Introduction
Diabetic neuropathy (DN) is an important
microvascular complication of diabetes. The principal pathological
alterations in DN include hypertrophic mesangial cells (MCs), the
abnormal deposition of the extracellular matrix (ECM) and renal
interstitial fibrosis (1).
Fibronectin (Fn) is an important component of the ECM. Fn is
primarily synthesized in the early stages of DN and is associated
with the local inflammatory reaction of the kidney (2). Collagen IV (Col IV) is one of the
major components of the basement membrane. Increased synthesis
leads to glomerulosclerosis (3).
Additionally, chronic inflammation is associated with abnormal ECM
deposition. These alterations are involved in the occurrence and
development of DN (4).
Platelet-activating factor (PAF) has been reported to be a strong
inflammatory factor and may damage renal tissue in a variety of
ways (5). A high glucose (HG) and
lysophosphatidylcholine (LPC) environment increases the expression
of inflammatory cytokines, leading to the stimulation of MCs and
endothelial cells to continuously synthesize PAF, ECM and protein
kinase C-β1 (PKC-β1) (5). PKC is
aberrantly activated in the diabetic kidney, which causes an
increase in PKC-β1 activity and the deposition of ECM proteins,
including Fn and Col IV (6).
In addition to their lipid lowering effects, statins
may inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase
(7). Studies have reported that
atorvastatin may significantly reduce the plasma levels of
C-reactive, interleukin (IL)-1β, IL-6, tumor necrosis factor-α and
other inflammatory markers in patients with Alzheimer's disease
(8). Furthermore, statins inhibit
HG- and LPC-induced secretion of the ECM (9). These notable effects of statins may
be present due to their lipid-lowering and anti-inflammatory
actions, in addition to their pleiotropic effects. As a result,
statins have been used not only in cardiovascular disease, but for
other conditions, including DN (10). However, more evidence in properly
designed trials is required to determine whether the protective
effect of atorvastatin on the kidney is associated with PAF and
PKC-β1. In the present study, it was demonstrated that atorvastatin
may reduce the secretion of Fn and Col IV in human (H)MCs in a HG
and LPC environment, by reducing the increase in PAF secretion via
inhibition of PKC-transforming growth factor-β1 (TGF-β1)
signaling.
Materials and methods
Cell culture
Immortalized HMCs (donated by Professor Sun Zilin,
Department of Endocrinology, Zhongda Hospital affiliated with
Southeast University, Nangjing, China) (11), were obtained by double transfection
with T-SV40 and H-ras proto-oncogene. These cells retain the basic
morphology and biological characteristics of normal human
glomerular MCs (12). The cells
were maintained in a sterile incubator with 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific
Inc.) in a humidified atmosphere with 5% CO2 at 37°C.
When cells had reached 90% confluency, cells were seeded into
6-well plates (6×105 cells/well) and then incubated at
37°C and 5% CO2 for 12 h.
Cells were then divided into three groups: A,
control [5.5 mmol/l D-glucose (Enzo Life Sciences, Inc.,
Farmingdale, NY, USA)] (11); B,
HG+LPC group [30 mmol/l D-glucose+20 mg/l LPC (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany)]; C, atorvastatin [30 mmol/l D-glucose+20
mg/l LPC+10 µmol/l atorvastatin (Pfizer Inc., New York, NY, USA)].
Group C was pretreated with atorvastatin for 1 h prior to the
addition of 30 mmol/l D-glucose and 20 mg/l LPC. Following ≥24 h,
the supernatant of all three groups was collected and the
experiments were repeated three times.
ELISA analysis
The expression levels of Fn, Col IV and PAF in the
supernatant of each group was determined by ELISA. Human FN ELISA
kit (NeoScientific, Cambridge, MA, USA; cat. no. HF0011), Human Col
IV ELISA kit (NeoScientific; cat. no. HC0787), Human PAF ELISA kit
(NeoScientific; cat. no. HP0596). The method was performed
according to the manufacturer's protocol Each group was set up in
three wells in order to repeat the experiment three times.
Detection of PAF receptor (PAF-R), GADPH, PKC-β1 and
TGF-β1 expression in HMCs by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). Total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The RT reaction was
performed using the Revert Aid First Strand cDNA Synthesis reagent
(Fermentas; Thermo Fisher Scientific, Inc.) at 42°C for 15 min and
95°C for 3 min, followed by 4°C for 5 min; according to the
manufacturer's protocol. PAF-R primer sequences were as follows:
Forward, 5′-TGCCCCTGCTACAGGCACCA-3′ and reverse,
5′-TGCTGTAAACAATCGGGAAGAG-3′. GAPDH primers sequences were as
follows: Forward, 5′-ACACCCACTCCTCCACCTTT-3′ and reverse,
5′-TTACTCCTTGGAGGCCATGT-3.
PKC-β1 primer sequences were as follows: Forward,
5′-GGGGGCGACCTCATGTAT-3′ and reverse, 5′-GCAATTTCTGCAGCGTAAAA-3′.
TGF-β1 primer sequences were as follows: Forward,
5′-ACTACTACGCCAAGGAGGTCAC-3′ and reverse,
5′-TGCTTGAACTTGTCATAGATTTCG-3′. Primers were designed using Premier
Oligo 5 and Primer 6.22 software (Premier Biosoft International,
Palo Alto, CA, USA). qPCR assays were performed using a QuantStudio
5 Real-Time PCR System (Thermo Fisher Scientific, Inc.) in a 20 µl
PCR volume using iQ SyBr Green Supermix for 10 sec at 95°C,
followed by 40 cycles of 56°C for 20 sec and 72°C for 10 sec. The
quantification cycle (2−ΔΔCq) method was used to
calculate the relative changes in expression levels (13). Each sample was prepared in
triplicate, and the results are expressed as the mean of three
independent experiments.
Western blotting
The cells were lysed using a HMC lysis buffer
(Promega Corporation, Madison, WI, USA). Lysates were transferred
into an Eppendorf tube and centrifuged at 12,000 × g for 15 min at
4°C. The supernatant was collected, and the concentration of
protein was determined by ultraviolet spectroscopy (14). Protein (50 µg) were separated by 5%
SDS-PAGE (Bio-Rad Laboratories Inc., Hercules, CA, USA) and
transferred onto a nitrocellulose membrane (Bio-Rad Laboratories,
Inc.). The membrane was incubated in TBS with 0.1% Tween-20 (TBST)
and 5% fat-free milk for 1 h at 37°C. The proteins were detected
with the addition of primary antibodies which were left to incubate
overnight at 4°C (Sigma-Aldrich; Merck KGaA). The dilution ratios
were as follows: TGF-β1 (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; cat. no. SC-130348; 1:2,000); PKC-β1 (Santa Cruz
Biotechnology, Inc.; cat. no. SC-8393; 1:400) and GAPDH (Santa Cruz
Biotechnology, Inc.; cat. no. SC-32233; 1:3,000). Following washing
with TBST, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.; cat. no. SC-516245; 1:5,000) for 1 h at 4°C,
followed by additional washes with TBST. Protein bands were
visualized by enhanced chemiluminescence (Pierce; Thermo Fisher
Scientific, Inc.). The Scion Image 4.03 system (National Institutes
of Health, Bethesda, MD, USA) was used to quantify band intensity,
and results are expressed as the mean of three independent
experiments.
