31 |
52.33±5.85a |
31.50±0.71d |
40.00±2.83c |
| AST (U/l) |
115.33±3.78 |
129.33±5.86a |
117.50±0.71c |
102.5±6.36d |
| BUN (mmol/l) |
6.33±0.21 |
8.17±0.21b |
6.70±0.28d |
5.80±0.56d |
| CR (µmol/l) |
10.77±1.63 |
11.67±2.57 |
11.45±0.64 |
10.75±1.91 |
| Uric acid
(µmol/l) |
177.92±14.37 |
143.23±1.67a |
179.09±0.26c |
150.805±13.14a |
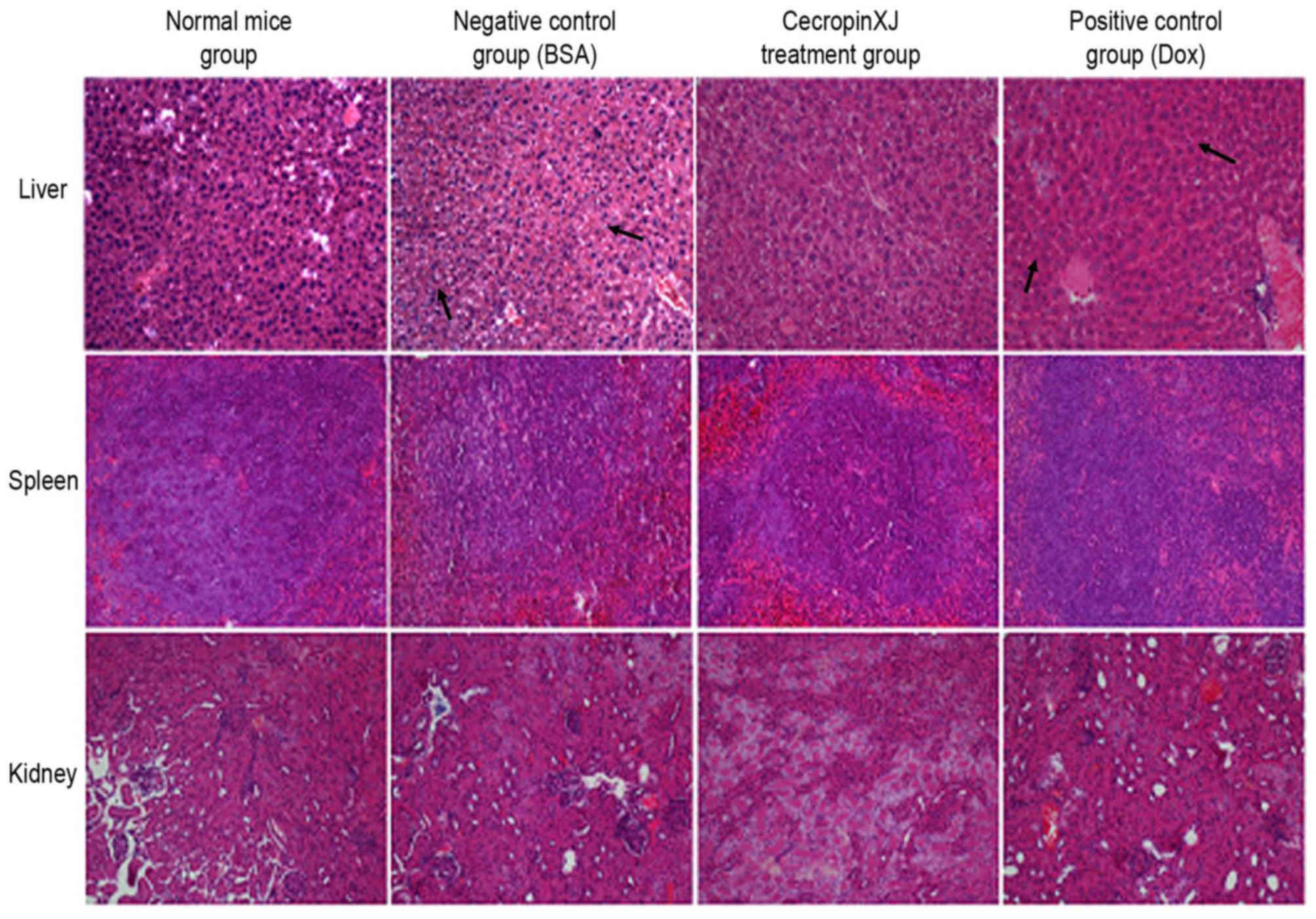
Effect of cecropinXJ on viscera of
mice
The abdominal viscera, including the liver, kidney
and spleen of sacrificed mice were subjected to hematoxylin and
eosin staining, and pathological factors, including infiltration,
inflammation, congestion, degradation and regeneration, were
determined. The results revealed that the livers of mice in the BSA
control and Dox groups exhibited cell necrosis, infiltration as a
result of central vein dilation and lymphocytosis (Fig. 5). By contrast, the kidney and
spleen of mice among all groups did not exhibit any significant
pathological alterations (Fig.
