Introduction
As the aging of population rises, the heart failure
has become a more and more serious social issue (1). It is true that the heart failure is
the final battlefield of nearly all heart diseases such as IHD,
valvular heart disease, hypertension, myocarditis, cardiomyopathy
and diabetes developing to their terminal stage (1,2). But
it is also widely acknowledged that various pathological changes
after the myocardial infarction, like changes in the myocardial
cell morphology, ECM remodeling, microvascular insufficiency and
ventricular dilatation, take up an important place in the
occurrence and development of the heart failure (3).
Myocardial infarction: Due to the effects of
multiple elements including mechanical stress, endocrine
stimulation and cytokine activation, the ventricular form and
structure have both changed, leading to a ventricular remodeling
(4). Such a change could affect
both the infarct area and the non-infarct area, demonstrating a
thinner, extended or dilated ventricular wall in the infarct area
(2). While the ventricular wall in
the non-infarct area is thickened and extended reactively,
eventually causing the ventricular dilatation and cardiac
insufficiency (5).
The inflammatory responses of heart could be
summarized as pure native immune reaction and/or the combination of
the native immune reaction and acquired immune reaction (6). The most typical feature of the native
immune reaction is to induce the production of inflammatory
factors. When myocardial ischemia occurs and even causes the heart
failure, usually the native immune reaction and inflammatory
responses both occur (7).
Myocardial infarction could cause the elevation in
the oxidative stress level of myocardium and further result in the
oxidative damage on the myocardium (8). As a secondary messenger, ROS could
deliver the stress signals and make various effects on cells.
Proper ROS level could effectively activate cells' redox sensitive
signal channels such as MAPKs and PI3K/AKt, with each channel being
interconnected (9). MAPKs and
PI3K/AKt adjust the transcription factors like HSF-1, P53 and NF-κB
through phosphorylation, thus inducing the expression of a series
of redox sensitive genes, to control the reactions of signals such
as growth, apoptosis or stress (8). However, overdose of ROS could cause
the cell apoptosis or necrosis. It is universally accepted that the
myocardial infarction will cause the oxidative stress and lead to
the pathologic myocardial remodeling and myocardial enlargement
(10).
TLRs expressed by the myocardial cells are majorly
TLR2, TLR3 and TLR4. According the combined protein, the TLRs
signal channels could be separated into two types: MyD88 channel
and Trif channel (11). MyD88
channel could activated by all TLRs except TLR3 while Trif channel
could be activated by TLR3 and TLR4. These two channels could both
activate NF-κB eventually, which is a pivot transcription factor
for activating the inflammatory responses (12). It has been reported that TLRs have
played a crucial rule in inducing the inflammatory responses of
heart diseases like myocardial infarction and viral myocarditis
(13).
Limonium sinense (Girard) Kuntze belongs to
the genus of Limonium, the family of plumbaginaceae, also
called sea spinach, sea Radix Paeoniae Rubra, spoon-like leaf grass
or salt cloud grass (14). As a
perennial salt-excretion medical herb, it is listed as the