Antibodies, immunoprecipitation and
immunoblotting
Cells (2×104 cells/ml) were cultured on
coverslips in 24-well plates and serum-starved for 24 h. The cells
were subsequently fixed with 4% paraformaldehyde for 5 min at −20°C
and blocked for 30 min in 0.2% Triton X-100 in PBS. The cells were
incubated with aforementioned primary antibodies (1:50) overnight
at 4°C, followed by fluorescein isothiocyanate-conjugated secondary
anti-mouse IgG antibodies (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) for 1 h in the dark at room temperature. Following
three washes in PBS, coverslips were placed on slides and
visualized by confocal microscopy (magnification, ×400).
Fluorescence intensity was analyzed using Image J software (version
1.48; National Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard
deviation. Data was analyzed using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA) for Windows. Multigroup comparisons of the means
were performed using one-way analysis of variance test followed by
the Newman-Keuls post hoc test. Differences between two groups were
assessed by Student's t-test. α=0.05 and P<0.05 was considered
to indicate a statistically significant difference.
Results
Atorvastatin inhibits the expression
of Fn and Col IV in HMCs
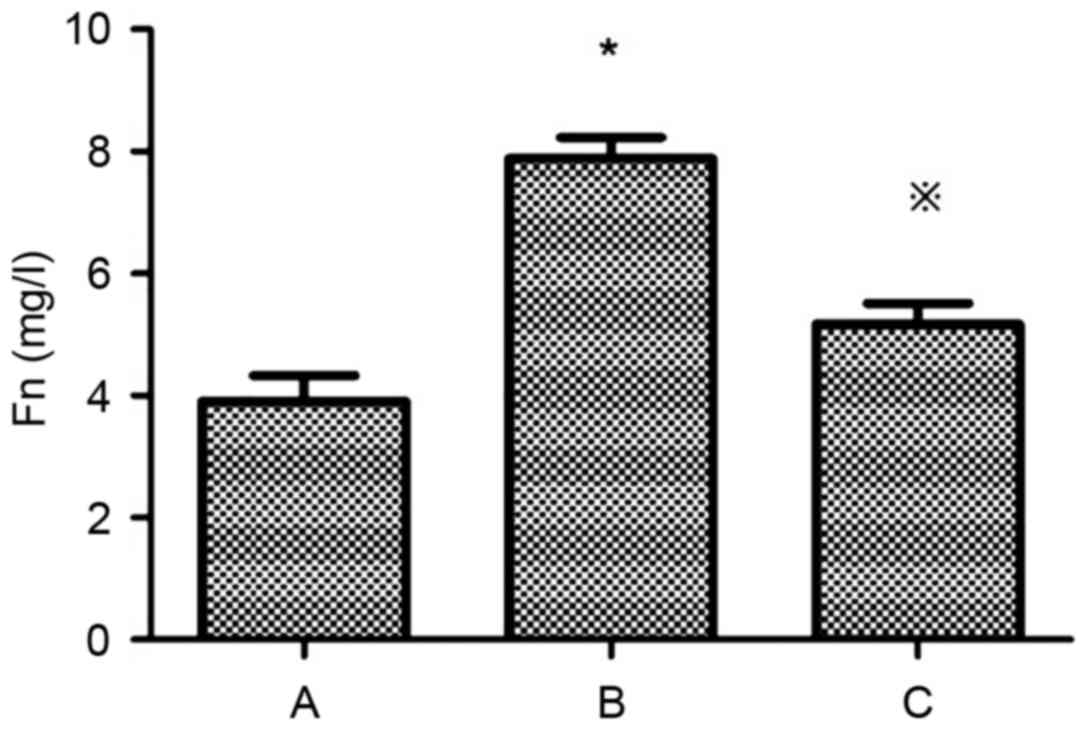
Levels of Fn and Col IV in the supernatant of the
HG+LPC group were significantly higher compared with the control
group (P<0.05). In the presence of atorvastatin their levels
were significantly decreased (Table
I; Figs. 1 and 2).
 | Table I.Effects of atorvastatin on Fn (mg/l)
and Col IV (µg/l) in human mesangial cells. |
Table I.
Effects of atorvastatin on Fn (mg/l)
and Col IV (µg/l) in human mesangial cells.
| Group | Fn (mg/l) | Col IV (µg/l) |
|---|
| Control |
3.90±0.43 |
4.54±0.74 |
| HG+LPC |
7.89±0.34a |
16.32±1.55a |
|
HG+LPC+atorvastatin |
5.17±0.34b |
10.80±1.70b |
Atorvastatin inhibits the expression
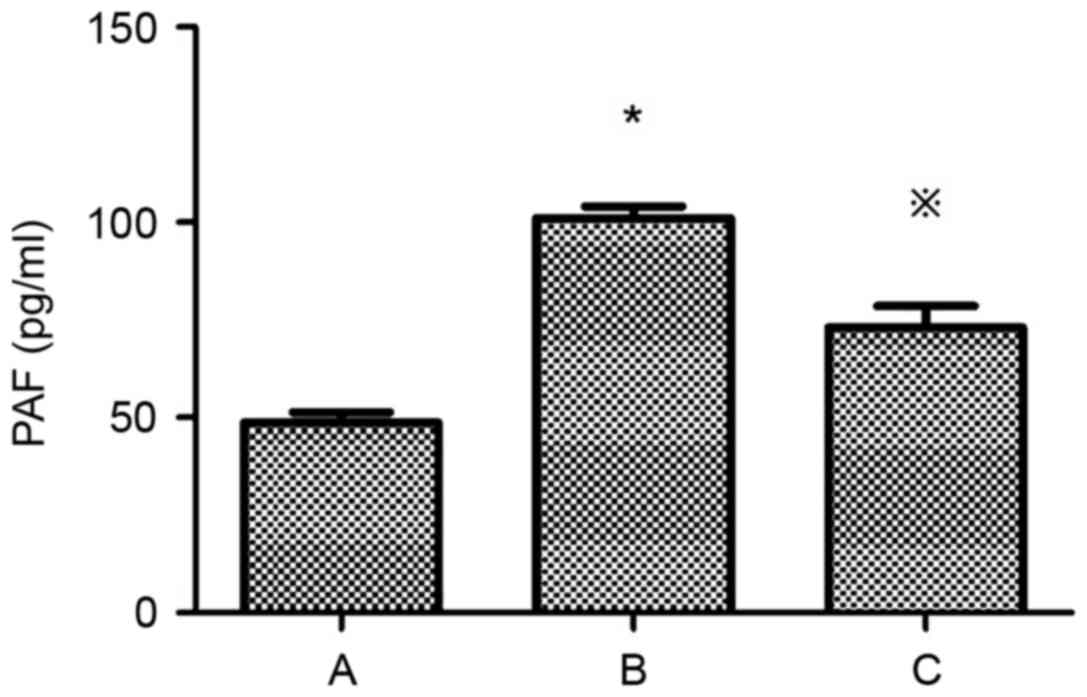
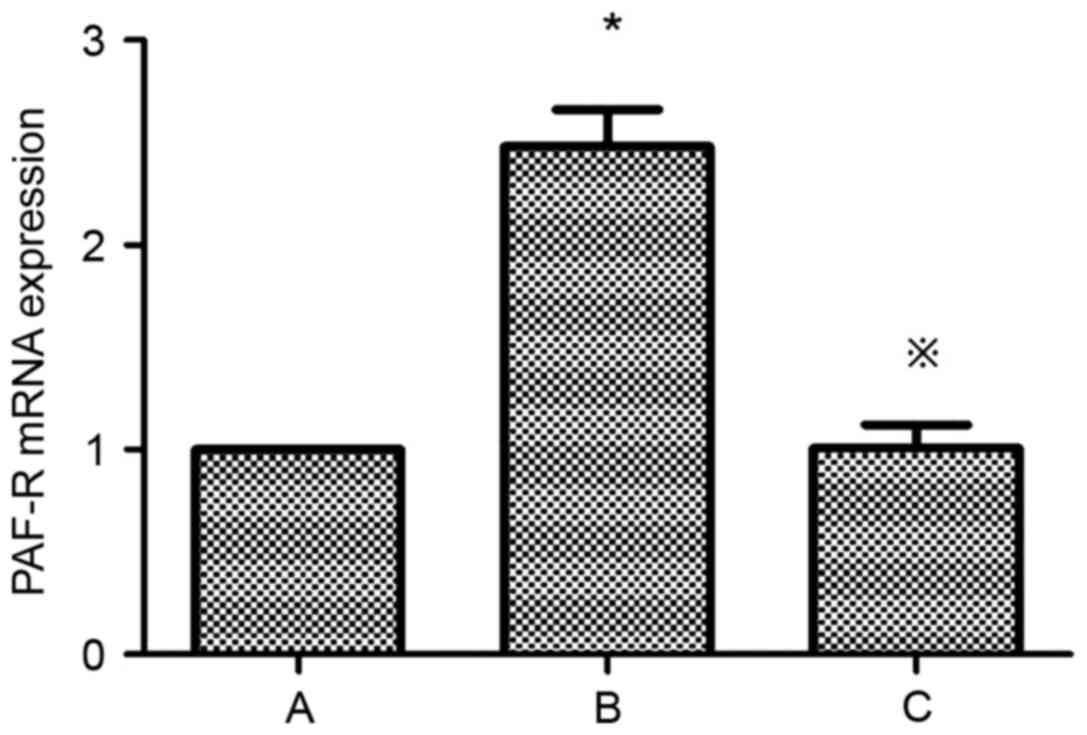
of PAF and PAF-R mRNA in HMCs
Compared with the control group, the expression of
PAF and PAF-R mRNA was significantly higher in the HG+LPC group
(P<0.05). Atorvastatin inhibited the increase in PAF content in
the supernatant of HMCs (P<0.05) (Table II; Fig. 3), in addition to the expression of
the PAF-R mRNA gene in the HG+LPC environment (P<0.05; Table II; Fig. 4).
 | Table II.Effects of atorvastatin on the
expression of PAF and PAF-R mRNA gene in human mesangial cells. |
Table II.
Effects of atorvastatin on the
expression of PAF and PAF-R mRNA gene in human mesangial cells.
