Introduction
Mast cells (MCs) are secretory cells that are
strategically located at host-environment interfaces, including the
skin, lungs and mucosal surfaces. MCs release granules following
activation and are part of the innate immune system, being involved
in the first line of defense against pathogens, including bacteria
and parasites (1). Activated MCs
produce a broad spectrum of inflammatory cytokines, chemokines,
lipid compounds and vasoactive amines, all of which are involved in
immune responses (2).
Lipopolysaccharide (LPS) released from Gram negative
bacteria is of great significance to mast cells, for the innate
immune response and, additionally, as it is able to aggravate an
existing inflammatory state in allergic airway inflammation via T
helper cell 2 (Th2) cytokine production (3). Toll-like receptor (TLR) 4 is a
receptor for LPS that serves an important role in inflammation and
is a risk factor for MC-mediated asthma. MCs directly respond to
LPS by producing inflammatory cytokines including interleukin-1β
(IL-1β), IL-6, IL-13 and tumor necrosis factor-α (TNF-α) (4). These mediators exert acute
inflammatory effects through endothelial cells and leukocytes.
Activation of TLR4 by LPS on MCs leads to the generation of Th2
cytokines (e.g. IL-4, IL-5 and IL-13), which enhances the allergic
inflammation that may occur in asthma (5,6). In
addition to allergic airway inflammation, MCs trigger inflammatory
responses in the skin when exposed to LPS. For example,
LPS-stimulated MCs serve an important role in edema formation by
releasing factors that increase vascular permeability, including
histamine and leukotrienes (LTs) (7).
Lipoxygenases (LOXs) are a family of enzymes that
catalyze the incorporation of oxygen into unsaturated fatty acids
(8). LOXs are expressed in immune
and epithelial cells and they are involved in a variety of
conditions, including inflammatory skin disease and allergic
asthma, wherein they generate LTs and proinflammatory mediators
(9,10). MCs were suggested to be one of the
principal cell types responsible for the action of 5-/12-LOX during
the progression of asthmatic inflammation due to their production
of LTs and IL-13 (11,12). Considering that LTs and IL-13 are
potent proinflammatory mediators, LOX may serve a role in allergic
inflammation mediated by MCs.
Previously, benzoxazoles have been reported to act
as 5-lipoxygenase or IL-6 inhibitors (13–16).
To investigate the effects of benzoxazole derivatives on the
LPS-induced inflammatory response in MCs, the expression of
proinflammatory cytokines, the levels of histamine secreted from
MCs under LPS stimulation, and the cell-surface localization of
co-stimulatory molecule in the presence or absence of benzoxazole
derivatives was assessed.
Materials and methods
Synthesis of benzoxazole
derivatives
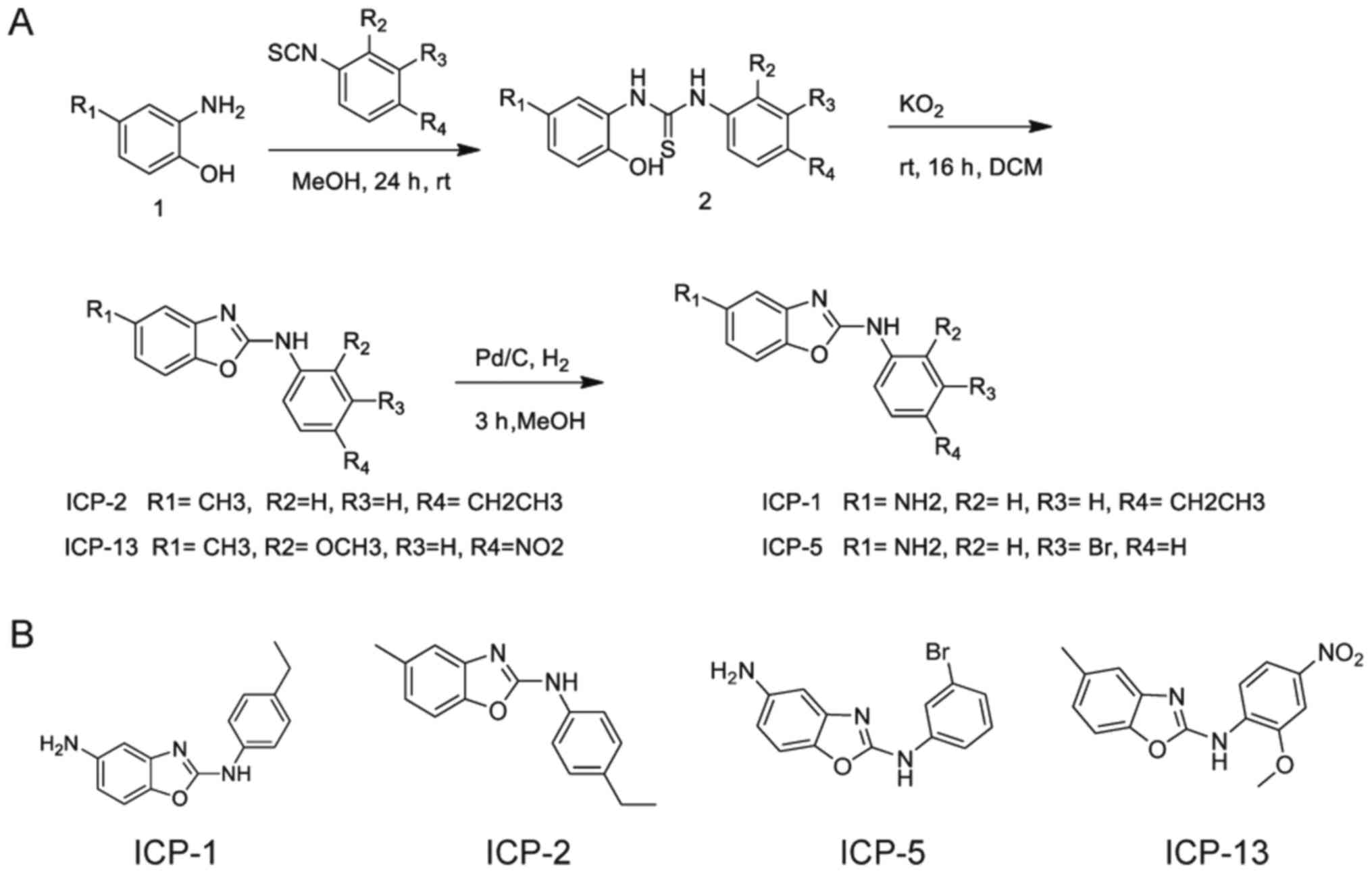
A total of four derivatives with the benzoxazole
moiety were synthesized starting from the commercially available
2-amino-4-nitro (or 4-methyl) phenol (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and substituted-phenyl isothiocyanate
(Sigma-Aldrich; Merck KGaA; Fig.
