Introduction
Macrophages are important immune cells, which are
involved in the stimulation of the innate immune system, including
the release of inflammatory cytokines and inflammatory molecules,
including tumor necrosis-factor (TNF)-α, interleukin (IL)-1β, IL-6
and nitric oxide (NO) (1,2). These cytokines and inflammatory
molecules are often regulated by the mitogen-activated protein
kinase (MAPK) signaling pathway (3,4).
TNF-α-induced protein-8 like 2 (TNFAIP8L2, also
known as TIPE2), is a novel member of the TNFAIP8 (TIPE) family.
TIPE2 was originally identified as a negative regulator of immune
homeostasis, being expressed mainly in lymphoid tissues,
inflammatory tissues and immune cells, including macrophages
(5).
Astragalus, a well-known Chinese traditional
medicine and edible food, has multiple effects in pharmacological
and biological processes. One of its bioactive components is
Astragalus polysaccharides (APS), which can improve cellular
and humoral immunity, and regulate certain cytokines (6–8).
Although it is well known that APS is an immune regulator, few
studies have reported on the molecular mechanism underlying the
effect of APS on the immune system. MAPKs are a family of
serine/threonine protein kinases, which are responsible for the
majority of cellular responses to cytokines and are crucial for
regulating the production of inflammation mediators. A small number
of natural extracts have been shown to inhibit the expression of
inflammatory genes by regulating the phosphorylation of MAPK
pathways (3–4,9). The
present study aimed to determine what effect TIPE2 has on
activating macrophages induced by APS in vitro.
Materials and methods
Drugs
APS (cat. no. 151001B) for clinical application with
an endotoxin content of <0.1 Eu/mg was purchased from
Pharmagenesis, Inc. (Palo Alto, CA, USA); it is a polysaccharide
with a molecular weight of 20,000-60,000, and the polysaccharides
consist of α-1, 4 (1,6) glucan, arabinose-galactose
polysaccharides, hamnose-galacturonic acid polysaccharides and
arabinose-galactose protein polysaccharides.
Cell line and culture
Murine macrophage RAW264.7 cells (Institute of
Biochemistry and Cell Biology, Shanghai, China) were grown in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with penicillin 100 IU/ml, streptomycin 100 IU/ml
and 2 mM/l-glutamine supplemented with 10% bovine serum albumin
(PPA, Ferlach, Austria). The cells were maintained subconfluent at
37°C in humidified air containing 5% CO2. The RAW264.7
cells were harvested by gentle scraping, washed in PBS, resuspended
in culture medium and plated. Non-adherent cells were removed by
repeated washing following incubation at 37°C for 4 h. Each
experimental unit comprised 5×105/ml cells.
RNA interference
The TIPE2-specific small interfering (si)RNA-517
(GenePharma Co., Ltd., Shanghai, China) sequences were as follows:
Sense 5′-GCAUCAGGCACGUGUUUGATT-3′ and antisense
5′-UCAAACACGUGCCUGAUGCTT-3′. The TIPE2-specific siRNA-166
(GenePharma Co., Ltd.) sequences were as follows: Sense
5′-CCGUGGCGCAUCUCUCUUUAUTT-3′ and antisense
5′-AUAAAGAGAUGCGCCACGGTT-3′. The TIPE2-specific siRNA-351
(GenePharma Co., Ltd.) sequences were as follows: Sense
5′-GCUACACGAUUUCGUCAGATT-3′ and antisense
5′-UCUGACGAAAUCGUGUAGCTT-3′. The control siRNA sequences
(GenePharma Co., Ltd.) were as follows: Sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. For each well, 8.4 ng of siRNA
duplexes was diluted into 100 µl of medium and mixed. INTERFERin (4
µl) was added to the mixture, which was immediately homogenize by
vortexing for 10 sec. It was then incubated for 10 min at room
temperature to allow transfection complexes to form between siRNA
duplexes and INTERFERin. During complex formation, the growth
medium was removed and 0.5 ml of fresh pre-warmed complete medium
added per well. Then, 100 µl of transfection mix was added to the
cells and homogenized by gently swirling the plate. The final
volume was 600 µl, and the siRNA concentration 20 nM. The plate was
incubated at 37°C and gene silencing measured after 48 h.
NO assay
The quantity of stable NO generated by activated
macrophages was determined using Griess reagent (Promega Corp.,
Madison, WI, USA). The TIPE2 siRNA- or control siRNA-transfected
RAW264.7 cells (5×105/ml) were treated with different
concentrations of APS (10, 100, 200 and 400 µg) and LPS (10 µg/ml)
at room temperature for different durations (4, 8, 16, 24, 32, 40
and 48 h). Subsequently, 50 µl of the cell culture supernatant was
mixed with an equal volume of Griess reagent and incubated at room
temperature for 15 min. The absorbance was measured at 550 nm using
a microplate reader, and a standard curve was plotted using a
serial known concentration of NO.