| Group | PAF (pg/ml) | PAF-R mRNA
expression |
|---|
| Control |
48.72±2.55 |
1.0±0 |
| HG+LPC |
101.1±3.00a |
2.48±0.18a |
|
HG+LPC+atorvastatin |
73.03±5.55b |
1.01±0.11b |
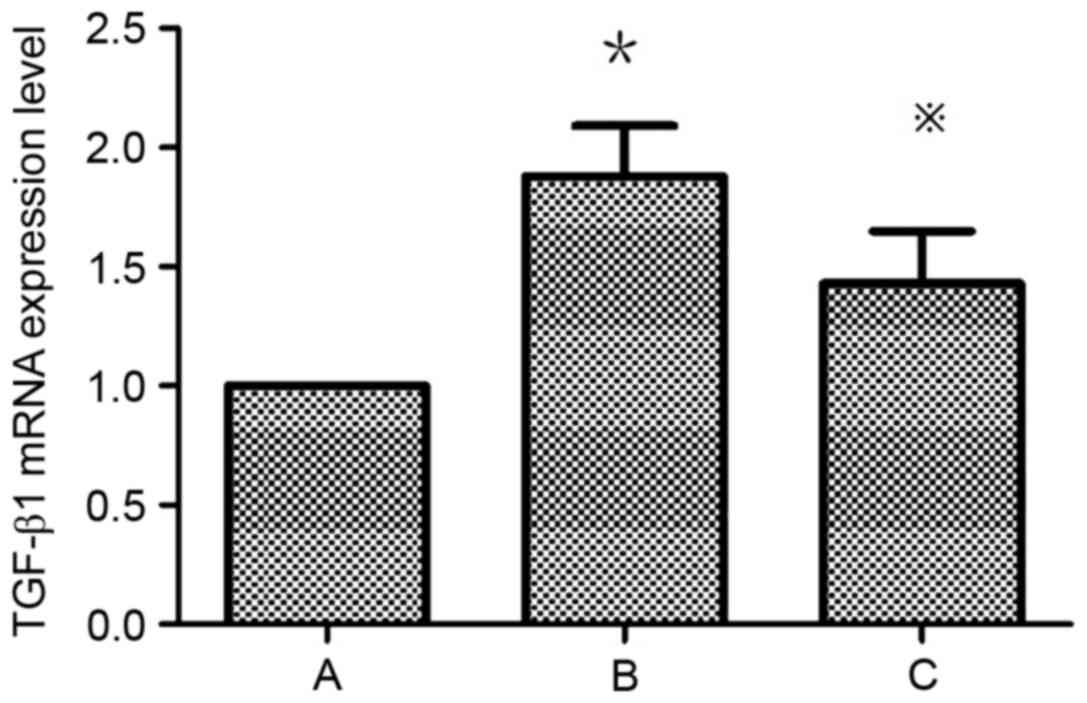
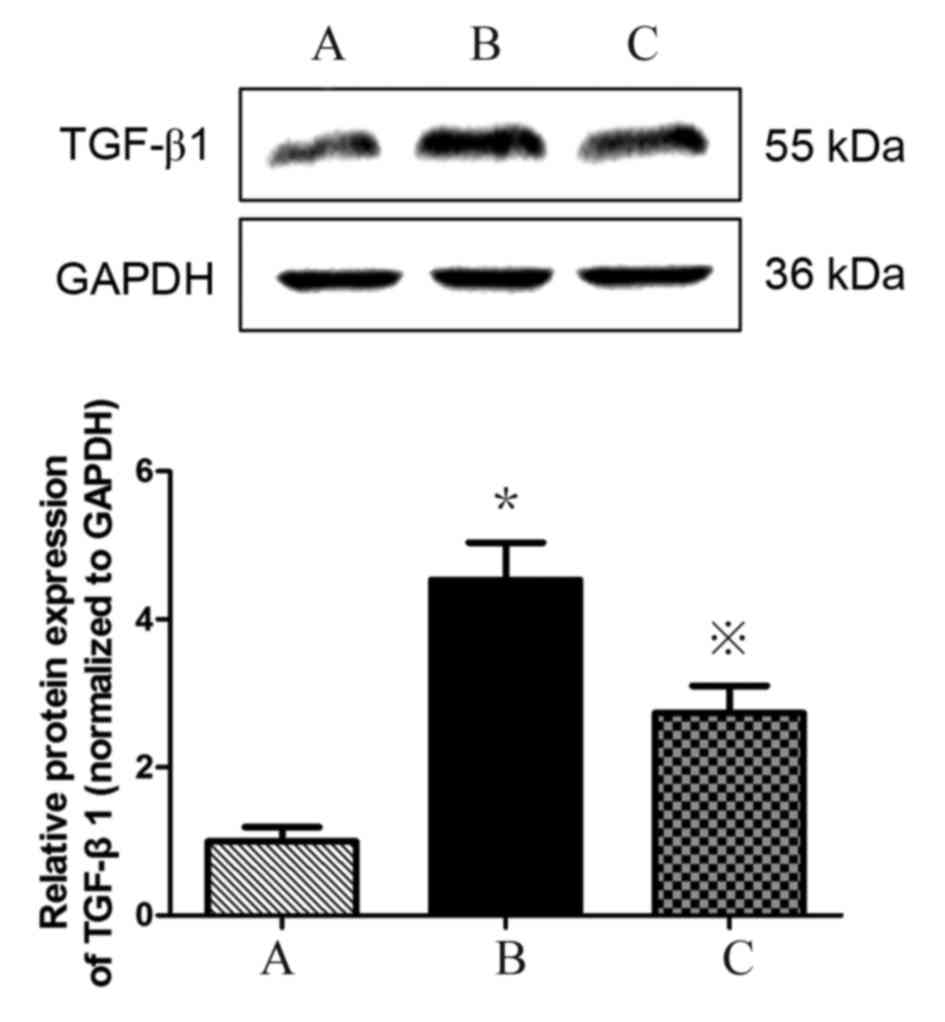
Atorvastatin inhibits the expression
of TGF-β1 in HMCs
TGF-β1 expression is upregulated in HMCs in the
presence of PAF, HG and LPC. TGF-β1 mRNA (Table III; Fig. 5) and protein (Fig. 6) expression levels were increased
in HMCs treated with HG+LPC relative to the control (P<0.05).
Expression was significantly lower in cells treated with
atorvastatin compared with those treated with HG+LPC only
(P<0.05).
 | Table III.Effects of atorvastatin on the
expression of TGF-β1 mRNA gene human mesangial cells. |
Table III.
Effects of atorvastatin on the
expression of TGF-β1 mRNA gene human mesangial cells.
| Group | TGF-β1 mRNA
expression level |
|---|
| Control |
1±0 |
| HG+LPC |
1.88±0.21a |
|
HG+LPC+atorvastatin |
1.43±0.22b |
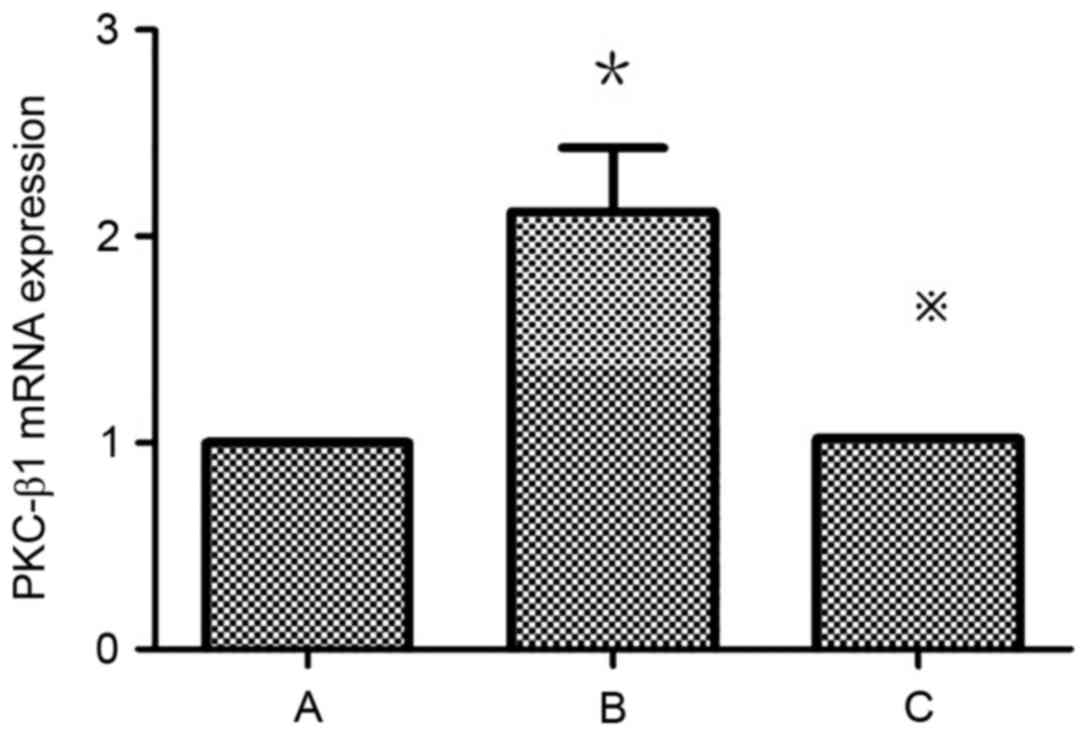
Atorvastatin inhibits the expression
of PKC-β1 in HMCs
Detection of PKC-β1 mRNA expression in HMCs in each
group by RT-qPCR revealed that mRNA levels were significantly
higher in the HG+LPC group compared with the control (P<0.05).