protected plant species by China in 2001 (15). Chinese Limonium sinense
possesses the function of clearing heat and removing toxicity,
removing blood stasis and hemostasis, dispelling the wind and
relieving inflammation as well as anticancer and anti-aging.
Studies have suggested that flavone is the one of the major
effective ingredients in the Chinese Limonium sinense and
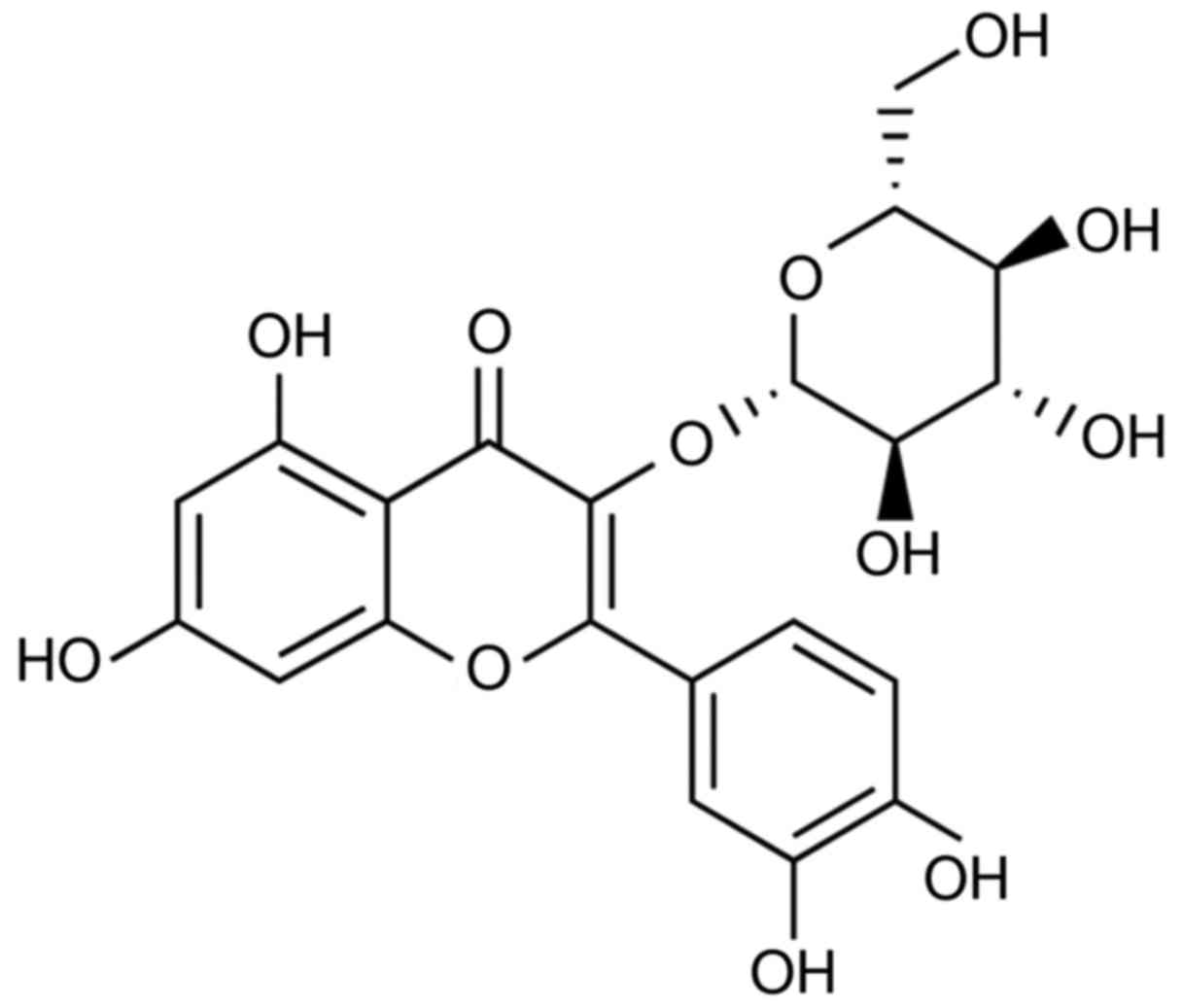
abundant literatures have attested that isoquercetin (Fig. 1) and morin are both found to be
engaged in the biological activities like anti-inflammation,
antioxidant and decompression; quercetin is identified with the
function of antioxidant, anticancer and antimutagen; luteolin
possesses the functions of controlling the proliferation of cancer
cells and inducing the cancer cells to apoptosis or the
sensitization of the apoptosis; and apigenin has been already found
to be clearly active in the antitumor in a mechanism (16,17).
These are all the major flavone active ingredients in the
Traditional Chinese Medicines (TCM) and the important indexes to
evaluate the quality of medicines (18). The aim of the present study was to
reveal the protective mechanisms and identify the effects of
isoquercetin on myocardial infarction in a rat model of acute
myocardial infarction.
Materials and methods
Animals, experimental design and
protocol
Male Sprague-Dawley (SD) rats (220–250 g, 6–8 weeks)
were provided by Qingdao University and housed in separated cages
with laboratory chow and tap water ad libitum. SD rats were
randomly allocated into three groups: Sham-operated control group
(control, n=6), myocardial infarction model group (MI model, n=6)
and isoquercetin group (Iso, n=6). Control and model groups
received an equal quantity of vehicle normal saline. SD rats were
anesthetized 30 mg/kg pentobarbital and left anterior descending
coronary artery was exposed and ligated with forceps at the
proximal left anterior descending coronary artery 2–3 mm from its
origin between the pulmonary artery conus. The heart was returned
and the thorax was closed. Rat of Iso group was gavaged with 80
mg/kg/day isoquercetin for 2 weeks. The present study was approved
by the Medical Ethics Committee of the Affiliated Hospital of
Qingdao University (Qingdao, China).
Measurement of myocardial infarct size
and histological analysis
Rat was sacrificed during anesthetization, and heart
was toke out and washed with PBS. Heart tissue was transversely cut
across the left ventricle and cutted 3 mm thick. Tissue samples
were incubated with 0.5% TTC 0.5% (Amresco LLC, Solon, OH, USA) in
phosphate buffer (Sangon Biotech Co., Ltd., Shanghai, China) at
37°C for 30 min, and fixed with 10% formalin. For histological
analysis, sections were stained with hematoxylin and eosin. The
tissue was observed using a microscope (BX53; Olympus Corporation,
Tokyo, Japan).
Measurement of cardiac marker enzyme
activity
Peripheral blood of rat was collected from eye
socket during anesthetization and serum was collected after
centrifuged at 1,000 × g for 20 min. Serum was used to analyze
creatine kinase (CK), CK-MB and LDH levels using commercial
diagnostic kits (Shanghai Kehua Medical Instruments Co., Ltd.,
Shanghai, China).
Enzyme-linked immunosorbent assay
(ELISA)
Peripheral blood of rat was collected from eye
socket during anesthetization and serum was collected after
centrifuged at 1,000 × g for 20 min. Serum was used to analyze
inflammation and oxidative stress using ELISA kit.