1). The thiourea 2 was cyclized to benzoxazole anti-itch agent
provided by Park [(ICP)-1, 2, 5, and 13, Laboratory of Dr Park,
Ewha Womans University, Seoul, Korea] by oxidation with 5 eq
KO2 (Alfa Aesar, Haverhill, MA, USA). This procedure to
prepare the benzoxazoles had an 80–90% yield (13–16).
For the preparation of ICP-1 and 5, the nitro group was reduced to
amino group with catalytic hydrogenation using 5% Pd/C (Sigma
Aldrich; Merck KGaA) as catalyst.
Generation of bone marrow-derived MCs
(BMMCs) and in vitro stimulation
For bone marrow cell isolation, 5 female C57BL/6
mice were purchased at 10 weeks of age (weight, 20–22 g) from
OrientBio, Inc., (Emsung, Korea). All animals were maintained at
21–23°C with 51–54% humidity under pathogen-free conditions on a
12-h light/dark cycle with free access to food and water. All
procedures were approved by the Ewha Womans University College of
Medicine Animal Care and Use Committee (Seoul, Korea; ESM 15–0309).
Bone marrow cells were isolated from the femurs of female C57BL/6
mice and grown in Iscove's Modified Dulbecco's Medium (IMEM;
Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum
(FBS; Welgene), 50 µM β-mercaptoethanol, 2 mM L-glutamine, 100
µg/ml streptomycin and 100 U/ml penicillin, and 10 ng/ml IL-13
(PeproTech, Inc., Rocky Hill, NJ, USA). Non-adherent cells were
transferred to fresh medium every 2–3 days to remove adherent
macrophages and fibroblasts. After 5 weeks, the cells were
identified as MCs using flow cytometric analyses of high affinity
immunoglobulin ε receptor subunit γ (FcεRI) and cluster of
differentiation (CD)117 (c-kit) expression and toluidine blue
staining. For toluidine blue staining cytospin slides were stained
for 3 min at room temperature. The image was captured by light
microscope with magnification, ×1,000 (Olympus BX50, Olympus
Corporation; Tokyo, Japan). MCs at 5–6 weeks following initiation
of culture were used for the experiments. For treatment with LPS,
BMMCs were seeded at a density of 6×105 cells/well in
96-well plates and stimulated with LPS (Sigma-Aldrich; Merck KGaA)
at a concentration of 1 µg/ml. A total of four types of benzoxazole
derivatives (ICP-1, 2, 5 and 13) were separately added to the BMMCs
at a concentration of 10 µM each to observe the effects on
LPS-induced BMMCs.
Flow cytometry
The surface expression of FcεRI and CD117 on BMMCs
following 5 weeks of culture was detected by staining with Alexa
488-conjugated anti-FcεRI antibody (Clone MAR-1; cat. no. 134329;
BioLegend, Inc., San Diego, CA, USA) and PerCP-conjugated
anti-CD117 antibody (Clone 2B8; cat. no. 105821; BioLegend, Inc.)
at a concentration of 5 mg/ml on ice for 30 min followed by washing
using PBS containing 0.5% FBS and 0.1% sodium azide. The surface
expression of CD80 and CD86 were additionally analyzed using
fluorescein isothiocyanate-conjugated anti-CD80 antibody (Clone
16–10A1; cat. no. 553768; BD Biosciences, San Jose, CA, USA) and
phycoerythrin-conjugated anti-CD86 antibody (Clone GL1; cat. no.
553692; BD Biosciences) at a concentration of 5 mg/ml on ice for 30
min. The level of nonspecific staining was performed using the
corresponding isotype antibody as described above; Armenian Hamster
IgG (cat. no. 400923; Biolegend, Inc.) for anti-FcεRI antibody, Rat
IgG2b, κ for anti-CD117 antibody (cat. no. 400629; Biolegend,
Inc.). Each sample was quantified on a NovoCyte™ Flow
Cytometer (ACEA Biosciences, San Diego, CA, USA) and the data were
analyzed using FlowJo software (version 8.7, FlowJo LLC, Ashland,
OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BMMCs with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Complementary DNA was synthesized by
incubation at 30°C for 10 min, at 42°C for 20 min and at 99°C for 5
min using the First Strand cDNA Synthesis kit (Toyobo Life Science,
Osaka, Japan). Amplification was performed in duplicate by 40
cycles of 15 sec denaturation step at 95°C and a 1 min
amplification and signal acquisition step at 60°C using StepOnePlus
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) using SYBR® green (Kapa
Biosystems, Inc., Wilmington, MA, USA).
All gene expression values were normalized to the
expression of the GAPDH gene using the following primers:
5′-GACCTTGTGTCCTCCGCTTAT-3′ forward, and 5′-CAACCGCAATTTGTGGCTC-3′
reverse for mouse perilipin 2 (Plin2; 157 bp);
5′-ATGTCTAGCAATGGTACAGATGC-3′ forward, and
5′-CGTGGAACTGATAAGAGGCAGG-3′ reverse for mouse Plin3 (114
bp); 5′-GCAACTGTTCCTGAACTCAACT-3′ forward, and
5′-ATCTTTTGGGGTCCGTCAACT-3′ reverse for mouse Il1b (89 bp);
5′-CTGCAAGAGACTTCCATCCAG-3′ forward, and
5′-AGTGGTATAGACAGGTCTGTTGG-3′ reverse for mouse Il6 (131
bp); 5′-CCTGGCTCTTGCTTGCCTT-3′ forward, and
5′-GGTCTTGTGTGATGTTGCTCA-3′ reverse for mouse I13 (116 bp);
5′-CCTGTAGCCCACGTCGTAG-3′ forward, and 5′-GGGAGTAGACAAGGTACAACCC-3′
reverse for mouse Tnfa (148 bp); and
5′-GGTAAAGTGGATATTGTTGCCATCAATG-3′ forward, and
5′-GGAGGGATCTCGCTCCTGGAAGATGGTG-3′ reverse for mouse GAPDH
(173 bp). The relative fold expression and changes were calculated
2−ΔΔCq method (17).
Immunoblotting
Cell culture supernatant from non-treated BMMCs,
BMMCs stimulated with LPS (1 µg/ml), and BMMCs stimulated with LPS
in the presence of the benzoxazole derivatives (ICP-1, 2, 5, and/or
13) were collected and prepared in a sample buffer [0.5 M Tris/HCl
(pH 6.8), 25% glycerol, 10% SDS, 500 mM DTT, 1% bromophenol blue;
Sigma-Aldrich; Merck KGaA].
