Introduction
Keloids are fibrotic tumors, which expand over
normal skin beyond the margins of the original wound. Keloids do
not revert spontaneously and are characterized by the proliferation
of dermal fibroblasts, overproduction of extracellular matrix
components, especially collagen, elastin, and proteoglycans,
fibronectin, an increased infiltration of inflammatory cells
(1).
Autosomal dominant with incomplete penetrance as
well as autosomal recessive modes of inheritance have been seen
among families with keloid disease. Variable gene expression
patterns are also implicated in the etiology of keloid disease, but
no single gene mutation has thus far been found to be responsible
(2). In particular, as reported in
multiple microarray studies, keloid-derived fibroblast cells
exhibit dysregulated genes involved in apoptosis, mitogen-activated
protein kinase, transforming growth factor-beta, interleukin-6 and
plasminogen activator inhibitor-1 pathways (3).
Epigenetic alterations in keloid tissue are also
involved. Actually, it has been reported that keloid fibroblast
cells have altered patterns of DNA methylation, microRNAs
deregulation and histone acetylation (4).
No single therapeutic modality is best for all
keloids.
Lately, it has been demonstrated that
ingenol-mebutate generates pro-apoptotic effects (5), even if the exact mechanism of action
is unknown. Multiple apoptotic mechanisms have pleiotropic effects
inducing tumor cell death or inhibiting the growth of cancer cells.
Due to such outcome, topical application of ingenol mebutate has
been successful for the treatment of precancerous skin; therefore,
we effectively treated the lesions from a patient showing recurrent
keloids with ingenol mebutate (6).
The main apoptotic effects are intermediate through
the induction of specific apoptotic target genes.
Multitudes of mechanisms are operated by p53 to
guarantee the induction of apoptosis in a stress signal and tissue
specific manner. p53 can lead the major steps in apoptotic
pathways: The intrinsic mitochondria pathways involving apoptosome
realization, the extrinsic death receptor signalling and the direct
caspase activation. Numerous of these effects are mediated through
the activation of specific p53-target genes.
Recently, it has been shown that components of p53
pathway are direct targets of microRNAs and their over-expression
lead to the induction of apoptosis, senescence and cell cycle
arrest (7–9).
MicroRNAs (miRNAs) deregulation is recognized as a
form of epigenetic regulation. MicroRNAs are small non-coding RNAs
approximately 22 nucleotides long able to control gene expression
post-transcriptionally through complementarity with a target mRNA,
(10). Aberrant microRNA
expression has been implicated in several cellular processes and
pathogenic pathways of a number of diseases (11–13).
Small non-coding RNAs termed microRNAs (miRNAs) can regulate
oxidative stress and several human diseases. Recent studies suggest
that microRNAs (miRNAs) might play critical roles in
pharmacogenomics (14,15). MicroRNA pharmacogenomics is to
study the decrease or increase the expressions of microRNAs, target
genes or to change the activities of binding microRNAs following to
a pharmacological treatment (16).
Since the molecular pathways underlying Ingenol
mebutate mechanism of action are still unclear, in the present
research, we aim to evaluate the expression levels of microRNAs and
differential expression of pro-apoptotic genes expression in keloid
fibroblasts following Ingenol-mebutate exposure.
Materials and methods
Cell cultures experiments and
treatment
Previously, fresh tissues were obtained from skin
lesions of 20 patients with keloids for establishing the primary
cell cultures. Primary cell cultures were performed in a previous
study (6), then used
retrospectively in the present study.
Fibroblasts from keloids were isolated from samples
following the same method established by Lim et al (17). All experiments used cells between
the second and third passage. No morphological and biochemical
differences were found with the passage. Fibroblast cells were
grown to confluence in Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (FBS), 10 µg/ml streptomycin, and
50 IU/ml penicillin in 5% carbon dioxide at 37°C.
From the second passage treatment time course
experiments were set up for 24, 48, and 72 h without serum
starvation, adding different concentrations (0.1, 0.3, 1 µM
respectively) of Ingenol Mebutate (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) dissolved in water.
RNA isolation
Total RNA was isolated from cultured cells using
TRIzol reagent (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's instructions. Quality of extracted RNA was
determined according to 260/280 absorbance ratio, measured by Nano
Drop spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
TaqMan Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
microRNA array
TaqMan Array Human microRNA Panel Kit (ABI, Forest
City, CA, USA) which includes 365 human microRNAs as well as 3
negative controls was used for microRNA expression profile.
For miR-34a analysis, reverse transcription was
performed using the TaqMan MicroRNA Reverse Transcription Kit
(Thermo Fisher Scientific, Inc.), and PCR products were amplified
from cDNA samples using TaqMan MicroRNA Assays (Thermo Fisher
Scientific, Inc.). The small nucleolar RNA human RNU48 were used as
endogenous controls for normalization of miR-34a expression levels.
Fold changes in microRNA expression were calculated using the ΔΔCq
method.
Gene ontology analysis (GO)
We analyzed the functional distribution of miR34a
target genes using the GO database: http://www.geneontology.org/.
Human apoptosis PCR array
We used the Human Apoptosis RT2 Profiler PCR Array
(Qiagen, Inc., Valencia, CA, USA) for the simultaneous human
pathway of 84 key genes involved in programmed cell death and the
Human Cellular Stress Responses RT2 Profiler PCR Array
profiles the expression of 84 genes involved in cellular stress
response according to the manufacturer's instructions.
mRNA RT-qPCR analyses
For mRNA analysis, reverse transcription was
performed using the High-Capacity Reverse-Transcription Kit (Thermo
Fisher Scientific, Inc.). 500 ng/RNA were used in
reverse-transcription reaction. RT-qPCR was performed using the
SYBR Green method (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Levels of expression were determined by normalization to the
housekeeping gene HPRT. PCR primers were purchased from Qiagen (RT2
qPCR Primer Assays).
Phase contrast microscopy
Phase contrast images of control and treated cells
were generated with the aid of a Zeiss Axiovert 40 CFL inverted
microscope equipped with a Canon Power Shot G6 digital camera.
Annexin V staining
For ANNEXIN V-PI staining, cells were washed with
ice-cold PBS, trypsinized, and resuspended in 1× binding buffer [10
mm HEPES/NaOH (pH 7.4), 140 mm NaCl, and 2.5 mm CaCl2]
at 1×106 cells/ml. After gentle vortex, the cells were
mixed with 5 µl annexin V/fluorescein isothiocyanate (FITC)
(Annexin V-FITC Apoptosis Detection kit 3, ABM Inc., USA) and 10 µl
propidium iodide (PI) stock solution (50 µg/ml in PBS) followed by
a 15 min incubation at room temperature in the dark for apoptosis
analysis, following manufacturer's instructions.
