Introduction
Fetal congenital heart disease (CHD) is a serious
heart defect with high incidence (1). Its incidence is about 1–40/1,000
worldwide (2). In China, there
~300,000 fetuses with CHD (3),
bringing a heavy burden to families and society (4). Therefore, prenatal detection of fetal
malformation and elucidating the molecular mechanism of fetal CHD
has important clinical significance and practical value (5). More importantly, prenatal examination
also has great significance to reduce fetal CHD birth rate and
promote prenatal and postnatal care (6).
Following science and technology developments,
increasing methods for early fetal CHD diagnosis have been applied
with high accuracy. Among them, color doppler ultrasound technique
is one of the most important screening methods for detecting fetal
CHD (1,7–9).
With the improvement of ultrasonic detecting instrument resolution,
color ultrasonic detection serves an important role in reducing
fetal CHD incidence and mortality (8,10).
Laboratory testing methods, especially polymerase
chain reaction, western blotting and gene sequencing, also have
critical roles in fetal cardiac function screening. At present,
laboratory testing methods have become an important supplement of
ultrasonography for fetal CHD (11–13).
Recent research suggested that the T-box transcription factor (TBX)
family of proteins, especially TBX1, has a close association with
fetal CHD occurrence and development (14,15).
TBX1 is a transcription factor expressed in several tissues but its
early expression in mesodermal tissue is particularly important for
normal development of second heart field-derived heart segments,
especially the outflow tract. Animal experiments have revealed that
TBX1 overexpression is positively associated with CHD, indicating
that TBX1 may be associated with fetal CHD occurrence (16–18).
The aim of the present study was to investigate the clinical
application value of prenatal ultrasonography combined with
molecular biology methods in the diagnosis of fetal CHD.
Materials and methods
Experimental subjects
According to the inclusion and exclusion criteria
(19,20), 1,000 cases of pregnant women who
had received fetal ultrasonography between June 2012 and June 2015
in Jining No. 1 People's Hospital (Jining, China) were enrolled.
The inclusion criteria consisted of pregnant women at 18–25
gestational weeks and aged 18–30 years old, with no CHD family
history or other serious disease history. Exclusion criteria were
the subjects unmatched to the inclusion criteria. All subjects and
families were informed of the purpose of the study and signed
informed consent was obtained. The present study was approved by
the ethics committee of Jining No. 1 People's Hospital. Ultrasounds
were performed at 18–25 gestational weeks. The mean age of pregnant
women was 24.9±4.2 years.
Ultrasonography
A GE Voluson E6 color doppler ultrasonic detector at
6–10 MHz was used for fetal cardiac ultrasonography (21). Routine inspection (blood test) was
applied before ultrasonography to test fetal development and
position. Special examination (electrocardiogram and
echocardiography) was then performed to test fetal heart. During
color doppler ultrasonography, the pregnant women took a supine
posture to reduce the probe pressure. Their posture was modulated
to obtain the best imagine and different view angle. Ultrasound
examination was conducted by the chief physician. Fetal heart
position, size, structure, function, and heart valve morphology and
function were examined. Fetal heart development condition was
analyzed, including the right ventricular outflow tract, left
ventricular outflow tract, pulmonary stenosis, atresia, aortic
abnormalities, endocardial defects, valve deformity, cardiac
neoplasm, tricuspid mobile and ventricular septal defects. Finally,
aortic arch detects, tetralogy of Fallot, aortic stenosis,
heterotopic heart, pericardial effusion and cardiothoracic ratio
were examined. All pregnant women received fetal CHD screening and
review in strict accordance with the world and domestic standards
(22).
Prenatal intervention method
For the cases diagnosed as fetal CHD, subjects and
their families were informed as soon as possible and accurately to
decide on the following examination. Termination was proposed for
the cases that were determined to be incurable (23). For the fetal CHD cases that
progressed to induction of labor, the families agreed to scientific
examination.
Pregnant women blood sample collection
and analysis
For the cases with fetal CHD, 3 ml venous blood was
extracted from the pregnant women with heparin anticoagulation
(24). Genomic DNA was extracted
using a DNA extraction kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol.
PCR
PCR was applied to test whether fetal CHD was
associated with TBX1 (25). TBX1
gene primer sequences were: Forward, 5′-AATTTGGCCTCACCTGTGTC-3′ and
reverse, 5′-ACTCAGCCCTGACTCAATAG-3′. β-actin (internal reference
control) primer sequences were: Forward, 5′-CTCACCCTGTCTGAATTTGG-3′
and reverse, 5′-AACCTTAACTAGGCGACTCC-3′. The primers were
synthetized by Sangon Biotech Co., Ltd. (Shanghai, China).
