Introduction
Diabetes is a chronic disease that is common
worldwide. According to its pathogenesis, diabetes can be broadly
divided into type 1 and type 2 diabetes (1). Type 1 diabetes is caused by the
destruction of β-cells, resulting in an inefficient level of
insulin secretion, while type 2 diabetes is due to insulin
resistance (2). There are a number
of potential causes of pancreatic β-cell damage, including hypoxia,
oxidative stress, glycosylation end-products and autoimmune
reactions (3–6). However, the detailed mechanism
underlying pancreatic β-cell injury in diabetes remains unclear.
Therefore, the present study aimed to investigate a novel drug
which may protect pancreatic β-cells against metabolic stress.
Decreasing pancreatic β-cell damage is an essential part of
treating type 1 diabetes.
Puerarin is an analogue of estrogen that was first
isolated from Pueraria lobata. Previous studies have
demonstrated that puerarin effectively decreased blood glucose in
rats with type 1 diabetes (7,8). An
additional previous study demonstrated that estrogen was able to
effectively promote the survival of the transplanted pancreatic
β-cells (9–11). Since puerarin is an analogue of
estrogen (12–14), and puerarin is able to decrease
blood glucose in type 1 diabetic rats and mice (15), it was hypothesized that puerarin
may be of benefit in protecting mouse insulinoma MIN6 cells from
external stress, while promoting cell survival. However, the
detailed mechanism underlying the role of puerarin in diabetes
remains unclear. Investigation of the mechanism of puerarin is
conducive to the treatment of type I diabetes.
Due to the requirement for pancreatic β-cells to
synthesize a large amount of insulin, pancreatic β-cells are
frequently exposed to oxidative stress (16,17).
Therefore, oxidative stress was considered to be among the primary
causes of pancreatic β-cell apoptosis. The present study used
H2O2 to induce intracellular oxidative
stress, in order to investigate the protective effect of puerarin
on MIN6 cells. In H2O2-induced apoptosis
experiments, it was observed that puerarin significantly decreased
apoptosis, and the levels of intracellular reactive oxygen species
(ROS) and mitochondrial superoxide (MitoSOX). It was additionally
observed that the ability of puerarin to protect MIN6 cells was
decreased by 6-aminonicotinamide (6AN). Puerarin in MIN6 cells may
promote the activity of glucose-6-phosphate dehydrogenase (G6PD),
thereby reducing intracellular oxidative stress; puerarin may
therefore protect against apoptosis induced by
H2O2 in pancreatic β-cells.
Materials and methods
Cell culture
MIN6 cells were purchased from ATCC (Manassas, VA,
USA) and cultured in a 37°C incubator (Sanyo, Osaka, Japan)
containing 5% CO2. MIN6 cells were grown in high glucose
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 15% fetal bovine serum
(HyClone; GE Healthcare Life Sciences), 1% penicillin-streptomycin
(HyClone; GE Healthcare Life Sciences) and 0.2% β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were
cultured overnight before treatment with puerarin,
H2O2 and 6AN. Puerarin, 6AN and
H2O2 were purchased from Sigma-Aldrich (Merck
KGaA). Briefly, prior to experiments, MIN6 cells (1×104
cells) were seeded in 96-well plates overnight. Then, puerarin (100
µM) was used to pre-treat the cells for 6 h during the experiments.
Finally, H2O2 (100 µM) and 6AN (50 µM) were
used to treat the cells for 24 h at the same time and cultured in
an incubator at 37°C.
Terminal deoxynucleotidyl transferase
dUTP nick end labelling (TUNEL) assay and cell viability
The cells were homogeneously seeded in 24-well
plates at 2×105 cells per well with slides and cultured.
Precooled 0.01 M PBS was used to wash the cells twice. MIN6 cells
were fixed with 4% paraformaldehyde for 30 min at room temperature.
Cells were subsequently permeabilized in 0.1% Triton-x 100 in 0.01
M PBS. TUNEL (Roche Diagnostics, Indianapolis, IN, USA) staining
materials were mixed according to the manufacturer's protocol, and
were subsequently added to the fixed cells in the incubator at 37°C
for 60 min. The cells were washed three times with 0.01 M PBS.
Finally, 0.3 mM DAPI was added for staining of the nuclei at room
temperature for 3 min. Each sample was observed in 5 different
fields, with a magnification of ×400. The cells were washed three
times with 0.01 M PBS. Cell viability was detected using a Cell
Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan).