DNA fragmentation analysis
The genomic DNA fragmentation was evaluated by
agarose gel electrophoresis of DNA. For this purpose, cells were
grown in the presence or absence of 3 µM Ingenol Mebutate up to 72
h. After counting and washing, cells were subjected to DNA
extraction. The DNA samples were carefully resuspended in TE
buffer; the nucleic acid concentration and purity were.
Measured using a NanoDrop® ND-1000
spectrophotometer (Thermo-Scientific, Wilmington, DE, USA). 2 µg of
each sample was loaded onto 1.5% TAE agarose gel; DNA laddering was
visualized on a UV transilluminator by ethidium bromide
staining.
Caspase-3 activity assay
Caspase-3 activity was determined in keloid cells
using a colorimetric kit from BioVision Inc., (Milpitas, CA, USA)
in accordance with the manufacturer's instructions.
The assay is based on the spectrophotometric
detection at 405 nm of the chromophore p-nitroaniline.
(pNA) after cleavage from the labeled substrate
DEVDpNA by caspase-3. After Ingenol Mebutate treatment for 48 and
72 h, cells were washed with ice-cold PBS, lysed, centrifuged for
10 min at 10,000 × g. Same volume of cell lysate was added to 50 µl
of reaction buffer and 5 µl of specific Caspase-3 substrate
(DEVD-pNA).
Statistical analyses
All results shown are mean ± standard deviation of
at least three separate experiments, measuring each parameter by
triplicate (n=3). Statistical significant differences were tested
by one way analysis of variance (ANOVA), and, when the F value was
significant, by Student-Newman-Keul's post hoc test. Analysis was
performed using UNISTAT version 10 software (Unistat Ltd.,
Highgate, London, England). P<0.05 was considered to indicate a
statistically significant difference.
Results
MicroRNAs differential expression in
keloid fibroblasts following Ingenol-mebutate treatment
We examined the microRNA profile in keloid
fibroblasts following ingenol-mebutate treatment.
Apoptosis was induced in a dose-dependent manner
with Ingenol Mebutate, and we showed the results at 1 microM
concentration since they were significant.
Among microRNAs assembled in the list of the TaqMan
MicroRNA Assays Human Panel, seven microRNAs (miR-16, −34a,
−34b, −34c, -146, -147, and -205) were
increased 2-fold or greater in IM-treated (1 Micromol, 72 h) keloid
fibroblast cells as compared with untreated cells.
The miR-34 family, miR-34a, −34b, and
-34c, were reproducibly induced over 2-fold after IM
treatment, whereas four other microRNAs (miR-16,
-146, -147, and -205) were not constantly
induced. The expression of 17 microRNAs in IM exposed keloid
fibroblast cells did not change compared with the untreated cells,
representing the microRNA not being responsive to IM.
Among differentially expressed microRNA, relevant
microRNAs, as miR-34a, miR-338-5p, mir-24 and let-7, were found
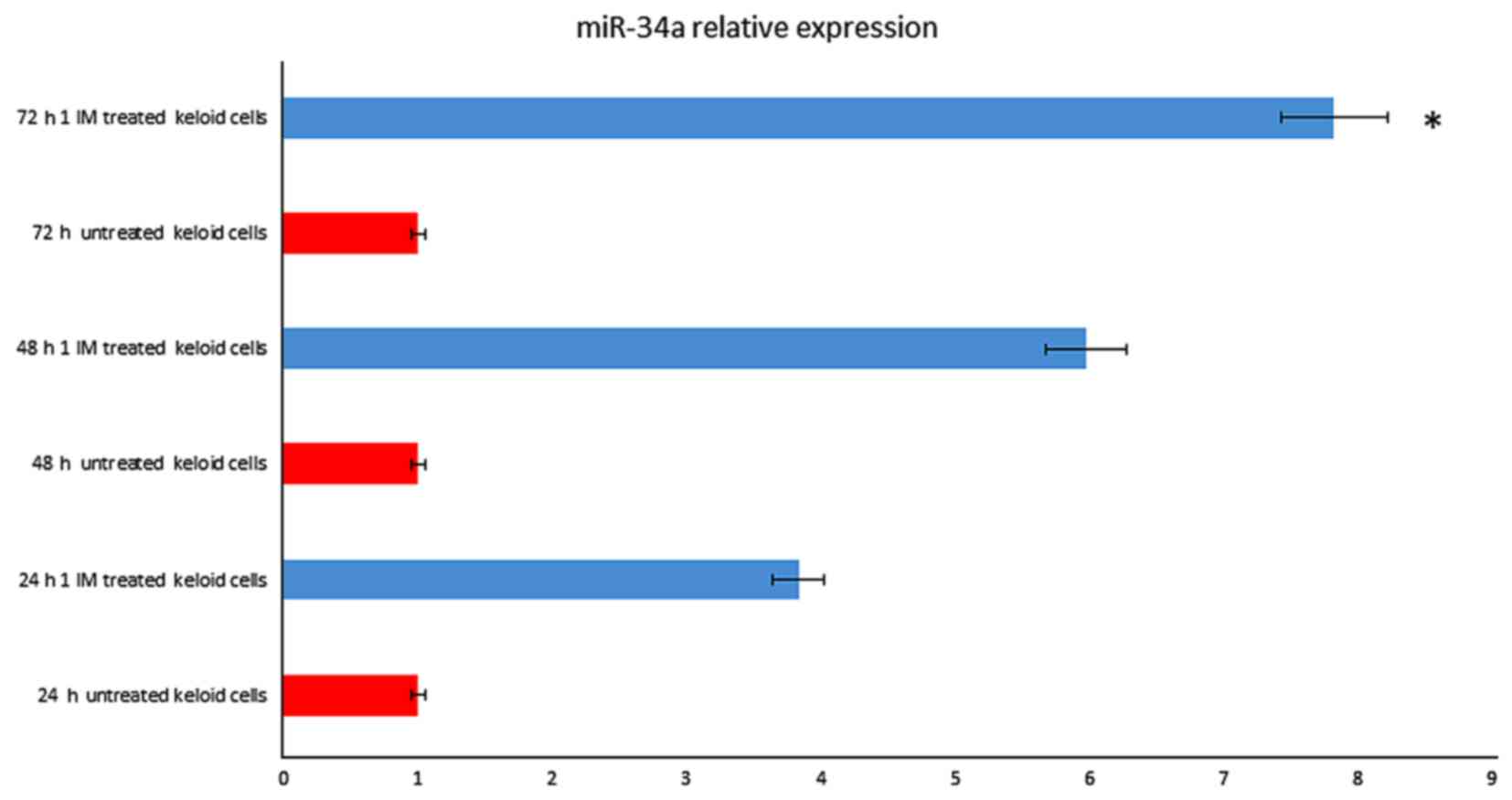
strongly up-regulated. MicroRNAs data analysis showed that miR-34a
(fold change=7.53; P=0.008) were up-regulated after 72 h treatment
(Fig. 1), as well miR-24 (fold
change=6.37; P=0.005) and let-7 (fold change=2.88; P=0.003 (data
not shown)).
