Introduction
Condyloma acuminatum (CA), also known as genital
warts, is a common sexually transmitted disease that causes
prolific lesions on the genitalia, perianal area, vagina and
cervix; CA is caused by certain types of human papillomavirus (HPV)
(1). Nearly all sexually active
individuals become infected with HPV at least once during their
lifetime (2). In females, HPV
infection can result in cervical and vaginal intraepithelial
neoplasia (3,4). Therefore, the prevention and
treatment of HPV infection is a public health issue.
Podophyllotoxin (POD), a cytotoxic compound
extracted from the roots and rhizomes of Berberidaceae, is
primarily used as an anti-tumor and anti-viral drug (5). POD tincture (POD-T; 0.5%) is commonly
used as a first-line agent for the treatment of CA (5). However, the tincture is prepared in
alcohol, which can cause severe irritation to the vagina, cervix
and other mucosae, limiting its application (6). In addition, epidermal uptake and
targeting of POD-T are poor and systemic absorption leads to severe
side effects, including gastrointestinal disturbances,
thrombocytopenia, leukopenia, abnormal liver function, ataxia and
peripheral nerve palsy (7,8). To overcome the side effects of POD-T
application on mucosae, it is necessary to develop novel
alternative formulations to prolong the local residence time in
cervical epithelial cells infected by HPV and reduce cervical
irritation and systemic toxicity.
At present, localized delivery of chemotherapeutic
drugs to the cervix is used for a number of reasons, including to
treat vaginal infections, as contraception and as HIV prevention,
with various formulations including gels, creams, suppositories,
films and nanoparticle drug carriers (NDCs) available (9). A previous study reported that NDCs
improve the physical and chemical properties of drugs, provide
sustained, targeted release and reduce systemic absorption
(10). Nanostructured lipid
carriers (NLCs) are a novel type of NDC developed from solid lipid
nanoparticles, in which a solid matrix is blended with a fluid
lipid to improve drug loading capacity and release properties
(10–12). NLCs are able to adhere to the skin
surface and transport drugs in a controlled manner, while causing
minimal irritation (13).
In the present study, a POD-NLC formula containing 5
mg/ml POD was prepared and optimized. Tibetan miniature pigs were
selected as an experimental animal model to investigate the
absorption and metabolism of POD-NLCs and the mucosal irritation
caused by POD-NLCs in the porcine cervix. The physicochemical
characteristics of this formula, including particle size,
entrapment efficiency and polydispersibility, were evaluated to
0.0005, 0.005, 0.05, 0.5 and 5 µg/ml with sterile water. The
stability of POD-NLCs was investigated at 4°C and at room
temperature (25°C). The release characteristics were investigated
in vitro and in vivo, and cervical mucosal tissue
irritation was investigated in vivo. The cytotoxicity of
POD-NLCs on VK2/E6E7 cells and cell cycle arrest were also
investigated.
Materials and methods
Chemicals
POD (purity 98%, high performance liquid
chromatography grade, lot no. J1208009) was purchased from Shanghai
Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). A 5 mg/ml
podophyllotoxin tincture was prepared using 75% ethanol.
POD loaded NLC preparation
A POD-NLC formula containing 5 mg/ml [POD-NLC
(0.5%)] was prepared using the emulsion-evaporation and low
temperature-solidification methods, as previously described
(14). The formulation was
optimized using orthogonal array testing. Briefly, 50 mg of POD,
glyceryl monostearate (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and octyl/decyl acid triglyceride (Sigma; Merck KGaA) were
dissolved in methylene chloride and lecithin (Sigma; Merck KGaA)
was dissolved by sonication in ethanol (≥99.7%) for 30 min at 4°C.
Methylene chloride and ethanol solutions were mixed to form an
organic phase. Subsequently, the organic phase was rapidly injected
into a poloxamer aqueous solution, which was subsequently stirred
(245 × g) in a water bath at 80°C (DF-101S; Xingshuo Instrument,
Guangzhou, China) for 3–4 h. Subsequently, a coarse emulsion was
rapidly mixed with 5 ml of water and ice mixture and sonicated (245
× g) at 4°C for 30 min.
Characterization of POD-NLC
The average particle size, ζ potential and
polydispersity index (PDI) were measured using a Malvern Zetasizer
3000HSA (Malvern Instruments, Ltd., Malvern, UK). All samples were
prepared in triplicate. The morphology of the POD-NLCs was observed
using transmission electron microscopy (TEM; Tecnai G2 Spirit T12;
FEI; Thermo Fisher Scientific, Inc., Waltham, MA, USA). To prepare
the sample for TEM examination, 50 µl POD-NSC was added into 5 ml
ethanol. Following ultrasonic dispersion for 15 min, the dispersion
was repeatedly scooped with a carbon film copper net sample for 5
min. Then, the dispersion was oven-dried at 60°C. The prepared
samples was used for TEM examination as follows: accelerating
voltage, 120 kV; dot resolution, 0.34 nm; line resolution, 0.20 nm;
magnification, ×50-800,000.
Entrapment efficiency (EE)
analysis
EE analysis was carried out as previously described
(15). The nanoparticles were
isolated using ultrafiltration filters (0.22 um) and centrifuged
for 30 min at 23,128 × g. Wfree is the amount of soluble
free drug in the supernatant and Wtotal is the amount of
drug added to the emulsion. The samples were analyzed in triplicate
and assessed using high-performance liquid chromatography (HPLC,
without internal standards; LC-20A; Shimadzu Corporation, Kyoto,
Japan) with a Ecosil C18 analytical column (4.6×250 mm; 5 µm) was
used and maintained at 40°C. The mobile phase contained methanol
and glacial acetic acid (52:48, v/v; flow rate, 1 ml/min). The
injection volume was 20 ml, the retention time was approximately 6
min and the detection wavelength was 292 nm. The recovery rate
ranged from 98.6 to 99.6%. The POD concentration, with a range of
1–500 µg/ml (r=correlation coefficient, 0.9995) was measured to
detect the POD formulations. The EE was calculated using the
following formula: EE (%) = [(Wtotal -
Wfree)/Wtotal] × 100.
In vitro release studies
The release kinetics of POD-NLC were studied using
Franz diffusion cells. Simulated vaginal fluid (SVF) was used as
the release medium and was prepared according to a previously
described method (16). Briefly,
0.5 ml tested solutions were added into donor chambers and filtered
through the cellulose acetate membrane (0.45 µm; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) and aliquots
(0.1 ml) were subsequently withdrawn from receptor chambers at 1,
2, 4, 6, 8, 10, 12, 24, 36, 48, 60, 72, 84 and 96 h. Samples were
diluted with methanol (99.9 %) six times and assessed by HPLC.
Animal model
A total of 15 female Tibetan mini-pigs (8 months
old; 25 kg) were purchased from the Center of Experimental Animals,
Southern Medical University (Guangzhou, China). The pigs were
maintained in large hog pens (n=7 per hog pen) in a natural
environment (25°C, 12 h dark cycle) with free access to food and
water. All the experimental animals and the protocol (permit number
for pigs: 44002100008963) used in the present study were approved
by the Experimental Animal Ethics Committee of the Southern Medical
University (Guangzhou, China).
