Introduction
Dexmedetomidine (DEX) has been widely used in
intensive care units (ICU) and clinical anesthesia due to its
anxiolytic and sedative effects without respiratory depression and
with minor adverse effects (1).
Studies have suggested that perioperative use of DEX reduced the
incidence of complications and mortality after cardiac surgery
(2). Oxidative stress plays an
important role in the development of many diseases, and previous
studies suggest that pretreatment with DEX protects against
oxidative stress injury and/or cell apoptosis of many organs
including lung, kidney, intestine and hippocampus. (3–5).
Yoshitomi et al (6)
reported that DEX exerted a direct protective effect against
ischemia/reperfusion (I/R) injury on the myocardium. However, it is
still unclear whether oxidative stress signaling pathway was
involved in the cardioprotective effect of DEX.
Reactive oxygen species (ROS) are generated in the
ischemic myocardium, especially after reperfusion, and the major
source of ROS in the I/R myocardium are mitochondria (7,8).
Organ I/R injury has been associated with significant increase in
ROS and depolarization of mitochondrial transmembrane potential
(ΔΨm), these factors play a key role in mitochondrial oxidative
stress-induced cell injury (9,10).
Fu et al (3) reported that
DEX attenuated lipopolysaccharide (LPS)-induced acute lung injury
through inhibiting oxidative stress and ROS. These studies suggest
that oxidative stress may be involved in the cardioprotective
effect of DEX. In addition, endoplasmic reticulum (ER) stress is
also one of the important pathways involved in oxidative stress,
ischemia, and other physiological and pathological conditions
(11,12). Unfolded protein response (UPR), the
key step in ER stress-mediated apoptosis, restored ER environment
in the early stage of ER stress or leads to changes in cell
function if UPR continues (13).
Based on literature review, this study aimed to
raise and investigate the hypothesis that DEX exerts a
cardioprotective effect through attenuating mitochondria and ER
stress-mediated injury and apoptosis in NRCMs.
Materials and methods
Reagents
Dexmedetomidine hydrochloride (DEX)
(C13H16N2·HCl, MW 236.7 g/mol) was
purchased from Singch Pharm (Jiangsu, China). DEX was added to DMEM
to obtain the desired concentration. H2O2
solution and collagenase type II were obtained from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany).
Cell culture and treatment
All protocols in this study were approved by the
Ethics Committee of Southwest Medical University. Neonatal rats
(2–3 days) was used for the isolation and culture of neonatal rat
cardiomyocytes (NRCMs) as we reported previously (14). Cultured NRCMs in DMEM with 10% FBS
were further divided into four groups by different treatments: i)
Control group: normal DMEM adding with 10% FBS, ii)
H2O2 group [with H2O2
for 6 h at 500 µM (15)], iii) DEX
group [with DEX at 5 µM (16)],
and iv) H2O2 + DEX group (with 5 µM DEX for 2
h before exposing to 500 µM H2O2 for 6 h).
Cells with different treatments then underwent serial analyses
including enzyme-linked immunosorbent assay (ELISA), western
blotting, flow cytometry (FCM) and fluorescent microscopy.
ELISA
ELISA was used in this study to detect the levels of
lactic dehydrogenase (LDH), glutathione (GSH) and the activities of
caspase 3, 8, 9 and 12 with ELISA kits according to the
instructions and our previous research (14).
FCM
The level of apoptosis, intracellular ROS level and
mitochondrial membrane potential (ΔΨm) in NRCMs were detected with
FCM (BD Biosciences, Franklin Lakes, NJ, USA) according to the
instructions and our previous research (14). The level of apoptosis in NRCMs was
analyzed with FITC-Annexin V Apoptosis Detection kit I (BD
Biosciences). Briefly, cells were harvested with cold PBS and
resuspended at a density of 1×106 cells/ml. Five µl of
FITC Annexin V and 10 µl propidium iodide (PI) were added in 100 µl
cell suspension and incubated at room temperature (25°C) for 15
min. Four hundred µl of 1X binding buffer was added to each tube
and apoptosis was tested with FCM within 1 h. To measure the
intracellular ROS level, NRCMs were washed with PBS and incubated
with the probe for ROS, membrane permeable
dichloro-dihydro-fluorescein diacetate (DCFH-DA) (final
concentration 10 µM) in PBS at 37°C for 20 min. At the end of
incubation, cells were washed with PBS and the level of ROS was
analyzed by flow cytometry. ΔΨm was also measured with FCM using
the JC-1 MitoScreen kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The ratio of monomeric JC-1 represented ΔΨm. The
increased ratio represented the depolarization of ΔΨm, while a
decreased ratio indicated the hyperpolarization of ΔΨm.