Cell ROS and MitoSOX detection
MIN6 cells were cultured in 24-well plates until 90%
confluence, the cells were washed with 0.01 M PBS. The
corresponding volumes (5 µl) of CellROS (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and Hoechst (Guangzhou Ribobio
Co., Ltd., Guangzhou, China) were added to MIN6 cells at 37°C for
30 min. Subsequently, the cells were washed three times with 0.01 M
PBS, observed and photographed under a microscope, with a
magnification of ×400. MitoSOX (Invitrogen; Thermo Fisher
Scientific, Inc.) and Hoechst were added to 24-well plates at 37°C
for 15 min, and cells were washed three further times with 0.01 M
PBS, observed and photographed under a microscope, with a
magnification of ×400.
Western blotting
MIN6 were cells grown in the 6-well plates and
washed by precooled 0.01 M PBS. The cells were lysed with
radioimmunprecipitation assay lysis buffer (EMD Millpore,
Billerica, MA, USA) and placed on ice for 30 min. Cells in lysis
buffer were centrifuged at 15,000 × g and 4°C for 15 min. The
supernatant was collected and the concentration of the total
protein in the supernatant was measured using a bicinchoninic acid
kit (Thermo Fisher Scientific, Inc.). Loading buffer was added to
the supernatant and boiled for 10 min. Protein samples (30 µg per
lane) were subjected to electrophoresis on a 10% SDS-PAGE gel, at
80 V and constant pressure. The total proteins in the gel were
transferred onto polyvinylidene fluoride (PVDF) membranes (EMD
Millipore) at 300 mA constant current for 90 min. The PVDF
membranes were blocked in 5% non-fat milk (Cell Signaling
Technology, Inc., Danvers, MA, USA) for 1 h at room temperature.
The primary antibodies against cleaved caspase3 (cat. no. 9664;
Cell Signaling Technology, Inc.), β-actin (cat. no. 3700; Cell
Signaling Technology, Inc.) and G6PD (cat. no. 12263; Cell
Signaling Technology, Inc.) were diluted 1:1,000. All the primary
antibodies were incubated at 4°C for 8 h. The secondary antibodies
Goat Anti-Rabbit IgG (cat. no. A9169; Sigma-Aldrich; Merck KGaA)
and Goat Anti-Mouse IgG (cat. no. A8924; Sigma-Aldrich; Merck KGaA)
were diluted 1:10,000. The secondary antibodies (HRP conjugated)
were incubated at 25°C for 1 h. Enhanced chemiluminescence liquid
(Lulong, Inc., Xiamen, China) was added to the membranes and images
were captured. The results of Western blots were quantified by
densitometry using ImageJ software version 1.41 (National
Institutes of Health, Bethesda, MA, USA).
Statistical analysis
All results were analyzed using GraphPad Prism
version 5.0 software (GraphPad Software, Inc. La Jolla, CA, USA).
All experiment results were analyzed using a one-way analysis of
variance followed by a post hoc Bonferroni test for multiple
comparisons. The results are expressed as the mean ± standard error
of the mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
H2O2 reduces
MIN6 cell viability
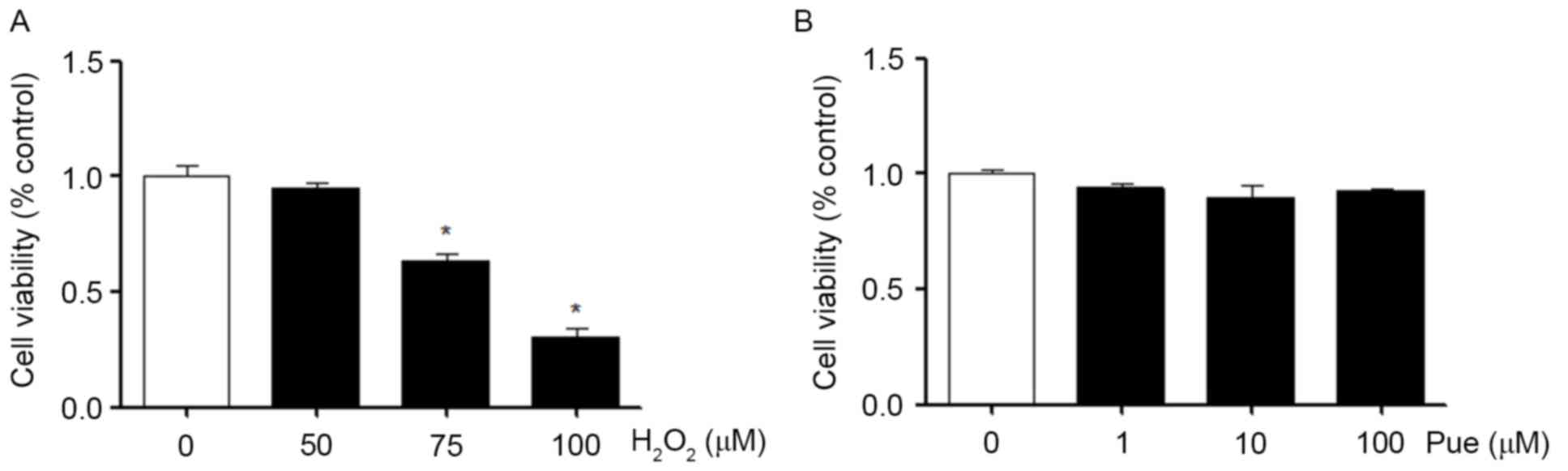
In MIN6 cells, the effects of different
concentrations of H2O2 on the viability of
the cells were measured. The results demonstrated that the
viability of MIN6 cells was significantly decreased as the
concentration of H2O2 increased (Fig. 1A). Similarly, we also examined the
effects of different concentrations of puerarin on the viability of
MIN6 cells. Puerarin exerted no apparent effects on the viability
of MIN6 cells under normal conditions (Fig. 1B). Puerarin neither promoted nor
inhibited cell viability.