Western blot analysis
Myocardial tissue (80 mg) was grinded in liquid
nitrogen and incubated with RIPA sassy and protein content was
quantitated using BCA sassy. Protein/lane (30 µg) was subjected by
8–12% SDS-PAGE and transferred onto nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 5%
bovine serum albumin in TBST and incubated with Bax (1:2,000),
Bcl-2 (1:2,000), eNOS (1:4,000), iNOS (1:4,000), TLR4, NF-κB
(1:3,000) and GAPDH (1:5,000) (all from Cell Signaling Technology,
Inc., Danvers, MA, USA) at 4°C overnight. Membranes were washed and
incubated with anti-rabbit horseradish peroxidase-conjugated
(1:5,000; Cell Signaling Technology, Inc.) in 5% nonfat dry milk in
washing buffer for 1 h at room temperature. Protein was detected
using ECL sassy and calculated using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are reported as the mean ± standard deviation.
The data were statistically analyzed using a one-way ANOVA for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isoquercetin ameliorates myocardial
infarct size
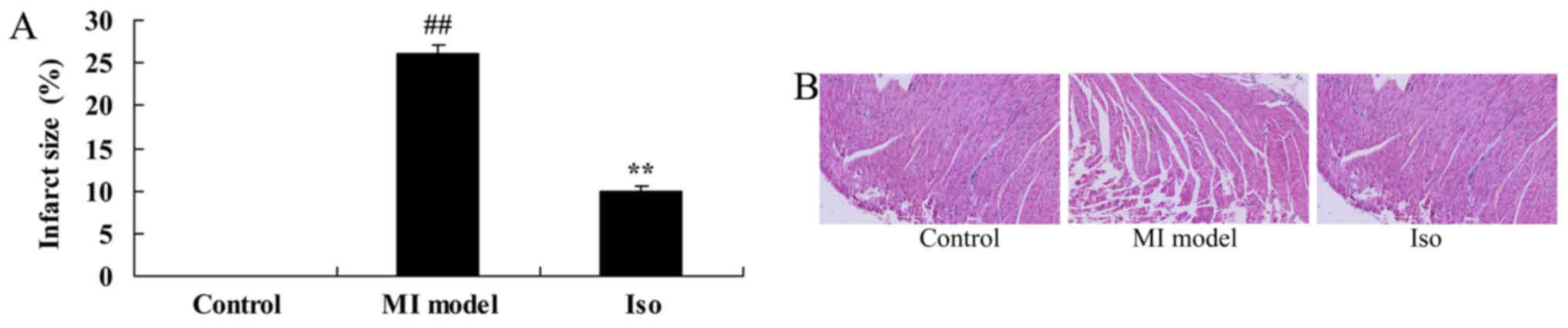
At the end of the experiments, myocardial infarct
size of MI rat was measured. We found that a significant increase
of myocardial infarct size in MI model rat, compared with control
group (Fig. 2A). However,
treatment with isoquercetin significantly inhibited the increase of