In vivo drug release studies in
cervical mucosal tissue and cervical mucus
For the purpose of dynamics analysis, 15 female
Tibetan mini-pigs were randomly divided into four subgroups and
administrated with 1, 5 and 20 mg/ml POD-T, and 5 mg/ml POD-NLC.
Cervical secretions were extracted from ophthalmic sponges using a
previously described method (17);
1, 5 and 20 mg/ml POD-T, and 5 mg/ml POD-NLC (0.5 ml) were
administrated on the cervix for one treatment. Cervical mucus was
collected at 2, 4, 6, 8 and 10 h using sponges held in the cervix
and left to stand for 5 min as previously described (18). All sponges were transferred
immediately into sterile tubes and stored at −20°C. Following
anesthesia, cervical mucosal tissue was collected by means of a
simple punch biopsy as previously described method (19) at 2, 4, 6, 8, 10 h following
administration of 1, 5 and 20 mg/ml POD-T, and 5 mg/ml POD-NLC (0.5
ml) as previous stated, and the coated cervical mucosa determined
to was 3.14 cm2. Subsequently, tissues were rinsed with
normal saline and stored at −20°C. POD was extracted from the
sponge and cervical tissue by homogenization in 1.0 ml methanol
followed by centrifugation (24,573 × g for 15 min at 4°C).
Cell culture
The immortalized vaginal epithelial cells VK2/E6E7
(ATCC® CRL-2616™) were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in an atmosphere
containing 5% CO2.
Histopathological detection and
evaluation of cervical mucous irritation
For the cervical mucous irritation study, 15 female
pigs were randomly assigned to 5 subgroups and administered with
the following treatments: i) Normal saline (0.5 ml/day; n=3); ii) 5
mg/ml POD-NLC (0.5 ml/day; n=3); iii) 5 mg/ml POD-NLC (1 ml/day;
n=3); iv) 5 mg/ml POD-NLC (2 ml/day; n=3); and v) 5 mg/ml POD-T
(0.5 ml/day; n=3). Following catheterization via an insemination
catheter (20), treatments were
administered for 3 consecutive days (once daily for three days in
total). The method of transcervical administration was adopted as
previously described (21). Pigs
were observed daily for clinical signs, including swelling,
redness, erosion and vaginal discharge. Cervical secretions of the
pigs were collected using ophthalmic sponges as previously
described (18). Porcine-specific
interleukin (IL)-lβ, −6 and −8 levels in the cervical mucus prior
to and following 24, 48 and 72 h drug administration were measured
using a Luminex instrument (Luminex 200; Luminex Corporation,
Austin, TX, USA) and immuno assays (ProcartaPlex Porcine Basic kit,
EPX010-60460-901; ProcartaPlex Porcine IL-1 beta Simplex,
EPX01A-66048-901; ProcartaPlex Porcine IL-8 (CXCL8) Simplex,
EPX01A-66052-901 and ProcartaPlex Porcine IL-6 Simplex,
EPX01A-66051-901; all Thermo Fisher Scientific, Inc.). On day 4,
cervical tissues (obtained by single punch biopsy) were cut into
6-mm sections using a slicer RM2235 (Leica Microsystems GmbH,
Wetzlar, Germany), and then fixed in 10% neutral-buffered formalin
(Beijing Solarbio Science & Technology Co., Ltd.) followed by
hematoxylin-eosin (Beijing Solarbio Science & Technology Co.,
Ltd.) staining (hematoxylin for 20 min and eosin for 5 min, each at
25°C) and complete examination under a light microscope
(BX-41-32H02; Olympus Corporation, Tokyo, Japan; magnification,
×10). The pathological scoring of mucosae was completed by two
blinded professional pathologists using a previously described
method with the following evaluation criteria: 0=absent, 1=minimal,
3=moderate, 4=marked (22).
Evaluation of inhibition of
proliferation
POD-NLC-induced inhibition of cell proliferation was
tested using VK2/E6E7 cells. Following attaching for 24 h, cells
were exposed to a series of doses (0.0005, 0.005, 0.05, 0.5 and 5
mg/ml) of POD-NLC or POD, which was diluted with sterile water. The
control group contained only the culture medium. Following
incubation for 24, 48 and 72 h, 10 µl Cell Counting kit-8 (CCK-8)
solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
was added to each well of the plate followed by incubation at 37°C
for 4 h. The absorbance was measured at a wavelength of 450 nm
using a Microplate Luminometer (Turner Designs, Sunnyvale, CA,
USA). All experiments were repeated three times. The proliferation
inhibition ratio of cells was calculated using the following
formula: Inhibition ratio = [1 - A(POD or POD-NLC group)/A(control
group)] × 100%, where A is the absorbance value at a wavelength of
450 nm.
Apoptosis assay
Cell apoptosis was detected using the Annexin
V-fluorescein isothiocyanate (FITC) kit (containing binding buffer,
Annexin V-FITC and propidium iodide; BD Biosciences, Franklin
Lakes, NJ, USA) according to a previously described method
(23). Briefly, 1×106
VK2/E6E7 cells were incubated with POD or POD-NLC at a dose of 0.05
µg/ml for 48 h. Cells were subsequently harvested and re-suspended
in PBS. The cells were counted and 1.5×105 cells were
re-suspended in 200 µl binding buffer and 5 µl Annexin V-FITC, and
stained for 10 min at room temperature. The cells were centrifuged
(120 × g for 5 min at 28°C) and re-suspended with 200 µl binding
buffer and then added 10 µl propidium iodide (PI) and stained for 5
min on ice. Finally, the stained cells were analyzed using a
NovoCyte flow cytometer (ACEA Biosciences, San Diego, CA, USA).
Data were analyzed with NovoExpress software (version 1.2.4; ACEA
Biosciences).
Cell cycle analysis
VK2/E6E7 cells (1×106) incubated with POD
or POD-NLC at a dose of 0.05 µg/ml for 48 h were harvested by
centrifuged (120 × g for 5 min at 28°C) then washed twice with cold
PBS. Following fixing with 75% ethanol for 24 h at 4°C, the cells
were washed twice with PBS and stained with 300 µl PI (100 g/ml)
for 30 min at 4°C in the dark. The cell cycle was analyzed using a
NovoExpress software (version 1.2.4; ACEA Biosciences) and the
percentage of cells in each phase was calculated with the ModFit
LT5.0 software (Verity Software House, Topsham, ME, USA).
Hoechst 33342 staining. 1×106 VK2/E6E7
cells incubated with POD or POD-NLC at a dose of 0.05 µg/ml for 48
h were fixed in 4% paraformaldehyde (Beyotime Institute of
Biotechnology, Beijing, China) for 15 min at room temperature.
Subsequently, the cells were stained with Hoechst 33342 (Beyotime
Institute of Biotechnology) for 15 min at room temperature; cells
were photographed by the fluorescence microscopic examination with
350 nm excitation wavelength (BX-41-32H02; Olympus Corporation,
magnification, ×20).