Detection of TNF-α, IL-6 and
IL-1β
The TIPE2 siRNA- or control siRNA-transfected
RAW264.7 cells treated with APS (100 µg/ml) were cultured for 16 h.
The levels of TNF-α (cat. no. BMS607), IL-6 (cat. no. BMS603) and
IL-1β (cat. no. BMS6002) in the supernatant were measured using the
ELISA method (Bender Medsystems, Vienna, Austria) according to the
manufacturer's protocol.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from cells using TRIzol
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. Single-strand cDNA (100 ng) was generated from total RNA
using reverse transcriptase (Toyobo Co., Ltd., Osaka, Japan).
RT-qPCR analysis, using 5 µl SYBR-Green detection chemistry mix
(Roche Diagnostics, Basel, Switzerland), was performed on a 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). For mouse TIPE2, 1 µl qPCR primers were: Forward,
5′-TCTCAGAAACATCCAAGGCC-3′ and reverse,
5′-TTTGAGCTGAAGGACTCCATG-3′. For mouse β-actin, the qPCR primers
were: Forward, 5′-AGTGTGACGTTGACATCCGT-3′ and reverse,
5′-GCAGCTCAGTAACAGTCCGC-3′. PCR amplification program was performed
under the following two steps. Stage 1: 50°C for 2 min, 95°C for 10
min (1 cycle). Stage 2: 95°C for 15 sec, 60°C for 35 sec (39
cycles). Relative expression fold change was calculated by the
2∆∆Cq method, and β-actin was used as the endogenous
reference gene to normalize the expression level of target
gene.
Western blot analysis
The cells were washed twice with cold PBS and lysed
with cell lysis buffer (Cell Signaling Technology, Inc., Boston,
MA, USA) supplemented with protease inhibitor mixture (Beyotime
Institute of Biotechnology, Shanghai, China). The protein
concentrations of the cell lysis extracts were measured using a BCA
assay (Pierce; Thermo Fisher Scientific, Inc.) and equalized with
the extraction reagent. Protein extracts (30 µg) were loaded and
subjected to separation on an 10% SDS-PAGE gel, following which
they were transferred onto a PVDF membrane. The membrane was
blocked with 5% skim milk in TBST for 1 h at 25°C and then
incubated with rabbit anti murine ERK (cat. no. 9102), JNK (cat.
no. 9252), P38 (cat. no. 8690), phosphorylated ERK (cat. no. 9101),
phosphorylated JNK (cat. no. 9251), and phosphorylated P38 antibody
(cat. no. 9215; Cell Signaling Technology, Inc.) for 1 h at 25°C.
Following washing three times in TBST for 10 min each time, the
membrane was incubated with HRP conjugated goat anti rabbit-IgG
(cat. no. 18-8816-33; Rockland, Gilbertsville, Philadelphia, PA,
USA) for 1 h and the antibody-specific protein was visualized by
using an ECL kit (Biological Industries, Kibbutz Beit Haemek,
Israel) in the enhanced chemiluminescence detection system as
previously described (10). The
intensities of the protein bands were analyzed by Gel-Pro Analyzer
software. All of the above antibodies were diluted to 1:1,000.