Immunofluorescence microscopy
Immunofluorescence microscopy of NRCMs was conducted
as previously described (14). In
brief, NRCMs were fixed in cold 4% paraformaldehyde, then blocked
with 5% BSA and treated with 0.1% Triton-X 100. NRCMs were further
incubated with the monoclonal primary antibody against α-actin
produced in mouse (1:100; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) overnight at 4°C and then incubated with DyLight
488-conjugated anti-mouse IgG secondary antibody (1:200; Abcam,
Cambridge, UK) for 1 h at room temperature. In addition, for the
staining of F-actin, NRCMs were treated with rhodamine phalloidin
(with final concentration, 100 nM) for 30 min at room temperature.
Images were acquired with a fluorescence microscope (Olympus IX-81;
Olympus Corp., Tokyo, Japan).
Tunnel asay
Tunnel assay was also used to investigate the effect
of DEX on H2O2 induced cell apoptosis. The
protocol was manipulated according to the instructions of tunnel
assay kit (Beyotime Institute of Biotechnology, Haimen, China). In
brief, NRCMs with different treatment was fixed with cold 4%
paraformaldehyde for 30 min and 0.3% Triton X-100 was treated for 5
min. The Tunnel detecting solution [including Terminal
Deoxynucleotidyl Transferase (TdT) and fluorescence solution] was
added and incubated for 60 min at 37°C. Images were acquired with a
fluorescence microscope (Olympus IX-81).
Western blot analysis
Western blotting was performed to determine the
protein expression level under various conditions. Lysates with 50
µg protein were separated by SDS-PAGE. The proteins were
transferred to PVDF membrane and blocked with 5% BSA in TBS-T
solution. The PVDF membrane was incubated with primary antibodies
against Grp78 (1:1,000; Abcam), IRE1α (1:1,000), Bcl-2 (1:1,000),
Bax (1:1,000; all Cell Signaling Technology, Inc., Danvers, MA,
USA) and GAPDH (1:10,000) from Sigma-Aldrich; Merck KGaA as
house-keeping protein overnight at 4°C, respectively. The membrane
was then incubated with HRP-conjugated IgG as a secondary antibody
(1:2,000; BBI, China) for 1 h at room temperature and the
immunoreactive bands were developed using an enhanced
chemiluminescent substrate (Engreen Biosystem, Ltd., Beijing,
China). Images of blotting bands and statistical analysis of the
gels were used Bio-Rad QuantityOne® software (Bio-Rad
Laboratories, Richmond, CA, USA).
Statistical analysis
The results obtained were presented as the mean ±
standard error of mean (SEM). Analysis of variance (ANOVA) was used
to statistical analysis for differences. Least Significant
Difference (LSD) was used for further multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DEX protects
H2O2-induced structural impairment of
NRCMs
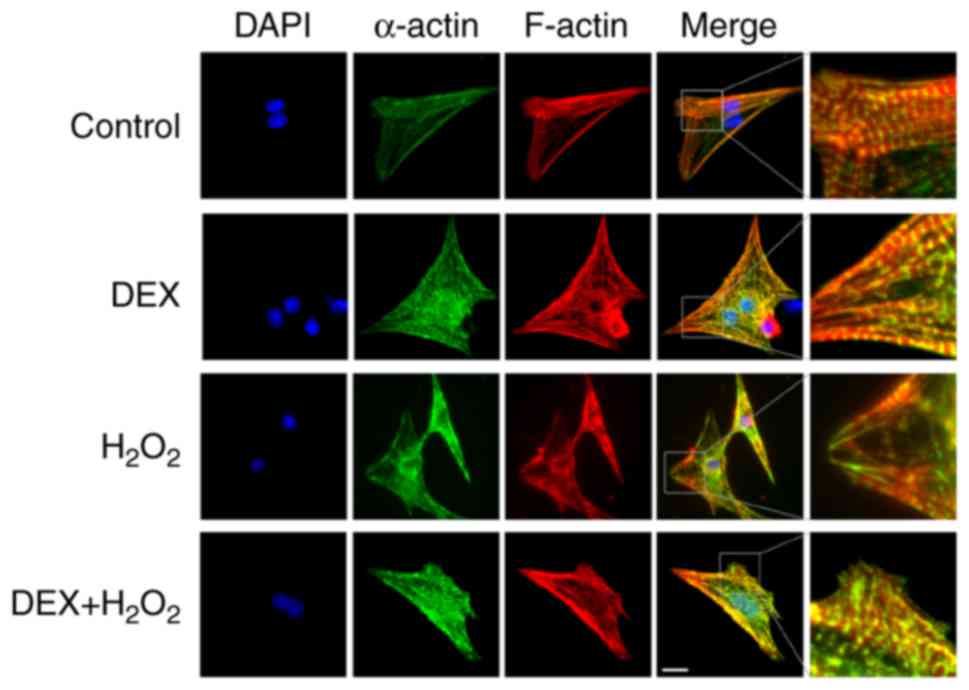
We detected the effects of DEX on
H2O2-induced injury of NRCMs. As shown in
Fig. 1, green colors indicated the
T-tube tagged with α-actin and red colors represented the
cytoskeleton tagged with F-actin in NRCMs. NRCMs without
H2O2 treatment exhibited clear and neatly
arranged myocardial stripes (green) and cytoskeleton
(red) (the first row in Fig.