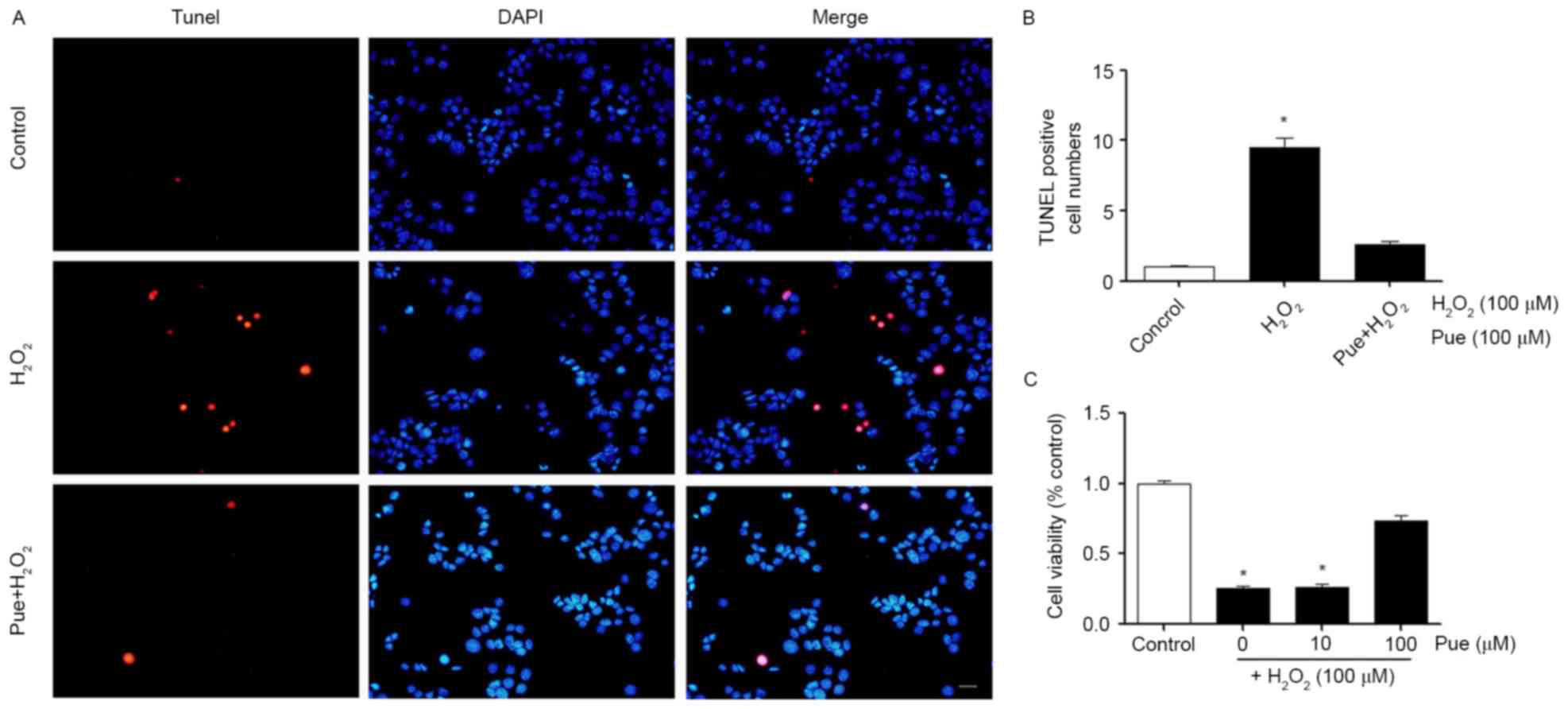
Puerarin decreases
H2O2-induced cellular apoptosis
According to the results of the CCK-8 assay, a
concentration of 100 µΜ H2O2 was selected for
use in subsequent experiments (Fig.