Equal amounts (40 µl) cell lysate were resolved by
10% SDS-PAGE and transferred to Immobilon-P polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 5% skim milk in TBS containing 0.1% Tween-20 (TBS-T)
solution for 1 h at room temperature and were subsequently
incubated with primary antibodies overnight at 4°C. The following
primary antibodies were used: IL-1β (1:200, diluted in 3% bovine
serum albumin (BSA; Bovogen Biologicals, Pty, Ltd., East Keilor,
Victoria, Australia) containing TBST; cat. no. sc-7884; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); and β-actin [1:3,000,
diluted in 3% BSA containing TBST; cat. no. A1978; mouse;
Sigma-Aldrich; Merck KGaA]. The membranes were washed 3 times for
10 min in TBST and incubated with anti-rabbit (cat. no. BR170-6515;
Bio-Rad Laboratories, Inc.) or anti-mouse (cat. no. BR170-6516;
Bio-Rad Laboratories, Inc.) horseradish peroxidase-conjugated
secondary antibodies (1:3,000, diluted in TBST) for 1 h at room
temperature. Following incubation, membranes were washed 3 times
for 10 min in TBST and developed using SuperSignal West Femto
Maximum Sensitivity Substrate (Pierce; Thermo Fisher Scientific,
Inc.). Images were obtained using ImageQuant LAS 4000 (GE
Healthcare Life Sciences, Little Chalfont, UK). The band pixel
densities of the precursor form of IL-1β and the active form of
IL-1β were divided by the pixel densities of the corresponding
β-actin bands for quantitation using UN-SCAN-IT-gel 6.1 software
(Silk Scientific Inc., Orem, UT, USA).
ELISA
For histamine measurements, 80 nM
phorbol-12-myristate-13-acetate (PMA) (Ebioscience; Thermo Fisher
Scientific, Inc.) and ionomycin (1 µM; Ebioscience; Thermo Fisher
Scientific, Inc.) were added in the presence of the benzoxazole
derivatives, and the cultures were maintained for 24 h at 37°C in a
humidified atmosphere of 5% CO2. Cell culture
supernatants were collected from non-treated BMMCs and BMMCs
stimulated with LPS (1 µg/ml) in the presence of the benzoxazole
derivatives (ICP-1, 2, 5, and/or 13). The levels of secreted
histamine were determined using a histamine ELISA kit in accordance
with the manufacturer's recommended protocol (Abnova; cat. no.
KA2589; Taipei, Taiwan).
Statistical analysis
Data are presented as the mean ± standard error of
the mean (n=3). Statistical significance was determined by one-way
analysis of variance (ANOVA) in conjunction with Dunnett's post hoc
test, or two-way ANOVA in conjunction with Sidak's post-hoc test
compared with the normal group using GraphPad Prism, version 7
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
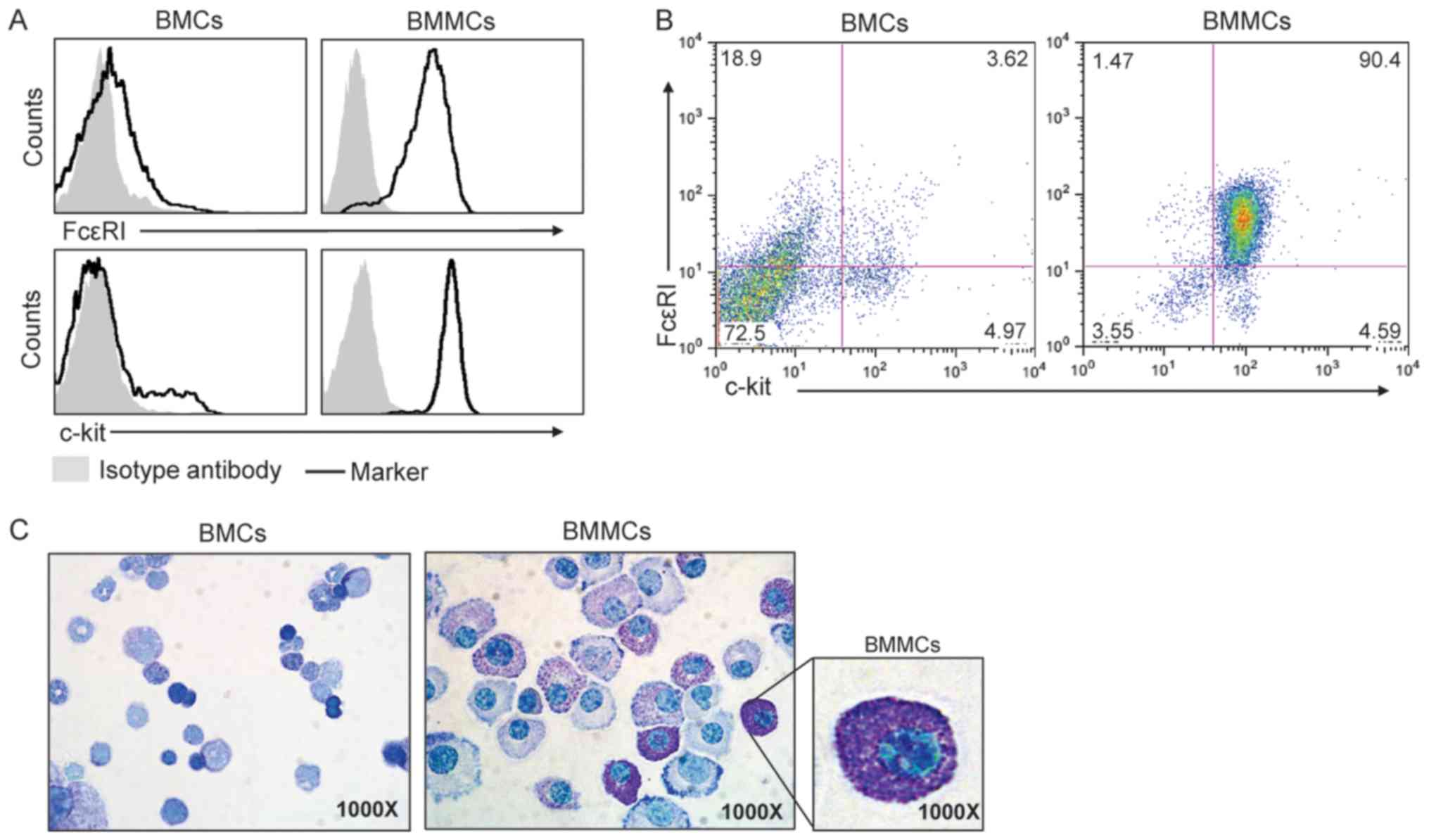
Generation of BMMCs from mice
BMMCs were generated from C57BL/6 mice. As exhibited
in Fig. 2A, low expression of the
mast cell markers FcεRI and c-kit was observed prior to BMMC
differentiation on the cell surface of fresh mouse bone marrow
cells. However, following 5 weeks of culture in the presence of
IL-3, >90% of the cells expressed FceRI and c-kit (Fig. 2B). In addition, toluidine blue
staining demonstrated differentiated MCs that contained granules in
the cytoplasm (Fig. 2C).