The PCR system contained 1 µl DNA template, 2.0 µl
10X PCR Buffer, 2.0 µl dNTP Mixture (2 mM), 1 µl primer 1 (10 µM),
1 µl primer 2 (10 µM), 0.25 µl Taq DNA Polymerase (Thermo Fisher
Scientific, Inc.), 1.75 µl MgCl2 (25 mM) and 16 µl
H2O. The PCR reaction conditions were: Initial
denaturation at 94°C for 7 min, followed by 28 cycles of
denaturation at 94°C for 30 sec, annealing at 50°C for 60 sec,
extension at 72°C for 30 sec and elongation at 4°C for 5 min.
Agarose gel electrophoresis
PCR products (500 ng/µl) 10 µl were mixed with
sample buffer and separated by 0.8% agarose gel electrophoresis at
80 mV for 15 min. The electrophoresis gel was stained by ethidium
bromide and analyzed using a gel imaging system (26).
Experimental results were analyzed using BandScan
680 software (Glyko; http://bandscan.software.informer.com/). All
electrophoresis bands were tested independently three times. TBX1
relative expression was defined as the ratio between TBX1 and
β-actin optical density.
PCR product sequencing and
analysis
TBX1 gene PCR products were sequenced by Sangon
Biotech Co., Ltd., following agarose gel electrophoresis detection
(27). TBX1 sequencing result was
analyzed and compared with TBX1 gene sequence in NCBI database
(www.ncbi.nlm.nih.gov/). The association
between TBX1 gene sequence and fetal CHD was analyzed.
Western blotting
Western blotting was performed to detect TBX1
expression in blood samples (28,29).
The protein was lysed by radioimmunoprecipitation assay lysis
buffer (Thermo Fisher Scientific, Inc.) and incubated on ice for 30
min followed by centrifugation at 13,000 × g for 30 min at 4°C. The
protein concentration was determined by Pierce bicinchoninic acid
Protein Assay kit (Thermo Fisher Scientific, Inc.). Then the
extracted proteins (40–60 µg) were separated by 8% SDS-PAGE at 60 V
for 30 min and 120 V for 90 min. Following this, the protein was
transferred to a polyvinylidene difluoride membrane at 300 mA for 3
h. Following blocking with 5% bovine serum albumin (Thermo Fisher
Scientific, Inc.)/PBS with Tween-20 (PBST) for 2 h at 37°C, the
membrane was incubated with anti-TBX1 (cat. no. ab18530; Abcam,
Cambridge, MA, USA) or anti-β-actin (cat. no. ab8227; Abcam)
primary antibodies (1:2,000) at room temperature for 2 h. Next, the
membrane was further incubated with horseradish
peroxidase-conjugated goat-anti-rabbit secondary antibody (cat. no.
ab6721; Abcam; 1:2,000) at room temperature for 1 h, after washing
with PBST three times. At last, the membrane was developed using
Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.,) and analyzed. The results were analyzed by BandScan 680
software. All electrophoresis bands were tested independently three
times. TBX1 relative expression was defined as the ratio between
TBX1 and β-actin optical density.