2). MIN6 cells were treated with 100 µΜ
H2O2 and different concentrations of puerarin
were added to the cells. Cell viability was detected using a CCK-8
assay. It was observed that low concentrations of puerarin were not
able to restore cell viability. A concentration of 100 µΜ puerarin
markedly restored the vitality of the cells (Fig. 2C). The effect of puerarin on the
apoptosis of MIN6 cells was additionally examined.
H2O2 induced apoptosis in MIN6 cells and
puerarin decreased cellular apoptosis (Fig. 2A). The results of the present study
indicated that a high concentration of puerarin decreased
H2O2-induced apoptosis and restored cell
viability (Fig. 2B).
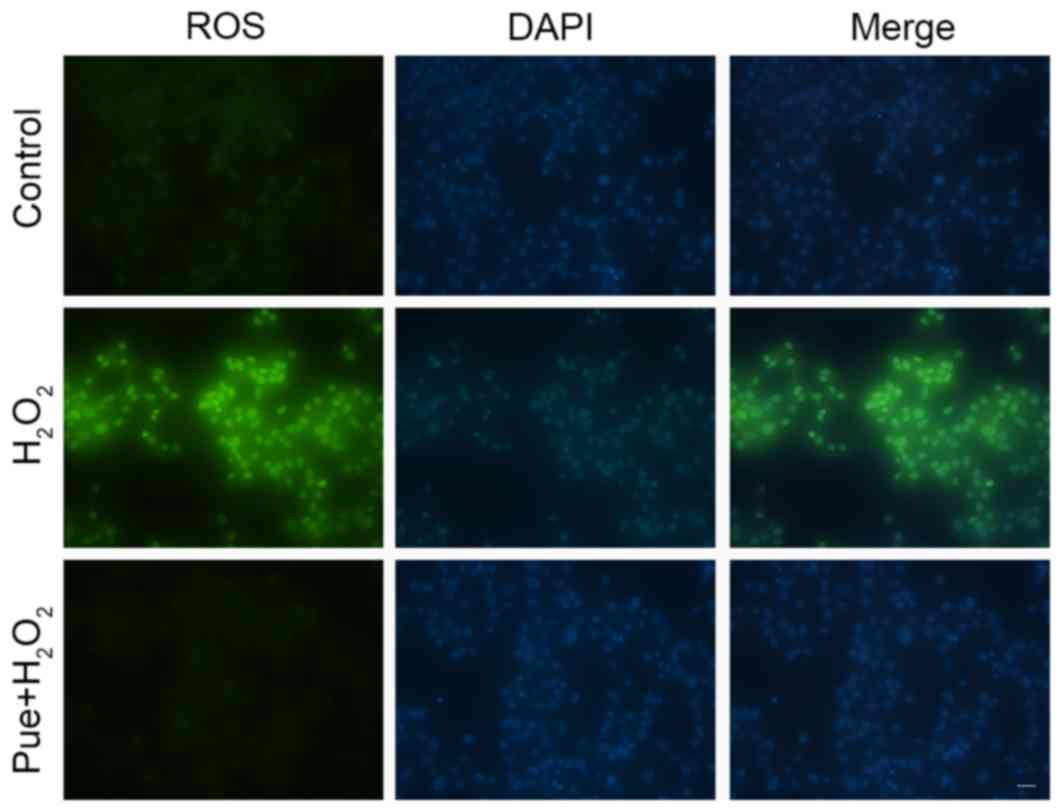
Puerarin decreases
H2O2-induced intracellular ROS levels
It is established that
H2O2-induced apoptosis is primarily caused by
intracellular oxidative stress. Puerarin can reduce
H2O2-induced apoptosis, suggesting that
puerarin can reduce oxidative stress in MIN6 cells. The stress
induced by exposure to H2O2 was detected by
measuring the intracellular levels of ROS, and it was observed that
intracellular ROS levels were increased in MIN6 cells following
treatment with H2O2 (Fig. 3). Puerarin markedly decreased ROS
levels in MIN6 cells. A small amount of ROS is beneficial for cells
(18), although excessive
production of ROS may cause cell damage, including protein
abnormalities, DNA damage and mitochondrial damage (19–21).