5).
Effect of cecropinXJ on HIS, KSI,
thymus index and spleen index
As demonstrated in Table III, the thymus and spleen indexes
in mice treated with Dox were not significantly different when
compared with the BSA control group. However, the thymus and spleen
indexes of mice treated with cecropinXJ were significantly
increased when compared with the BSA group. When compared with the
normal healthy control group, the thymus index of mice in Dox group
was decreased, however, this did not reach statistical significance
(Table III). Mice in the
cecropinXJ treatment group displayed the highest thymus and spleen
indexes among the tumor-bearing mice.
 | Table III.Effect cecropinXJ treatment on immune
organ indexes. |
Table III.
Effect cecropinXJ treatment on immune
organ indexes.
| Parameter | Healthy mice
group | Negative control
group (BSA) | CecropinXJ
treatment group | Positive control
group (Dox) |
|---|
| Number | 10 | 10 | 10 | 10 |
| HSI (%) | 6.36±1.82 | 8.66±0.39 | 8.08±1.57 | 6.70±0.40 |
| KSI (%) | 0.90±0.40 | 1.32±0.43 | 1.89±0.20 | 1.32±0.09 |
| Thymus index | 19.72±3.73 | 12.28±2.75 |
33.06±1.15d,e | 17.27±3.53 |
| Spleen index | 60.77±3.94 |
150.82±1.29b |
190.35±1.44c | 130.78±1.31 |
Discussion
Malignant ascites and peritoneal metastases develop
due to metastasis of malignant tumor cells to the peritoneum. It is
known that ~50% patients with advanced or recurrent malignant
tumors develop varying degrees of malignant ascites (19). Once this occurs, the survival of
patients is usually ~5–7 months (20). For gastroenteric tumors, peritoneal
or abdominal visceral metastasis is the primary cause of tumor
recurrence and is a key prognostic factor. The survival rate for
these patients is only 3 months (21). Therefore, it is of value to
identify novel drugs to enhance the survival and quality of life of
patients.
CecropinXJ may effectively inhibit the proliferation
of human gastric cancer BGC823 cells (12). Studies investigating the effect of
cecropinXJ on the growth of malignant ascites tumors are scarce. In
the present study, an ascites model of gastric cancer was
established by inoculating BGC823 cells into the peritoneal cavity
of mice. In this model, cecropinXJ was observed to inhibit the
growth and progression of ascites tumors in the peritoneal cavity,
and induce tumor cell apoptosis in ascites fluid. These results
suggested that intraperitoneal injection of cecropinXJ in mice
demonstrated an anti-tumor effect, and that the cytotoxic effects
may have been due to increased apoptosis.
Traditional chemotherapy and radiotherapy drugs
frequently have the toxic effects that may be observed by changes
in blood parameters (22) and
viscera (23), which may affect
the quality of life of patients. Important factors used to
determine the efficacy of anti-cancer drugs include patient
survival, the degree of tumor growth inhibition and the reduction
in tumor cell numbers (24,25).
The results of the present study suggest that cecropinXJ may
significantly prolong the survival of tumor-bearing mice, and
demonstrated limited toxicity to vital organs. Bone marrow
suppression and anemia, as indicated by a decrease in hemoglobin
and red blood cells, are frequently observed in patients with
ascites tumors (26). There is a
high incidence of anemia in patients with gastrointestinal cancer
(27). White blood cells,
including neutrophils, lymphocytes and monocytes, are key cells of
the immune system. Under pathological conditions, the numbers of
white blood cells are enhanced and an inflammatory reaction is
induced. In the present study, intraperitoneal injection of
cecropinXJ significantly increased hemoglobin levels and the number
of red blood cells, while the number of white blood cells was
decreased, when compared with tumor-bearing mice treated with BSA.
In addition, the level of red blood cells in the cecropinXJ groups
were similar to healthy mice. These results suggested that
cecropinXJ may protect hematological activity without inducing bone
marrow toxicity, which is the most common side effect of
chemotherapy drugs. But the above results need to be confirmed and
validated furher.