myocardial infarct size in MI rat (Fig. 2A). Meanwhile, hematoxylin and eosin
(H&E) staining showed that MI model rat appeared augmentation
and loose arrangement, compared with control group (Fig. 2B). Treatment with isoquercetin
significantly reduced augmentation and loose arrangement in MI rat
(Fig. 2B).
Isoquercetin ameliorates CK, CK-MB and
LDH activity
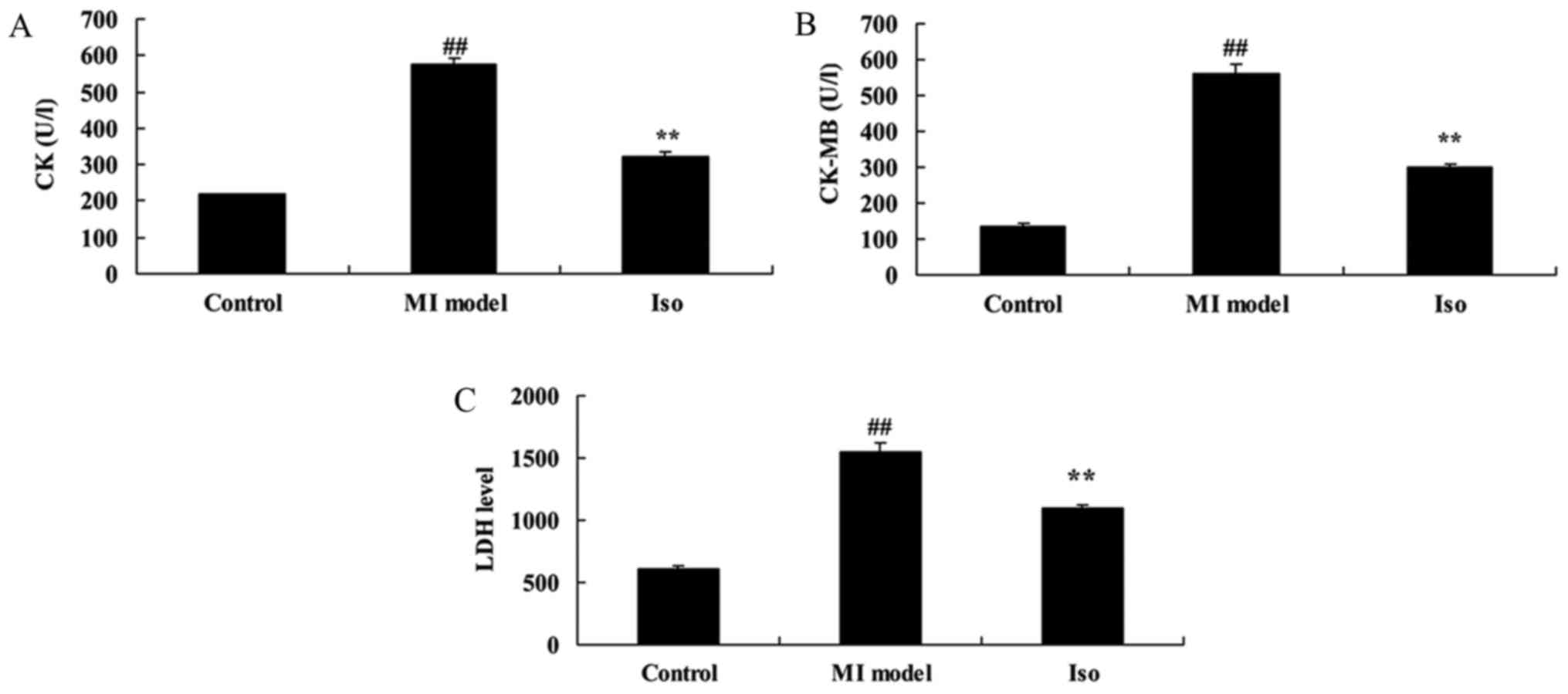
Instead, we report significant increases in CK,
CK-MB and LDH activity of MI model rat, compared with control group
(Fig. 3). Fig. 3 showed that isoquercetin
significantly reduced CK, CK-MB and LDH activity in MI rat.
Isoquercetin inhibited
inflammation
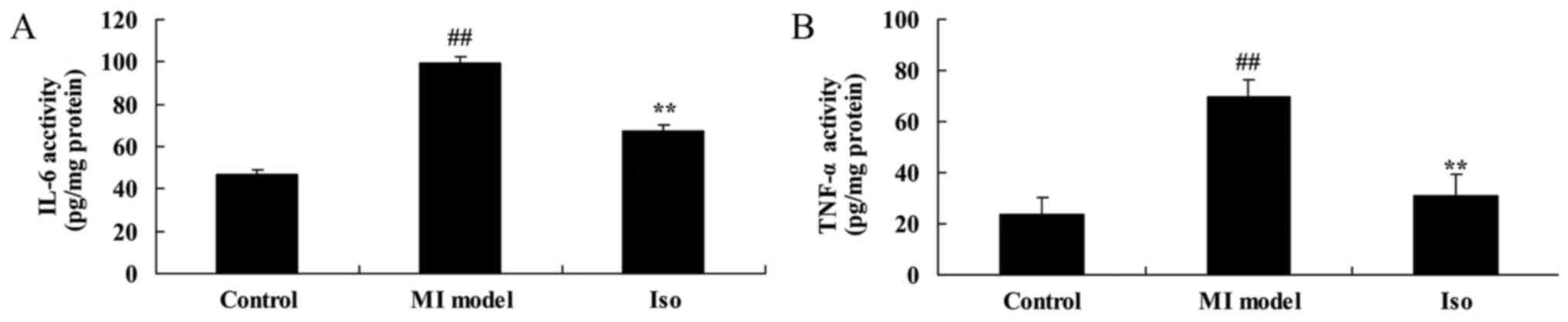
We explored the anti-inflammation effects of
isoquercetin in MI model. The results of the ELISA assay indicate
that IL-6 and TNF-α levels increased significantly in the model
group, compared with control group (Fig. 4). These elevations were
significantly inhibited by isoquercetin in MI rat (Fig. 4).
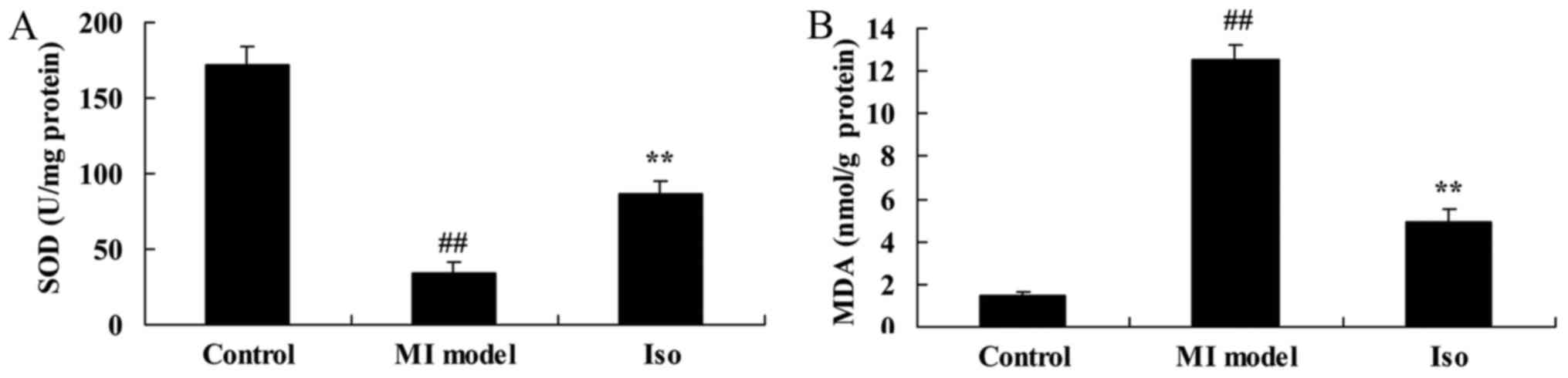
Isoquercetin inhibited oxidative
stress
We analyzed MDA and SOD levels in MI rat using ELISA
kits. As showed in Fig. 5, the
inhibition of SOD level and increase of MDA level were effectively
observed in MI model group, compared with control group. After MI
rat by isoquercetin, MDA level was inhibited and SOD level was
increased in MI rat (Fig. 5).