1). Treatment with DEX alone did not significantly affect the
structure of NRCMs (the second row in Fig. 1). Treatment of
H2O2 significantly changed the shape of NRCMs
from typical fusiform cell to irregularly shaped cell and
H2O2 impaired the structure of NRCMs with
disordered a-actin and F-actin as shown in Fig. 1 (the third row). Pre-treatment with
DEX (5 µM) and H2O2 significantly attenuated
the impairment of cell structure induced by
H2O2 (the fourth row in Fig. 1). The right column in Fig. 1 showed that the effect of DEX on
the structure of NRCMs with more details.
DEX alleviates
H2O2-induced apoptosis of NRCMs
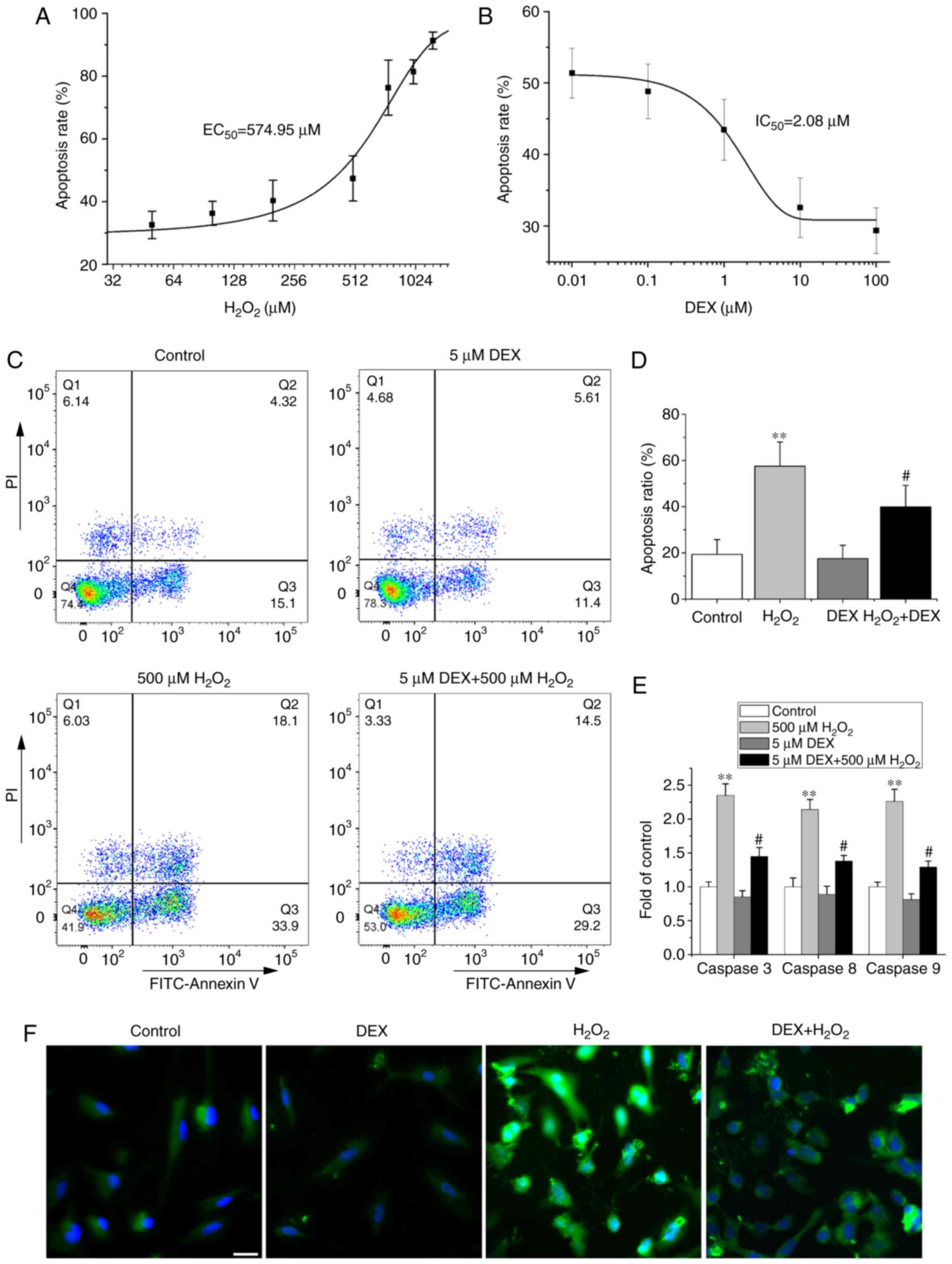
We investigated the effect of DEX on apoptosis of
NRCMs induced by H2O2 with FCM, and results
were shown in Fig. 2. We first
tested the dose-dependent effect of H2O2
(0–1250 µM) on apoptosis of NRCMs and results showed that
H2O2 concentration-dependently increased the
cell apoptosis ratio with an EC50 of 574.95 µM (Fig. 2A). Therefore,
H2O2 at 500 µM was used in this study in the
later study. We further found that DEX (0.01–100 µM) reduced
H2O2 induced apoptosis of NRCMs with an
IC50 of 2.08 µM, and thus 5 µM DEX was used in later
study (Fig. 2B). The concentration
of DEX was similar with that used in a previous report (16).
H2O2 increased the ratio of