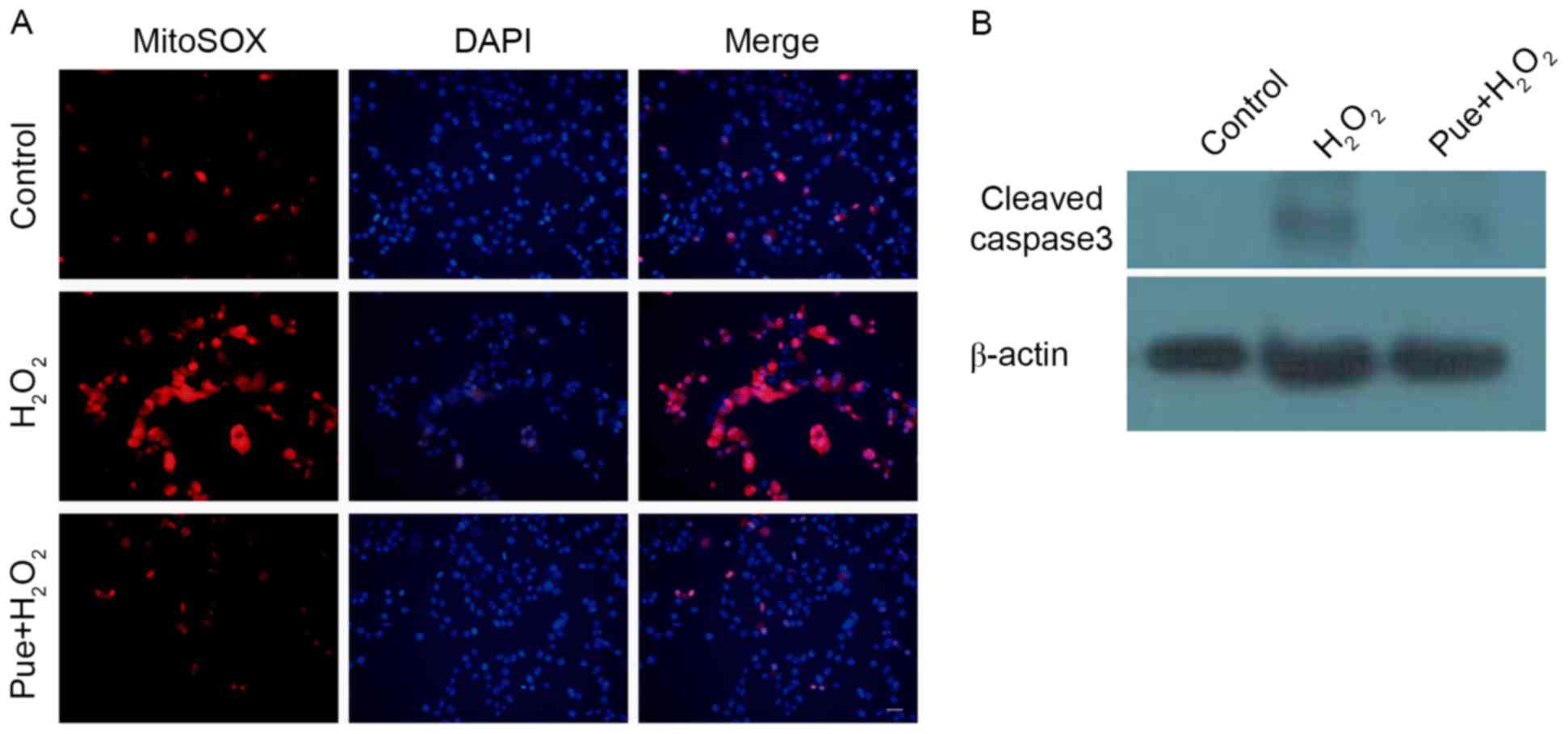
Following examination of the production of MitoSOX, it was observed
that H2O2 caused an increase in MitoSox
levels (Fig. 4A). ROS may cause
mitochondrial damage. The expression of cleaved caspase 3 was
subsequently detected, due to mitochondrial injury-induced caspase
3 activation. Consistent with expectations, puerarin decreased the
expression of cleaved caspase 3 (Fig.