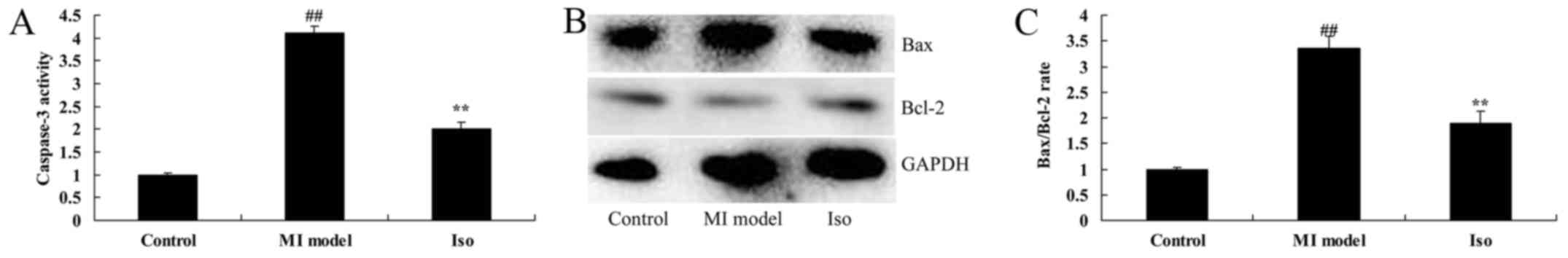
Isoquercetin inhibited heart cell
apoptosis
We probed that the anti-apoptosis effects of
isoquercetin in MI rat, we measured caspase-3 activity and
Bax/Bcl-2 protein expression. Caspase-3 activity and Bax/Bcl-2
protein expression were significantly increased in MI model rat,
compared with control group (Fig.
6). Isoquercetin significantly suppressed caspase-3 activity
and Bax/Bcl-2 protein expression in MI rat (Fig. 6).
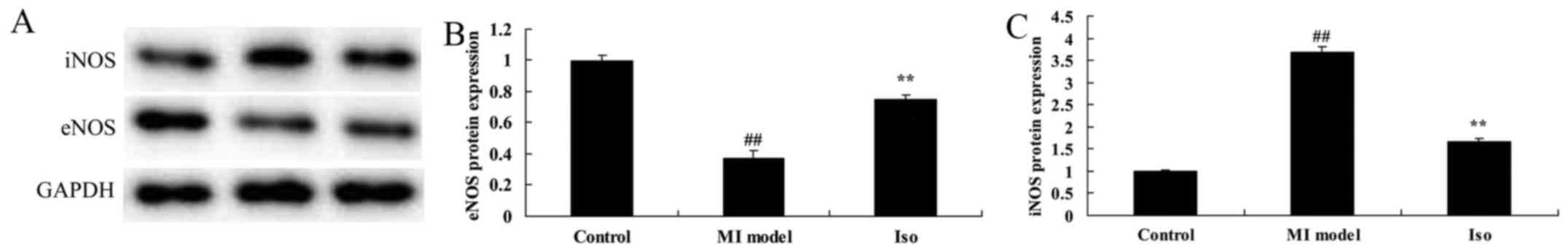
Isoquercetin affects on NO levels
We sought iNOS and eNOS protein expression using
Western blot analysis for cardioprotection. iNOS protein expression
was significantly induced, and eNOS protein expression was
significantly reduced in MI model rat, compared with control group
(Fig. 7). Treatment with
isoquercetin significantly suppressed iNOS protein expression and
induced eNOS protein expression in MI rat (Fig. 7).
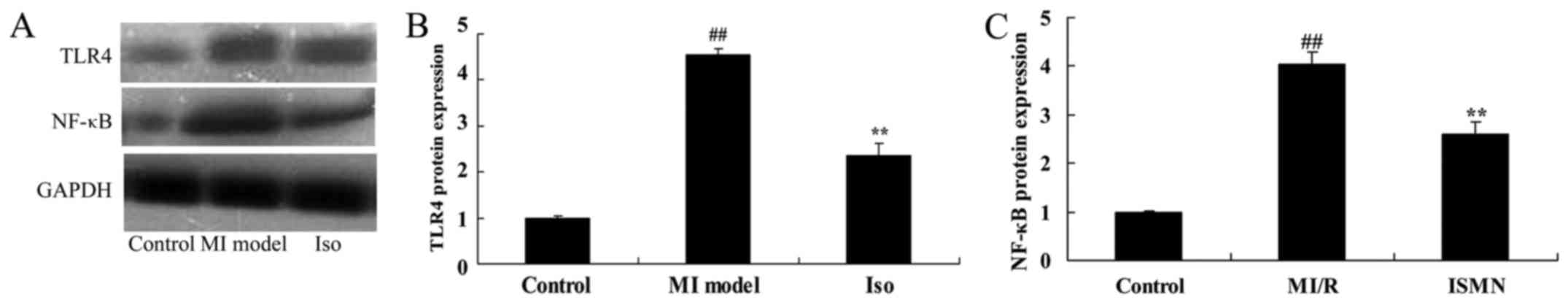
Isoquercetin affects on Toll-like
receptor 4-nuclear factor (TLR4-NF)-κB signal pathway
The western blot analysis results indicated that
TLR4 and NF-κB protein expression was significantly promoted in MI
model group, compared with control group (Fig. 8). Isoquercetin significantly
suppressed TLR4 and NF-κB protein expression in MI rat (Fig. 8).