Statistical analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used
for data analysis. All data are presented as the mean ± standard
deviation. One-way analysis of variance with the Newman-Keuls
multiple comparison post-hoc analysis was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Basic information
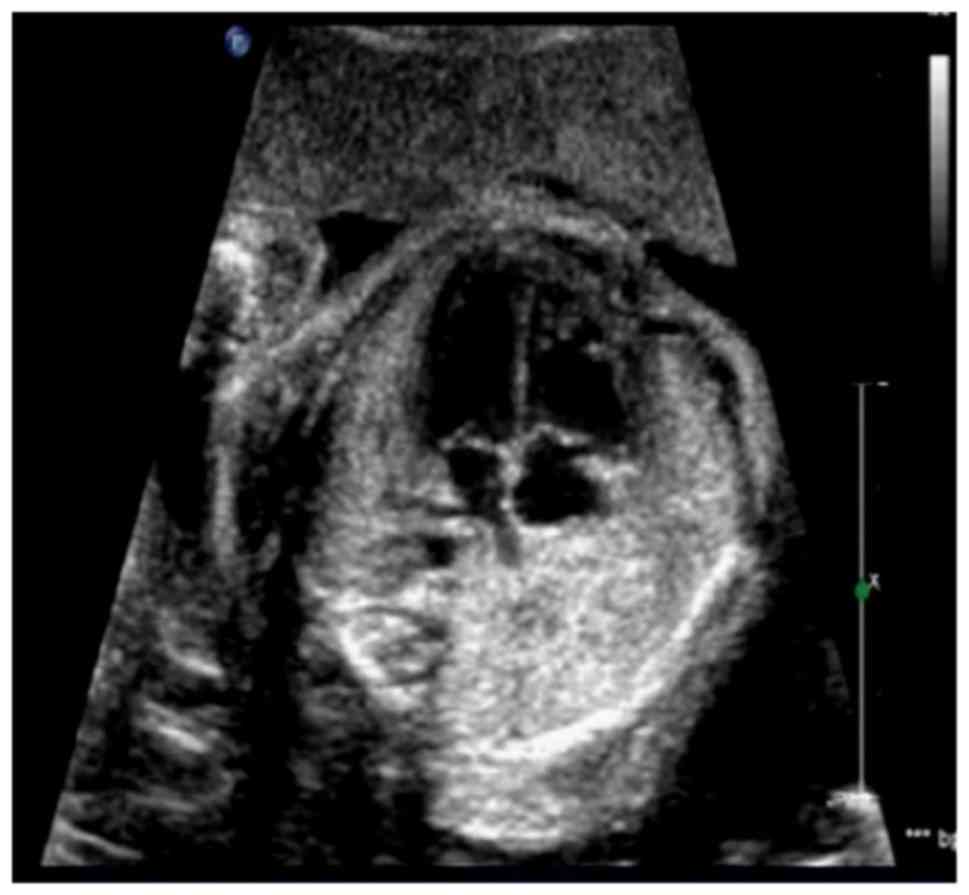
Fig. 1 presents a
representative fetal ultrasonography result. In the present study,
10 fetuses were identified to have CHD (0.99%), of which ultrasound
detected 9 cases. The specificity and sensitivity of ultrasound
were 100 and 90%, respectively.
Comparison between ultrasound
examination and pathological result
Of the 9 cases of CHD detected by prenatal
ultrasound screening, 2 cases were endocardial cushion defect, 1
case was pulmonary stenosis combined right ventricular dysplasia, 1
case was tetralogy of Fallot combined cleft lip and palate, 2 cases
were ventricular septal defect, 1 case was single ventricle defect,
1 case was Ebstein and 1 case had a triatrial heart. One case of
ventricular septal defect was missed prior to delivery (Tables I and II).
 | Table I.Ultrasound examination and
pathological result comparison. |
Table I.
Ultrasound examination and
pathological result comparison.
| CHD type | Pathological
result | Ultrasound
examination |
|---|
| Triatial heart | 1 | 1 |
| Ebstein | 1 | 1 |
| Single ventricle | 1 | 1 |
| Ventricular septal
defect | 3 | 2 |
| Tetralogy of
Fallot | 1 | 1 |
| Pulmonary
stenosis | 1 | 1 |
| Endocardial cushion
defect | 2 | 2 |
| Total | 10 | 9 |
 | Table II.Cases of ultrasound examination and
pathological result comparison (n=9). |
Table II.
Cases of ultrasound examination and
pathological result comparison (n=9).