Benzoxazole derivatives attenuate the
LPS-mediated gene expression of Il1b Il6, Il13, Tnfa, Plin2 and
Plin3 in BMMCs
A number of studies have indicated that MCs produce
proinflammatory cytokines, including IL-1β, IL-6, IL-13 and TNF-α
when exposed to LPS (4,18–20).
LOX activation positively regulates inflammatory responses,
including LPS-induced inflammation (21,22)
and LPS is a direct inducer of 5-LOX in immune cells, including
monocytes (23). As it was
reported that benzoxazoles are 5-LOX and IL-6 inhibitors (13), benzoxazole derivatives were
synthesized and LPS-stimulated BMMCs were treated with the
inhibitors for 24 h. Benzoxazole derivatives had an inhibitory
effect on the expression of the Il1b, Il6, Il13 and
Tnfa genes in BMMCs. In particular, a higher fold increase
in Il1b and Tnfa expression, compared with Il6
and Il13, following LPS stimulation was observed. A total of
three types of cytokines (Il1b, Il13 and Tnfa) were
significantly downregulated by all four types of benzoxazole
derivatives (ICP-1, 2, 5 and 13; P<0.05; Fig. 3A).
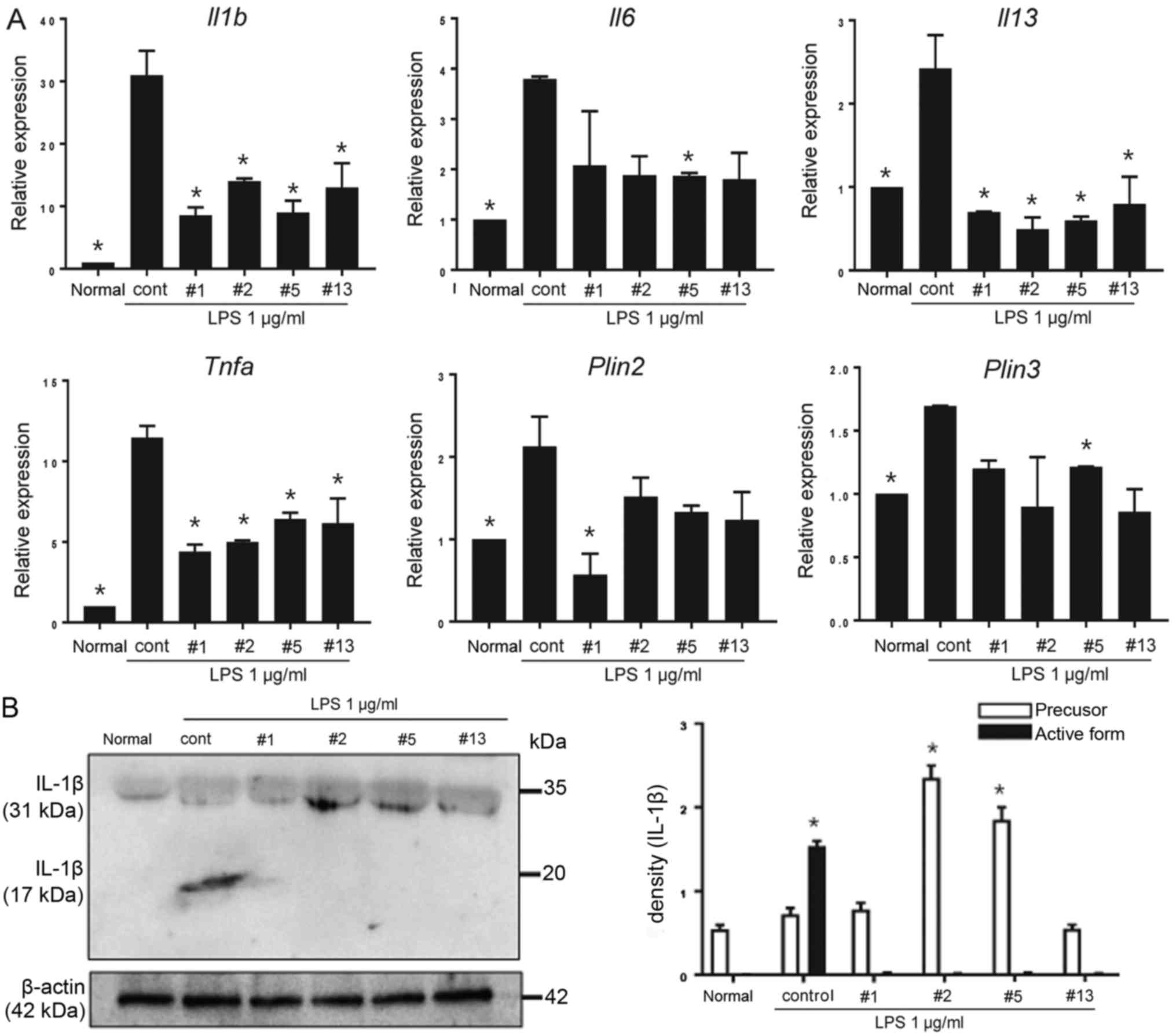 | Figure 3.Gene expression profiles in BMMCs.