Discussion
After myocardial infarction, the progressive
enlargement and the shape change will occur to the ventricle, which
is called the ‘ventricular remodeling’ after infarction, remaining
as a major pathologic basis for the chronic heart failure after
myocardial infarction (19). The
ventricular remodeling includes the changes in the myocardial cells
and the changes in the extracellular matrix (20). The dynamic balance between the
synthesis and degradation of the fibrin collagen in the
extracellular matrix plays an important role in the process of
ventricular remodeling (21). The
balance is broken after the myocardial infarction and leads to the
myocardial interstitial fibrosis, limiting the myocardial
activities and causing the decrease in the ventricular compliance
and the myocardial contractility, which could further develop into
the heart failure (22).
Meanwhile, the collagen deposition could also slow down the evoked
response passing through the myocardium, which damages the
electrical stability of myocardial cells and leads to arrhythmia
(3). Therefore, it was speculated
for the purposes of the present study that isoquercetin
significantly inhibited the increase of myocardial infarct size,
and reduced CK, CK-MB and LDH activity in MI rat.
Myocardial infarction is the most common inducement
for the chronic heart failure and even causes extensive
inflammatory responses of the heart (23). Many studies, including this one,
have observed that the expression of inflammatory factors in the
ischemic area and distal area of the heart has increased after the
myocardial infarction (7,23). The inflammation in the infarct area
could be explained as that the stress reaction occurring in the
myocardial damage and concrescence in the infarct area causes the
inflammation, accompanied by the infiltration of inflammatory cells
(7). However, what is worth
noticing is a report revealing that the distal area is still
staying at an obvious inflammatory status for seven weeks since the
myocardial infarction happened. In our study we observed
isoquercetin significantly inhibited inflammation in a rat with
acute myocardial infarction (AMI). Wang et al reported that
isoquercetin ameliorates via anti-oxidative, anti-inflammatory, and
anti-apoptotic effects in cerebral impairment (14).
Multiple studies have pointed out that the
myocardial infarction leads to the necrosis and dissolution of
abundant myocardial cells as well as the infiltration of
inflammatory cells, which further causes the decreased functioning
of the heart (24). The myocardial
infarction results in the abnormal intracellular environment due to
the insufficient energy supply (25). The increased compensatory
contraction of the heart could cause the elevation in the level of
the reactive oxygen species (ROS) due to the NADPH. More severely,
the elevation of the ROS level will trigger the mitochondria to
produce abundant ROS (the mechanism of ‘oxygen triggering oxygen
releasing’ inside the mitochondria) (26). Oxidative stress will not only
attack the cell membrane and organelle, but even interact with
inflammatory factors to initiate inflammatory responses, further
aggravating the myocardial damage caused by the infarction
(27). So increasing the oxidase
level and lowering the content of ROS are often seen as an
important instruction to treat the myocardial infarction (28). In this study, we found that
isoquercetin significantly reduced oxidative stress, iNOS
expression and heart cell apoptosis, and induced eNOS expression in
a rat with AMI. Lu et al showed that isoquercetin
ameliorates tunicamycin-induced apoptosis through suppressing
ROS-dependent stress (18).
It has been reported that the TLR4 receptor in the
myocytes could be activated by the ligands PAMP and DAMP (12). LPS from gram-negative bacteria is a
typical TLR4 specificity PAMP ligand and often used as the
instrumental medicine to activate TLR4 (29). HSP60 is the DAMP ligand of TLR4,
derived from the myocardial ischemia. Studies have indicated that
in the detached mouse cardiac myocytes, LPS could activate TLR4,
increase the transcriptional activity of NF-κB, induce the
production of cellular factors and lower the contractility of
myocytes (30). NF-κB is a widely
distributed nuclear transcription factor with extensive functions.
It can be combined with the specific sequence in the promoter or
enhancer regions of specific gene to initiate the transcription of
the gene (30). Usually, NF-κB is
combined with IκB to be an inactive alien complex existing in the
cytoplasm (29). Under the effect
of extracellular stimulation signals, IκB is phosphorylated and
then processed through ubiquitination (29). Based on the effect of 26S protease,
it will release NF-κB P50/P65 into the nucleus and combine with κB
on the target gene, leading to the conformation change in the DNA
here and thus initiating or enhancing the target gene transcription
(31). In the present study, we
found that isoquercetin significantly suppressed TLR4 and NF-κB
protein expression in MI rat. Wang et al showed that
isoquercetin protects induced injury of cortical neurons in
oxygen-glucose deprivation-reperfusion via suppression of
TLR4-NF-κB signal pathway (32).