The expression was significantly higher in the HG+LPC group
compared with cells treated with HG+LPC+atorvastatin (P<0.05).
Thus, atorvastatin may inhibit the expression of PKC-β1 mRNA in
HMCs (Table IV; Fig. 7).
 | Table IV.PKC-β1 mRNA expression in each group
(2‒ΔΔCq). |
Table IV.
PKC-β1 mRNA expression in each group
(2‒ΔΔCq).
| Group | PKC-β1 mRNA
expression level |
|---|
| Control |
1±0 |
| HG+LPC |
2.12±0.31a |
|
HG+LPC+atorvastatin |
1.02±0.001b |
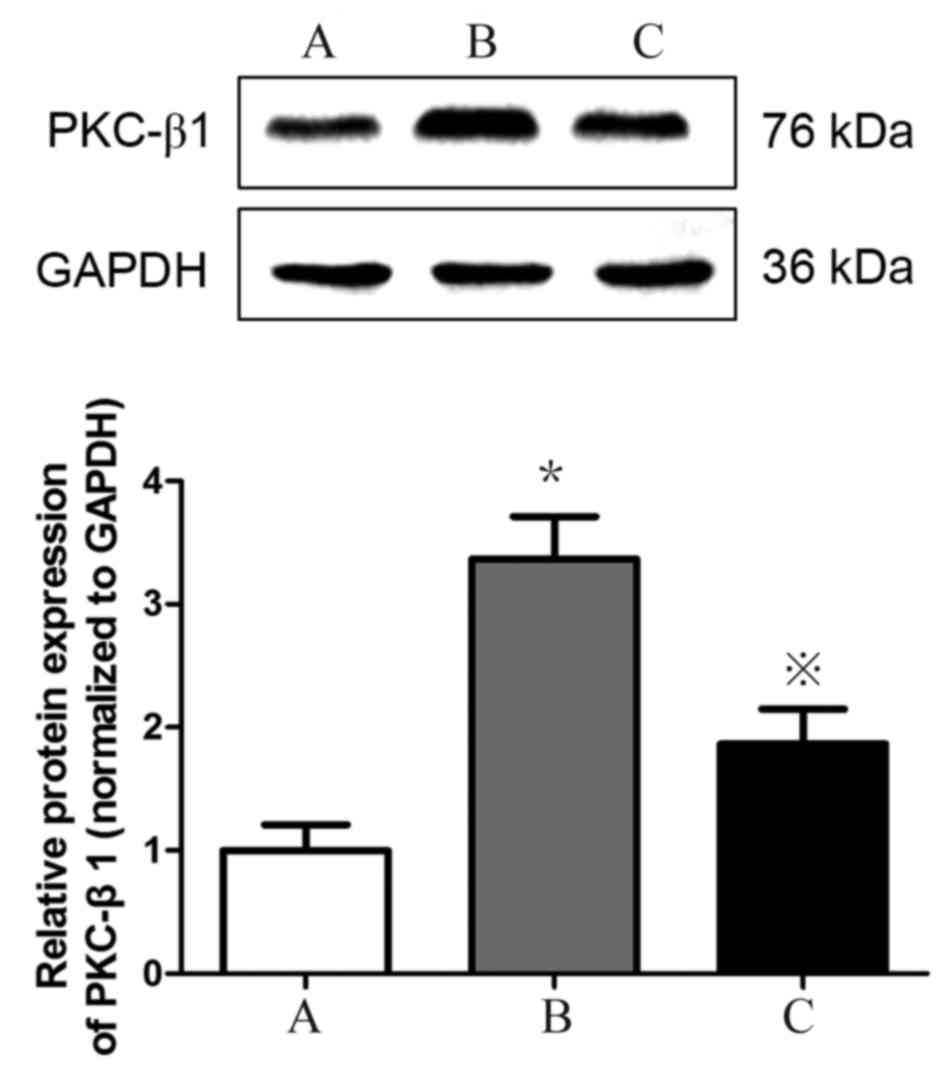
Detection of PKC-β1 protein expression
in HMCs in each group by western blotting
PKC-β1 protein (Fig.
8) expression levels were significantly increased in HMCs
treated with HG+LPC relative to the control (P<0.05). Expression
levels were significantly lower in cells treated with atorvastatin
compared with those treated with HG+LPC only (P<0.05).
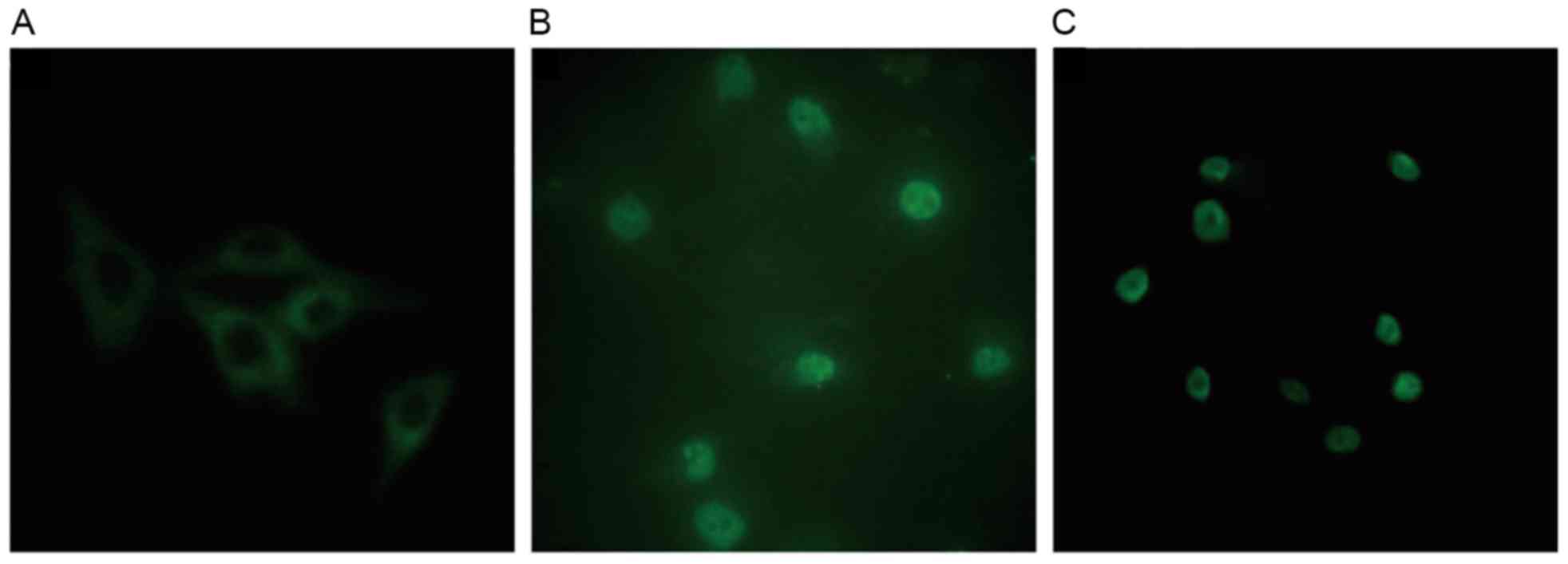
Effect of atorvastatin on the
expression and localization of PKC-β1 in HMCs
In control cells, PKC-β1 immunofluorescence was
diffusely distributed throughout the cytoplasm, with no membrane or
nuclear localization. Treatment with HG and LPC increased PKC-β1
protein expression levels, and induced the translocation of the
protein from the cytoplasm to the nucleus. These effects were
reduced in cells treated with atorvastatin (Fig. 9).
