Introduction
Alzheimer's disease (AD) is a chronic
neurodegeneration with symptoms that affect language and
motivation, and cause behavioral and orientational disorders that
gradually lead to patient mortality. The average life expectancy
following AD diagnosis is usually within 10 years (1). There are ~48 million patients with AD
worldwide, most of whom are >65 years old. Increasing evidence
indicates that AD is partially caused by amyloid plaques in the
brain (2). Amyloid plaques,
composed of amyloid fibers, are extracellular deposits and the
primary toxicant in AD brains. Amyloid β (Aβ) is the major
component of amyloid plaques and has a central role in AD pathology
(3). Aβ levels are elevated in AD
brains, contributing to cerebrovascular lesions (4). Of the various Aβ oligomers,
Aβ1–42 is the most amyloidogenic and fibrillogenic form
of the peptide due to its hydrophobic nature, and therefore has a
higher association with AD (5,6). The
majority of previous studies have focused on the toxicity of
Aβ1–42 on neurons (7–10).
However, fewer studies have focused on the toxicity of
Aβ1–42 on astrocytes, which also have important roles in
AD.
Astrocytes are star-shaped macroglial cells, the
most abundant cells in the central nervous system (CNS). They are
responsible for ion exchange and uptake with neurons. Astrocytes
also regulate the trophic factors, transmitters and transporters in
CNS, modulating the normal neuron functions, synaptic activity and
neuronal homeostasis. Astrocytes have critical roles in CNS energy
provision, blood supply and synaptic activity regulations,
homeostasis and remodeling in the brain. Dysfunctions of astrocytes
are associated various brain diseases, including neurodegeneration
(11,12). As the most neural toxic peptide,
Aβ1–42 is highly associated with AD. However, the
detailed effects and regulation pathway of Aβ1–42 on
astrocytes are yet to be established.
As a double membrane-bound organelle and source of
chemical energy (adenosine triphosphate; ATP) that is present in
all eukaryotic organisms, mitochondria are involved in cell growth
and apoptosis in the CNS. Mitochondrial dysfunction is implicated
in several types of human neurodegeneration (13–15).
Cytochrome P450 reductase (CPR), a 678-amino acid microsomal
flavoprotein, is the obligate redox partner for all microsomal P450
cytochromes. CPR is required in all microsomal P450-catalyzed
monooxygenase reactions, which catalyze the majority of the
chemical metabolism in the human body (16–18).
In the present study, the U87 human glioblastoma
cell line was used as astrocytes and treated with different doses
of Aβ1–42 for different durations. The effects of
Aβ1–42 on U87 cells and the regulation of CPR were
detected. Furthermore, in vivo detection of Aβ1–42 levels
and astrocytes was performed in AD and wild-type (WT) mice. The
current study meets ethical guidelines and all research was
approved by the Research Management Office and Experimental Animal
Center of Suzhou Vocational Health College (Suzhou, China).
Materials and methods
Cells and chemicals
The U87MG ATCC human glioblastoma cell line, termed
U87 throughout the manuscript, was kept in our laboratory for a
long time. The original cell line was purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). U87 cells were kept in Thermo Scientific incubator at 37°C,
5% CO2 with saturated humidity and cultured in
Dulbecco's modified Eagle's medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Shanghai ExCell Biology, Inc., Shanghai, China), 100 U/ml
penicillin and 0.1 mg/ml streptomycin (Hyclone; GE Healthcare Life
Sciences). The sequence of Aβ1–42 peptide (molecular
weight, 4,514.08 Da; Sangon Biotech Co., Ltd., Shanghai, China) was
as follows: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA.
Aβ1–42 was initially dissolved at a concentration of 1
mM in dimethyl sulfoxide, and 100 µl Aβ1–42 was combined
with 900 µl PBS to generate 100 µM Aβ1–42. The product
was incubated at 37°C for 7 days prior to use.
Detection on the cellular
proliferation
Cellular proliferation was recorded using MTT
reagent (Beyotime Institute of Biotechnology, Haimen, China)
following the manufacturer's protocol. Briefly, U87 cells were
seeded at a density of 3×103 cells per well, attached on
the well and cultured with 0, 0.1, 0.5, 1, 5, 10, 20 and 50 µM
Aβ1–42 at 37°C for 24 and 48 h. Subsequently, 20 µl MTT
(5 mg/ml) was added to each well and incubated for 4 h at 37°C.
Medium was removed and 150 µl formazan solvent was added for 15
min. The absorbance at 590 nm was recorded. This experiment was
repeated three times.
Detection of glial fibrillary acidic
protein (GFAP) expression
The expression of the astrocyte marker GFAP on U87
cells (at a density of 5.0×104 viable
cells/cm2) cultured with 0, 1, 10 and 20 µM
Aβ1–42 for 48 h at 37°C was detected using
immunofluorescence. Cells were washed with 1X PBS three times,
fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature
and treated with 1X PBS containing 0.5% Triton-X-100 for 15 min on
ice. Subsequently, blocking was performed using 10% normal goat
serum (ab7481; Abcam, Cambridge, UK) for 1 h at room temperature.