apoptosis-positive cells located in the first (Q2 area in Fig. 2C) and fourth quadrants (Q3 area in
Fig. 2C) of the FCM plots from the
control group 19.36±6.38 to 57.49±10.52% (Fig. 2C and D), while DEX replenishment
decreased the ratio to 39.86±9.35%. Treatment with DEX alone did
not affect the apoptosis of NRCMs. Consistent with the FCM results,
pretreatment with DEX also significantly attenuated the
H2O2-induced activity increase of caspases 3,
8, and 9, the important apoptosis-inducing molecules. Treatment of
DEX alone did not change the baseline activities of caspases 3, 8,
and 9 (Fig. 2E). Tunnel assay
shown the similar results of DEX on cell apoptosis as that with FCM
(Fig. 2F). These results indicate
that DEX attenuates H2O2-induced apoptosis of
NRCMs.
DEX suppresses
H2O2-induced mitochondrial oxidative stress
injury in NRCMs
To evaluate the effect of DEX on oxidative stress
induced by H2O2, we further determined the
levels of ROS, GSH and LDH, the important indices of oxidative
stress. Fig. 3A showed that ROS
level was evaluated with FCM in NRCMs with different treatments. As
shown in Fig. 3B and C,
H2O2 treatment increased the levels of ROS
and LDH and reduced GSH release. Pretreatment with DEX alleviated
H2O2-induced increment of ROS and LDH and
refreshed H2O2-induced reductions of GSH
significantly.