Functional categorization for miR-34a
gene targets and Gene-expression analysis
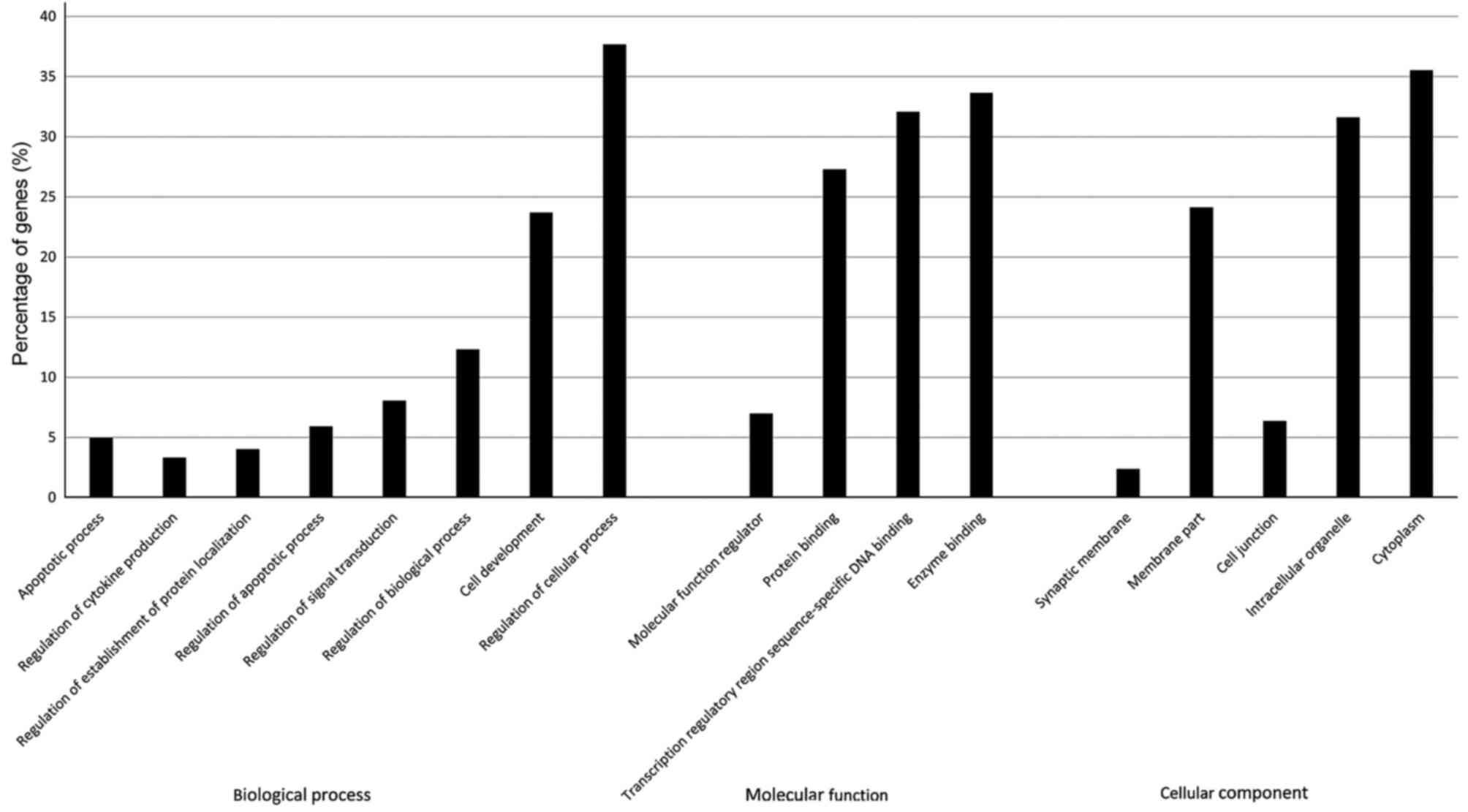
As shown in Fig. 2,
the most significantly enriched GO terms identified after screening
with a threshold of false discovery rate (FDR) of <0.05.
Functional categorization of the GO terms demonstrated that the
miR-34a gene targets were strongly associated with biological
processes as regulation of biological process, apoptotic process
and regulation of apoptotic process. Based on the analysis above,
we assumed that the apoptosis pathway is one of the main target for
mir-34a (9).
Beside, it has reported that Ingenol Mebutate induce
apoptosis in keratosis actinic cells. Therefore, we aimed to
understand if similar molecular mechanism is involved in the
treatment of keloids with Ingenol Mebutate. Later, in future
research, we aim to investigate other molecular pathway too.
Therefore, we explored the consequences of such
microRNA up-regulation in Ingenol Mebutate (1 µM after 72 h)
treated keloid fibroblasts on 84 genes of the apoptosis pathway
using a PCR array. miR-34a overexpression resulted related to a
significant up regulation of tp53 and genes that plays a central
role in regulation and activation of apoptosis such as tp53bp2, a
regulator that plays a central role in regulation of apoptosis and
cell growth via its interactions with TP53 (Fig. 3).