Statistical analysis
The experimental groups were compared with the
control groups via one-way analysis of variance with repeated
measures and the Fisher's Least Significant Difference. P<0.05
was considered to indicate a statistically significant difference.
Data are presented as the mean ± standard error of the mean. All
calculations were performed using SPSS software (version 19.0; IBM
Corp., Armonk, NY, USA).
Results
Characterization of POD-NLC
In the present study, 0.5% POD-NLC was prepared
using emulsion-evaporation and low temperature-solidification
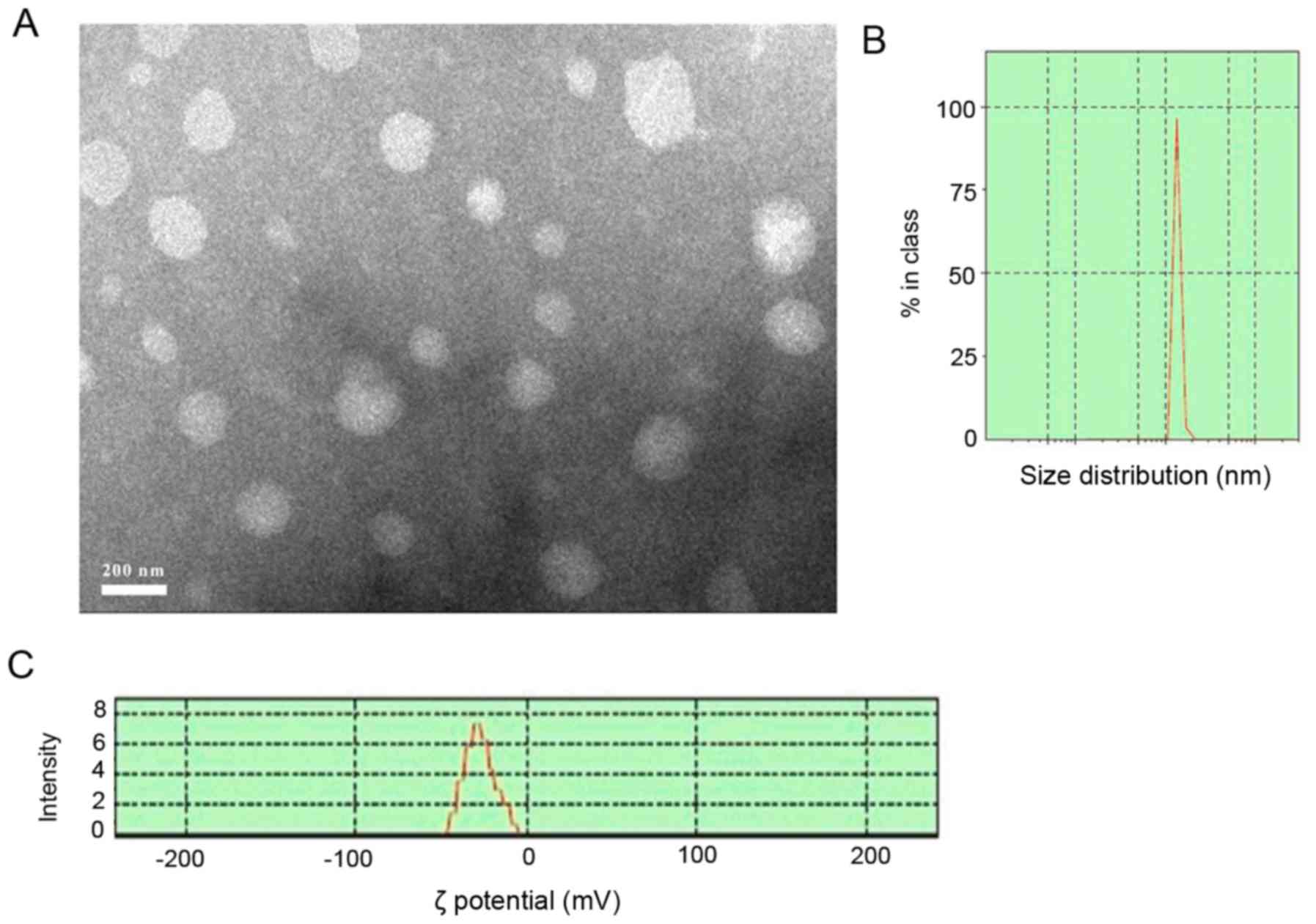
methods. The POD-NLCs were spherical or ellipsoidal (Fig. 1A) and the mean particle size was
178.5±20 nm (Fig. 1B) with a PDI
of 0.18±0.01 (data not shown), which implies that the prepared
POD-NLCs were monodisperse. The ζ potential was −27±0.5 mV
(Fig. 1C), and the pH was
6.20±0.04 (Table I). The
preparation's entrapment efficiency were calculated using the
following formula: EE (%) = [(Wtotal -
Wfree)/Wtotal] × 100. The POD entrapment
efficiency in the nanoparticles was determined to be 82.9±2% by
HPLC (Table I). The final results
showed POD-NLC (5 mg/ml) has a really high entrapment efficiency.
The POD-NLC formulation was stable when stored at 4°C for 6 months
or at room temperature for 3 months, with no stratification,
sedimentation or POD crystallization observed.
 | Table I.Podophyllotoxin-nanostructured lipid
carriers (5 mg/ml). |
Table I.
Podophyllotoxin-nanostructured lipid
carriers (5 mg/ml).
|
| pH | Polydispersity
index | Entrapment
efficiency (%) |
|---|
| A | 6.22 | 0.187 | 84.90 |
| B | 6.16 | 0.168 | 80.90 |
| C | 6.23 | 0.184 | 83.00 |
POD-NLCs sustain drug release and
extend the local action time in vitro and in vivo
To investigate the release of POD-NLCs in
vitro, the medium was collected from Franz diffusion cells
following POD-NLC and POD-T treatment and analyzed using HPLC. At 6
h following treatment, nearly 69% of POD was released from POD-T,
compared with 8.8% from POD-NLCs (Fig.
2A). At 24 h following treatment, 30.98% of the encapsulated
POD was released from POD-NLCs and 64.90% was released following 72
h (Fig. 2A). Furthermore, in
vivo release studies were performed with cervical mucus and
mucosal tissues. The results demonstrated that the concentration of
POD in cervical mucus, released from the POD-NLC (5 mg/ml)
formulation, decreased gradually following administration but
increased compared with the POD-T (5 mg/ml) group at each time
point and increased compared with the POD-T (20 mg/ml) group at 4 h
(115.3 vs. 95.8 µg/ml, respectively; P<0.05). The POD-NLC group
maintained an increased concentration at 10 h compared with POD-NLC
group (189.6 vs. 34.2 µg/ml; Fig.