In conclusion, our data show isoquercetin has a
clear role in modulating inflammation and oxidative stress and is
effective for MI through TLR4-NF-κB signal pathway. Further
clinical research on the protective effects of isoquercetin on
human MI cells is required.
References
|
1
|
Morita Y, Maeda K, Kondo T, Ishii H,
Matsudaira K, Okumura N, Mitsuhashi H, Shibata R and Murohara T:
Nagoya Acute Myocardial Infarction Study (NAMIS) Group: Impact of
adiponectin and leptin on long-term adverse events in Japanese
patients with acute myocardial infarction. Results from the Nagoya
Acute Myocardial Infarction Study (NAMIS). Circ J. 77:2778–2785.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jensen LJ, Munk K, Flyvbjerg A, Bøtker HE
and Bjerre M: Soluble receptor of advanced glycation end-products
in patients with acute myocardial infarction treated with remote
ischaemic conditioning. Clin Lab. 61:323–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka S, Masuda T, Kamiya K, Hamazaki N,
Akiyama A, Kamada Y, Maekawa E, Noda C, Yamaoka-Tojo M and Ako J: A
Single session of neuromuscular electrical stimulation enhances
vascular endothelial function and peripheral blood circulation in
patients with acute myocardial infarction. Int Heart J. 57:676–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Høfsten DE, Kelbæk H, Helqvist S,
Kløvgaard L, Holmvang L, Clemmensen P, Torp-Pedersen C, Tilsted HH,
Bøtker HE, Jensen LO, et al: The third DANish study of optimal
acute treatment of patients with ST-segment elevation myocardial
infarction: Ischemic postconditioning or deferred stent
implantation versus conventional primary angioplasty and complete
revascularization versus treatment of culprit lesion only:
Rationale and design of the DANAMI 3 trial program. Am Heart J.
169:613–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uysal H and Ozcan Ş: The effect of
individual education on patients' physical activity capacity after
myocardial infarction. Int J Nurs Pract. 21:18–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Li Y, Zhang T, Miao W and Su G:
Comparison of the influence of ticagrelor and clopidogrel on
inflammatory biomarkers and vascular endothelial function for
patients with ST-segment elevation myocardial infarction receiving
emergency percutaneous coronary intervention: Study protocol for a
randomized controlled trial. Trials. 17:752016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stefanadi E, Tousoulis D, Antoniades C,
Katsi V, Bosinakou E, Vavuranakis E, Triantafyllou G, Marinou K,
Tsioufis C, Papageorgiou N, et al: Early initiation of low-dose
atorvastatin treatment after an acute ST-elevated myocardial
infarction, decreases inflammatory process and prevents endothelial
injury and activation. Int J Cardiol. 133:266–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng L, Jin Z, Zhao R, Ren K, Deng C and
Yu S: Resveratrol attenuates inflammation and oxidative stress
induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE
pathway. Int J Clin Exp Med. 8:10420–10428. 2015.PubMed/NCBI
|
|
9
|
Cheng XY, Gu XY, Gao Q, Zong QF, Li XH and
Zhang Y: Effects of dexmedetomidine postconditioning on myocardial
ischemia and the role of the PI3K/Akt-dependent signaling pathway
in reperfusion injury. Mol Med Rep. 14:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Börekçi A, Gür M, Türkoğlu C, Selek Ş,
Baykan AO, Şeker T, Harbalıoğlu H, Özaltun B, Makça İ, Aksoy N, et
al: Oxidative stress and spontaneous reperfusion of infarct-related
artery in patients with ST-segment elevation myocardial infarction.
Clin Appl Thromb Hemost. 22:171–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elmas E, Ahmad-Nejad P, Weiss C, Neumaier
M and Borggrefe M: Plasminogen activator inhibitor-1 (PAI-1),
toll-like receptor 4 (TLR4), factor II (FII), FXIII and fibrinogen
polymorphisms are not associated with the prevalence of sudden
death due to ventricular fibrillation during myocardial infarction.