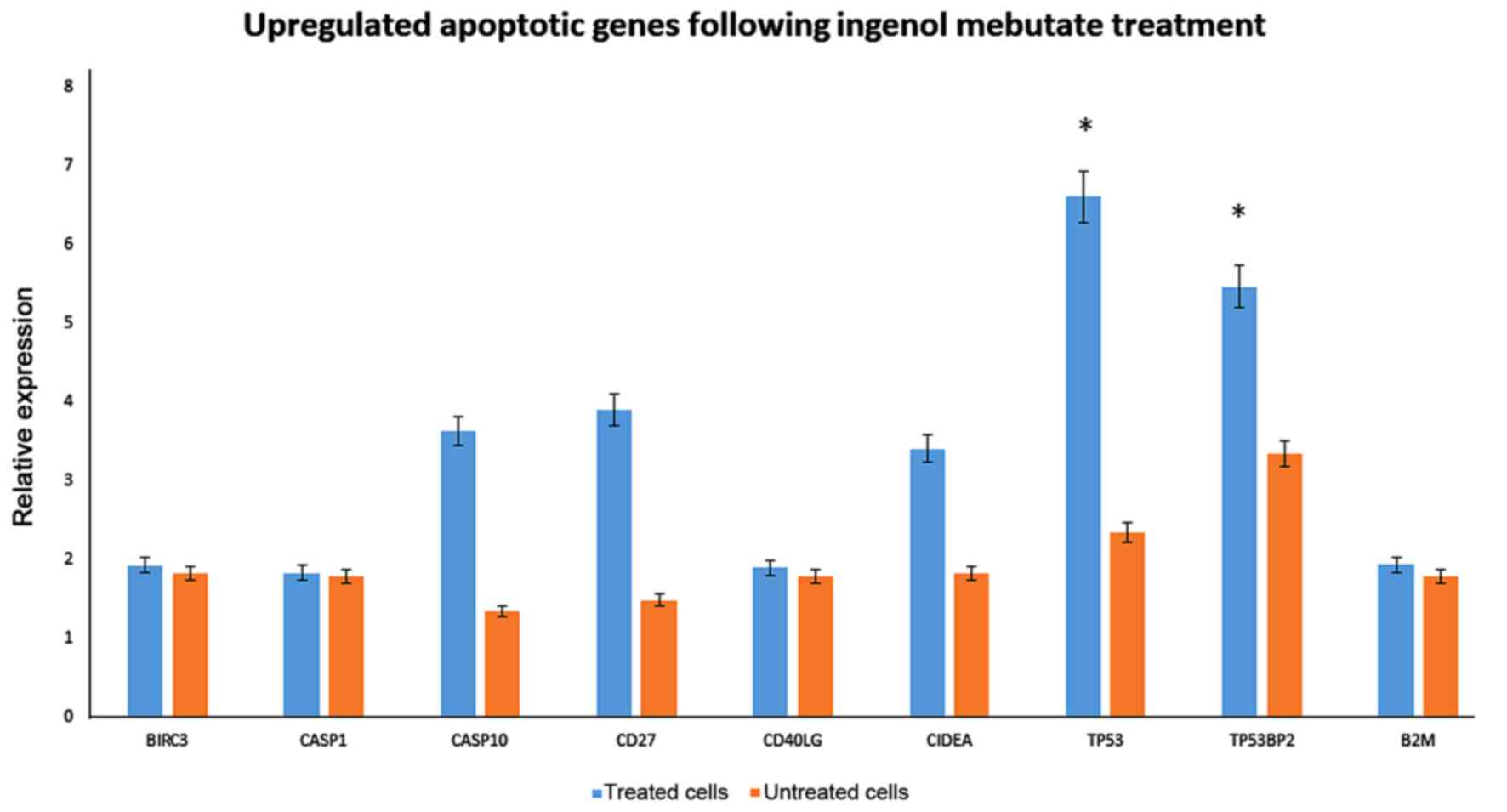 | Figure 3.Upregulated apoptotic genes in keloid
fibroblasts following IM treatment. Significant upregulation of the
expression of apoptotic genes, was evaluated by quantitative
polymerase chain reaction in 1 µmol IM treated keloid fibroblasts
following 72 h of treatment. *P<0.05 vs. untreated cells. IM,
Ingenol Mebutate; BIRC3, baculoviral IAP repeat-containing protein
3; CASP, caspase; CD, cluster of differentiation; CD40LG, CD40
ligand; CIDEA, cell death inducing DFFA-like effector A; TP53,
tumor protein p53; TP53BP2, TP53 binding protein 2; B2 M,
β-2-microglobulin. |
Among the up-regulated genes, birc3, casp1, casp10,
and CIDEA were detected. Interestingly, CD40 was up-regulated,
which is expressed on cultured human fibroblasts, but also shifted
fibroblasts into the G0/G1 phase of the cell cycle.
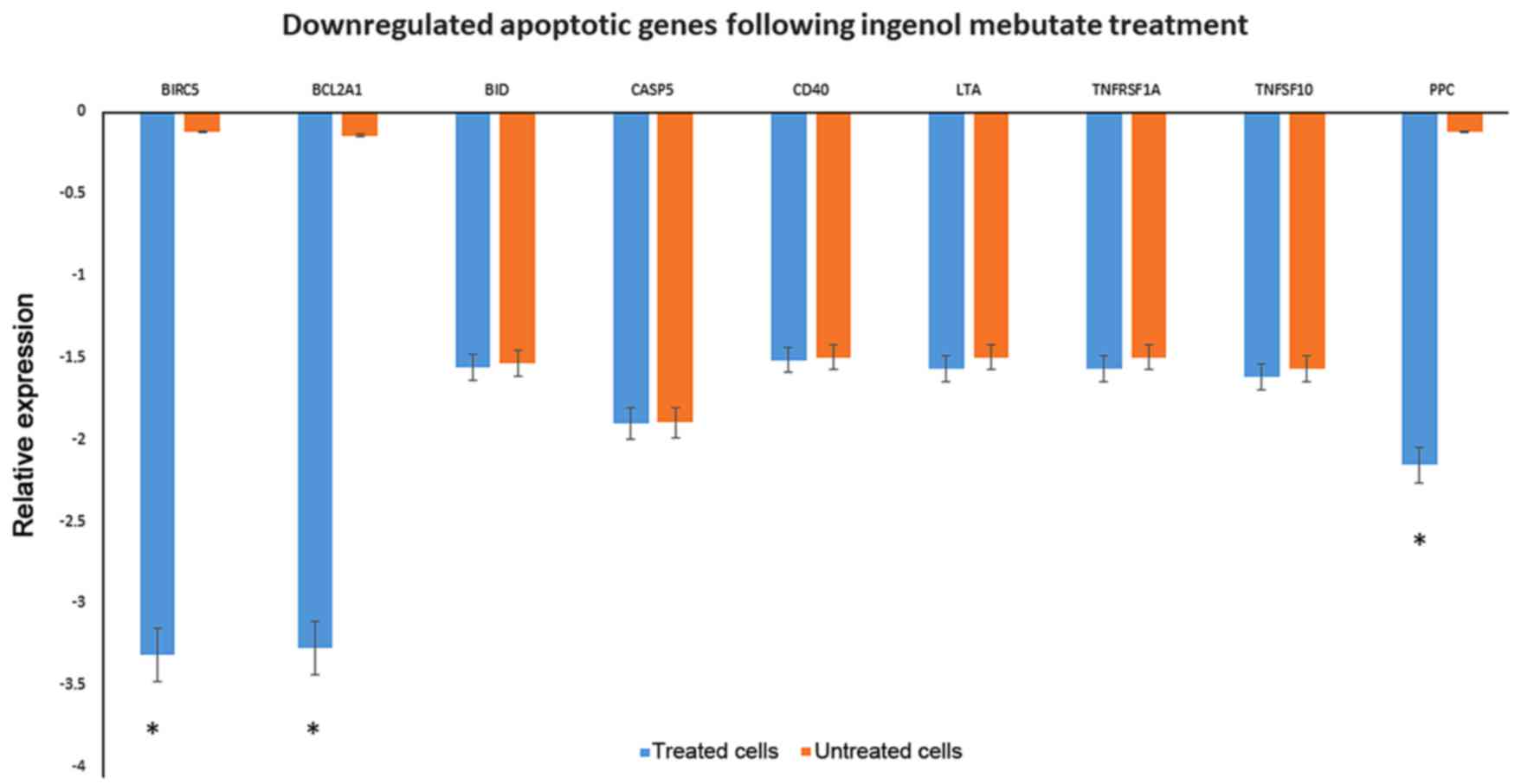
The down-regulation of Bid, casp5, cd40, cd70, and
members of Tumor necrosis factor receptor superfamily as TNFRSF1A
and TNFSF10, leading to signalling complexes that activate both the
apoptotic caspase cascade and the NF-κB and JNK anti-apoptotic
pathways, were observed among the list. Genes associated with the
inhibition of apoptosis as birc5 and bcl2a1 were highly
down-regulated (Fig. 4).