In the present study, the pathological alterations
in visceral organs of tumor-bearing mice treated with cecropinXJ
were analyzed following hematoxylin and eosin staining. The results
revealed that cecropinXJ treatment was associated with low-level
toxicity to visceral organs, including the liver, spleen and
kidney. In addition, cecropinXJ exhibited selective cytotoxicity to
tumor cells. Therefore, cecropinXJ may be useful as an anti-tumor
agent for ascites in patients with gastric cancer.
Evasion of the immune system is beneficial to tumor
development. The thymus is a key immune organ and the spleen is an
important peripheral immune organ. The thymus and spleen indexes
may be used to directly reflect the level of immune function in
humans. When ascites tumors form in mice, abnormalities in immune
organ function is indicated by a decrease in spleen and thymus
indexes (28). In the present
study, the thymus index in the Dox group was decreased compared
with healthy control group, indicating that the immune function of
mice was suppressed and may have been the cause of the reduced
survival in these mice. Compared with the mice in the Dox and BSA
groups, the thymus index and spleen index of the mice in the
cecropinXJ treatment group were significantly enhanced. This
suggested that cecropinXJ may have reduced injury to viscera in
tumor-bearing mice, and was not toxic to the thymus and spleen.
This may facilitate maintenance of immune function, inhibit tumor
growth, increase immunity and improve quality of life.
In conclusion, the results of the current study
demonstrated that cecropinXJ may effectively inhibit ascites
formation in mice bearing tumors derived from gastric cancer cells.
In addition, cecropinXJ may prolong survival and exhibit anti-tumor
effects via induction of apoptosis and enhanced immunity. Notably,
cecropinXJ demonstrated no obvious side effects in tumor-bearing
mice. Therefore, cecropinXJ may be useful as an anti-cancer agent
for the treatment of ascites in gastric cancer patients.
Acknowledgements
We thank Professor Youyong Lv for providing the
gastric cancer BGC823 cells.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31500752), the
Doctoral Start-up Fund of Xinjiang University (grant no. BS150241)
and the High-Tech Research and Development Program of Xinjiang
(grant no. 201110101).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJX analyzed and interpreted the data of the present
study, and was a major contributor in writing the manuscript. YLW
performed the histological examination of the mouse, and
physiological and biochemical analysis. JM and FCZ made substantial
contribution to the study conception and design. FCZ was involved
in the drafting of the manuscript and gave the final approval to be
published. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study is licensed under a Creative
Commons Attribution-NonCommercial-NoDerivatives 4.0 International
(CC BY-NC-ND 4.0) License.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
References
|
1
|
Parsons SL, Watson SA and Steele RJ:
Malignant ascites. Br J Surg. 83:6–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Runyon BA: Care of patients with ascites.
N Engl J Med. 330:337–342. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasako M: Principles of surgical treatment
for curable gastric cancer. J Clin Oncol. 21 23 Suppl:274s–275s.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Becker G, Galandi D and Blum HE: Malignant
ascite: Systematic review and guideline for treatment. Eur J
Cancer. 42:589–597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith EM and Jayson GC: The current and
future management of malignant ascites. Clin Oncol (R Coll Radiol).
15:59–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cruciani RA, Barker JL, Zasloff M, Chen HC
and Colamonici O: Antibiotic magainins exert cytolytic activity
against transformed cell lines through channel formation. Proc Natl
Acad Sci USA. 88:pp. 3792–3796. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin XB, Li XB, Zhu JY, Lu XM, Shen J, Chu
FJ and Mei HF: The target of Musca domestica cecropin on human
hepatocellular carcinoma BEL-7402 cells. Zhongguo Ji Sheng Chong
Xue Yu Ji Sheng Chong Bing Za Zhi. 29:271–273. 2011.(In Chinese).
PubMed/NCBI
|
|
8
|
Jin XB, Wang YJ, Liang LL, Pu QH, Shen J,
Lu XM, Chu FJ and Zhu JY: Cecropin suppresses human hepatocellular
carcinoma BEL-7402 cell growth and survival in vivo without
side-toxicity. Asian Pac J Cancer Prev. 15:5433–5436. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Zhang F, Cai L, Zhao G and Wang B:
Studies on the properties of cecropin-XJ expressed in yeast from
Xinjiang silkworm. Wei Sheng Wu Xue Bao. 43:635–641. 2003.(In
Chinese). PubMed/NCBI
|
|
10
|
Xia L, Zhang F, Liu Z, Ma J and Yang J:
Expression and characterization of cecropinXJ, a bioactive
antimicrobial peptide from Bombyx mori (Bombycidae,
Lepidoptera) in Escherichia coli. Exp Ther Med.