2B). In the cervical mucosal tissue, POD-T administrations at
different concentrations (1, 5 and 20 mg/ml) reached the maximum
concentration of POD 4 h following administration, followed by a
decline. In the 5 mg/ml POD-NLC group, however, the POD
concentration in the cervical mucosal tissue continued to increase
up to 8 h following administration. At 10 h following
administration, the POD concentration in mucosal tissues was not
markedly elevated in the 5 mg/ml POD-NLC group compared with the 5
mg/ml POD-T group (Fig. 2C). These
results demonstrated that, compared with POD-T administration, the
POD-NLC exhibited a more sustained release of POD, which may extend
the local action time in vitro and in vivo.
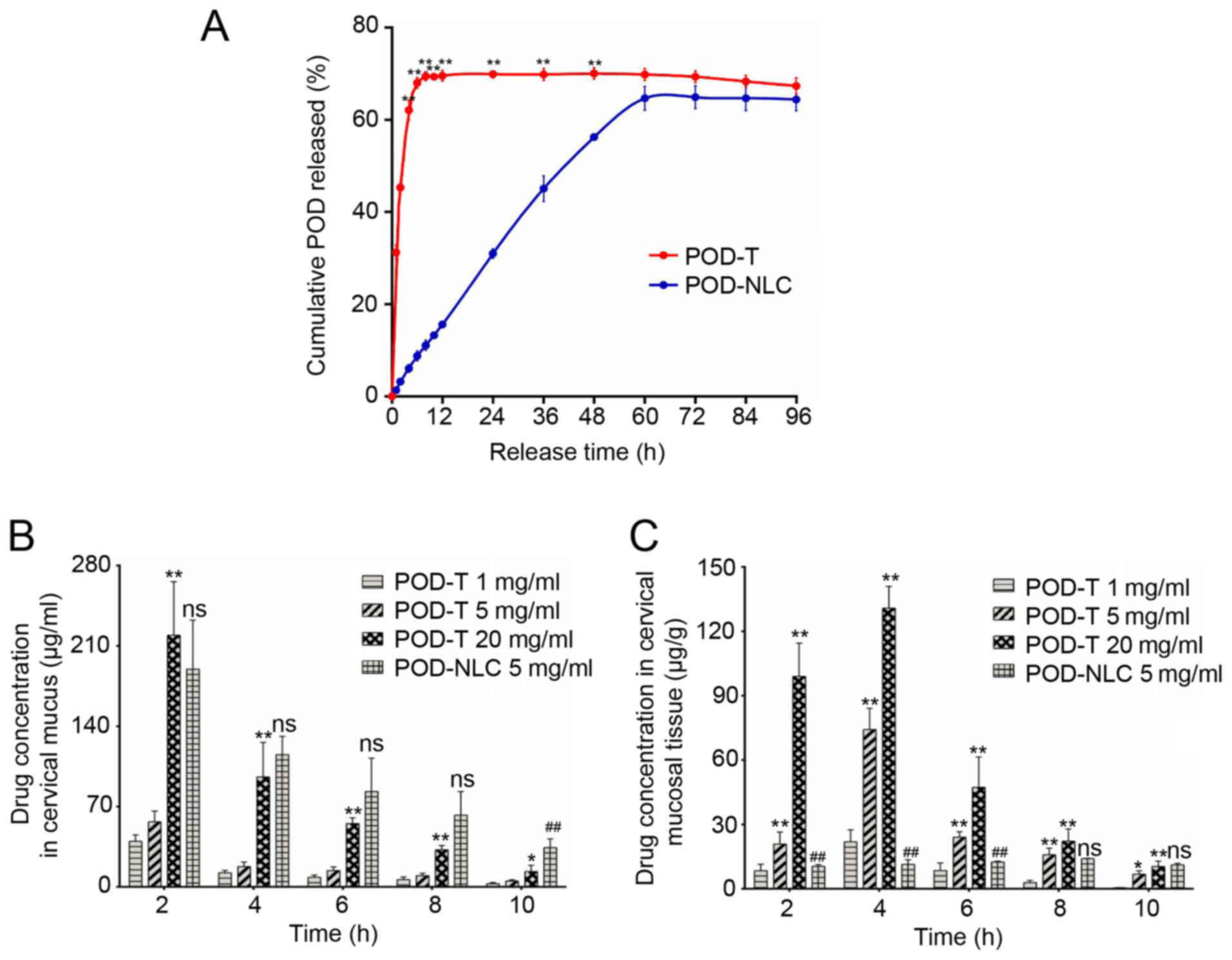 | Figure 2.POD-NLC sustains the release of the
drug and extends the local action time in vitro and in
vivo. (A) In vitro POD release profiles of 0.5% POD-NLC
and 0.5% POD-T in simulated vaginal fluid. The release kinetics of
POD-NLC were studied using Franz diffusion cells (pH 4.2 at
37±0.5°C). The cumulative release rate of POD was detected at 1, 2,
4, 6, 8, 10, 12, 24, 36, 48, 60, 72, 84 and 96 h. (B) The
concentrations of POD in cervical mucus treated with POD-NLC (5
mg/ml) and different concentrations (1, 5 and 20 mg/ml) of POD-T,
were detected at 2, 4, 6, 8 and 10 h. (C) Concentration of POD in
cervical mucosa treated with POD-NLC (5 mg/ml) and different
concentrations (1, 5, and 20 mg/ml) of POD-T, were detected at 2,
4, 6, 8 and 10 h. *P<0.05, **P<0.01 vs. the POD-T (1 mg/ml)
group; ns, no significant difference vs. the POD-T (20 mg/ml)
group; ##P<0.01 vs. the POD-T (20 mg/ml) group.
POD-NLC, podophyllotoxin-loaded nanostructured nanolipid
carriers. |
POD-NLC prevents injury and
inflammatory cytokine production in the cervical mucosal tissue
compared with POD-T
Following administration of the formulations, damage
to the cervical mucosa was assessed for 72 h. In the low-dose
POD-NLC group (0.5 ml/day), the cervical mucosal epithelium
consisted of 5–10 layers of cells with structural integrity. Ulcers
and hemorrhaging with neutrophil and mononuclear cell infiltrations
were observed in the high-dose POD-NLC and POD-T groups. In
addition, necrosis of the mucosal epithelium was observed in the
POD-T group (Fig. 3A). In the
lamina propria of the cervical mucosal tissue, no inflammatory
infiltration was observed in the low-dose POD-NLC group (Fig. 3A-g), whereas a large number of
infiltrating neutrophils and mononuclear cells were observed in the
high-dose POD-NLC and POD-T groups (Fig. 3A-i and -j, respectively).
Furthermore, submucosal vessels in the low-dose POD-NLC group
appeared normal (Fig. 3A-l);
however, in the high-dose POD-NLC group, capillary congestion,
hemorrhaging and infiltration of mononuclear cells into the
perivascular region were observed. In the POD-T group, thrombosis
of partial vessels occurred (Fig.
3A-o). Histopathological scores were calculated by assessing
the results in multiple regions of the cervical mucosal tissue.