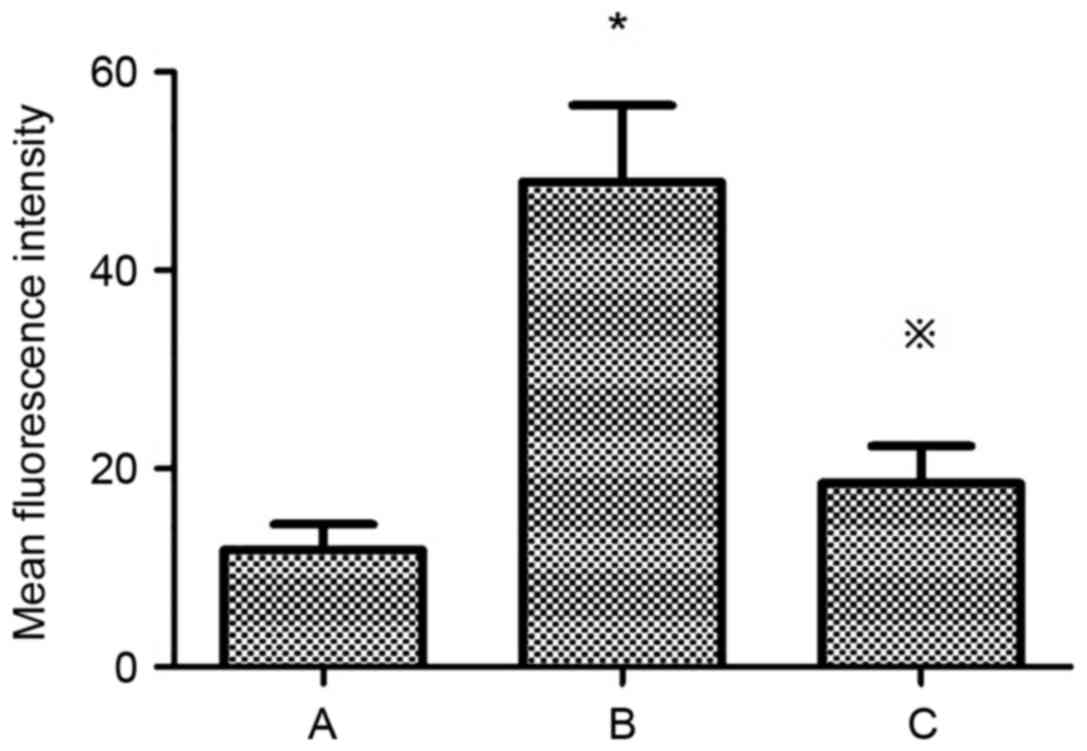
Mean fluorescence intensity of PKC-β1
in HMCs under various treatment conditions
The mean fluorescence intensity of PKC-β1 in HMCs
was significantly increased in the HG+LPC group compared with the
control (P<0.05). The addition of atorvastatin to the HG+LPC
environment significantly reduced the mean fluorescence intensity
(P<0.05; Table V; Fig. 10).
 | Table V.Mean fluorescence intensity of PKC-β1
in human mesangial cells under various treatment conditions. |
Table V.
Mean fluorescence intensity of PKC-β1
in human mesangial cells under various treatment conditions.
| Group | Mean fluorescence
intensity |
|---|
| Control |
11.80±2.57 |
| HG+LPC |
48.92±7.70a |
|
HG+LPC+atorvastatin |
18.53±3.74b |
Discussion
Statins have the potential to be used to treat DN.
This disease is thought to be triggered by abnormal ECM deposition
and a decrease in the glomerular filtration rate. Morphometric
studies on HMCs in a HG environment provide convincing evidence
that Col IV and Fn are increased, which partially explains the
occurrence of glomerular sclerosis and renal failure (15,16).
Genetic and environmental factors contribute to renal failure. For
example, patients with diabetes may have increased concentrations
of PAF, a potent pro-inflammatory factor implicated in the
pathogenesis of DN (17). Under
certain stimuli, various cell types may secrete PAF, including
platelets, endothelial cells and MCs (18). The primary source of PAF, however,
is the kidney, in which 20–25% of PAF is secreted by MCs (19). MCs are the principal component of
the glomerular filtration barrier and excessive expression of PAF
may cause damage to this interface. A previous study reported that
PAF is able to promote the secretion of the ECM (20). Excessive secretion of ECM
deposition in MCs results in the development of glomerular
sclerosis (20). The PAF-R is a G
protein coupled receptor that is expressed on MCs. The binding of
PAF triggers downstream signaling pathways resulting in the
production of effector molecules, including arachidonic acid,
prostacyclin and TGF-β1 which, in turn, regulate ECM secretion
(5,21). The expression of PAF-R mRNA has
been detected in all cells of the kidney, and its levels depend on
the amount of PAF present. In a model of unilateral ureteral
obstruction, PAF-R expression increased by 70-fold (22), revealing that an inflammatory
environment may stimulate the kidney to secrete PAF, and the
increase in PAF secretion, in turn, increases the expression of the
PAF-R. In a mixed culture model of rat and human MCs, it was
demonstrated that PAF was able to upregulate ECM secretion
(23); a corresponding increase in
Fn and Col IV was additionally observed in this model (24). To support this observation, it was
confirmed in the present study that pretreatment of the HMCs with
HG and LPC significantly increased Col IV and Fn via the induction
of PAF.
Inflammatory factors promote the expression of
TGF-β1 by activating the PKC-dependent pathway; this stimulates the
synthesis of Fn (16). In line
with this statement, a rise in PAF activity in the cells exposed to
HG+LPC was observed in the present study, which was concomitant
with an increase in Fn, Col IV and PKC-β1. PAF has detrimental
effects in MCs, and this may be through the activation of the
PKC-β1 signaling pathway, which leads to increased expression of
ECM proteins (16). It has been
previously reported that HG may activate the PKC-β1 pathway and
promote the secretion of TGF-β1 in MCs. TGF-β1 may further
stimulate the synthesis of collagen III and IV, Fn and other ECM
components (15). Consistent with
these findings, the present study demonstrated that an increase in
PAF expression in cells exposed to HG+LPC was associated with
increased levels of PKC-β1 and TGF-β1. This finding may indicate
that PAF induces a molecular cascade that activates PKC-β1, leading
to ECM accumulation and glomerular sclerosis (21).