5:1745–1751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia L, Liu Z, Ma J, Sun S, Yang J and
Zhang F: Expression, purification and characterization of cecropin
antibacterial peptide from Bombyx mori in Saccharomyces
cerevisiae. Protein Expr Purif. 90:47–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu YL, Xia LJ, Li JY and Zhang FC:
CecropinXJ inhibits the proliferation of human gastric cancer
BGC823 cells and induces cell death in vitro and in vivo. Int J
Oncol. 46:2181–2193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia L, Wu Y, Kang S, Ma J, Yang J and
Zhang F: CecropinXJ, a silkworm antimicrobial peptide, induces
cytoskeleton disruption in esophageal carcinoma cells. Acta Biochim
Biophys Sin (Shanghai). 46:867–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Xia L and Zhang F: Inhibition of
CecropinXJ on proliferation of human gastric cancer AGS cells. Chin
J Cell Biol. 36:1355–1361. 2014.(In Chinese).
|
|
15
|
Fung KY, Ooi CC, Zucker MH, Lockett T,
Williams DB, Cosgrove LJ and Topping DL: Colorectal carcinogenesis:
A cellular response to sustained risk environment. Int J Mol Sci.
14:13525–13541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lettre H: Tests of compounds against the
Ehrlich mouse ascites tumor. Cancer Res. 2 Suppl:S125–S128.
1955.
|
|
17
|
Sugiura K: Effect of various compounds on
the Ehrlich ascites carcinoma. Cancer Res. 13:431–441.
1953.PubMed/NCBI
|
|
18
|
Dagistan Y, Dagistan E and Citisli V:
Evaluation of simple blood counts as inflammation markers for brain
tumor patients. Neurol Neurochir Pol. 50:231–235. 2016.PubMed/NCBI
|
|
19
|
Sedláková O, Sedlák J, Hunáková L,
Jakubíková J, Duraj J, Sulíková M, Chovancová J and Chorváth B:
Angiogenesis inhibitor TNP-470: Cytotoxic effects on human
neoplastic cell lines. Neoplasma. 46:283–289. 1999.PubMed/NCBI
|
|
20
|
Yoshizawa J, Mizuno R, Yoshida T, Hara A,
Ashizuka S, Kanai M, Kuwashima N, Kurobe M and Yamazaki Y:
Inhibitory effect of TNP-470 on hepatic metastasis of mouse
neuroblastoma. J Surg Res. 93:82–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oey RC, van Buuren HR and de Man RA: The
diagnostic work-up in patients with ascites: Current guidelines and
future prospects. Neth J Med. 74:330–335. 2016.PubMed/NCBI
|
|
22
|
Sostelly A, Henin E, Chauvenet L,
Hardy-Bessard AC, Jestin-Le Tallec V, Kirsher S, Leyronnas C,
Ligeza-Poisson C, Ramdane S, Salavt J, et al: Can we predict
chemo-induced hematotoxicity in elderly patients treated with
pegylated liposomal doxorubicin? Results of a population-based
model derived from the DOGMES phase II trial of the GINECO. J
Geriatr Oncol. 4:48–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zolfagharzadeh F and Roshan VD:
Pretreatment hepatoprotective effect of regular aerobic training
against hepatic toxicity induced by Doxorubicin in rats. Asian Pac
J Cancer Prev. 14:2931–2936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hogland HC: Hematologic complications of
cancer chemotherapy. Semin Oncol. 9:95–102. 1982.PubMed/NCBI
|
|
25
|
Arora S, Jain J, Rajwade JM and Paknikar
KM: Cellular responses induced by silver nanoparticles: In vitro
studies. Toxicol Lett. 179:93–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maseki M, Nishigaki I, Hagihara M, Tomoda
Y and Yagi K: Lipid peroxide levels and lipids content of serum
lipoprotein fractions of pregnant subjects with or without
pre-eclampsia. Clin Chim Acta. 115:155–161. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verhaeghe L, Bruyneel L, Stragier E,
Ferrante M, Dierickx D and Prenen H: The effectiveness of
intravenous iron for iron deficiency anemia in gastrointestinal
cancer patients: A retrospective study. Ann Gastroenterol.
30:654–663. 2017.PubMed/NCBI
|
|
28
|
Dong JF, Zheng XQ and Rui HB: Effect of
taurine on immune function in mice with T-cell lymphoma during
chemotherapy. Asian Pac J Trop Med. 10:1090–1094. 2017. View Article : Google Scholar : PubMed/NCBI
|