(A) Non-treated normal BMMCs, BMMCs cultured with LPS, and BMMCs
cultured with LPS in the presence of benzoxazole derivatives (10 µM
each) were collected, and the mRNA expression of Il1b, Il6,
Il13, Tnfa, Plin2 and Plin3 was analyzed by reverse
transcription-quantitative polymerase chain reaction. The
benzoxazole derivatives ICP-1, ICP-2, ICP-5 and ICP-13 are
indicated as #1, #2, #5 and #13, respectively. Data are presented
as the mean ± SEM. *P<0.05 vs. control. (B) The level of
secreted IL-1β from non-treated normal BMMCs, and BMMCs cultured
with LPS stimulation and each benzoxazole derivative (10 µM each)
were compared using the cell culture supernatant. The precursor
form of IL-1β was indicated by the 31 kDa band and the active form
of IL-1β was indicated by the 17 kDa band (upper). The pixel
densities of each band were divided by the corresponding β-actin
bands for normalization (lower). Data are presented as the mean ±
SEM. *P<0.05 vs. normal. SEM, standard error of the mean; BMMCs,
bone marrow derived mast cells; LPS, lipopolysaccharide;
Il/IL, interleukin; Tnfa, tumor necrosis
factor-α; cont, control; Plin, perilipin. |
In addition, lipid droplets (LD) from MCs are
involved in cell signaling and the generation of biologically
active lipid mediators (e.g. prostaglandin D2, leukotriene B4 and
C4) evoked by inflammatory and infectious conditions (24,25).
The most well-known LD proteins are members of the PLIN family. Out
of the five PLINs (PLIN1, PLIN2, PLIN3, PLIN4 and PLIN5), PLIN2 and
PLIN3 are expressed in developing and mature human MCs (26), and the constitutive expression of
the Plin2 and Plin3 genes in BMMCs was confirmed in
the present study (data not shown). A modest, although significant,
inhibition and induction of Plin2 and Plin3,
respectively by ICP-1 and 5, following treatment with LPS was
observed (P<0.05; Fig. 3A).
As the activation of MCs via the NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) inflammasome may be linked to
IL-1β production (27), the
protein expression levels of IL-1β in the cell culture supernatant
were tested. Following 24 h of treatment with LPS in the presence
or absence of benzoxazole derivatives, the secreted active form of
IL-1β was most abundant in the BMMC supernatant; however,
benzoxazole derivatives suppressed the secretion of the protein.
Instead, a precursor form of IL-1β (pro-IL-1β) accumulated,
particularly under treatment with the benzoxazole derivatives ICP-2
and ICP-5 (Fig. 3B).
Benzoxazole derivatives suppress
histamine production in BMMCs
LPS-induced inflammation was demonstrated to
increase histamine secretion, possibly by directly stimulating
histamine-forming enzyme (histidine decarboxylase) activity
(28). Therefore, it was
hypothesized that MC activation by LPS may be followed by histamine
secretion. Furthermore, the suppressive effect of the benzoxazole
derivatives was additionally investigated. The secretion of
histamine from BMMCs was triggered by PMA and ionomycin as a
positive control. As exhibited in Fig.
4A, LPS stimulation alone for 24 h promoted histamine
production in BMMCs. Although the increases in histamine production
with ICP-1, 2 and 5 were not statistically significant, benzoxazole
derivative ICP-13 significantly inhibited the increase (P<0.05).
The BMMCs treated with PMA and ionomycin generated significantly
increased histamine levels, which was suppressed by all the
inhibitors (P<0.05; Fig.
4B).
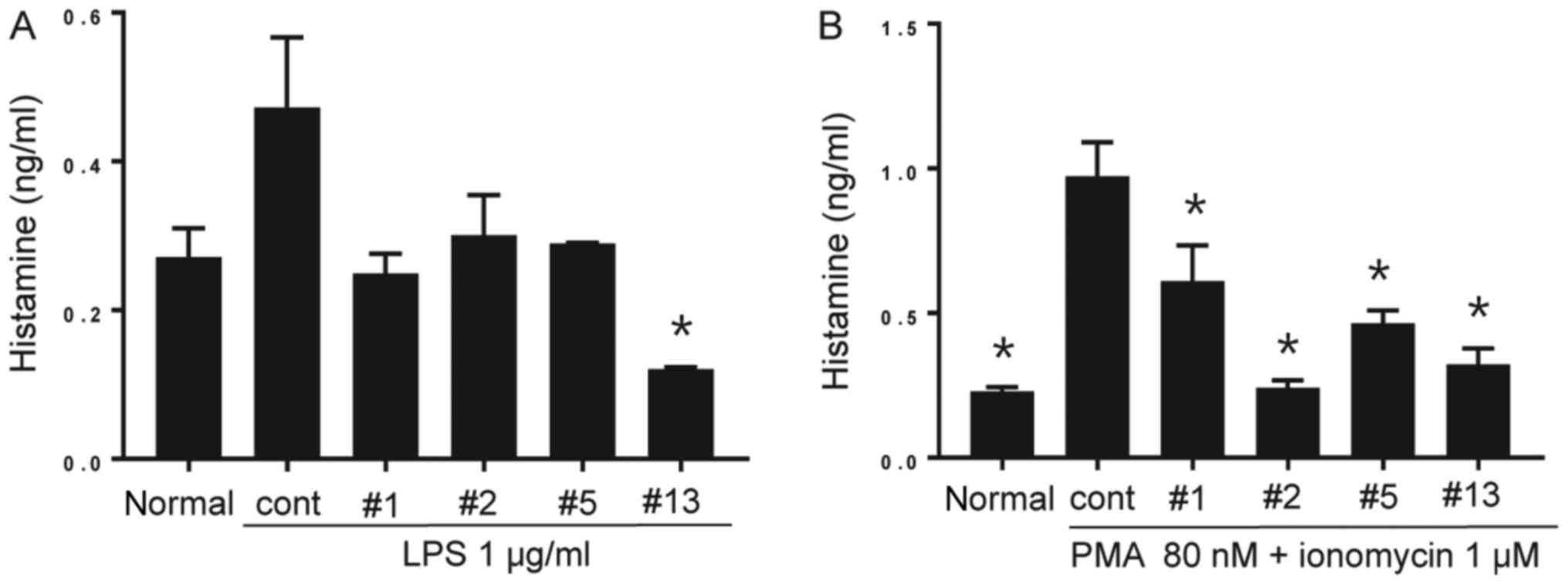 | Figure 4.Histamine production of BMMCs. (A)
The cell culture supernatant from non-treated normal BMMCs, BMMCs
treated with LPS, and BMMCs treated with LPS in the presence of
each of the benzoxazole derivatives (10 µM each) for 24 h was
collected and the amount of secreted histamine was measured by
ELISA. The benzoxazole derivatives ICP-1, ICP-2, ICP-5 and ICP-13
are indicated as #1, #2, #5 and #13, respectively. Data are
presented as the mean ± SEM. *P<0.05 vs. cont. (B) Cell culture
supernatants from non-treated normal BMMCs, BMMCs treated with PMA
and ionomycin, BMMCs treated with PMA and ionomycin in the presence
of each benzoxazole derivative (10 µM each) for 24 h were
collected, and the amount of secreted histamine was measured by
ELISA. Data are presented as the mean ± SEM. *P<0.05 vs. cont.