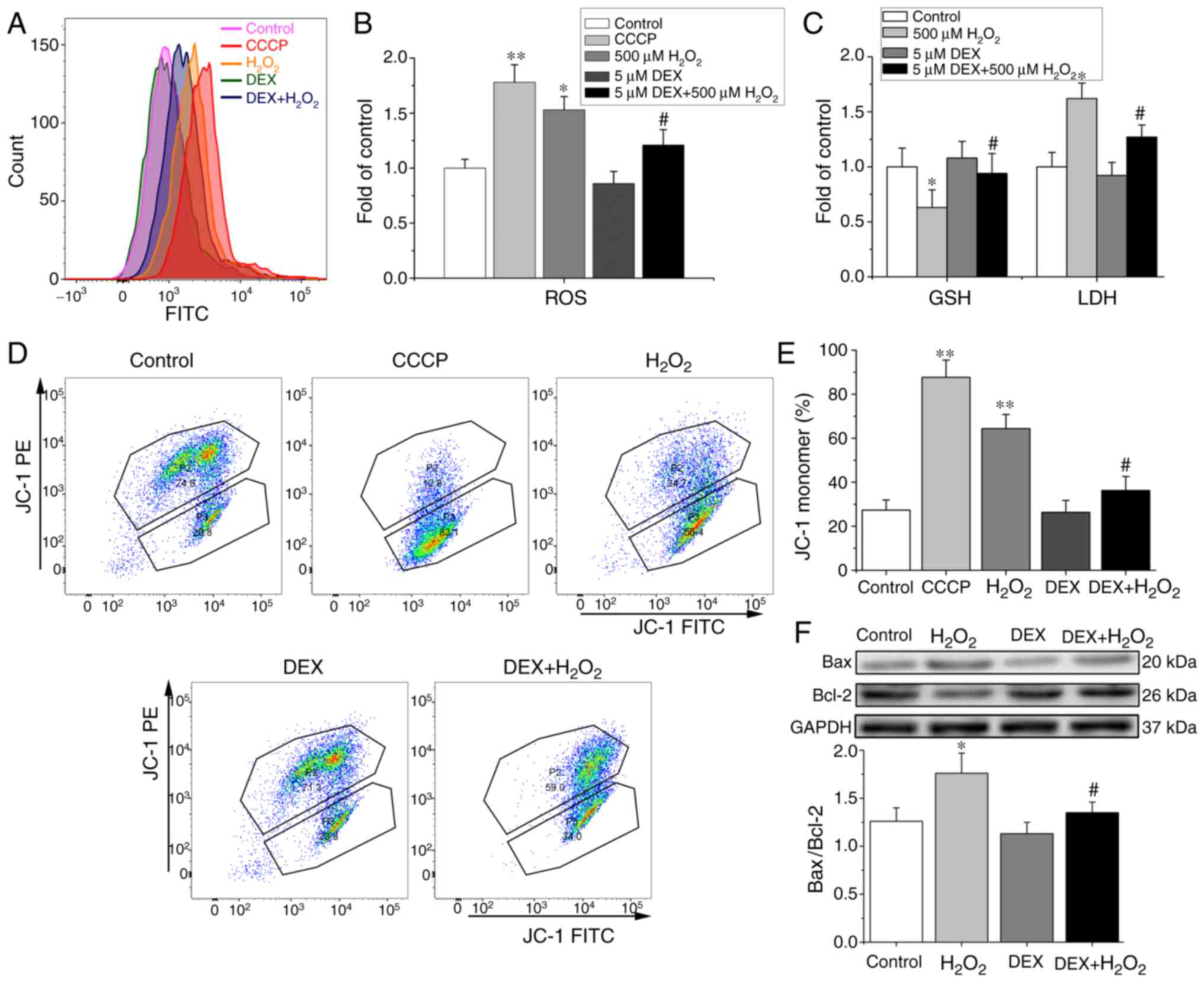 | Figure 3.Effects of DEX on
H2O2-induced mitochondrial oxidative stress
injury and apoptosis of NRCMs. (A) The original flow cytometry
histogram showing the evaluation the ROS level in NRCMs with
different treatments. (B) Effects of DEX on ROS in
H2O2-challenged NRCMs. n=5. (C) Effects of
DEX on LDH and GSH in H2O2-challenged NRCMs.
n=5. (D) The original flow cytometry plot showing the evaluation
the ΔΨm level in NRCMs. The percentage of JC-1 monomer in P3 area
indicating the level of ΔΨm. (E) Flow cytometry showing that DEX
inhibited H2O2-induced ΔΨm depolarization
monitored by JC-1 dye and indicated by JC-1 monomer (n=5). CCCP, a
mitochondrial uncoupler, was used as a positive control drug to
induce depolarization of ΔΨm. (F) Western blotting showing the
protein levels of Bax and Bcl-2 (upper) and the ratio of Bax/Bcl2
(lower) (n=5). *P<0.05, **P<0.01 vs. control.
#P<0.05 vs. H2O2. DEX,
dexmedetomidine; NRCMs, neonatal rat cardiomyocytes ROS, reactive
oxygen species; LDH, lactic dehydrogenase; GSH, glutathione; ΔΨm,
mitochondrial transmembrane potential; CCCP, carbonyl cyanide 3
chlorophenylhydrazone. |
ΔΨm was also an important oxidative stress index. We
investigated whether DEX affects the ΔΨm monitored with JC-1 dye.
The percentage of monomeric JC-1 represented ΔΨm and typical
recording with FCM was shown in Fig.
3D. As shown in Fig. 3E,
H2O2 significantly depolarized the ΔΨm, while
pretreatment with DEX suppressed the ΔΨm depolarizing effect of
H2O2.