4B). Puerarin prevented mitochondrial damage induced by
H2O2 in MIN6 cells.
Protective role of puerarin is
inhibited by 6AN
Puerarin protected MIN6 cells against oxidative
stress caused by H2O2. The present study
further explored the mechanism underlying the effect of puerarin
against oxidative stress. NADPH is considered to be the major
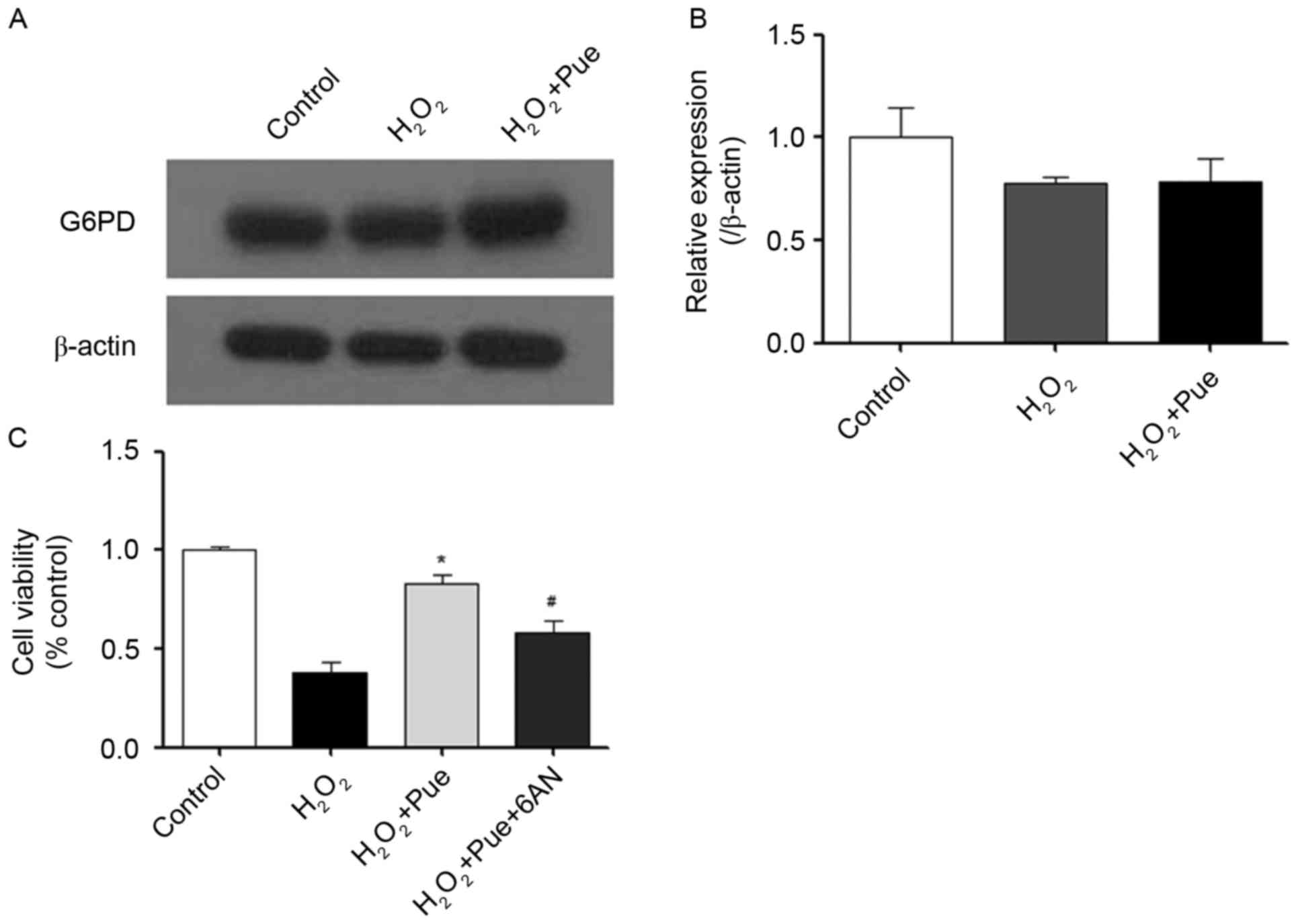
intracellular reductive force. NADPH is produced by G6PD (22). Therefore, the effect of puerarin on
G6PD expression was examined. It was observed that the expression
of G6PD did not alter following treatment with puerarin (Fig. 5). Puerarin did not promote the
expression of G6PD protein levels. 6AN was used to inhibit G6PD
activity (23). The ability of
puerarin to resist oxidative stress was reduced significantly by
6AN (Fig. 5C). Therefore, it may
be hypothesized that puerarin serves a role in the promotion of
G6PD enzymatic activity, which may facilitate resistance to
intracellular oxidative stress.