| Case | Gestational week | Factor | Ultrasound
diagnosis | Pathological
result |
|---|
| 1 | 21 | None | Endocardial cushion
defect | Endocardial cushion
defect |
| 2 | 22 | Abortion | Ebstein | Ebstein |
| 3 | 23 | Family history | Ventricular septal
defect | Ventricular septal
defect |
| 4 | 23 | None | Single ventricle | Single ventricle |
| 5 | 24 | Influenza | Tetralogy of
Fallot | Tetralogy of
Fallot |
| 6 | 24 | None | Ventricular septal
defect | Ventricular septal
defect |
| 7 | 25 | None | Pulmonary
stenosis | Pulmonary
stenosis |
| 8 | 25 | None | Endocardial cushion
defect | Endocardial cushion
defect |
| 9 | 26 | Poison contact | Triatial heart | None |
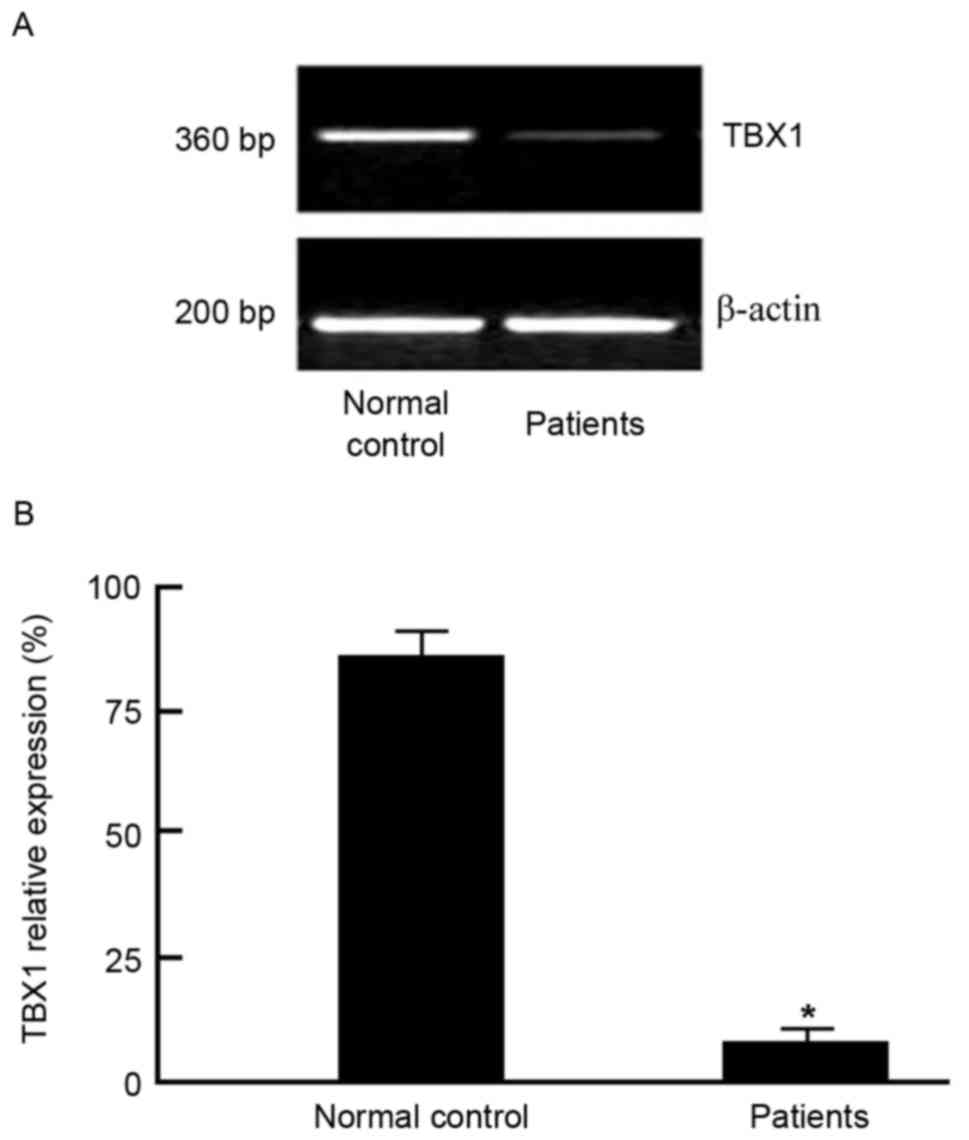
TBX1 gene PCR product agarose gel
electrophoresis result
TBX1 was PCR amplified by using primer based on exon
1. PCR products were separated by agarose gel electrophoresis. The
TBX1 gene PCR product was 360 bp in length (Fig. 2).