Statistical analysis
The results are expressed as the mean ± standard
deviation of the indicated number of experiments. The statistical
significance of difference was estimated using a t-test for
unpaired observations. All statistical analysis was performed using
SPSS software version 15.0 (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
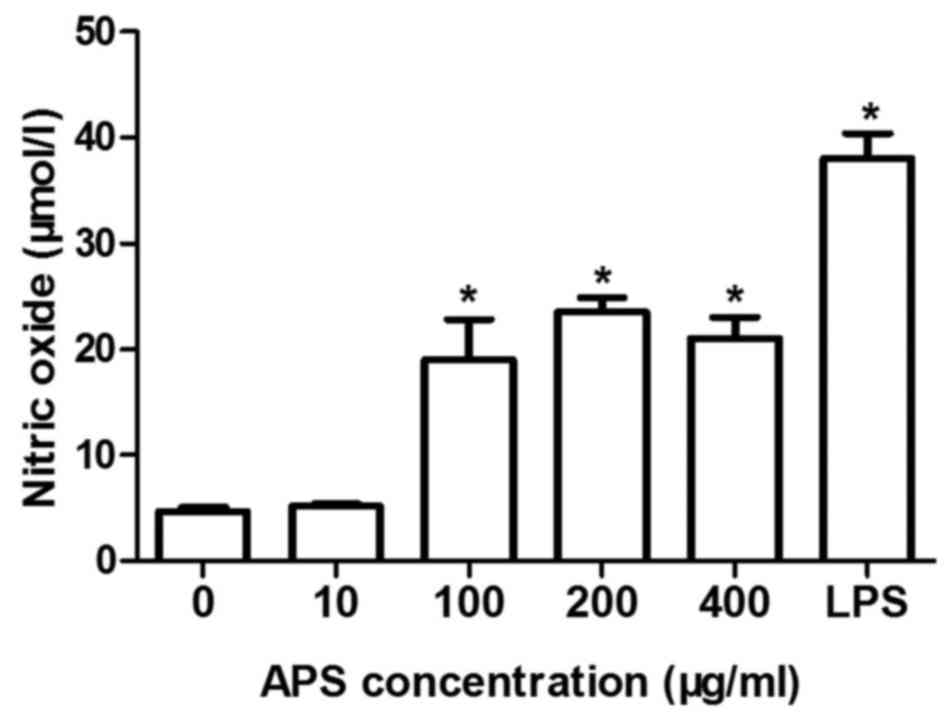
APS induces the production of NO in a
dose-dependent manner
The production of NO in the RAW264.7 cells treated
with different concentrations of APS (10, 100, 200 and 400 µg/ml),
LPS (10 µg/ml) or negative control for 16 h was detected. As shown
in Fig. 1, the production of NO
(18.9±1.5 µmol/l; P<0.01) was induced significantly 16 h
following treatment with APS (100 µg/ml). When treated with 200
µg/ml of APS, the production of NO in the RAW264.7 cells reached a
peak and then began to weaken.
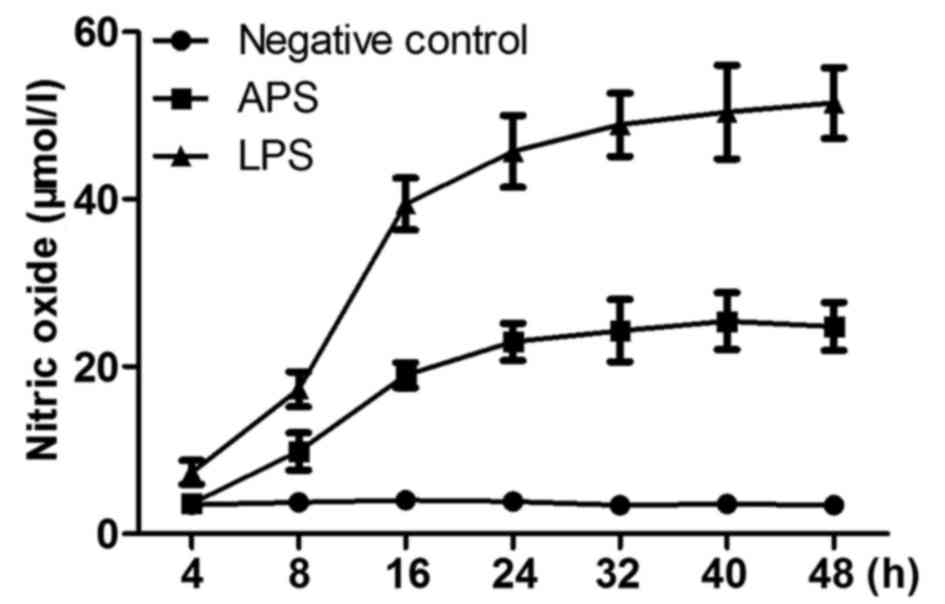
APS induces the production of NO in
time-dependent manner
The production of NO in RAW264.7 cells treated with
APS (100 µg/ml), LPS (10 µg/ml) or negative control for different
durations are shown in Fig. 2. It
was found that the production of NO was significantly induced
(9.87±2.23 µmol/l; P<0.01) 8 h following treatment with APS.
After 16 h, the rate of NO production slowed down (18.9±1.5 µmol/l;
P<0.01). In the negative control group, only minimal NO was
released and there were no significant difference at any time
point.