Discussion
Puerarin is able to effectively decreased blood
glucose in type 1 diabetic mice and rats (8,15).
However, the detailed mechanism underlying the protective effect of
puerarin in pancreatic β-cells remains to be elucidated. Therefore,
the present study investigated the mechanism of puerarin in MIN6
cells treated with H2O2. Using
H2O2 to induce oxidative stress in MIN6
cells, it was observed that puerarin significantly decreased
intracellular ROS levels and superoxide in the mitochondria. The
protective effect of puerarin was attenuated by 6AN. It was
determined that puerarin was able to decrease the intracellular ROS
level, in part by regulating the activity of G6PD.
Type 1 diabetes is a disorder caused by damage to
pancreatic β-cells, primarily. There have been a number of
hypotheses formulated to explain the pathophysiology of pancreatic
β-cell injury; these include hypoxia, oxidative stress and
autoimmunity. However, the detailed mechanism through which
pancreatic β-cells become damaged remains unclear. Ameliorating
pancreatic β-cell damage may be beneficial in treating the
development and course of type 1 diabetes. Previously, it has been
demonstrated that puerarin may decrease blood glucose in type 1
diabetic rats (7). A previous
study additionally demonstrated that puerarin may decreased blood
glucose in type 1 diabetic mice and decrease hypoxia-induced MIN6
cell damage via activity in the phosphatidylinositol 3-kinase/RAC-α
serine/threonine protein kinase signaling pathway (15). However, the detailed mechanism
underlying the function of puerarin in promoting MIN6 cell survival
during treatment with H2O2 remains unclear.
The present study demonstrated that puerarin promoted the activity
of G6PD and decreased the apoptosis of MIN6 cells induced by
H2O2. The results of the present study
suggested that the promotion of G6PD activity in MIN6 cells or
additional NADPH expression in MIN6 cells may reduce cell damage.
At present, there is no cure for type 1 diabetes except islet
transplantation; the limitation of islet transplantation is largely
due to the number of available donors required to provide islets.
In islet cells transplantation, the use of puerarin as an adjuvant
may prove useful in promoting the survival of viable transplant
pancreatic β-cell tissue.
In addition, when G6PD activity was inhibited,
puerarin continued to exert a partial protective effect on MIN6
cells. This result suggested that puerarin may exhibit other
functions in addition to promoting G6PD activity. Puerarin may
promote other intracellular oxidoreductase activity or expression.
Detection of the influence of puerarin on intracellular
oxidoreductases may enhance the understanding of puerarin, which
may serve as a means to provide a novel method for protecting
pancreatic β-cells. In particular, stimulating the activity of a
different variety of reductase within cells may effectively
ameliorate the damage to pancreatic β-cells. Autophagy may also
decrease the levels of intracellular ROS. Puerarin may promote
autophagy to decrease intracellular oxidative stress. A more
detailed mechanism of puerarin in pancreatic β-cells requires
further investigation. Additionally, the function of puerarin in
type 2 diabetes remains unknown, and future studies may examine
this in more detail.
Acknowledgements
The authors would like to thank Professor Yunwu
Zhang in the Fujian Provincial Key Laboratory of Neurodegenerative
Disease and Aging Research, Institute of Neuroscience, Medical
College, Xiamen University for providing the opportunity to perform
this work.
Funding
The present study was supported by grants from the
National Natural Science Foundation to SY (grant no. 30973912), SL
(grant no. 81270901), CH (grant no. 81673661), and XL (grant no.
81570770), the Key Project of Fujian Provincial Science and
Technology Planning Programs (grant no. 2012D60) and the Xiamen
Innovation Program for Outstanding Youth Scientist (grant no.
2011S0446) to SL, and the Xiamen Science and Technology Bureau
(Xiamen Research Platform for Systems Biology of Metabolic Disease;
grant no. 3502Z20100001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW, SL and SY conceived and designed the study. YL,
HM, CH, WW and ZX performed the experiments. TW and HM wrote the
paper. XL and SL analyzed the raw data. XL, SL and SY reviewed and
edited the manuscript. SY agrees to be accountable for all aspects
of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes care. 1 29
Suppl:S43–S48. 2006.
|
|
2
|
Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen
S and Eizirik DL: Mechanisms of pancreatic beta-cell death in type
1 and type 2 diabetes: Many differences, few similarities.