We further checked the Bax/Bcl2 ratio which reflects
the status of mitochondrial-mediated apoptosis. As shown in
Fig. 3F, compared with that in
control group, H2O2 significantly increased
the ratio of Bax/Bcl2, while pretreatment of DEX reduced the ratio
compared with H2O2 challenge.
DEX attenuates
H2O2-induced ER oxidative stress injury in
NRCMs
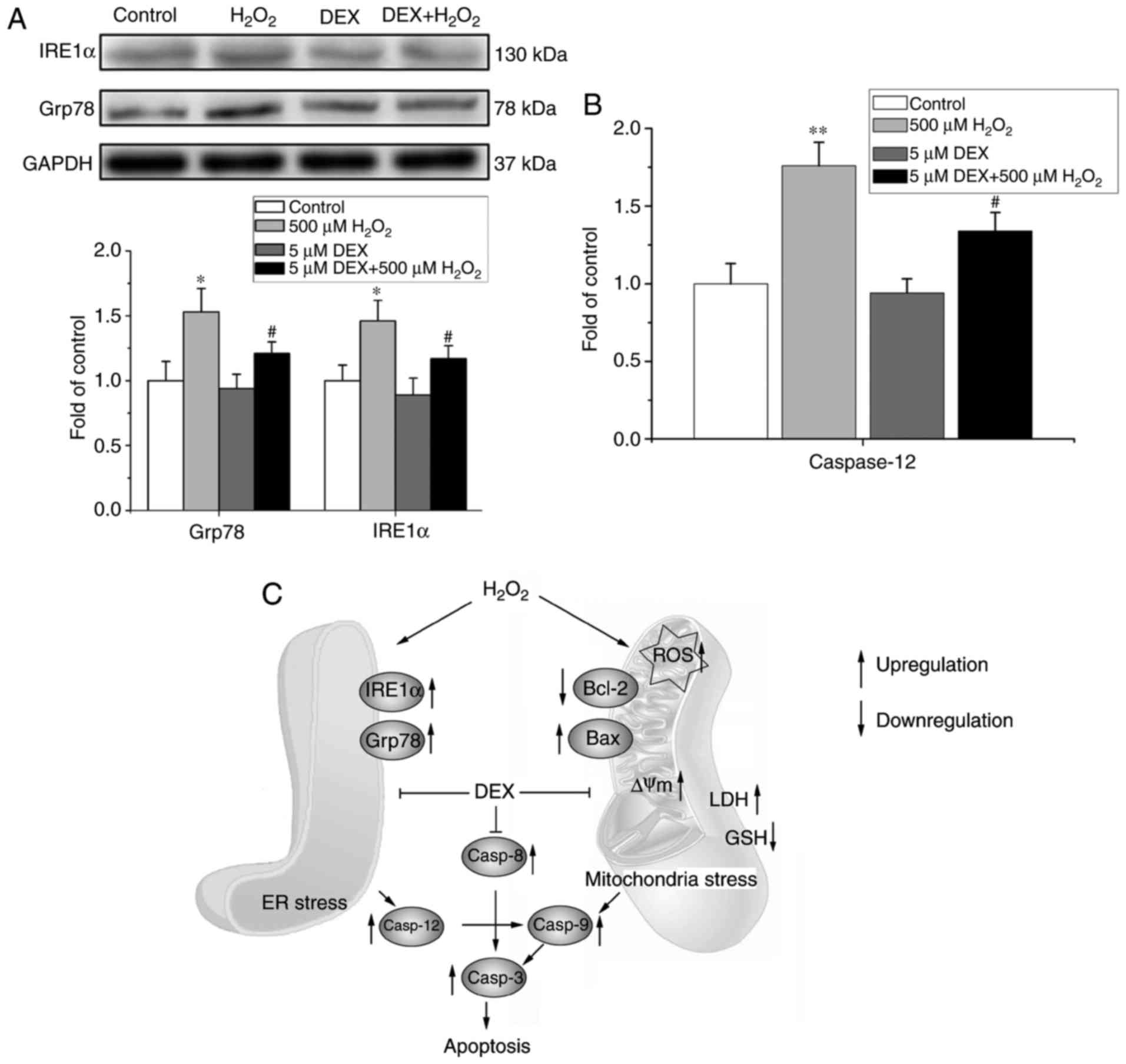
In addition, to determine whether ER-mediated
apoptosis pathway was also involved in the effect of DEX on NRCMs,
the expression of Grp78 and IRE1α and activity of caspase 12 were
measured in NRCMs with different treatment. As shown in Fig. 4A and B, treatment of
H2O2 significantly increased the expression
levels of Grp78 and IRE1α and the activity of caspase 12, while
these effects of H2O2 were abolished by
pretreatment of DEX. Treatment of DEX alone did not change the
expression of Grp78 and IRE1α and the activity of caspase 12
compared with the control group significantly (Fig. 4A and B).