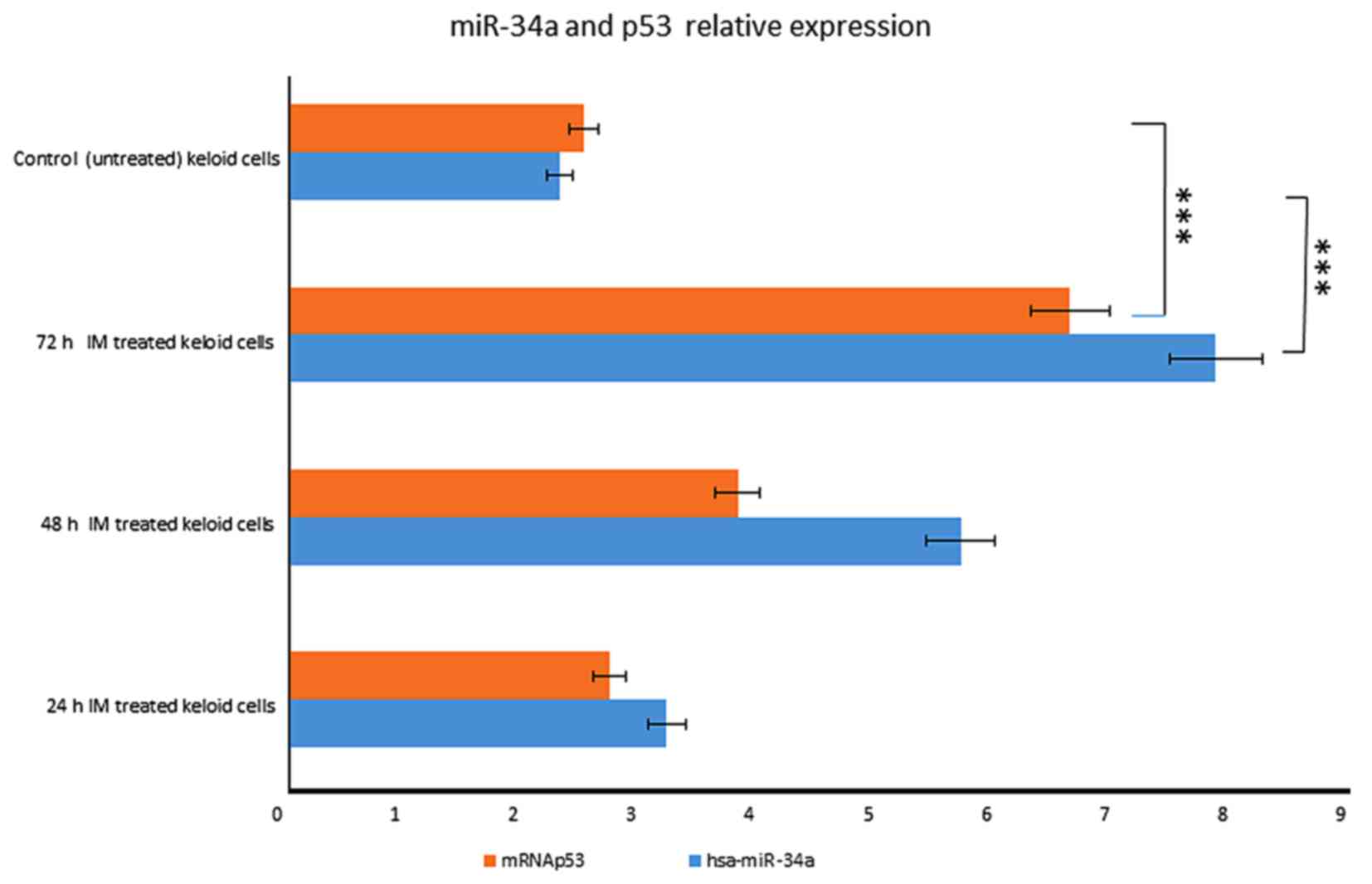
miR-34a induction depends on p53
activation
In particular, miR-34a expression was
increased in a time-dependent manner after IM treatment, rising
3.2-fold at 24 h until 7 fold at 72 h (Fig. 1). We also observed that p53 started
to increase at 24 h, respectively, and the increasing continued
until 72 h after treatment (Fig.
5).
These results indicate that miR-34a is
induced in a p53-dependent manner after IM treatment.
Ingenol mebutate induces
apoptosis-like phenotypes
We observed that introduction of Ingenol Mebutate in
keloid fibroblast cells caused apoptosis-like phenotypes showing
similar morphological changes with enlarged cellular size.