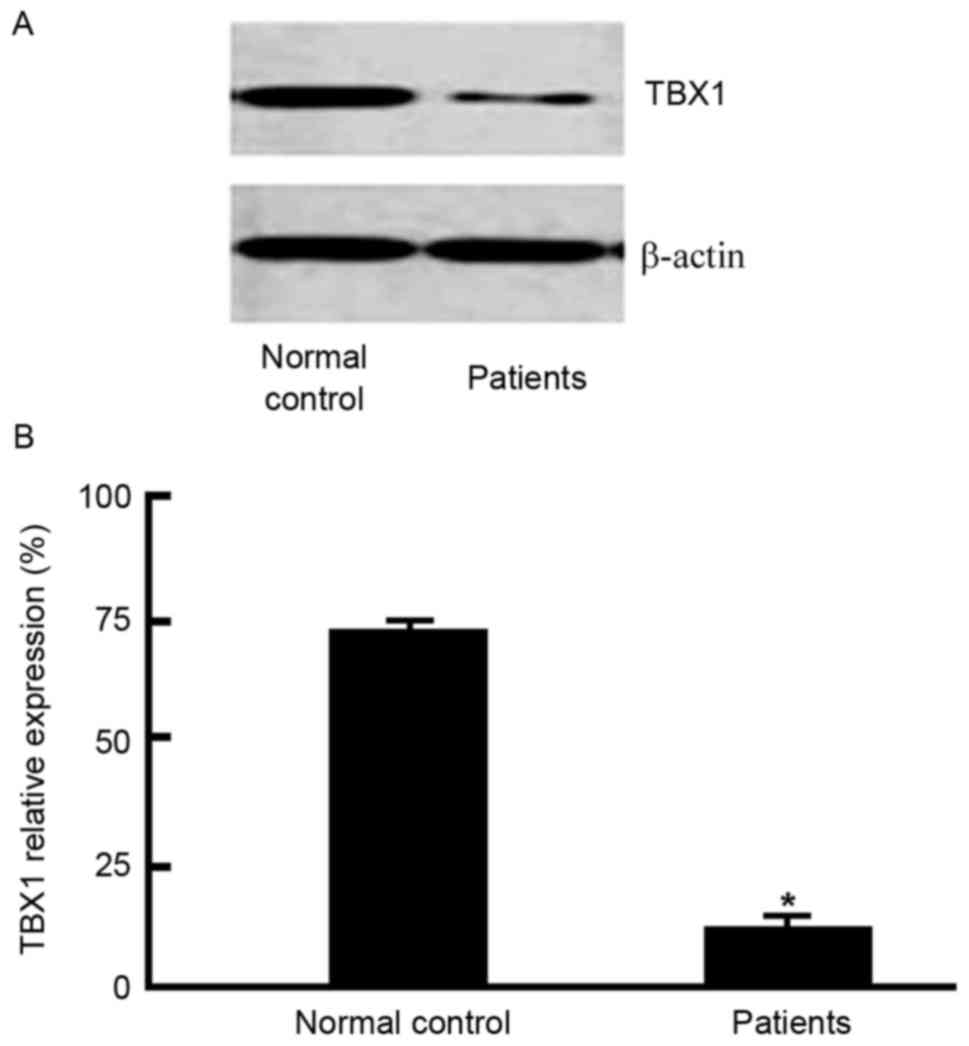
TBX1 western blotting result
Protein expression levels were analyzed by BandScan
680 software. Western blotting revealed that TBX1 protein
expression levels were reduced in blood samples from pregnant women
with fetal CHD (Fig. 3),
suggesting that TBX1 reduction may be associated with fetal CHD
occurrence and development.
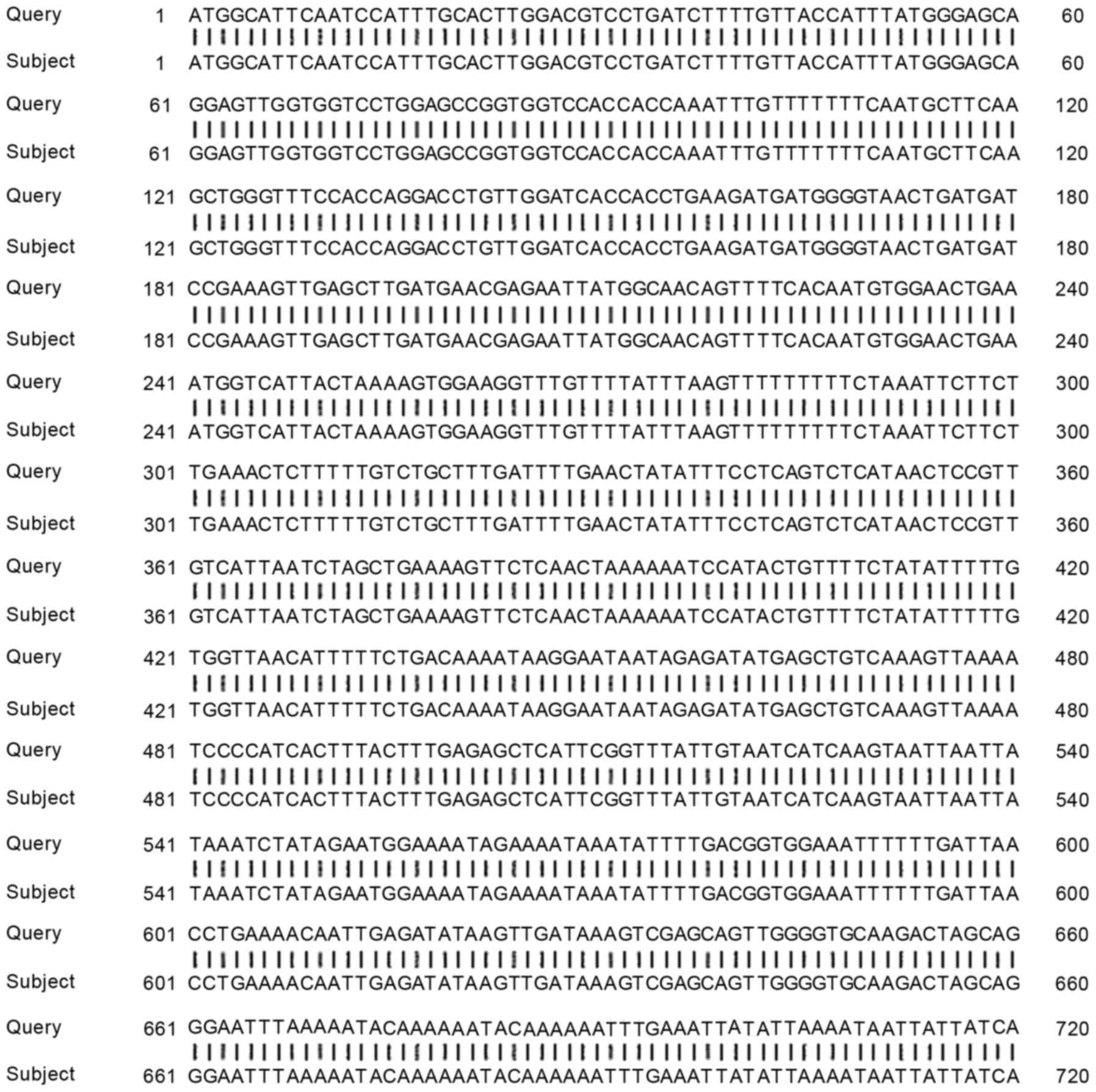
TBX1 gene PCR amplification product
sequencing comparison
The sequencing result of the TBX1 gene was compared
with the TBX1 gene in NCBI database. The PCR product sequencing
results of the present study coincided with the TBX1 gene sequence,
and the sequence similarity achieved 100% (Fig. 4).
Discussion
With technology progression, prenatal examination is
an important aspect to secure fetal safety and health. The present
study investigated clinical application value of fetal cardiac
ultrasound combined with molecular biology methods, such as PCR and
western blot, in detecting fetal CHD.
Ultrasonic inspection is widely used for fetuses and
has made a significant contribution for prenatal and postnatal
care; however, the etiology of fetal CDH has various reasons. The
present study verified ultrasonic diagnosis at the gene and protein
level. The results demonstrated that consistent with previous
studies (16–18), ultrasound scanning can effectively
detect fetal CHD.
Previous studies have demonstrated that the
transcription factor TBX1 has a close association with CHD