Histopathological scores of the low-dose and medium-dose POD-NLC
group were significantly lower compared with the POD-T group
(4.3±0.8 vs. 29.3±29.3; P<0.05). No significant difference was
observed between the low-dose POD-NLC and saline groups (4.3±0.8
vs. 3.3±1.0; P>0.05; Fig. 3B).
Furthermore, the levels of IL-6, −8 and −1β were significantly
lower in the low-dose POD-NLC group compared with the high-dose
group (P<0.01), and were not significantly different compared
with the NS group (Fig. 3C-E). The
levels of inflammatory cytokines in the POD-T group were
significantly increased compared with the low-medium- and high-dose
POD-NLC groups (Fig. 3C-E). Taken
together, these results demonstrate that POD-NLCs cause less injury
and inflammatory cytokine production in the cervical mucosal tissue
compared with POD-T.
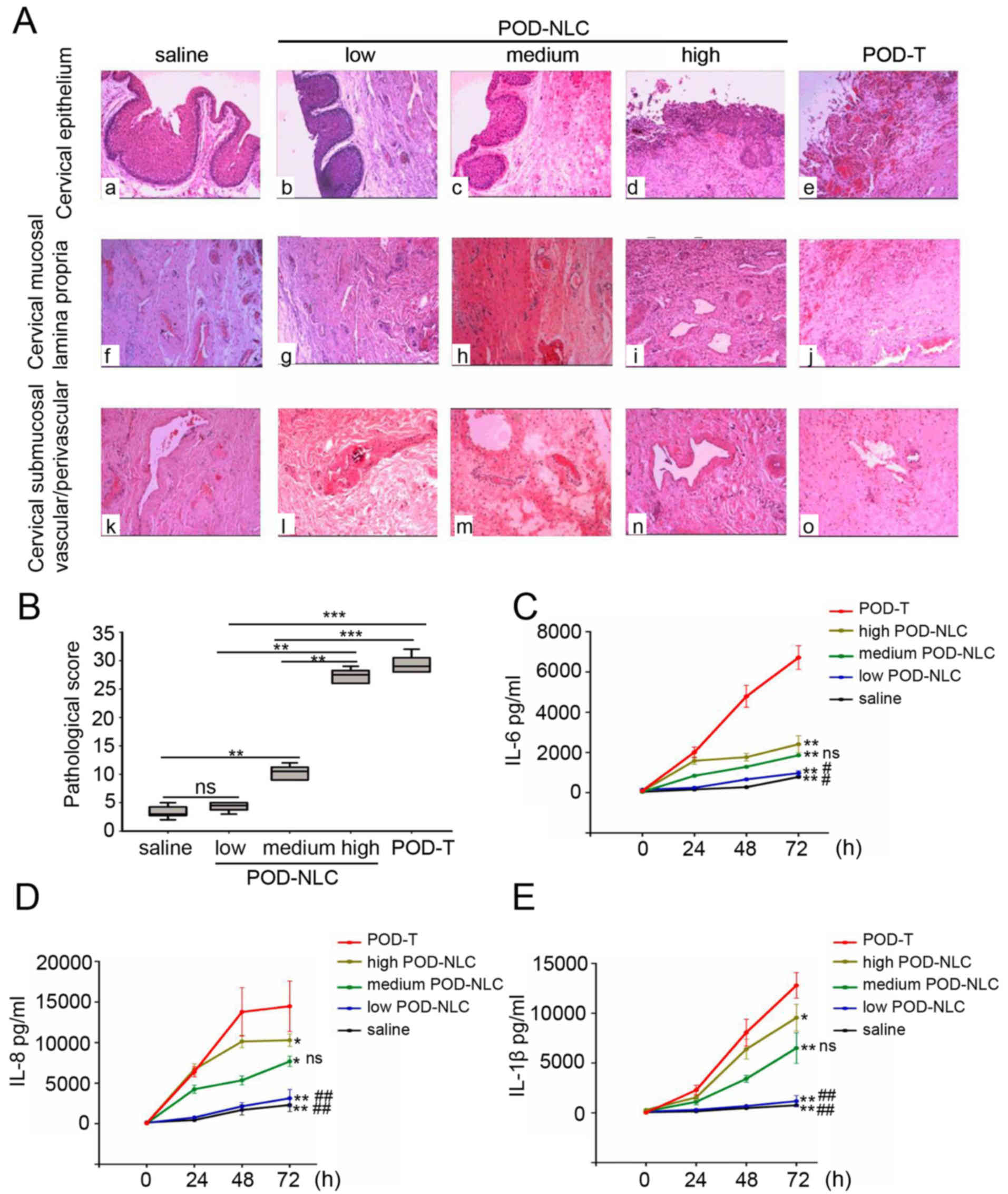 | Figure 3.POD-NLC causes decreased injury and
expression of inflammatory cytokines in cervical mucous. (A)
Histological alterations of the cervical mucosa following treatment
with 0.5 ml normal saline (a, f and k), 0.5 ml 0.5% POD-NLC (b, g
and l), 1.0 ml 0.5% POD-NLC (c, h, and m), 2.0 ml 0.5% POD-NLC (d,
i and n) and 0.5 ml 0.5% POD-T (e, j, and o) were detected by
hematoxylin and eosin staining (magnification, ×40). (B)
Histopathological score of cervical mucosa treated with 0.5 ml
normal saline, 0.5 ml 0.5% POD-T and 0.5% POD-NLC at different
doses (0.5, 1.0 and 2.0 ml). ns, no significant difference;
**P<0.01 and ***P<0.0001. Concentrations of inflammatory
cytokines in cervical secretions were detected by Luminex
instrument at 24, 48 and 72 h following treatment. The
concentrations of (C) IL-6, (D) IL-8 and (E) IL-1β were detected in
cervical secretions of pigs treated with 0.5 ml normal saline, 0.5
ml 0.5% POD-NLC, 1.0 ml 0.5% POD-NLC, 2.0 ml 0.5% POD-NLC and 0.5
ml 0.5% POD-T, respectively. *P<0.05 and **P<0.01 vs. the
POD-T group. ns, no significant difference; #P<0.05
and ##P<0.01 vs. high-dose POD-NLC group. POD-NLC,
podophyllotoxin-loaded nanostructured nanolipid carriers; IL,
interleukin. |
POD-NLC inhibits the proliferation of
VK2/E6E7 cells
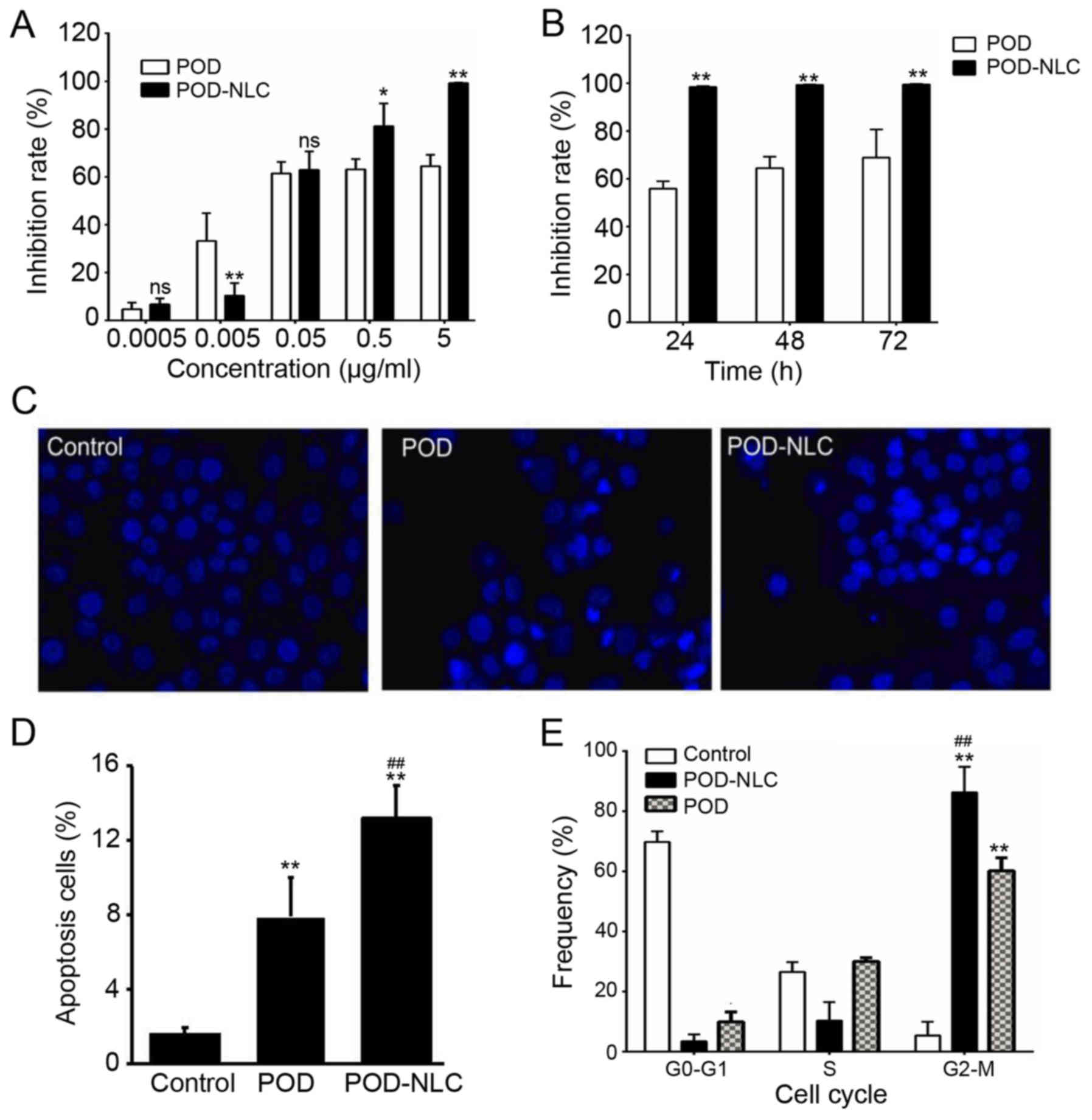
To evaluate the CA-inhibitory activity of POD-NLCs,
the effect of POD-NLC on the proliferation of VK2/E6E7 cells was
investigated. Compared with POD, POD-NLC resulted in decreased cell
viability at high concentration (0.5 and 5 µg/ml). The
proliferation inhibition rate of VK2/E6E7 cells treated with
POD-NLC reached 99% at a dose of 5 µg/ml (Fig. 4A). However, the inhibition of POD
was 64.5% at a dose of 5 µg/ml and did not increase further with
prolonged exposure time (Fig. 4B).
The inhibition of POD-NLCs reached 98.4% at 24 h and persisted at
>98% from 24 to 72 h (Fig. 4B),
which is increased compared with the POD treatment group.
Hoechst 33342 staining was performed to assess the
morphology of the nuclei (Fig.
4C). It was determined that POD-NLC-treated VK2/E6E7 cells had
apoptotic morphology, including cell membrane shrinkage, cell
shedding and chromatin condensation. POD-treated cells exhibited a
similar pattern (Fig. 4C). In the
POD-NLC- and POD-treated groups, the apoptosis rates increased by