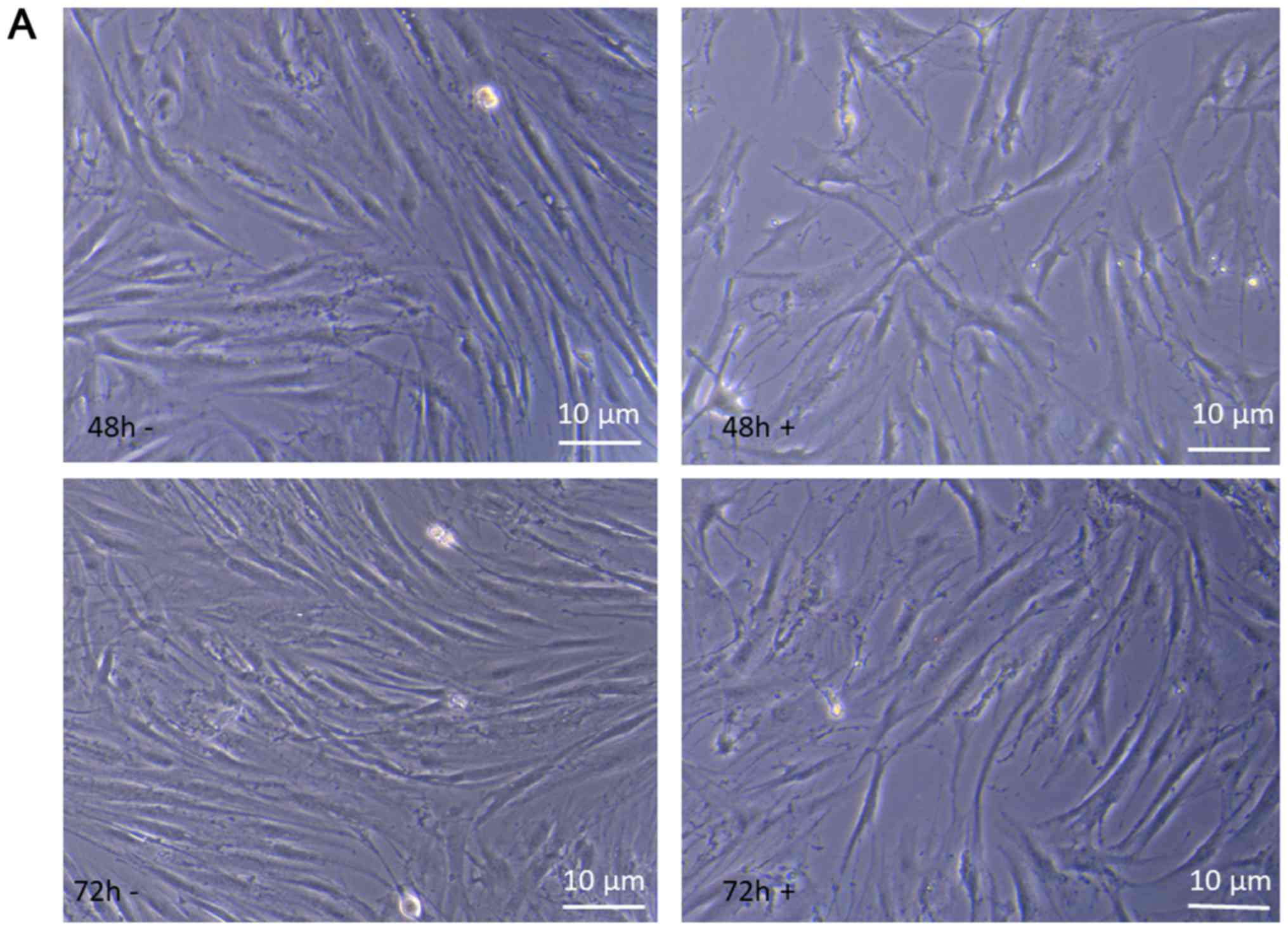
The behavior of keloid fibroblasts over the 72 h
period of treatment with 1 µM Ingenol Mebutate was monitored by
phase-contrast microscopy and high enlargement pictures analyzed.
As shown in Fig. 6A at 48 and 72 h
of incubation time, comparing treated to untreated keloid
fibroblasts morphological changes are evident that strongly suggest
alterations in the cytoskeleton organization, impairment of cell
growing, differentiation and development. These results suggest
that suppression of cell proliferation by Ingenol Mebutate could be
mainly associated with the induction of apoptosis-like phenotypes.
Therefore, we evaluated apoptosis with Annexin V assay. As shown in
Fig. 6B, staining revealed Annexin
V-positive/PI negative, apoptotic, keloid cells after 72 h ingenol
mebutate treatment. Quantification of the annexin was not
assayed.
Ingenol mebutate induces DNA
fragmentation
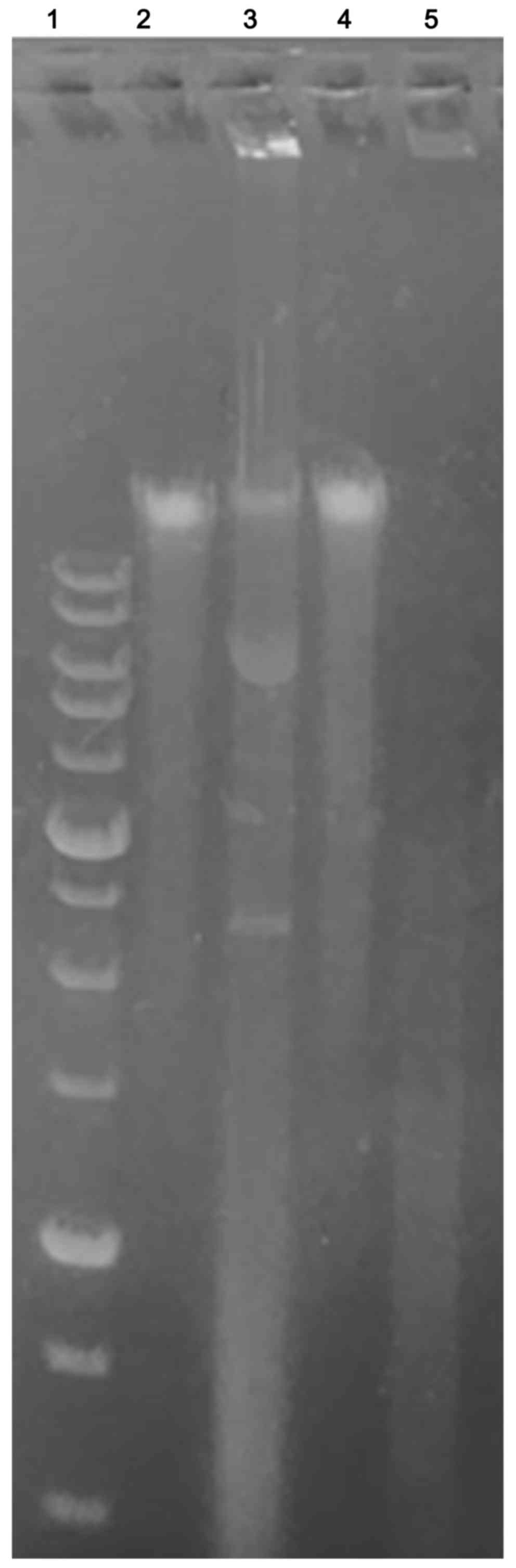
To verify if Ingenol Mebutate could induce DNA
fragmentation and thus to confirm whether apoptosis occurred,
keloid fibroblasts exposed to treatment were assessed for DNA
laddering by agarose gel electrophoresis (Fig. 7). We found that treated keloid
fibroblasts after 48 and 72 h of incubation with 1 Micromol Ingenol
mebutate, showed apoptotic DNA fragmentation profiles. On the
opposite, no nucleic acid fragmentation was observed in negative
controls represented by untreated cells.