SEM, standard error of the mean; BMMCs, bone marrow derived mast
cells; LPS, lipopolysaccharide; PMA,
phorbol-12-myristate-13-acetate; cont, control; ICP, anti-Itch
agent by Park. |
Benzoxazole derivatives do not affect
the surface expression of co-stimulatory molecules on BMMCs
In addition to their contribution to host defense
via innate mechanisms, MCs additionally promote adaptive immune
responses through interactions with CD4+ and
CD8+ T cells (29). It
was demonstrated that MCs may be primed to express functional class
II major histocompatibility complex molecules and co-stimulatory
molecules, and may serve as antigen presenting cells for
CD4+ T lymphocytes, including helper T cells (30). Therefore mouse BMMCs were examined
by flow cytometry for the surface expression of members of the B7
protein family, CD80 and CD86, which are potent co-stimulatory
proteins for T cell activation. BMMCs highly expressed both CD80
and CD86 even in the absence of stimulation, and their expression
was not altered following 24 h of treatment with LPS. Similarly,
simultaneous treatment with benzoxazole derivatives and LPS did not
affect the expression level of these surface proteins. However,
ICP-5 downregulated the percentages of CD80-positive BMMCs
(Fig. 5), by modifying CD80
expression rather than CD86 expression.
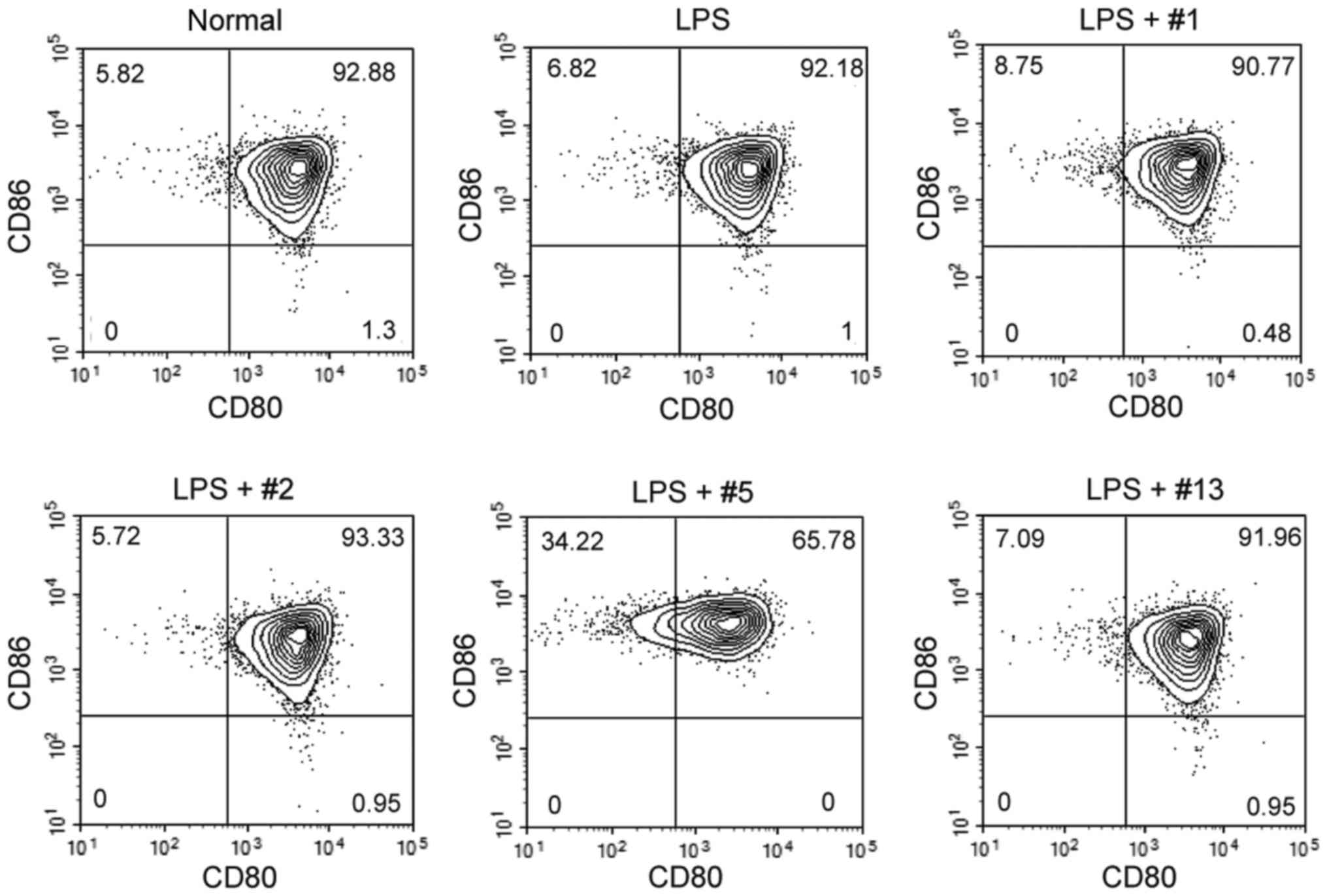 | Figure 5.Surface expression of co-stimulatory
molecules in BMMCs. Non-treated normal BMMCs, and BMMCs cultured in
the presence of LPS (1 µg/ml) and each benzoxazole derivative (10
µM each) for 24 h were collected, and the surface expression of
CD80 and CD86 was analyzed by flow cytometry. The benzoxazole
derivatives ICP-1, ICP-2, ICP-5 and ICP-13 are indicated as #1, #2,
#5 and #13, respectively. BMMCs, bone marrow derived mast cells;
LPS, lipopolysaccharide; CD, cluster of differentiation, ICP,
anti-Itch agent by Park. |
Discussion
Previously, the authors reported that benzoxazoles
act as 5-LOX or IL-6 inhibitors (13–16).
As part of the authors' ongoing study on the use of benzoxazoles as
anti-inflammatory agents, benzoxazole derivatives were synthesized
and their effects on MCs were determined. In the present study,
benzoxazole derivatives were demonstrated to attenuate LPS-induced
BMMC activation. Specifically, it was demonstrated that the
LPS-mediated gene expression of Il1b, Il6, Il13, Tnfa, Plin2
and Plin3 in BMMCs was downregulated by four benzoxazole
derivatives. Furthermore, histamine secretion from BMMCs exposed to
LPS or PMA/ionomycin was suppressed in the presence of benzoxazole
derivatives. However, the inhibition of the surface expression of
co-stimulatory molecules, including CD80 and CD86, was less
evident.