Statins have been widely used as lipid-lowering
drugs to exert a protective effect on endotheliocytes. Statins
reduce cardiovascular morbidity and mortality in patients with
end-stage renal disease (25).
This effect of statins may be due to their lipid-lowering effects,
in addition to their pleiotropic effects, including the ability to
promote endothelial repair, and reduce inflammation and oxidative
stress (26). Atorvastatin
inhibits the HG-induced secretion of Col IV and Fn, mesangial
matrix deposition and mesangial cell proliferation (27,28).
Atorvastatin has been reported to have anti-fibrotic effects in
chronic kidney disease (9).
Essentially, atorvastatin has the ability to reduce glomerular
mesangial ECM deposition, and thus delay the progression of
glomerular sclerosis (29). A
previous study demonstrated that statins inhibit the production of
PAF, and this action may be through the inhibition of TGF-β1 and
reactive oxygen species as well as the expression of other
cytokines, in addition to mothers against decapentaplegic homolog
protein expression in glomerular MCs, thus inhibiting Fn and Col IV
secretion (9). A recent report
observed that statins may suppress PAF production (30), and they may therefore effectively
treat cardiovascular diseases, and conditions including diabetes
(31) and DN (32). The present study revealed that
atorvastatin reduced the PAF content in MCs in a HG and LPC
environment. This may be associated with the upregulation of
PAF-acetylhydrolase activity (33), leading to an increase in PAF
degradation and thus reducing the renal protective effect of ECM
secretion in the culture model. The present study additionally
demonstrated that the expression of the PAF-R gene in the HMCs in
the atorvastatin-pretreated group was significantly decreased
compared with the HG and LPC group. This suggested that
atorvastatin may inhibit the expression of the PAF-R gene in HMCs
induced by HG and LPC. The expression of the PAF-R gene in MCs is
associated with nuclear factor-κB (NF-κB) activity. HG and LPC may
activate the PKC-NF-κB pathway in MCs, which subsequently increases
the transcription of the PAF-R gene (34). However, the present study
identified that when the PAF-R gene was significantly increased,
PKC-β1 and TGF-β1 were increased. Therefore, it may be hypothesized
that atorvastatin may inhibit the expression of the PAF-R gene in
HG and LPC induced HMCs by inhibiting the activation of TGF-β1, and
inhibiting the PKC pathway. However, the specific mechanism remains
unclear and requires further investigation.
In the present study, the expression of PKC-β1 mRNA
and protein in HMCs pretreated with atorvastatin was significantly
lower compared with groups treated with HG and LPC alone.
Immunofluorescence indicated that the expression of PKC-β1 in the
cytoplasm and nucleus was additionally reduced, suggesting that
atorvastatin may inhibit the activation of the PKC pathway under
the conditions of HG and LPC. Simvastatin inhibits inflammatory and
oxidative stress by inhibiting the activation of the PKC pathway,
thus exerting a protective effect in patients with chronic kidney
disease (35). Atorvastatin may
inhibit the expression of TGF-β1, which may alleviate the local
inflammatory reaction of the kidney (36). In the present study, suppression of
PKC-β1 signaling by atorvastatin is partly responsible for the
reduced levels of PAF and ECM. These results all suggest that
atorvastatin may alter the activation of the PKC-β1/TGF-β1 pathway
to reduce ECM secretion in HMCs stimulated by HG and LPC, although
the specific mechanism remains unclear and further research is
required.
In conclusion, the results of the present study
suggested that atorvastatin may reduce the secretion of ECM
proteins (Fn and Col IV) by inhibiting the secretion of PAF, the
expression of PAF-R, and the activation of the PKC-β1/TGF-β1
signaling pathway in HMCs under HG and LPC conditions.
Additionally, these findings revealed that atorvastatin may delay
glomerular fibrosis by inhibiting the PKC-β1/TGF-β1 signaling
pathway in HMCs. This mechanism may contribute to the efficiency of
atorvastatin for the treatment of DN.
Acknowledgements
The authors gratefully acknowledge Professor Sun
Zilin from the Zhongda Hospital Affiliated with Southeast
University (Nangjing, China), for the donation of immortalized
human mesangial cells. The authors would also like to thank the
Science and Research Center, Guilin Medical University (Guilin,
China) for their technical support.
Funding
The present study was supported by the National
Natural Science Foundation of China. Grant: Research on the
relationship between insulin resistance of podocytes in diabetic
neuropathy (grant no. 81560148).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SXZ designed the study. YHX and XYH performed the
experiments, and YHX was the major contributor in the writing of
the manuscript. QH and FY analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shao Y, Lv C, Wu C, Zhou Y and Wang Q:
Mir-217 promotes inflammation and fibrosis in high glucose cultured
rat glomerular mesangial cells via Sirt1/HIF-1α signaling pathway.
Diabetes Metab Res Rev. 32:534–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang ZL, Beezhold DH, Personius CD and
Shen ZL: Fibronectin cell-binding domain triggered transmembrane
signal transduction in human monocytes. J Leukoc Biol. 53:79–85.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Wang Z, Yin W, Li Q, Cai M, Zhang
C, Xiao J, Hou H, Li H and Zu X: Severe insulin resistance and
moderate glomerulosclerosis in a minipig model induced by
high-fat/high-sucrose/high-cholesterol diet. Exp Anim. 56:11–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yiu WH, Lin M and Tang SC: Toll-like
receptor activation: From renal inflammation to fibrosis. Kidney
Int Suppl (2011). 4:20–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fragopoulou E, Iatrou C, Antonopoulou S,
Ruan XZ, Fernando RL, Powis SH, Moorhead JF and Varghese Z:
Platelet-activating factor (PAF) increase intracellular lipid
accumulation by increasing both LDL and scavenger receptors in
human mesangial cells. J Lab Clin Med. 147:281–289. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koya D, Jirousek MR, Lin YW, Ishii H,
Kuboki K and King GL: Characterization of protein kinase C beta
isoform activation on the gene expression of transforming growth
factor-beta, extracellular matrix components, and prostanoids in
the glomeruli of diabetic rats. J Clin Invest. 100:115–126. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banach M, Dinca M, Ursoniu S, Serban MC,
Howard G, Mikhailidis DP, Nicholls S, Lip GY, Glasser S, Martin SS,
et al: A PRISMA-compliant systematic review and meta-analysis of
randomized controlled trials investigating the effects of statin
therapy on plasma lipid concentrations in HIV-infected patients.