The above results showed that DEX alleviated
H2O2-induced oxidative stress injury through
both mitochondria and ER stress-mediated apoptosis pathways and a
putative signaling network was shown in Fig. 4C. For the mitochondria mediated
oxidative stress pathway, treatment of H2O2
first induced oxidative stress injury with increment of
intracellular ROS level. Mitochondria oxidative stress injury and
increased ROS induced a series of cascade reactions, including ΔΨm
depolarization, increased Bax/Bcl-2 ratio, increment of LDH release
and decrement of GSH. Pretreatment of DEX reversed the
H2O2 induced mitochondrial oxidative stress
injury. In addition, H2O2 also induced ER
stress with increment of Grp78 and IRE1α, the important molecular
indexes for reflexing ER stress with increment of UPR, while
pretreatment of DEX also attenuated H2O2
induced ER stress. Both mitochondria and ER stress induced cell
apoptosis through downstream reaction including activation of
caspase 9 and caspase 12 then activation of caspase 3, which are
the important markers for cell apoptosis. Pretreatment of DEX
attenuated H2O2 induced cell apoptosis
through decreasing the alleviating the mitochondria and ER mediated
oxidative stress.
Discussion
It is well-documented that increased oxidative
stress (OS) leads to dysfunction of relative signaling pathways and
is involved in many cardiovascular diseases, such as myocardial I/R
injury (8,17). Therefore, the oxidative stress
signaling pathway may provide therapeutic targets against cardiac
I/R injury. In the present study, we found that DEX, a drug widely
used in clinical anesthesia, protects NRCMs against oxidative
stress injury and apoptosis through modulating the mitochondria and
ER stress-mediated apoptosis signaling pathways.
Mitochondria are the important cellular components
for biological oxidation in eukaryotic cells (9). In the present study, DEX attenuated
H2O2-induced increment of intracellular ROS
level and LDH release, and restored the reduction of GSH induced by
H2O2 in NRCMs and inhibited
H2O2-induced depolarization of ΔΨm, which are
the important molecular for reflexing the mitochondria oxidative
stress status. These results support that mitochondria are the
important target organelle of DEX for its cardioprotective action.
Fu et al (3) reported that
DEX attenuates LPS-induced acute lung injury of rats by inhibiting
oxidative stress, mitochondrial dysfunction and apoptosis. These
data suggested that oxidative stress signaling pathway is an
important target for the organ protection of DEX. Therefore, the
present study supports our hypothesis that mitochondria-mediated
signaling pathway is involved in the protective effect of DEX on
cardiomyocyte.
ER stress-mediated apoptosis is an alternative
important signaling pathway in oxidative stress injury and Grp78,
caspase 12, CHOP and IRE1α are the important molecules in this
signaling pathway (18–20). Wang et al (21) reported that DEX produced
cardioprotection against myocardial apoptosis injury through
modulating expression of Grp78, CHOP and caspase 12 in grave
scalding rat model. In this study, we also showed that DEX produced
myocardial protection through inhibiting H2O2
induced increment of expression or activity of Grp78 and caspase
12. In addition, UPR signals are transmitted across the ER membrane
to the nucleus via the proximal sensor IRE1α-a transmembrane
serine/threonine kinase in the ER membrane (22). This study further demonstrated that
pretreatment of DEX reduced the increased expression of IRE1α,
suggesting that ER was another target of DEX on cardiac
protection.
In summary, the present study supports our
hypothesis that preventive treatment with DEX protects NRCMs
against H2O2-induced injury and apoptosis
through both mitochondria and ER stress-mediated signaling
pathways. Oxidative stress (including mitochondria and ER stress)
is the important target for the cardioprotective effect of DEX. The
present study may encourage the widespread use of DEX in clinical
anesthesia and ICU especially in high risk cardiovascular patients
undergoing surgery operation such as cardiopulmonary bypass (CPB)
or patients with cardiovascular diseases undergoing noncardiac
surgery.
Acknowledgements
The authors would like to thank Professor Ji-Min Cao
from the Chinese Academy of Medical Sciences (Shanghai, China) for
critical reading of the manuscript.
Funding
The research was supported by the National Natural
Science Foundation of China (grant no. 81670310) and Southwest
Medical University Scientific Research Fund (grant no.
2014QN-003).