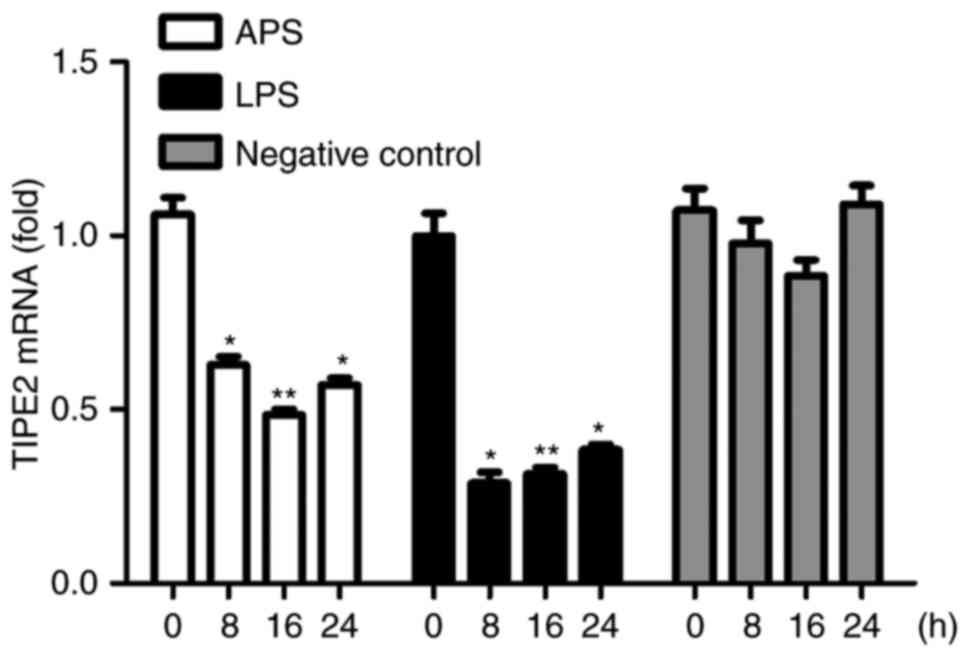
Downregulated expression of TIPE2 is
induced by APS in vitro
The expression of TIPE2 in RAW264.7 cells treated
with APS (100 µg/ml), LPS (10 µg/ml) or negative control for
different durations were examined using RT-qPCR analysis, as shown
in Fig. 3. It was found that the
expression of TIPE2 was significantly reduced following treatment
of the cells with APS and LPS. The downregulation of TIPE2 upon APS
stimulation suggested that TIPE2 may function as a regulator of the
APS-induced immune response in macrophages.
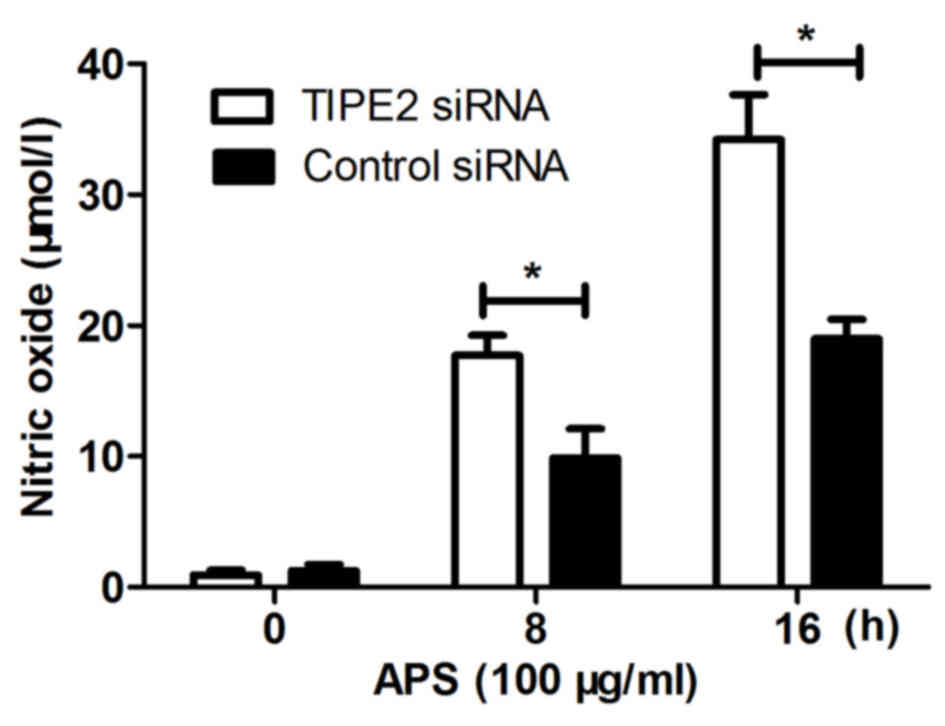
Effect of TIPE2 siRNA on the
production of NO induced by APS
In the present study, RAW264.7 cells were
transfected with TIPE2 siRNA or control siRNA and, 48 h later, the
cells were stimulated with APS (100 µg/ml). The supernatants were
collected at different time points to detect the production of NO.