Apoptotic effect mediated through
caspase-3
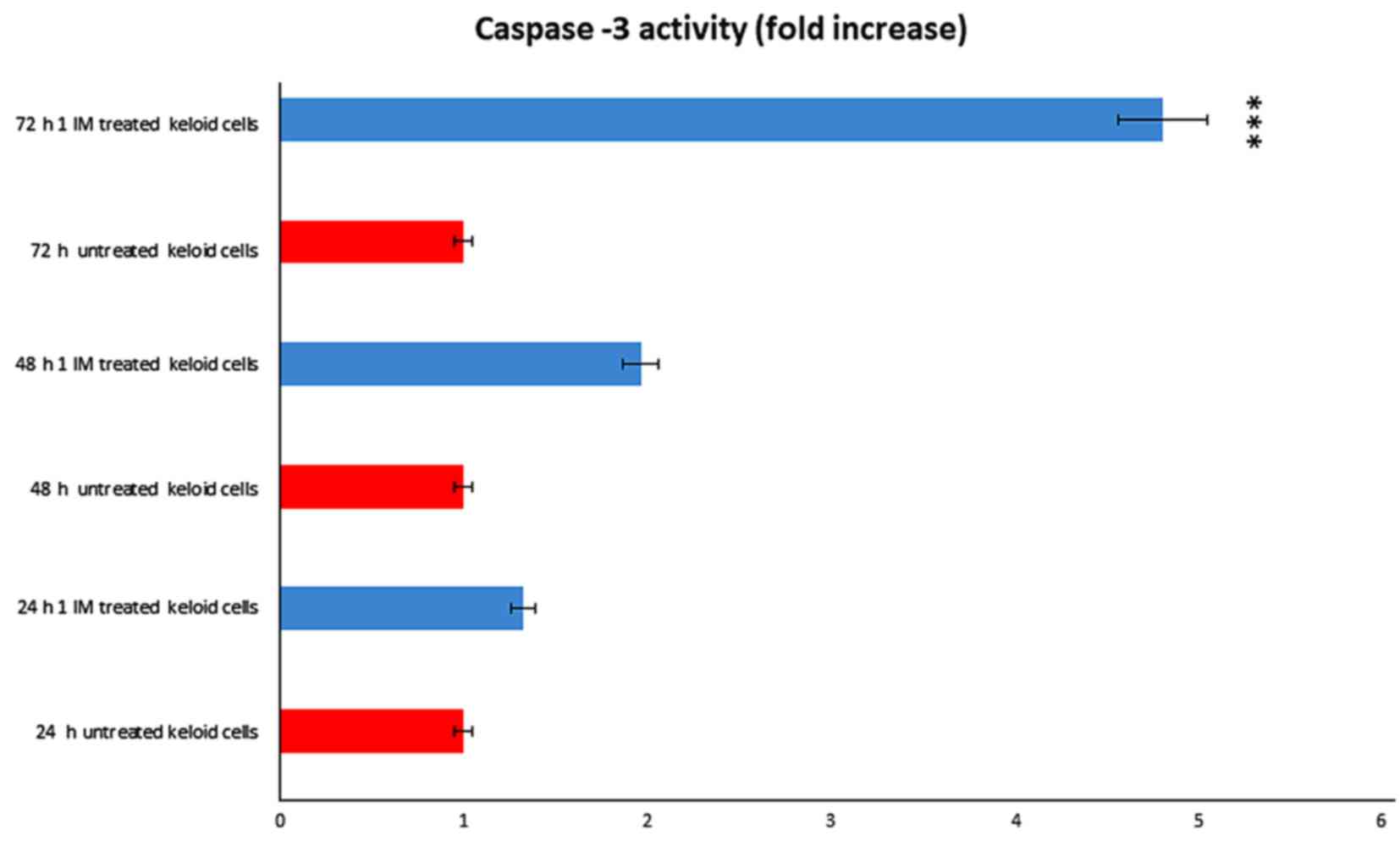
We evaluated the potential contribution of caspase-3
in Ingenol mebutate treated keloid cells. We obtained that
caspase-3 was significantly activated after treatment (Fig. 8). We observed a dose and a time
dependent effect, obtaining a significant result after 72 h of
incubation with 1 Micromol Ingenol mebutate.
Discussion
Keloids are benign tumors that resist all clinical
therapies and often recur, compromising the quality of life of many
patients. Since pro-apoptotic effects of this drug have been
demonstrated in several malignant cells (5), in a previous research we successfully
treated a woman with a large keloid with Ingenol mebutate (6). So far, there is no molecular study on
its effect on keloid fibroblasts.
Here, to explore such novel treatment modalities for
keloids and to elucidate the molecular mechanism of IM treatment in
keloid fibroblasts, we analyzed the microRNA and gene apoptosis
expression patterns in keloid fibroblasts following exposure to
Ingenol mebutate, by array strategy.
Current studies have suggested that microRNAs
regulates gene expression and play important roles in mediating
responses of drug molecules (18).
microRNAs can regulate the expression of drug transporters and drug
targets. Following Ingenol-mebutate exposure, we identified miR-34a
as a major microRNA that is induced in in keloid fibroblasts.
miR-34 is a direct targets of p53 and their
over-expression results in the induction of apoptosis, cell cycle
arrest and senescence (7,9,19).
Beside, miR-34a could partially replace p53 in inducing apoptosis
or senescence. p53 directly can activate the expression of miR-34
genes, which play an important role in p53-mediated apoptotic
pathway (20). Activation of
miR-34a by p53 may contribute to induction of apoptosis. The
miR-34a gene itself may be a tumor suppressor gene since it
maps to a region on chromosome 1p36 which is commonly deleted in
neuroblastoma.
The p53 tumor-suppressor protein activates a mass of
genes encoding proteins with roles in apoptosis, DNA repair,
senescence, and cell-cycle control. miR-34 might be key effectors
of p53 tumor-suppressor function, and the inactivation might
contribute to definite cancers.
Due to IM exposure in keloid fibroblasts, genotoxic
stress activated p53 transcription of many genes, leading to cell
cycle arrest, apoptosis or senescence (21). microRNAs, as the miR-34 family, are
transcriptionally activated by p53. In turn microRNAs regulate
expression of many p53-induced genes.
However, the outcome of miR-34a overexpression on
p53 levels and role depends on cellular setting (7). In order to evaluate the association
between the regulation of miR-34 with pro-apoptotic gene expression
in Ingenol Mebutate treated keloid fibroblast, we measured
expression levels of the apoptosis pathway gene was here
investigated using a PCR array. We found a positive correlation
between the increase of miR-34 and p53 expression.