MCs are particularly abundant in the skin, airway
and gut mucosa, where they are strategically located for optimal
interaction with the environment. MCs are heterogeneous in terms of
morphology, receptor expression, mediator content and reactivity
towards various stimulants, according to their localization in the
body and among animal species. Therefore, cellular pathways acting
on MCs to produce key mediators involved in inflammation,
regardless of the heterogeneity of cells, may be efficient targets
to modulate MC responses (31).
MCs have been demonstrated to express the majority of TLRs, and to
respond to their agonists by secreting cytokines, chemokines and
lipid mediators. There are similarities and differences between the
cytokines produced upon stimulation with different TLRs. For
example, TLR2 and TLR4 trigger the production of the Th2 cytokines
IL-4, IL-5, IL-13, IL-6 and TNF-α from MCs via the c-Jun N-terminal
kinase and p38 pathways (4).
Additionally, the benzoxazole derivatives, particularly ICP-1, used
in the present study were reported to suppress the production of
interferon-γ, IL-17, IL-4, IL-5 or IL-13 via suppression of the
IL-6-signal transducer and activator of transcription 3 signaling
pathway (16).
LPS stimulation of BMMCs induced the expression of
Il1b mRNA in addition to the induction of the active form of
IL-1β, which was suppressed by the benzoxazole derivatives.
Notably, treatment with benzoxazole derivatives failed to induce
expression of the cleaved form of active IL-1β. This result
suggested that the benzoxazole derivatives used in the present
study may inhibit the activation of the NLRP3 signaling pathway
directly or indirectly, via a decrease in TNF-α (32) or PLIN (33) from BMMCs. Activation of the NLRP3
inflammasome requires two signals: Signal 1 from microbial
components (e.g. LPS) or endogenous cytokines (e.g. TNF-α), and
signal 2 from adenosine triphosphate or bacterial toxin stimulation
of the NLRP3 inflammasome. Signal 1 leads to the upregulation of
NLRP3 in addition to pro-IL-1β expression via nuclear factor
(NF)-κB activation through TLR4. Signal 2 leads to the activation
of the NLRP3 inflammasome followed by activation of caspase 1,
which cleaves pro-IL-1β to the active form of IL-1β. Therefore, the
benzoxazole derivatives that were tested possibly inhibited the
NF-κB pathway and/or inflammasome activation, either by a direct
effect on the NF-κB signaling pathway or an indirect effect by
suppressing 5-LOX activity (13).
Inflammation is a fundamental protective response in
higher eukaryotes against a variety of external stimuli, including
environmental toxins, pathogens and allergens. These stimuli are
encountered by immune cells including neutrophils, MCs or
macrophages, which mediate the initial defense reaction. Therefore,
the response of these cells upon activation is indispensable for
maintaining host defenses and homeostasis. However, excessive or
unresolved activation may induce pathophysiological processes
resulting in the development of inflammatory disease. Inflammation
is closely associated with oxidative processes and, therefore,
oxidative enzymes, including 5-LOX, that are known to serve key
roles in inflammation (34). 5-LOX
generates a number of lipid and pro-inflammatory mediators that
directly act on inflammation. Furthermore, the mediators generated
by 5-LOX including LTs are involved in the activation of
pro-inflammatory signal transduction pathways including the NF-κB
signaling pathway that potentiates inflammatory status. Indeed,
5-LOX-generated pro-inflammatory products have been implicated in a
number of human acute and chronic inflammatory diseases, including
asthma, atherosclerosis, rheumatoid arthritis, inflammatory bowel
diseases, urticaria and atopic dermatitis.
It was reported that 5-LOX enzymatic activity is
inhibited by phenolic antioxidants including nordihydroguaiaretic
acid and caffeic acid, suggesting a beneficial role of dietary
polyphenol intake (35). Synthetic
drugs that act on LOX are currently relatively limited. The 5-LOX
inhibitor zileuton has been used successfully for the control of
asthma. Currently, 5-LOX inhibitors are being developed by
pharmaceutical companies (36).
These inhibitors prevent the transportation of 5-LOX from the
nucleus to the cytoplasm, leading to the suppression of
5-hydroperoxyeicosatetraenoic acid production.
In conclusion, the present study demonstrated that
benzoxazole derivatives (ICP-1, 2, 5 and 13) suppressed the
production of proinflammatory cytokines of BMMCs and LD proteins,
including PLINs, following LPS stimulation. Therefore, agents,
including benzoxazole derivatives that are able to inhibit acute
inflammation resulting from the activation of MCs in allergic
reactions or urticaria may be therapeutically important. Further
studies are required to refine the mode of action of benzoxazole
derivatives and their therapeutic effects in vivo to
determine the most effective therapeutic strategy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Institute of
Clinical Medicine Research of Bucheon St. Mary's Hospital, Research
Fund, BCMC12AH08. In addition, the present study was supported by
the RP-Grant 2018 of Ewha Womans University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KAC performed experiments and wrote the manuscripts.
MP and YHK performed experiments and analyzed data. HP and KHL
designed the experiments and wrote the manuscripts.
Ethics approval and consent to
participate
All procedures were approved by the Ewha Womans
University College of Medicine Animal Care and Use Committee
(Seoul, Korea; ESM 15-0309).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Sismanopoulos N, Delivanis DA,
Alysandratos KD, Angelidou A, Therianou A, Kalogeromitros D and
Theoharides TC: Mast cells in allergic and inflammatory diseases.
Curr Pharm Des. 18:2261–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wernersson S and Pejler G: Mast cell
secretory granules: Armed for battle. Nat Rev Immunol. 14:478–494.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamashita M and Nakayama T: Progress in
allergy signal research on mast cells: Regulation of allergic
airway inflammation through toll-like receptor 4-mediated
modification of mast cell function. J Pharmacol Sci. 106:332–335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masuda A, Yoshikai Y, Aiba K and
Matsuguchi T: Th2 cytokine production from mast cells is directly
induced by lipopolysaccharide and distinctly regulated by c-Jun
N-terminal kinase and p38 pathways. J Immunol. 169:3801–3810. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murakami D, Yamada H, Yajima T, Masuda A,
Komune S and Yoshikai Y: Lipopolysaccharide inhalation exacerbates
allergic airway inflammation by activating mast cells and promoting
Th2 responses. Clin Exp Allergy. 37:339–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nigo YI, Yamashita M, Hirahara K,
Shinnakasu R, Inami M, Kimura M, Hasegawa A, Kohno Y and Nakayama
T: Regulation of allergic airway inflammation through Toll-like
receptor 4-mediated modification of mast cell function. Proc Natl
Acad Sci USA. 103:pp. 2286–2291. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shiba E, Izawa K, Kaitani A, Isobe M,
Maehara A, Uchida K, Maeda K, Nakano N, Ogawa H, Okumura K, et al:
Ceramide-CD300f binding inhibits lipopolysaccharide-induced skin
inflammation. J Biol Chem. 292:2924–2932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuhn H and O'Donnell VB: Inflammation and
immune regulation by 12/15-lipoxygenases. Prog Lipid Res.