13.2 and 7.9%, respectively, compared with the control group
(Fig. 4D). Cell cycle distribution
was analyzed using flow cytometry. Following treatment for 48 h,
VK2/E6E7 cells in the POD- and POD-NLC-treated groups were
predominantly in the G2/M phase, and fewer cells were observed in
the G0/G1 phase (Fig. 4E). POD-NLC
treatment induced G2/M arrest to a greater extent compared with the
POD treatment (0.863±0.085 vs. 0.602±0.043, respectively;
P<0.01; Fig. 4E). Collectively,
these results demonstrate that POD-NLC has a more prominent effect
on VK2/E6E7 cell viability compared with the POD treatment.
Discussion
POD-T (0.5%) has been reported to be an effective
initial drug for the treatment of CA (24). However, it has been demonstrated to
cause severe irritation when applied to the vagina, cervix or other
mucosae (25). Recently, POD-NLCs
have been proposed as an attractive delivery system for the
treatment of HPV and associated diseases with low systemic adverse
effects (14,15). Previous studies have reported that
nanoparticles of small diameter (<100 nm) would be more likely
to be hindered or immobilized by cervical mucus compared with
nanoparticles of larger diameter (200 nm) (26,27).
In the present study, 0.5% POD-NLCs were prepared with an average
diameter of 178.5±20 nm, which is within the optimal range for
mucosal drug delivery.
The mucosae of the reproductive tract secrete
appropriate amounts of mucus, mainly composed of mucins, which are
responsible for mucoadhesion and elasticity (28). Ramineni et al (29) proposed that the characteristics of
mucus could cause it to act as a barrier limiting drug penetration.
Additionally, the retention time of traditional formulations
(including solutions, suspensions and emulsions) in the
reproductive tract surface is insufficient, making it difficult to
achieve the desired therapeutic effect (30). Nanoparticles with mucoadhesive
properties have previously been used to facilitate drug
administration to the mucosa and improve the active effects of
transmucosal administration (31).
In the present study, in vitro experimentation indicated
that the residence time of POD-NLCs was increased compared with
POD-T. POD-NLCs enable sustained drug delivery for 60 h, consistent
with results of a previous study, which used POD-NLCs for targeted
drug delivery (12). In the
present study, the in vivo drug release from POD-NLCs in
Tibetan miniature pigs was monitored by detecting the amount of POD
in the cervical mucus and mucosal tissue. The results demonstrated
that the concentration of POD in the mucus of the POD-T group
decreased rapidly following transmucosal administration. The amount
of POD in the mucus and the mucosal tissue was increased in the
POD-NLC group compared with the POD-T group. This demonstrates
that, although POD-T penetrates the mucus and mucosa more rapidly,
POD-NLCs are able to persist in the cervical mucus and on the
mucosal surface and exhibit sustained-release for 10 h and
controlled-release properties as high levels of POD were
maintained. These results suggest that POD-NLCs possesses
mucoadhesive abilities and provides controlled drug release, which
is more suitable for the treatment of CA through the cervical
mucosal tissue compared with POD-T.