(20). The PCR and western
blotting results of the present study demonstrated that TBX1 may be
associated with CHD at the gene and protein level. Therefore, TBX1
downregulation maybe associated with CHD occurrence. However, the
underlying mechanism of TBX1 in CHD requires further
investigation.
The present study had three primary results.
Firstly, 10 fetuses were identified to CHD (0.99%), of which
ultrasound detected 9 cases. The specificity and sensitivity of
ultrasound were 100 and 90%, respectively. Secondly, of the 9 cases
detected prenatal ultrasound screening, 2 cases had endocardial
cushion defect, 1 case had pulmonary stenosis combined right
ventricular dysplasia, 1 case had tetralogy of Fallot combined with
a cleft lip and palate, 2 cases had a ventricular septal defect, 1
case had a single ventricle defect, 1 case had Ebstein and 1 case
had a triatrial heart. One case of ventricular septal defect was
missed prior to delivery. Lastly, PCR and western blotting
demonstrated TBX1 expression may be associated with CHD. This
implicates TBX1 as a biomarker of CHD.
However, the present study had several limitations.
Firstly, the enrolled sample size was relatively small. Since the
incidence of fetal CHD was low, a larger sample size is required in
the future. Secondly, a single-blind method of analysis was used;
multicenter double-blind clinical trials are required to
investigate the association between TBX1 expression and CHD.