45:334–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han H, Liang X, Ekberg M, Kritikou JS,
Brunnström Å, Pelcman B, Matl M, Miao X, Andersson M, Yuan X, et
al: Human 15-lipoxygenase-1 is a regulator of dendritic-cell
spreading and podosome formation. FASEB J. 31:491–504. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Claesson HE: On the biosynthesis and
biological role of eoxins and 15-lipoxygenase-1 in airway
inflammation and Hodgkin lymphoma. Prostaglandins Other Lipid
Mediat. 89:120–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ro M, Lee AJ and Kim JH:
5-/12-lipoxygenase-linked cascade contributes to the IL-33-induced
synthesis of IL-13 in mast cells, thus promoting asthma
development. Allergy. 73:350–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mashima R and Okuyama T: The role of
lipoxygenases in pathophysiology; new insights and future
perspectives. Redox Biol. 6:297–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song H, Oh SR, Lee HK, Han G, Kim JH,
Chang HW, Doh KE, Rhee HK and Choo HY: Synthesis and evaluation of
benzoxazole derivatives as 5-lipoxygenase inhibitors. Bioorg Med
Chem. 18:7580–7585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH, An MH, Choi EH, Choo HYP and Han
G: A facile synthesis of 2-acyl and 2-alkylaminobenzimidazoles for
5-lipoxygenase inhibitors. Heterocycles. 70:571–580. 2006.
View Article : Google Scholar
|
|
15
|
Yoon JH, Song H, Kim SW, Han G and Choo
HYP: A facile synthesis of 2-aminothiazolo [5,4-b]pyridines and
2-aminobenzoxazoles via cyclization of thioureas. Heterocycles.
65:2729–2740. 2005. View Article : Google Scholar
|
|
16
|
Kim D, Won HY, Hwang ES, Kim YK and Choo
HP: Synthesis of benzoxazole derivatives as interleukin-6
antagonists. Bioorg Med Chem. 25:3127–3134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiba N, Masuda A, Yoshikai Y and
Matsuguchi T: Ceramide inhibits LPS-induced production of IL-5,
IL-10, and IL-13 from mast cells. J Cell Physiol. 213:126–136.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hochdörfer T, Tiedje C, Stumpo DJ,
Blackshear PJ, Gaestel M and Huber M: LPS-induced production of
TNF-α and IL-6 in mast cells is dependent on p38 but independent of
TTP. Cell Signal. 25:1339–1347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sandig H and Bulfone-Paus S: TLR signaling
in mast cells: Common and unique features. Front Immunol.
3:1852012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lopes DEM, Jabr CL, Dejani NN, Saraiva AC,
de Aquino SG, Medeiros AI and Junior CR: Inhibition of
5-lipoxygenase (5-Lo) attenuates inflammation and bone resorption
in lipopolysaccharide (Lps)-induced periodontal disease. J
Periodontol. 1–18. 2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rossaint J, Nadler JL, Ley K and Zarbock
A: Eliminating or blocking 12/15-lipoxygenase reduces neutrophil
recruitment in mouse models of acute lung injury. Crit Care.
16:R1662012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SJ, Seo KW and Kim CD: LPS increases
5-LO expression on monocytes via an activation of Akt-Sp1/NF-αB
pathways. Korean J Physiol Pharmacol. 19:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Avila H, Maya-Monteiro CM and Bozza PT:
Lipid bodies in innate immune response to bacterial and parasite
infections. Int Immunopharmacol. 8:1308–1315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bozza PT, Bakker-Abreu I, Navarro-Xavier
RA and Bandeira-Melo C: Lipid body function in eicosanoid
synthesis: An update. Prostaglandins Leukot Essent Fatty Acids.
85:205–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dichlberger A, Schlager S, Lappalainen J,
Käkelä R, Hattula K, Butcher SJ, Schneider WJ and Kovanen PT: Lipid
body formation during maturation of human mast cells. J Lipid Res.
52:2198–2208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura Y, Franchi L, Kambe N, Meng G,
Strober W and Núñez G: Critical role for mast cells in
interleukin-1β-driven skin inflammation associated with an
activating mutation in the nlrp3 protein. Immunity. 37:85–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shoji N, Yoshida A, Yu Z, Endo Y and
Sasano T: Lipopolysaccharide stimulates histamine-forming enzyme
(histidine decarboxylase) activity in murine dental pulp and
gingiva. Arch Oral Biol. 51:856–860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hershko AY and Rivera J: Mast cell and T
cell communication; amplification and control of adaptive immunity.
Immunol Lett. 128:98–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kambayashi T, Allenspach EJ, Chang JT, Zou
T, Shoag JE, Reiner SL, Caton AJ and Koretzky GA: Inducible MHC
class II expression by mast cells supports effector and regulatory
T cell activation. J Immunol. 182:4686–4695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Espinosa E and Valitutti S: New roles and
controls of mast cells. Curr Opin Immunol. 50:39–47. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamura Y and Kambe N: Linkage of
bacterial colonization of skin and the urticaria-like rash of
NLRP3-mediated autoinflammatory syndromes through mast cell-derived
TNF-α. J Dermatol Sci. 71:83–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho KA and Kang PB: PLIN2 inhibits
insulin-induced glucose uptake in myoblasts through the activation
of the NLRP3 inflammasome. Int J Mol Med. 36:839–844. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wisastra R and Dekker FJ: Inflammation,
cancer and oxidative lipoxygenase activity are intimately linked.
Cancers (Basel). 6:1500–1521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Werz O: Inhibition of 5-lipoxygenase
product synthesis by natural compounds of plant origin. Planta Med.
73:1331–1357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pettersen D, Davidsson Ö and Whatling C:
Recent advances for FLAP inhibitors. Bioorg Med Chem Lett.
25:2607–2612. 2015. View Article : Google Scholar : PubMed/NCBI
|