Availability of data and materials
The datasets and materials used and/or analyzed
during the current study are available from the corresponding
author on reasonable request.
Authors' contributions
XT, XL, TL and LC designed the experiments; XL, TL,
LC, YY, LLC, XF and BY performed in the experiments; XT, XL, TL and
LC analyzed the data; XT, XL and TL wrote the paper.
Ethics approval and consent to
participate
All protocols in the present study were approved by
the Ethics Committee of Southwest Medical University (no.
20160063).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arcangeli A, D'Alò C and Gaspari R:
Dexmedetomidine use in general anaesthesia. Curr Drug Targets.
10:687–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji F, Li Z, Nguyen H, Young N, Shi P,
Fleming N and Liu H: Perioperative dexmedetomidine improves
outcomes of cardiac surgery. Circulation. 127:1576–1584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu C, Dai X, Yang Y, Lin M, Cai Y and Cai
S: Dexmedetomidine attenuates lipopolysaccharide-induced acute lung
injury by inhibiting oxidative stress, mitochondrial dysfunction
and apoptosis in rats. Mol Med Rep. 15:131–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang H, Liu HZ, Wang HB, Zhong JY, Yang
CX and Zhang B: Dexmedetomidine protects against cisplatin-induced
acute kidney injury in mice through regulating apoptosis and
inflammation. Inflamm Res. 66:399–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie C, Li Y, Liang J, Xiao J, Zhao Z and
Li T: The effect of dexmedetomidine on autophagy and apoptosis in
intestinal ischemia reperfusion-induced lung injury. Zhonghua Jie
He He Hu Xi Za Zhi. 38:761–764. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Yoshitomi O, Cho S, Hara T, Shibata I,
Maekawa T, Ureshino H and Sumikawa K: Direct protective effects of
dexmedetomidine against myocardial ischemia-reperfusion injury in
anesthetized pigs. Shock. 38:92–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rajendran P, Nandakumar N, Rengarajan T,
Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J and
Nishigaki I: Antioxidants and human diseases. Clin Chim Acta.
436:332–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burgoyne JR, Mongue-Din H, Eaton P and
Shah AM: Redox signaling in cardiac physiology and pathology. Circ
Res. 111:1091–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Addabbo F, Montagnani M and Goligorsky MS:
Mitochondria and reactive oxygen species. Hypertension. 53:885–892.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Groenendyk J, Agellon LB and Michalak M:
Coping with endoplasmic reticulum stress in the cardiovascular
system. Annu Rev Physiol. 75:49–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Zoubeidi A, Beraldi E and Gleave ME:
GRP78 regulates clusterin stability, retrotranslocation and
mitochondrial localization under ER stress in prostate cancer.
Oncogene. 32:1933–1942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Cao L, Li T, Chen LL, Yu YY, Huang
WJ, Liu L and Tan XQ: Propofol attenuates H2O2-induced oxidative
stress and apoptosis via the mitochondria- and ER-medicated
pathways in neonatal rat cardiomyocytes. Apoptosis. 22:639–646.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ichinose M, Yonemochi H, Sato T and
Saikawa T: Diazoxide triggers cardioprotection against apoptosis
induced by oxidative stress. Am J Physiol Heart Circ Physiol.
284:H2235–H2241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan M, Dai H, Ding T, Dai A, Zhang F, Yu
L, Chen G and Chen Z: Effects of dexmedetomidine on the release of
glial cell line-derived neurotrophic factor from rat astrocyte
cells. Neurochem Int. 58:549–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Von Harsdorf R, Li PF and Dietz R:
Signaling pathways in reactive oxygen species-induced cardiomyocyte
apoptosis. Circulation. 99:2934–2941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pluquet O, Pourtier A and Abbadie C: The
unfolded protein response and cellular senescence. A review in the
theme: Cellular mechanisms of endoplasmic reticulum stress
signaling in health and disease. Am J Physiol Cell Physiol.
308:C415–C425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang LH and Zhang X: Roles of GRP78 in
physiology and cancer. J Cell Biochem. 110:1299–1305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann
N Y Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Zhang S, Xu S and Zhang L: The
efficacy and mechanism of dexmedetomidine in myocardial apoptosis
via the renin-angiotensin-aldosterone system. J Renin Angiotensin
Aldosterone Syst. 16:1274–1280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hetz C, Martinon F, Rodriguez D and
Glimcher LH: The unfolded protein response: Integrating stress
signals through the stress sensor IRE1α. Physiol Rev. 91:1219–1243.
2011. View Article : Google Scholar : PubMed/NCBI
|