Pharmacol Res. 111:343–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Zhao Q, Zhou Y, Zhao Y and Wan Q:
Atorvastatin may correct dyslipidemia in adult patients at risk for
Alzheimer's disease through an anti-inflammatory pathway. CNS
Neurol Disord Drug Targets. 15:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song CY, Kim BC and Lee HS: Lovastatin
inhibits oxidized low-density lipoprotein-induced plasminogen
activator inhibitor and transforming growth factor-beta1 expression
via a decrease in Ras/extracellular signal-regulated kinase
activity in mesangial cells. Transl Res. 151:27–35. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kom GD, Schwedhelm E, Maas R, Schneider L,
Benndorf R and Böger RH: Impact of atorvastatin treatment on
platelet-activating factor acetylhydrolase and
15-F(2trans)-isoprostane in hypercholesterolaemic patients. Br J
Clin Pharmacol. 63:672–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou SX, Lei MX, Zhao JJ and Chen HL: The
study of the effects of platelet activating factor (PAF) on the
relation between the endothelial cell and mesangial cells exposed
to high glucose and high lysophosphatidylcholine. Chin J Diabetes.
18:591–593. 2010.(In Chinese).
|
|
12
|
Sraer JD, Delarue F, Hagege J, Feunteun J,
Pinet F, Nguyen G and Rondeau E: Stable cell lines of T-SV40
immortalized human glomerular mesangial cells. Kidney Int.
49:267–270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu D, Qian F, Wu Y, Jones DS, Rowe C,
Narum DL, Duffy P, Miller LH and Saul A: Determination of protein
concentration for protein-protein conjugates using ultraviolet
absorption. J Immunol Methods. 387:317–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meier M, Park JK, Overheu D, Kirsch T,
Lindschau C, Gueler F, Leitges M, Menne J and Haller H: Deletion of
protein kinase C-beta isoform in vivo reduces renal hypertrophy but
not albuminuria in the streptozotocin-induced diabetic mouse model.
Diabetes. 56:346–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tahara A, Tsukada J, Tomura Y, Yatsu T and
Shibasaki M: Vasopressin increases type IV collagen production
through the induction of transforming growth factor-beta secretion
in rat mesangial cells. Pharmacol Res. 57:142–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davignon J: Emphasis on pleiotropic
effects, a new paradigm shift? Coron Artery Dis. 15:223–225. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alfaro V: Role of histamine and
platelet-activating factor in allergic rhinitis. J Physiol Biochem.
60:101–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsoupras AB, Fragopoulou E, Nomikos T,
Iatrou C, Antonopoulou S and Demopoulos CA: Characterization of the
de novo biosynthetic enzyme of platelet activating factor,
DDT-insensitive cholinephosphotransferase, of human mesangial
cells. Mediators Inflamm. 2007:276832007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reznichenko A and Korstanje R: The role of
platelet-activating factor in mesangial pathophysiology. Am J
Pathol. 185:888–896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Honda ZI, Ishii S and Shimizu T:
Platelet-activating factor receptor. J Biochem. 131:773–779. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asano KK, Taniguchi SS, Nakao AA, Watanabe
TT and Kurokawa KK: Distribution of platelet activating factor
receptor mRNA along the rat nephron segments. Biochem Biophys Res
Commun. 225:352–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruiz-Ortega M, Bustos C, Plaza JJ and
Egido J: Overexpression of extracellular matrix proteins in renal
tubulointerstitial cells by platelet-activating-factor stimulation.
Nephrol Dial Transplant. 13:886–892. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruiz-Ortega M, Largo R, Bustos C,
Gómez-Garre D and Egido J: Platelet-activating factor stimulates
gene expression and synthesis of matrix proteins in cultured rat
and human mesangial cells: Role of TGF-beta. J Am Soc Nephrol.
8:1266–1275. 1997.PubMed/NCBI
|
|
25
|
Kopecky C, Genser B, Drechsler C, Krane V,
Kaltenecker CC, Hengstschläger M, März W, Wanner C, Säemann MD and
Weichhart T: Quantification of HDL proteins, cardiac events, and
mortality in patients with type 2 diabetes on hemodialysis. Clin J
Am Soc Nephrol. 10:224–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tremoulet AH: The role of statins in
inflammatory vasculitides. Autoimmunity. 48:177–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolavennu V, Zeng L, Peng H, Wang Y and
Danesh FR: Targeting of RhoA/ROCK signaling ameliorates progression
of diabetic nephropathy independent of glucose control. Diabetes.
57:714–723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mooradian AD and Haas M: Statins
ameliorate glomerular permeability changes in
streptozotocin-induced diabetic rats. Am J Ther. 14:41–45. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song Y, Li C and Cai L: Fluvastatin
prevents nephropathy likely through suppression of connective
tissue growth factor-mediated extracellular matrix accumulation.
Exp Mol Pathol. 76:66–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaikumkao K, Pongchaidecha A, Chattipakorn
N, Chatsudthipong V, Promsan S, Arjinajarn P and Lungkaphin A:
Atorvastatin improves renal organic anion transporter 3 and renal
function in gentamicin-induced nephrotoxicity in rats. Exp Physiol.
101:743–753. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pahan K: Lipid-lowering drugs. Cell Mol
Life Sci. 63:1165–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsoupras AB, Iatrou C, Frangia C and
Demopoulos CA: The implication of platelet activating factor in
cancer growth and metastasis: Potent beneficial role of
PAF-inhibitors and antioxidants. Infect Disord Drug Targets.
9:390–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noto H, Hara M, Karasawa K, Iso-O N, Satoh
H, Togo M, Hashimoto Y, Yamada Y, Kosaka T, Kawamura M, et al:
Human plasma platelet activating factor acetylhydrolase binds to
all them urine lipoproteins, conferring protection again
stoxidatives tress. Arterioscler Thromb Vasc Biol. 23:829–835.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park CW, Kim JH, Lee JH, Kim YS, Ahn HJ,
Shin YS, Kim SY, Choi EJ, Chang YS and Bang BK: High
glucose-induced intercellular adhesion molecule-1(ICAM-1)
expression through an osmotic effect in rat mesangial cells is
PKC-NF-kappa B-dependent. Diabetologia. 43:1544–1553. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang F, Sun D, Chen J, Guan N, Huo X and
Xi H: Simvastatin attenuates angiotensin II-induced inflammation
and oxidative stress in human mesangial cells. Mol Med Rep.
11:1246–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vincent JL, Spapen H, Bakker J, Webster NR
and Curtis L: Phase II multicenter clinical study of the
platelet-activating factor receptor antagonist BB-882 in the
treatment of sepsis. Crit Care Med. 28:638–642. 2000. View Article : Google Scholar : PubMed/NCBI
|