As shown in Fig. 4, the production
of NO was significantly increased in the TIPE2 siRNA group,
compared with that in the control siRNA group at the different time
points. These results showed that TIPE2 negatively regulated the
APS-induced production of NO in macrophages.
Effect of TIPE2 siRNA on the
production of inflammatory cytokines induced by APS
The RAW264.7 cells were transfected with TIPE2 siRNA
or control siRNA and, 48 h later, the cells were stimulated with
APS (100 µg/ml). The supernatants were collected at different time
points to detect the production of inflammatory cytokines,
including TNF-α, IL-6 and IL-1β. It was found that the expression
of inflammatory cytokines TNF-α (Fig.
5A), IL-6 (Fig. 5B) and IL-1β
(Fig. 5C) were upregulated in the
TIPE2-silenced RAW264.7 cells infected with APS at different time
points. Taken together, these results demonstrated that TIPE2
negatively regulated the APS-induced production of inflammatory
cytokines in macrophages.
 | Figure 5.Silencing of TIPE2 promotes the
production of inflammatory cytokines in RAW264.7 cells following
stimulation with APS for indicated durations. RAW264.7 cells were
transfected with control siRNA or TIPE2 siRNA, as indicated, at a
final concentration of 20 nM. After 48 h, the cells were stimulated
with or without APS (100 µg/ml) for the indicated durations. Levels
of (A) TNF-α, (B) IL-6 and (C) IL-1β were measured using ELISA
following stimulation. Data are presented as the mean ± standard
deviation of three independent experiments. *P<0.05. TIPE2,
tumor necrosis factor-α-induced protein 8-like-2; APS,
Astragalus polysaccharides; siRNA, small interfering RNA;
TNF-α, tumor necrosis factor-α, IL, interleukin. |
Effect of TIPE2 on the MAPK signaling
pathway in RAW264.7 cells treated with APS
To further examine the possible mechanism of TIPE2
on the regulation of inflammatory cytokine expression induced by
APS, western blot analysis was performed to determine the effect of
TIPE2 on the MAPK signaling pathway. As shown in Fig. 6, the results revealed found that
the phosphorylation of P38, c-Jun N-terminal kinase (JNK) and
extracellular signal-regulated kinase (ERK) were significantly
activated following APS challenge following interference of
endogenous TIPE2 in macrophages. Therefore, it was hypothesized
that TIPE2 may inhibit the secretion of inflammatory cytokines when
stimulated with APS by inhibiting the activation of the MAKP
signaling pathway.
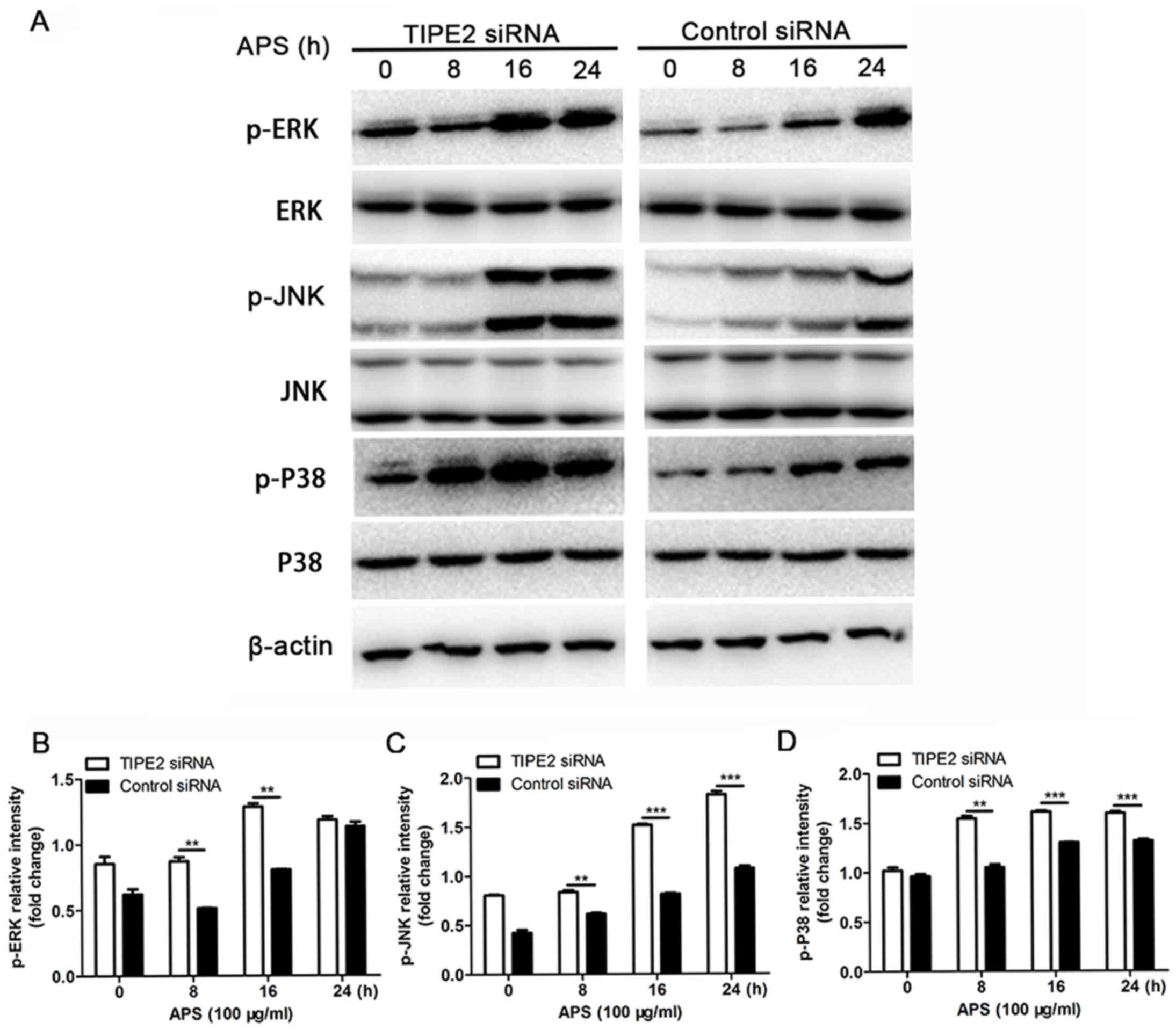 | Figure 6.(A) Effect of silenced TIPE2 on
APS-induced signaling pathway activation in RAW264.7 cells.