Finally, the subjects were not classified by nationality, ethnicity
and family history, which may have influenced the results.
In conclusion, ultrasonography combined with
laboratory examination may represent efficient, economic and safe
methods for fetal CHD detection. These methods may be significant
to improve the rate of CHD diagnosis, and require further
investigation.
References
|
1
|
Sargos P, Guerif S, Latorzeff I, Hennequin
C, Pommier P, Lagrange JL, Crehange G, Chapet O, de Crevoisier R,
Azria D, et al: Definition of lymph node areas for radiotherapy of
prostate cancer: A critical literature review by the French
Genito-Urinary Group and the French Association of Urology
(GETUG-AFU). Cancer Treat Rev. 41:814–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nicholson A, Mahon J, Boland A, Beale S,
Dwan K, Fleeman N, Hockenhull J and Dundar Y: The clinical
effectiveness and cost-effectiveness of the PROGENSA(R) prostate
cancer antigen 3 assay and the prostate health index in the
diagnosis of prostate cancer: A systematic review and economic
evaluation. Health Technol Assess. 19(i–xxxi): 1–191. 2015.
View Article : Google Scholar
|
|
3
|
Woodrum DA, Kawashima A, Gorny KR and
Mynderse LA: Magnetic resonance-guided thermal therapy for
localized and recurrent prostate cancer. Magn Reson Imaging Clin N
Am. 23:607–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmad AE and Finelli A: Should prebiopsy
multiparametric magnetic resonance imaging be offered to all
Biopsy-naïve men undergoing prostate biopsy? Eur Urol. 69:426–427.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westphalen AC, Noworolski SM, Harisinghani
M, Jhaveri KS, Raman SS, Rosenkrantz AB, Wang ZJ, Zagoria RJ and
Kurhanewicz J: High-resolution 3-T endorectal prostate MRI: A
multireader study of radiologist preference and perceived
interpretive quality of 2D and 3D T2-weighted fast Spin-Echo MR
images. AJR Am J Roentgenol. 206:86–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amanullah MM, Hamid M, Hanif HM, Muzaffar
M, Siddiqui MT, Adhi F, Ahmad K, Khan S and Hasan Z: Effect of
steroids on inflammatory markers and clinical parameters in
congenital open heart surgery: A randomised controlled trial.
Cardiol Young. 26:506–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Shen A, Li X, Jiao W, Zhang X and
Li Z: T-box transcription factor TBX20 mutations in Chinese
patients with congenital heart disease. Eur J Med Genet.
51:580–587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Butler TL, Esposito G, Blue GM, Cole AD,
Costa MW, Waddell LB, Walizada G, Sholler GF, Kirk EP, Feneley M,
et al: GATA4 mutations in 357 unrelated patients with congenital
heart malformation. Genet Test Mol Biomarkers. 14:797–802. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirk EP, Sunde M, Costa MW, Rankin SA,
Wolstein O, Castro ML, Butler TL, Hyun C, Guo G, Otway R, et al:
Mutations in cardiac T-box factor gene TBX20 are associated with
diverse cardiac pathologies, including defects of septation and
valvulogenesis and cardiomyopathy. Am J Hum Genet. 81:280–291.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Han Q, Li C, Li W, Fan H, Xing Q
and Yan B: Genetic analysis of the TBX1 gene promoter in indirect
inguinal hernia. Gene. 535:290–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Töpf A, Griffin HR, Glen E, Soemedi R,
Brown DL, Hall D, Rahman TJ, Eloranta JJ, Jüngst C, Stuart AG, et
al: Functionally significant, rare transcription factor variants in
tetralogy of Fallot. PLoS One. 9:e954532014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monroy-Muñoz IE, Pérez-Hernández N,
Rodríguez-Pérez JM, Muñoz-Medina JE, Angeles-Martínez J,
García-Trejo JJ, Morales-Ríos E, Massó F, Sandoval-Jones JP,
Cervantes-Salazar J, et al: Novel mutations in the transcriptional
activator domain of the human TBX20 in patients with atrial septal
defect. Biomed Res Int. 2015:7187862015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Sun F, Fu J and Zhang H:
Association of TBX20 gene polymorphism with congenital heart
disease in Han Chinese neonates. Pediatr Cardiol. 36:737–742. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin SB, Xie YJ, Chen Z, Zhou Y, Wu JZ,
Zhang ZQ, Shi SS, Chen BJ and Fang Q: Improved assay performance of
single nucleotide polymorphism array over conventional karyotyping
in analyzing products of conception. J Chin Med Assoc. 78:408–413.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henderson DR, de Souza NM, Thomas K,
Riches SF, Morgan VA, Sohaib SA, Dearnaley DP, Parker CC and van As
NJ: Nine-year follow-up for a study of diffusion-weighted magnetic
resonance imaging in a prospective prostate cancer active
surveillance cohort. Eur Urol. 69:1028–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You JY, Lee HJ, Hwang SI, Bae YJ, Kim H,
Hong H and Choe G: Value of T1/T2-weighted magnetic resonance
imaging registration to reduce the postbiopsy hemorrhage effect for
prostate cancer localization. Prostate Int. 3:80–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biglino G, Capelli C, Wray J, Schievano S,
Leaver LK, Khambadkone S, Giardini A, Derrick G, Jones A and Taylor
AM: 3D-manufactured patient-specific models of congenital heart
defects for communication in clinical practice: Feasibility and
acceptability. BMJ Open. 5:e0071652015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Sundquist J, Hamano T, Zoller B and
Sundquist K: Neighbourhood deprivation, individual-level and
familial-level socio-demographic factors and risk of congenital
heart disease: A nationwide study from Sweden. Int J Behav Med.