Clin Chem Lab Med. 46:1329–1331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Zhang J, Xu Y, Huang Y and Wu C:
Effect of carvedilol on cardiomyocyte apoptosis in a rat model of
myocardial infarction: A role for toll-like receptor 4. Indian J
Pharmacol. 45:458–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W,
Deng C, Fan C, Di S, Sun Y and Yi W: The emerging role of Toll-like
receptor 4 in myocardial inflammation. Cell Death Dis. 7:e22342016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CP, Shi YW, Tang M, Zhang XC, Gu Y,
Liang XM, Wang ZW and Ding F: Isoquercetin ameliorates cerebral
impairment in focal ischemia through anti-oxidative,
anti-inflammatory and anti-apoptotic effects in primary culture of
rat hippocampal neurons and hippocampal CA1 region of rats. Mol
Neurobiol. 54:2126–2142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhatia N, Kaur G, Soni V, Kataria J and
Dhawan RK: Evaluation of the wound healing potential of
isoquercetin-based cream on scald burn injury in rats. Burns
Trauma. 4:72016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vongsak B, Sithisarn P and Gritsanapan W:
Simultaneous determination of crypto-chlorogenic acid,
isoquercetin, and astragalin contents in moringa oleifera leaf
extracts by TLC-densitometric method. Evid Based Complement
Alternat Med. 2013:9176092013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang R, Yao Y, Wang Y and Ren G:
Antidiabetic activity of isoquercetin in diabetic KK-Ay mice. Nutr
Metab (Lond). 8:852011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu T, Zhang C, Chai M and An Y:
Isoquercetin ameliorates tunicamycin-induced apoptosis in rat
dorsal root ganglion neurons via suppressing ROS-dependent
endoplasmic reticulum stress. Biomed Pharmacother. 80:343–351.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao S, Wang L, Ouyang W, Zhou Y, Qi J, Guo
L, Zhang M and Hinek A: Traditional Chinese medicine, Danlou
tablets alleviate adverse left ventricular remodeling after
myocardial infarction: Results of a double-blind, randomized,
placebo-controlled, pilot study. BMC Complement Altern Med.
16:4472016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eitel I, Pöss J, Jobs A, Eitel C, de Waha
S, Barkhausen J, Desch S and Thiele H: Left ventricular global
function index assessed by cardiovascular magnetic resonance for
the prediction of cardiovascular events in ST-elevation myocardial
infarction. J Cardiovasc Magn Reson. 17:622015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ketchum ES, Dickstein K, Kjekshus J, Pitt
B, Wong MF, Linker DT and Levy WC: The Seattle Post Myocardial
Infarction Model (SPIM): Prediction of mortality after acute
myocardial infarction with left ventricular dysfunction. Eur Heart
J Acute Cardiovasc Care. 3:46–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kohl LP, Leimberger JD, Chiswell K, Jones
WS, Thiele H, Smalling RW, Chandra P, Cohen M, Perera D, Chew DP,
et al: Clinical characteristics and outcomes after unplanned
intraaortic balloon counterpulsation in the counterpulsation to
reduce infarct size Pre-PCI acute myocardial infarction trial. Am
Heart J. 174:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prondzinsky R, Unverzagt S, Lemm H,
Wegener N, Heinroth K, Buerke U, Fiedler M, Thiery J, Haerting J,
Werdan K and Buerke M: Acute myocardial infarction and cardiogenic
shock: Prognostic impact of cytokines: INF-γ, TNF-α, MIP-1β, G-CSF,
and MCP-1β. Med Klin Intensivmed Notfmed. 107:476–484. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raish M: Momordica charantia
polysaccharides ameliorate oxidative stress, hyperlipidemia,
inflammation, and apoptosis during myocardial infarction by
inhibiting the NF-κB signaling pathway. Int J Biol Macromol.
97:544–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basili S, Tanzilli G, Mangieri E,
Raparelli V, Di Santo S, Pignatelli P and Violi F: Intravenous
ascorbic acid infusion improves myocardial perfusion grade during
elective percutaneous coronary intervention: Relationship with
oxidative stress markers. JACC Cardiovasc Interv. 3:221–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aldakkak M, Stowe DF, Heisner JS, Riess ML
and Camara AK: Adding ROS quenchers to cold K+ cardioplegia reduces
superoxide emission during 2-hour global cold cardiac ischemia. J
Cardiovasc Pharmacol Ther. 17:93–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng Q, Lu C, Wang L, Song L, Li C and
Uppada RC: Effects of renal denervation on cardiac oxidative stress
and local activity of the sympathetic nervous system and
renin-angiotensin system in acute myocardial infracted dogs. BMC
Cardiovasc Disord. 17:652017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sedlic F, Muravyeva MY, Sepac A, Sedlic M,
Williams AM, Yang M, Bai X and Bosnjak ZJ: Targeted modification of
mitochondrial ROS production converts high glucose-induced
cytotoxicity to cytoprotection: Effects on anesthetic
preconditioning. J Cell Physiol. 232:216–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Wang H, Li J, Wang Q, Zhang S, Feng
N, Fan R and Pei J: κ-Opioid receptor stimulation modulates
TLR4/NF-κB signaling in the rat heart subjected to
ischemia-reperfusion. Cytokine. 61:842–848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Huang J and Song K: BET protein
inhibition mitigates acute myocardial infarction damage in rats via
the TLR4/TRAF6/NF-κB pathway. Exp Ther Med. 10:2319–2324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Li C, Cheng K, Zhang R, Narsinh K,
Li S, Li X, Qin X, Zhang R, Li C, et al: Activation of liver X
receptor improves viability of adipose-derived mesenchymal stem
cells to attenuate myocardial ischemia injury through TLR4/NF-κB
and Keap-1/Nrf-2 signaling pathways. Antioxid Redox Signal.
21:2543–2557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CP, Li JL, Zhang LZ, Zhang XC, Yu S,
Liang XM, Ding F and Wang ZW: Isoquercetin protects cortical
neurons from oxygen-glucose deprivation-reperfusion induced injury
via suppression of TLR4-NF-κB, signal pathway. Neurochem Int.
63:741–749. 2013. View Article : Google Scholar : PubMed/NCBI
|