RAW264.7 cells (1×106) were transfected with control
siRNA or TIPE2 siRNA, as indicated, at a final concentration of 20
nM. After 48 h, the cells were stimulated with APS (100 µg/ml) for
different durations, as indicated. Cell lysates were analyzed using
western blot analysis for phosphorylated mitogen-activated protein
kinase (ERK, JNK and P38). β-actin was used as the loading control.
Data shown are from one representative experiment of three. Fold
change in the expression of (B) p-ERK, (C) p-JNK and (D) p-P38.
Data are presented as the mean ± standard deviation of three
independent experiments. **P<0.05 and ***P<0.01. TIPE2, tumor
necrosis factor-α-induced protein 8-like-2; APS, Astragalus
polysaccharides; siRNA, small interfering RNA; p-, phosphorylated;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase. |
Discussion
Several preliminary studies have shown that
polysaccharides isolated from Astragalus possess a wide
range of biological functions (11–13).
In the present study, APS with a molecular weight of 20,000-60,000
was selected due to its specificity in binding to macrophages.
Macrophages are important in immune defense, immune stability and
immune surveillance. Activated macrophages can identify and kill
pathogenic microorganisms, and clean up apoptotic cells and mutant
cells, and can also initiate acquired immunity by their efficient
antigen-presenting ability to activate T cells and B cells. NO is
considered to be a key molecule in regulating the immune response
(14), which is associated with
the cytolytic function of macrophages against a variety of tumor
cells, and the increase of NO synthesis can interfere with tumor
cell growth (15). One of the most
prominent characteristics of TNF-α is its ability to induce the
apoptosis of tumor-associated endothelial cells, resulting in the
necrosis of tumor cells (16).
IL-6 and IL-1β can be secreted by macrophages, which act as
inflammatory and anti-inflammatory cyokines (17). The data obtained in the present
study showed that the basal levels of NO, TNF-α, IL-6 and IL-1β
were relatively low in resting macrophages; however, APS induced
the activation of macrophages, resulting in significant increases
in the production of IL-1β, TNF-α, IL-6 and NO, which may be
beneficial for resisting pathogen invasion. Of note, the present
study also found that the production of NO induced by APS occurred
in a dose-dependent manner and a time-dependent manner. These
results suggested the possibility that APS may be administrated to
the human body to activate macrophages for immunological
activities.