23:112–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaynor JW, Stopp C, Wypij D, Andropoulos
DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS,
et al: Neurodevelopmental outcomes after cardiac surgery in
infancy. Pediatrics. 135:816–825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwiatkowski D, Czarny P, Galecki P,
Bachurska A, Talarowska M, Orzechowska A, Bobińska K,
Bielecka-Kowalska A, Pietras T, Szemraj J, et al: Variants of base
excision repair genes MUTYH, PARP1 and XRCC1 in Alzheimer's disease
risk. Neuropsychobiology. 71:176–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knopp C, Rudnik-Schöneborn S, Eggermann T,
Bergmann C, Begemann M, Schoner K, Zerres K and Ortiz Brüchle N:
Syndromic ciliopathies: From single gene to multi gene analysis by
SNP arrays and next generation sequencing. Mol Cell Probes.
29:299–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Warnes CA, Williams RG, Bashore TM, Child
JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP Jr,
Hijazi ZM, et al: ACC/AHA 2008 Guidelines for the management of
adults with congenital heart disease: Executive summary: A report
of the American college of cardiology/american heart association
task force on practice guidelines (writing committee to develop
guidelines for the management of adults with congenital heart
disease). Circulation. 118:2395–2451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weaver JK, Kim EH, Vetter JM, Fowler KJ,
Siegel CL and Andriole GL: Presence of magnetic resonance imaging
suspicious lesion predicts gleason 7 or greater prostate cancer in
Biopsy-Naive patients. Urology. 88:119–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tay KJ, Gupta RT, Brown AF, Silverman RK
and Polascik TJ: Defining the incremental utility of prostate
multiparametric magnetic resonance imaging at standard and
specialized read in predicting extracapsular extension of prostate
cancer. Eur Urol. 70:211–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang YJ, Huang CY, Hou WH, Wang CC, Lan
KH, Chen CH, Yu HJ, Lai MK, Cheng AL, Liu SP, et al: The outcome
and prognostic factors for lymph node recurrence after node-sparing
definitive external beam radiotherapy for localized prostate
cancer. World J Surg Oncol. 13:3122015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Engelhard K, Kühn R, Osten A, Bogner K,
Dworak A, Lübke L and Schneider F: Impact of magnetic resonance
imaging-guided prostate biopsy in the supine position on the
detection of significant prostate cancer in an inhomogeneous
patient cohort. Scand J Urol. 50:110–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thompson JE, van Leeuwen PJ, Moses D,
Shnier R, Brenner P, Delprado W, Pulbrook M, Böhm M, Haynes AM,
Hayen A and Stricker PD: The diagnostic performance of
multiparametric magnetic resonance imaging to detect significant
prostate cancer. J Urol. 195:1428–1435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia JB, Houshyar R, Verma S, Uchio E and
Lall C: Prostate cancer on computed tomography: A direct comparison
with multi-parametric magnetic resonance imaging and tissue
pathology. Eur J Radiol. 85:261–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Fan X, Medved M, Pineda FD, Yousuf
A, Oto A and Karczmar GS: Arterial input functions (AIFs) measured
directly from arteries with low and standard doses of contrast
agent, and AIFs derived from reference tissues. Magn Reson Imaging.
34:197–203. 2016. View Article : Google Scholar : PubMed/NCBI
|