Tp53 plays a central role in regulation and
activation of apoptosis in Ingenol Mebutate treated keloid
fibroblasts, since was up-regulated as well as tp53bp2, a regulator
that plays a central role in regulation of apoptosis and cell
growth via its interactions with TP53. Among the up-regulated
genes, more apoptotic genes as birc3, casp1, casp10, and CIDEA were
identified. Such genes plays a central role in the execution-phase
of cell apoptosis and regulate also inflammatory signaling and
immunity.
miR-34 family were shown to suppress tumor growth
and metastasis by promoting processes that inhibit carcinogenesis,
such as apoptosis and senescence. MiR-34 regulate these processes
through downregulation of their target mRNAs. As of today, more
than 77 miR-34 targets have been confirmed (22). Among miR-34 target gene, we
detected birc5 and bcl2a1, genes associated with the inhibition of
apoptosis, that were down-regulated.
The expressions of more miR34a target genes, such as
Notch1, Delta1, Bcl2 or E2F3 in Ingenol Mebutate treated cells,
will be examined in next researches.
The molecular results have been confirmed by the
behavior of keloid fibroblasts over the 72 h period of treatment
with 1 µM Ingenol Mebutate that was monitored by phase-contrast
microscopy. Microscope observations showed an apoptosis-like
phenotype such as accumulation of typical membrane blebbing,
containing cytoplasm and encircled by multilayered lamellar
structures, as well as fingerprint profiles and apparent
mitochondrial swelling. DNA fragmentation Ingenol treated
fibroblasts is also a hallmark of cell apoptosis. Apoptotic effect
of Ingenol Mebutate effect on keloid cells was confirmed by
Annexin-V staining and caspase-3 assay.
All together, these results indicate that Ingenol
mebutate induce fibroblasts growth inhibition by the promotion of
apoptosis.
Our results show, for the first time, that Ingenol
Mebutate treatment induce DNA damage, which is able to generate the
apoptotic response in keloid fibroblasts with higher expression of
miR-34, probably because of a strong interaction between p53 and
miR-34, which is a direct transactivational target of p53, inducing
apoptosis, cell cycle arrest and senescence. Following Ingenol
Mebutate treatment, miR-34 may modulates the pro-apoptotic genes
expression promoting apoptosis in a p53-independent manner and in a
p53-dependent manner.
These events all promote cell apoptosis. Our
findings show that the miR-34a regulates cell-cycle progression and
apoptosis together with p53 following Ingenol mebutate exposure. We
put down a groundwork for further experiments in order to progress
these challenges in the future.
Acknowledgements
The authors thank Professor Paolo Mondola
(University of Naples ‘Federico II’, Naples, Italy), who greatly
encouraged us to continue this study.
Funding
The present study was supported partly by a research
grant from the University of Naples II, Italy (grant no. Grant L.R.
N.5 2007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BDF performed all of the experiments and wrote the
manuscript. CG and MS performed microscope observations. FM
interpreted the data, corrected the English and helped draft the
manuscript. MN designed the present study, critically revised the
manuscript for intellectual content and gave final approval of the
version to be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miR/miRNA
|
microRNA
|
|
p53
|
tumor protein p53
|
References
|
1
|
Halim AS, Emami A, Salahshourifar I and
Kannan TP: Keloid scarring: Understanding the genetic basis,
advances and prospects. Arch Plast Surg. 3:184–189. 2012.
View Article : Google Scholar
|
|
2
|
Shih B and Bayat A: Genetics of keloid
scarring. Arch Dermatol Res. 5:319–339. 2010. View Article : Google Scholar
|
|
3
|
Chen W, Fu X, Sun X, Sun T, Zhao Z and
Sheng Z: Analysis of differentially expressed genes in keloids and
normal skin with cDNA microarray. J Surg Res. 113:208–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russell SB, Russell JD, Trupin KM, Gayden
AE, Opalenik SR, Nanney LB, Broquist AH, Raju L and Williams SM:
Epigenetically altered wound healing in keloid fibroblasts. J
Invest Dermatol. 130:2489–2496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stahlhut M, Bertelsen M, Hoyer-Hansen M,
Svendsen N, Eriksson AH, Lord JM, Scheel-Toellner D, Young SP and
Zibert JR: Ingenol mebutate: Induced cell death patterns in normal
and cancer epithelial cells. J Drugs Dermatol. 11:1181–1192.
2012.PubMed/NCBI
|
|
6
|
De Felice B, Guida M, Boccia L and Nacca
M: Ingenol mebutate treatment in keloids. BMC Res Notes. 8:4662015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Navarro F and Lieberman J: miR-34 and p53:
New insights into a complex functional relationship. PLoS One.
10:e01327672015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tarasov V, Jung P, Verdoodt B, Lodygin D,
Epanchintsev A, Menssen A, Meister G and Hermeking H: Differential
regulation of microRNAs by p53 revealed by massively parallel
sequencing: miR-34a is a p53 target that induces apoptosis and
G1-arrest. Cell Cycle. 3:1586–1593. 2007. View Article : Google Scholar
|
|
9
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Fortin K and Mourelatos Z:
MicroRNAs: Biogenesis and molecular functions. Brain Pathol.
18:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Felice B, Guida M, Guida M, Coppola C,
De Mieri G and Cotrufo R: A miRNA signature in leukocytes from
sporadic amyotrophic lateral sclerosis. Gene. 508:35–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rukov JL and Shomron N: MicroRNA
pharmacogenomics: Post-transcriptional regulation of drug response.
Trends Mol Med. 17:412–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koturbash I, Tolleson WH, Guo L, Yu D,
Chen S, Hong H, Mattes W and Ning B: MicroRNAs as pharmacogenomics
biomarkers for drug efficacy and drug safety assessment. Biomark
Med. 9:1153–1176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shomron N: MicroRNAs and pharmacogenomics.
Pharmaco-genomics. 11:629–632. 2010.
|
|
17
|
Lim IJ, Phan TT, Song C, Tan WT and
Longaker MT: Investigation of the influence of keloid-derived
keratinocytes on fibroblast growth and proliferation in vitro.
Plastic Reconstr Surg. 107:797–808. 2001. View Article : Google Scholar
|
|
18
|
He Y, Chevillet JR, Liu G, Kim TK and Wang
K: The effects of microRNA on the absorption, distribution,
metabolism and excretion of drugs. Br J Pharmacol. 172:2733–2747.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang TC, Wentzel EA, Kent OA,
Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M,
Ferlito M, Lowenstein CJ, et al: Transactivation of miR-34a by p53
broadly influences gene expression and promotes apoptosis. Mol
Cell. 26:745–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raver-Shapira N, Marciano E, Meiri E,
Spector Y, Rosenfeld N, Moskovits N, Bentwich Z and Oren M:
Transcriptional activation of miR-34a contributes to p53-mediated
apoptosis. Mol Cell. 26:731–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oren M: Decision making by p53: Life,
death and cancer. Cell Death Differ. 10:431–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rokavec M, Li H, Jiang L and Hermeking H:
The p53/miR-34 axis in development and disease. J Mol Cell Biol.
6:214–230. 2014. View Article : Google Scholar : PubMed/NCBI
|