TIPE2, a novel protein of the TNFAIP8 family, has
attracted increasing attention (18). TIPE2 was initially identified as an
essential protein, which is important in maintaining immune
homeostasis by negatively regulating innate and adaptive immunity
(5,19–23).
TIPE2-knockout and knockdown macrophages are hypersensitive to
toll-like receptor stimulation, and TIPE2 can inhibit the secretion
of inflammatory cytokine IL-6 in mouse macrophages (11). In the present study, the expression
of TIPE2 was markedly reduced in RAW264.7 cells. TIPE2 siRNA was
used to inhibit the endogenous expression of TIPE2 in RAW264.7
cells, following which they were stimulated with APS. It was found
that TIPE2 negatively regulated the APS-induced production of
inflammatory cytokines (IL-1β, TNF-α and IL-6) and NO in
macrophages. However, the molecular mechanisms, particularly the
signaling pathway of TIPE2 involved in macrophage activation by
APS, remain to be fully elucidated. It has been reported that the
expression of TIPE2 is closely associated with MAPK signal
transduction (24,25). Therefore, the present study focused
on the effect of TIPE2 on the MAPK signaling pathway activated by
APS. It was shown that stimulation of the RAW264.7 cells with APS
resulted in activation of the MAPK signaling pathway, and TIPE2
inhibited the MAPK signaling pathway in the RAW264.7 cells
activated by APS. These results suggested that TIPE2 negatively
regulated the production of inflammatory cytokines and NO, which
were induced by APS, in macrophages via inhibiting the MAPK
signaling pathway.
There were severe limitations in the present study.
First, the experiments did not examine the secretion of various
inflammatory factors following the activation of macrophages by APS
under the overexpression of TIPE2. Second, TIPE2-deficient mice
were not included in the present study, leading to the lack of
relevant investigations in primary peritoneal macrophages. The
investigation of these factors is likely to provide more direct
evidence for the role of TIPE2. Finally, further investigations are
required to determine which molecules were directly bound with
TIPE2.
APS has been widely used clinically as an injection
in patients in China to improve immune functions. The present study
provided a clearer understanding of the possible mechanism
involved, compared with previous reports. According to the results
of the present study, APS was effective in inducing the activation
of macrophages and TIPE2 was identified as a novel molecule with a
negative effect on the APS-induced immune response via the MAPK
signaling pathway. These findings provide insights into the novel
function of TIPE2 in APS-induced immunity and its associated
clinical significance.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by grants
from the Zhejiang Medicine and Health Research Fund (grant no.
2013KYB014) and the National Natural Science Foundation (grant no.
81401301). The funders had no role in study design, data collection
and analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ designed the study and interpreted experiment
data. YZ was responsible for cell culture and RNA interference. FF
was responsible for the NO assay. GW was responsible for detection
of TNF-α and IL-1β. ZX was responsible for western blot analysis.
HZ was responsible for preparation of the study.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APS
|
Astragalus polysaccharides
|
|
TIPE2
|
tumor necrosis factor-α-induced
protein 8-like-2
|
|
NO
|
nitric oxide
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Ma H, Liu G, Ding W, Wu Y, Cai L and Zhao
Y: Diabetes-induced alteration of F4/80+ macrophages: A study in
mice with streptozotocin-induced diabetes for a long term. J Mol
Med (Berl). 86:391–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marcato LG, Ferlini AP, Bonfim RC,
Ramos-Jorge ML, Ropert C, Afonso LF, Vieira LQ and Sobrinho AP: The
role of Toll-like receptors 2 and 4 on reactive oxygen species and
nitric oxide production by macrophage cells stimulated with root
canal pathogens. Oral Microbiol Immunol. 23:353–359. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie C, Kang J, Ferguson ME, Nagarajan S,
Badger TM and Wu X: Blueberries reduce pro-inflammatory cytokine
TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB
activation and the MAPK pathway. Mol Nutr Food Res. 55:1587–1591.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu X, Ci X, He J, Wei M, Yang X, Cao Q,
Li H, Guan S, Deng Y, Pang D and Deng X: A novel anti-inflammatory
role for ginkgolide B in asthma via inhibition of the ERK/MAPK
signaling pathway. Molecules. 16:7634–7648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei W, Xiao HT, Bao WR, Ma DL, Leung CH,
Han XQ, Ko CH, Lau CB, Wong CK, Fung KP, et al: TLR-4 may mediate
signaling pathways of Astragalus polysaccharide RAP induced
cytokine expression of RAW264.7 cells. J Ethnopharmacol.