Previous reports have demonstrated that certain
antimicrobial agents used in the female reproductive tract can
cause damage to the mucosal barrier; drugs that alter the
reproductive tract mucosal tissue environment and disrupt this
barrier can increase the risk of infection by sexually transmitted
pathogens, including human immunodeficiency virus (HIV), HPV and
Chlamydia trachomatis (32–34).
When the cervical mucosal tissue is stimulated, induced epithelial
cell hyperactivity leads to the release of a series of chemokines
and cytokines, eliciting an immune response through the
transmission of signals to submucosal cells and tissues (24). Cytokines, including IL-1β, IL-8 and
IL-6, are used as markers for the potential toxicity of drugs
administered to the reproductive tract mucosa (20). NLCs can be prepared using both
solid and liquid lipids, which induce minimal skin irritation
(35). In the present study, the
irritation caused to the cervical mucosal tissue of Tibetan mini
pigs following repeated administrations of different doses of
POD-NLC was evaluated. In the POD-T and high-dose and medium-dose
POD-NLC-treated groups, severe destruction to the cervical mucosal
tissue and elevated levels of IL-1β, −6 and −8 were observed,
suggesting induction of inflammatory responses. However, the
low-dose POD-NLC group did not result in any apparent pathological
alteration in the mucosal epithelium. Therefore, POD-NLCs can
maintain the integrity of the cervical mucosal epithelial barrier
whilst also preventing an increase in the release of
pro-inflammatory cytokines. The results of the present study are in
agreement with previous studies, which demonstrated that the
intermittent use of small doses of POD-NLCs is an efficient
therapeutic method for preventing irritation of the cervical
mucosal tissue (36).
E6 and E7 are oncoproteins encoded by HPV that
transform cells by cooperatively targeting diverse cellular
pathways involved in the regulation of cell cycle control,
apoptosis and cell polarity control networks (37). Therefore, the HPV-transformed
VK2/E6E7 cell line was used to investigate the effects of POD-NLC
treatment on the viability of cells. Cytotoxic effects of POD were
previously reported to influence different stages of cell cycle,
including inhibition of RNA and DNA synthesis and polymerization,
and prevention of cell division through inhibition of microtubule
assembly (38). In a previously
published study, the authors of the present study reported that POD
increases the intracellular calcium concentration and upregulates
the expression of 78 kDa glucose-regulated protein homolog, glucose
requlated protein 94 and calpain2 mRNA in VK2/E6E7 cells (19). To determine the effect of POD-NLCs
on VK2/E6E7 cells, proliferation inhibition rate and cytotoxicity
studies were performed. The results of the present study
demonstrated that more efficient inhibition of VK2/E6E7 cell
proliferation was achieved following treatment with POD-NLCs
compared with the POD group. POD-NLC treatment induced a
proliferation inhibition rate of >99% compared with 68.9%
achieved by POD treatment. The elevated proliferation inhibition
rate induced by POD-NLC treatment may be due to increased uptake of
POD into VK2/E6E7 cells. In addition, compared with the POD
treatment, POD-NLC resulted in increased proportion of cells in the
G2/M stage and elevated apoptosis of VK2/E6E7 cells. Although the
mechanism underlying these results remains to be elucidated, the
observations indicate that POD-NLCs demonstrated more effective
anti-HPV activity compared with free POD.
In conclusion, POD-NLCs demonstrate favorable
physicochemical characteristics for transmucosal delivery,
providing sustained release and controlled delivery in the cervical
mucosal tissue. POD-NLCs can inhibit the growth of VK2/E6E7 cells
via G2/M arrest and inhibit cell proliferation more efficiently
compared with the POD. The present study demonstrated that NLCs is
a promising delivery system for POD in the treatment of CA.
Acknowledgements
The authors of the present study would like to thank
Mr. Zeng and Ms. Wang for technical assistance.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81673067), the Natural
Science Foundation of Guangdong Province (grant no. 2014A030313319)
and the President Funding of Southern Medical University Nanfang
Hospital (grant no. 2016C030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors were involved in acquisition of data,
analysis and interpretation of data. ZH, QL, LL were involved in
revising the manuscript critically for important intellectual
content. YG and KZ were involved in drafting the manuscript. KZ was
involved conception and design of the manuscript.
Ethics approval and consent to
participate
All the experimental animals and the protocol
(permit number for pigs: 44002100008963) used in the present study
were approved by the Experimental Animal Ethics Committee of the
Southern Medical University (Guangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ball SL, Winder DM, Vaughan K, Hanna N,
Levy J, Sterling JC, Stanley MA and Goon PK: Analyses of human
papillomavirus genotypes and viral loads in anogenital warts. J Med
Virol. 83:1345–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park IU, Introcaso C and Dunne EF: Human
papillomavirus and genital warts: A review of the evidence for the
2015 centers for disease control and prevention sexually
transmitted diseases treatment guidelines. Clin Infect Dis. 61
Suppl 8:S849–S855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamos C, Mihaljevic C, Aulmann S, Bruckner
T, Domschke C, Wallwiener M, Paringer C, Fluhr H, Schott S, Dinkic
C, et al: Detection of human papillomavirus infection in patients
with vaginal intraepithelial neoplasia. PLoS One. 11:e01673862016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu Xi L, Schiffman M, Ke Y, Hughes JP,
Galloway DA, He Z, Hulbert A, Winer RL, Koutsky LA and Kiviat NB:
Type-dependent association between risk of cervical intraepithelial
neoplasia and viral load of oncogenic human papillomavirus types
other than types 16 and 18. Int J Cancer. 140:1747–1756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gordaliza M, Garcia PA, del Corral JM,
Castro MA and Gómez-Zurita MA: Podophyllotoxin: Distribution,
sources, applications and new cytotoxic derivatives. Toxicon.
44:441–459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lacey CJ, Woodhall SC, Wikstrom A and Ross
J: 2012 European guideline for the management of anogenital warts.
J Eur Acad Dermatol Venereol. 27:e263–e270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karuppaiya P and Tsay HS: Therapeutic
values, chemical constituents and toxicity of Taiwanese Dysosma
pleiantha-a review. Toxicol Lett. 236:90–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng JF, Huang CC and Lee YJ:
Toxicokinetic parameters in the management of poisoning: An example
of podophyllotoxin intoxication. J Toxicol Sci. 23 Suppl
2:S205–S208. 1998. View Article : Google Scholar
|
|
9
|
Karimunnisa S and Atmaram P: Mucoadhesive
nanoliposomal formulation for vaginal delivery of an antifungal.