Diabetes. 2 54 Suppl:S97–S107. 2005. View Article : Google Scholar
|
|
3
|
Coskun O, Kanter M, Korkmaz A and Oter S:
Quercetin, a flavonoid antioxidant, prevents and protects
streptozotocin-induced oxidative stress and beta-cell damage in rat
pancreas. Pharmacol Res. 51:117–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sato Y, Endo H, Okuyama H, Takeda T,
Iwahashi H, Imagawa A, Yamagata K, Shimomura I and Inoue M:
Cellular hypoxia of pancreatic beta-cells due to high levels of
oxygen consumption for insulin secretion in vitro. J Biol Chem.
286:12524–12532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim M, Park L, Shin G, Hong H, Kang I and
Park Y: Induction of apoptosis of Beta cells of the pancreas by
advanced glycation end-products, important mediators of chronic
complications of diabetes mellitus. Ann N Y Acad Sci. 1150:311–315.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Atkinson MA and Eisenbarth GS: Type 1
diabetes: New perspectives on disease pathogenesis and treatment.
Lancet. 358:221–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen WC, Hayakawa S, Yamamoto T, Su HC,
Liu IM and Cheng JT: Mediation of beta-endorphin by the isoflavone
puerarin to lower plasma glucose in streptozotocin-induced diabetic
rats. Planta Med. 70:113–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Kilic G, Meyers MS, Navarro G, Wang
Y, Oberholzer J and Mauvais-Jarvis F: Oestrogens improve human
pancreatic islet transplantation in a mouse model of insulin
deficient diabetes. Diabetologia. 56:370–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Le May C, Wong WP, Ward RD, Clegg
DJ, Marcelli M, Korach KS and Mauvais-Jarvis F: Importance of
extranuclear estrogen receptor-alpha and membrane G protein-coupled
estrogen receptor in pancreatic islet survival. Diabetes.
58:2292–2302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tiano JP, Delghingaro-Augusto V, Le May C,
Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar
SM, et al: Estrogen receptor activation reduces lipid synthesis in
pancreatic islets and prevents β cell failure in rodent models of
type 2 diabetes. J Clin Invest. 121:3331–3342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malaivijitnond S, Tungmunnithum D,
Gittarasanee S, Kawin K and Limjunyawong N: Puerarin exhibits weak
estrogenic activity in female rats. Fitoterapia. 81:569–576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang YP, Kim HG, Hien TT, Jeong MH, Jeong
TC and Jeong HG: Puerarin activates endothelial nitric oxide
synthase through estrogen receptor-dependent PI3-kinase and
calcium-dependent AMP-activated protein kinase. Toxicol Appl
Pharmacol. 257:48–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D, Liu Y, Han J, Zai D, Ji M, Cheng
W, Xu L, Yang L, He M, Ni J, et al: Puerarin suppresses invasion
and vascularization of endometriosis tissue stimulated by
17β-estradiol. PLoS One. 6:e250112011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Shangguan Z, Liu Y, Wang J, Li X,
Yang S and Liu S: Puerarin protects pancreatic β-cell survival via
PI3K/Akt signaling pathway. J Mol Endocrinol. 53:71–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carlsson PO and Palm F: Oxygen tension in
isolated transplanted rat islets and in islets of rat
whole-pancreas transplants. Transpl Int. 15:581–585. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carlsson PO, Liss P, Andersson A and
Jansson L: Measurements of oxygen tension in native and
transplanted rat pancreatic islets. Diabetes. 47:1027–1032. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Devasagayam TP, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: Current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.PubMed/NCBI
|
|
19
|
Simon H-U, Haj-Yehia A and Levi-Schaffer
F: Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wiseman H and Halliwell B: Damage to DNA
by reactive oxygen and nitrogen species: Role in inflammatory
disease and progression to cancer. Biochem J. 313:17–29. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ballinger SW, Patterson C, Yan CN, Doan R,
Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman
BA and Runge MS: Hydrogen peroxide-and peroxynitrite-induced
mitochondrial DNA damage and dysfunction in vascular endothelial
and smooth muscle cells. Circ Res. 86:960–966. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida A: Hemolytic anemia and G6PD
deficiency. Science. 179:532–537. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Köhler E, Barrach H and Neubert D:
Inhibition of NADP dependent oxidoreductases by the
6-aminonicotinamide analogue of NADP. FEBS Lett. 6:225–228. 1970.
View Article : Google Scholar : PubMed/NCBI
|