179:243–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang WJ and Frei B: Astragaloside IV
inhibits NF-κB activation and inflammatory gene expression in
LPS-treated mice. Mediators Inflamm. 2015:2743142015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denzler KL, Waters R, Jacobs BL, Rochon Y
and Langland JO: Regulation of inflammatory gene expression in
PBMCs by immunostimulatory botanicals. PLoS One. 5:e125612010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH
and Kang JJ: Shikonin derivatives inhibited LPS-induced NOS in RAW
264.7 cells via downregulation of MAPK/NF-kappaB signaling. J
Ethnopharmacol. 120:264–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meyer KA, Neeley CK, Baker NA, Washabaugh
AR, Flesher CG, Nelson BS, Frankel TL, Lumeng CN, Lyssiotis CA,
Wynn ML, et al: Adipocytes promote pancreatic cancer cell
proliferation via glutamine transfer. Biochem Biophys Rep.
7:144–149. 2016.PubMed/NCBI
|
|
11
|
Kallon S, Li X, Ji J, Chen C, Xi Q, Chang
S, Xue C, Ma J, Xie Q and Zhang Y: Astragalus polysaccharide
enhances immunity and inhibits H9N2 avian influenza virus in vitro
and in vivo. J Anim Sci Biotechnol. 4:222013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Zhong Y, Li H, Zhang N, Ma W, Cheng
G, Liu F, Liu F and Xu J: Enhancement of Astragalus
polysaccharide on the immune responses in pigs inoculated with
foot-and-mouth disease virus vaccine. Int J Biol Macromol.
49:362–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdullahi AY, Kallon S, Yu X, Zhang Y and
Li G: Vaccination with astragalus and ginseng
polysaccharides improves immune response of chickens against H5N1
avian influenza virus. Biomed Res Int. 2016:15102642016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiao L, Li X, Li T, Jiang P and Zhang L,
Wu M and Zhang L: Characterization and anti-tumor activity of
alkali-extracted polysaccharide from Enteromorpha
intestinalis. Int Immunopharmacol. 9:324–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palmer RM, Ashton DS and Moncada S:
Vascular endothelial cells synthesize nitric oxide from L-arginine.
Nature. 333:664–666. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lejeune FJ, Liénard D, Matter M and Rüegg
C: Efficiency of recombinant human TNF in human cancer therapy.
Cancer Immun. 6:62006.PubMed/NCBI
|
|
17
|
Park JY, Chung TW, Jeong YJ, Kwak CH, Ha
SH, Kwon KM, Abekura F, Cho SH, Lee YC, Ha KT, et al: Ascofuranone
inhibits lipopolysaccharide-induced inflammatory response via
NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7
macrophages. PLoS One. 12:e01713222017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan YC, Wang N, Sun YY, Xiao XY and Wang
K: TIPE2 mRNA level in PBMCs serves as a novel biomarker for
predicting short-term mortality of acute-on-chronic hepatitis B
liver failure: A prospective single-center study. Medicine
(Baltimore). 94:e16382015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, Liang X, et al: Roles of TIPE2 in hepatitis B
virus induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong L, Liu K, Zhang YZ, Jin M, Wu BR,
Wang WZ, Li W, Nan YM and Chen YH: Downregulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
chronic hepatitis C. Hepatol Int. 7:844–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Song L, Fan Y, Li X, Li Y, Chen J,
Zhu F, Guo C, Shi Y and Zhang L: Down-regulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
systemic lupus erythematosus. Clin Immunol. 133:422–427. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lou Y, Sun H, Morrissey S, Porturas T, Liu
S, Hua X and Chen YH: Critical roles of TIPE2 protein in murine
experimental colitis. J Immunol. 193:1064–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Wei X, Liu L, Liu S, Wang Z,
Zhang B, Fan B, Yang F, Huang S, Jiang F, et al: TIPE2, a novel
regulator of immunity, protects against experimental stroke. J Biol
Chem. 287:32546–32555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu MW, Liu R, Wu HY, Zhang W, Xia J, Dong
MN, Yu W, Wang Q, Xie FM, Wang R, et al: Protective effect of
Xuebijing injection on D-galactosamine- and
lipopolysaccharide-induced acute liver injury in rats through the
regulation of p38 MAPK, MMP-9 and HO-1 expression by
increasingTIPE2 expression. Int J Mol Med. 38:1419–1432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Zhu T, Liu W, Qu X, Chen Y, Ren
P, Wang Z, Wei X, Zhang Y and Yi F: TIPE2 acts as a negative
regulator linking NOD2 and inflammatory responses in myocardial
ischemia/reperfusion injury. J Mol Med (Berl). 93:1033–1043. 2015.
View Article : Google Scholar : PubMed/NCBI
|