Drug Dev Ind Pharm. 39:1328–1337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puglia C, Blasi P, Rizza L, Schoubben A,
Bonina F, Rossi C and Ricci M: Lipid nanoparticles for prolonged
topical delivery: An in vitro and in vivo investigation. Int J
Pharm. 357:295–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu D, Liu Z, Wang L, Zhang C and Zhang N:
Nanostructured lipid carriers as novel carrier for parenteral
delivery of docetaxel. Colloids Surf B Biointerfaces. 85:262–269.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Piao X, Shi X, Si A, Zhang Y and
Feng N: Podophyllotoxin-loaded nanostructured lipid carriers for
skin targeting: In vitro and in vivo studies. Molecules. 21:pii:
E1549. 2016. View Article : Google Scholar
|
|
13
|
Rajinikanth PS and Chellian J: Development
and evaluation of nanostructured lipid carrier-based hydrogel for
topical delivery of 5-fluorouracil. Int J Nanomedicine.
11:5067–5077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Wang J and Pan J: Baicalin-loaded
PEGylated lipid nanoparticles: Characterization, pharmacokinetics,
and protective effects on acute myocardial ischemia in rats. Drug
Deliv. 23:3696–3703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Zhao B, Wang S, Liang Q, Cai Y,
Yang F and Li G: Formulation and evaluation of novel glycyrrhizic
acid micelles for transdermal delivery of podophyllotoxin. Drug
Deliv. 23:1623–1635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owen DH and Katz DF: A vaginal fluid
simulant. Contraception. 59:91–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holt JD, Cameron D, Dias N, Holding J,
Muntendam A, Oostebring F, Dreier P, Rohan L and Nuttall J: The
sheep as a model of preclinical safety and pharmacokinetic
evaluations of candidate microbicides. Antimicrob Agents Chemother.
59:3761–3770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koshiol J, Sklavos M, Wentzensen N, Kemp
T, Schiffman M, Dunn ST, Wang SS, Walker JL, Safaeian M, Zuna RE,
et al: Evaluation of a multiplex panel of immune-related markers in
cervical secretions: A methodologic study. Int J Cancer.
134:411–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Han K, Li X, Xiao Y and Zeng K:
Role of endoplasmic reticulum stress pathway in podophyllotoxin
nanostructured lipid carriers-induced apoptosis of VK2/E6E7 cells.
Nan Fang Yi Ke Da Xue Xue Bao. 34:832–836. 2014.(In Chinese).
PubMed/NCBI
|
|
20
|
D'Cruz OJ, Erbeck D and Uckun FM: A study
of the potential of the pig as a model for the vaginal irritancy of
benzalkonium chloride in comparison to the nonirritant microbicide
PHI-443 and the spermicide vanadocene dithiocarbamate. Toxicol
Pathol. 33:465–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pachman DR, Barton DL, Clayton AC,
McGovern RM, Jefferies JA, Novotny PJ, Sloan JA, Loprinzi CL and
Gostout BS: Randomized clinical trial of imiquimod: An adjunct to
treating cervical dysplasia. Am J Obstet Gynecol. 206:42.e1–7.
2012. View Article : Google Scholar
|
|
22
|
Fichorova RN, Mendonca K, Yamamoto HS,
Murray R, Chandra N and Doncel GF: A quantitative multiplex
nuclease protection assay reveals immunotoxicity gene expression
profiles in the rabbit model for vaginal drug safety evaluation.
Toxicol Appl Pharmacol. 285:198–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olerile LD, Liu Y, Zhang B, Wang T, Mu S,
Zhang J, Selotlegeng L and Zhang N: Near-infrared mediated quantum
dots and paclitaxel co-loaded nanostructured lipid carriers for
cancer theragnostic. Colloids Surf B Biointerfaces. 150:121–130.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gutierrez-Xicotencatl L, Salazar-Piña DA,
Pedroza-Saavedra A, Chihu-Amparan L, Rodriguez-Ocampo AN,
Maldonado-Gama M and Esquivel-Guadarrama FR: Humoral immune
response against human papillomavirus as source of biomarkers for
the prediction and detection of cervical cancer. Viral Immunol.
29:83–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bestehorn K, Bestehorn M and Fleck E:
Influence of different approaches of aortic valve replacement on
the incidence of post-operative delirium in intermediate risk
patients-a matched pair analysis. Curr Med Res Opin. 31:2157–2163.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
das Neves J, Araújo F, Andrade F, Amiji M,
Bahia MF and Sarmento B: Biodistribution and pharmacokinetics of
dapivirine-loaded nanoparticles after vaginal delivery in mice.
Pharm Res. 31:1834–1845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai SK, O'Hanlon DE, Harrold S, Man ST,
Wang YY, Cone R and Hanes J: Rapid transport of large polymeric
nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci
USA. 104:pp. 482–1487. 2007;
|
|
28
|
Khutoryanskiy VV: Advances in mucoadhesion
and mucoadhesive polymers. Macromol Biosci. 11:748–764. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramineni SK, Dziubla TD, Cunningham LL Jr
and Puleo DA: Local delivery of imiquimod in hamsters using
mucoadhesive films and their residence time in human patients. Oral
Surg Oral Med Oral Pathol Oral Radiol. 118:665–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Franasiak JM and Scott RT Jr: Reproductive
tract microbiome in assisted reproductive technologies. Fertil
Steril. 104:1364–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Chang X, Du D, Liu W, Liu J, Weng
T, Yang Y, Xu H and Yang X: Podophyllotoxin-loaded solid lipid
nanoparticles for epidermal targeting. J Control Release.
110:296–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fichorova RN, Bajpai M, Chandra N, Hsiu
JG, Spangler M, Ratnam V and Doncel GF: Interleukin (IL)-1, IL-6,
and IL-8 predict mucosal toxicity of vaginal microbicidal
contraceptives. Biol Reprod. 71:761–769. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwata T, Fujii T, Morii K, Saito M,
Sugiyama J, Nishio H, Morisada T, Tanaka K, Yaguchi T, Kawakami Y
and Aoki D: Cytokine profile in cervical mucosa of Japanese
patients with cervical intraepithelial neoplasia. Int J Clin Oncol.
20:126–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Passmore JA, Jaspan HB and Masson L:
Genital inflammation, immune activation and risk of sexual HIV
acquisition. Curr Opin HIV AIDS. 11:156–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghate VM, Lewis SA, Prabhu P, Dubey A and
Patel N: Nanostructured lipid carriers for the topical delivery of
tretinoin. Eur J Pharm Biopharm. 108:253–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
von Krogh G: Podophyllotoxin in serum:
Absorption subsequent to three-day repeated applications of a 0.5%
ethanolic preparation on condylomata acuminata. Sex Transm Dis.
9:26–33. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peuster M, Fink C, Reckers J, Beerbaum P
and von Schnakenburg C: Assessment of subacute inflammatory and
proliferative response to coronary stenting in a porcine model by
local gene expression studies and histomorphometry. Biomaterials.
25:957–963. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakurai H, Miki T, Imakura Y, Shibuya M
and Lee KH: Metal- and photo-induced cleavage of DNA by
podophyllotoxin, etoposide, and their related compounds. Mol
Pharmacol. 40:965–973. 1991.PubMed/NCBI
|