Following blocking, cells were incubated with rabbit monoclonal
anti-GFAP (1:600; ab33922; Abcam) at 4°C overnight and were washed
three times with 1X TPBS for 10 min. Goat anti-rabbit IgG H&L
(Alexa Fluor 488; 1:400; ab150077; Abcam) secondary antibody was
added for 1 h in a dark room at room temperature, followed by
washing three times with 1X TPBS for 10 min. Photos were taken
using a fluorescence microscope at ×100 magnification (Eclipse
Ti-S; Nikon Corporation, Tokyo, Japan).
In vivo detection of Aβ1–42
and astrocytes
APPswe/PSEN1dE9 male mice were purchased from the
Model Animal Research Center of Nanjing University (Nanjing,
China). These double transgenic mice, originally generated in
Jackson Laboratory (Bar Harbor, ME USA), are well referred to and
widely used as AD mice studies. A total of 3 male, 8-month-old mice
(weighing 25.4±2.9 g) were used to represent early stage AD and 3
12-month-old mice (weighing 26.1±4.5 g) were used for a
later/progressed stage of AD. Then 3 8-month-old C57BL/6J mice
(weighing 24.7±3.4 g) and 3 12-month-old C57BL/6J mice (weighing
24.3±4.0 g) were used as WT mice. The mice were raised in an
environment with a temperature of 24±2°C, humidity of 55±15% and a
12-h light/dark cycle. Mice were given ad libitum access to
food and water. Experiments and surgeries on the mice were
performed according to the Institutional Animal Care and Use
Committee (IACUC) guidelines. Mouse brain cortex tissues were
homogenized in cell lysis buffer which contains 25 mM HEPES, 125 µM
DTT, 1 mM PMSF, 100 µg/ml Leupeptin and 20 µg/ml Aprotinin.
Concentration of Aβ1–42 in mouse brains was detected
using an Amyloid β 42 Mouse ELISA kit (KMB3441; Novex; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The final results were calculated by comparing to the standard
curve and presented in ng/mg protein. Mice brains were embedded in
OTC on dry ice and cut into 10 µm frozen sections. Slides were
placed in 4% paraformaldehyde (PFA) for 20 min at room temperature,
then in 10% normal goat serum for 20 min also at room temperature.
Astrocytes in the hippocampus region of mice were detected using
GFAP primary antibody (ab7260; 1:200; Abcam) at 4°C overnight and
goat anti-rabbit IgG H&L (Alexa Fluor 488; ab150077; 1:400;
Abcam) secondary antibody at room temperature for 2 h. The present
study was approved by the Experimental Animal Center of Suzhou
Vocational Health College.
Detection of apoptosis by DAPI
staining
U87 cells at a density of 5.0×104 viable
cells/cm2 were cultured in the incubator and treated
with 10 µM Aβ1–42 at 37°C for 48 h. Apoptosis was
detected using DAPI staining (Beyotime Institute of Biotechnology).
Normal cells exhibit a round nucleus uniformly stained with clear
margin, while apoptotic cells exhibit abnormal nucleus margin and
the condensed chromosomes. Following fixation with 4% PFA for 20
min at room temperature and treatment with 1X TPBS (0.5%
Triton-X-100) for 10 min on ice, cells were stained with DAPI
solution for 15 min at room temperature. Images were acquired using
a fluorescence microscope at ×100 and ×200 magnification (Eclipse
Ti-S; Nikon Corporation).
Detection of apoptosis and CPR by
western blot analysis
U87 cells were treated with 10 µM Aβ1–42
in an incubator at 37°C for 48 h. Protein was extracted from U87
cells using the above mentioned cell lysis buffer and quantified
with Pierce BCA Protein Assay Kit (23225; Thermo Fisher Scientific,
Inc.). Protein (25 µg per lane) was detected using western blot
analysis. Following electrophoresis and transfer to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA), the membrane was blocked with 5% fat-free milk for 1 h at
room temperature and incubated with anti-Bcl-2 (rabbit polyclonal
IgG; 12789-1-AP; 1:1,000; ProteinTech Group, Inc., Chicago, IL,
USA,), anti-cleaved caspase-3 (9661; 1:800; Cell Signaling
Technology, Inc.) and anti-CPR (rabbit polyclonal IgG; ab13513;
1:1,000; Abcam) primary antibodies at 4°C overnight. Subsequently,
membranes were incubated with goat anti-rabbit IgG secondary
antibody (ab6721; 1:5,000; Abcam) at room temperature for 2 h.
GAPDH (ab9485; 1:2,500; Abcam,) was used as a loading control
indicator.
Detection of CPR activity
U87 cells at a seeding density of 5.0 ×
104 viable cells/cm2 were treated with 10 µM
Aβ1–42 in a cell culture incubator at 37°C for 48 h. The
microsome of U87 cells was homogenized on ice in 10 mM PBS (pH 7.7)
containing 250 mM sucrose, 1 mM EDTA and 0.5 mM PMSF for 15 sec
then centrifuged for 20 min at 10,000 × g at 4°C. The supernatant
was collected and centrifuged for 1 h at 40,000 × g at 4°C, then
the supernatant was decanted. The pellet was re-suspended in 0.1 M
PBS containing 1 mM EDTA, 1mM dithiothreitol, 30% glycerol and
protease inhibitors (pH 7.25). The activity of CPR in microsome was
detected in the reduction of cytochrome c determined in reactions.
Briefly, 80 µl of a 0.5-mM solution of the horse heart cytochrome c
(in 10 mM PBS, pH 7.7) was added into a glass with an aliquot of
sample. Then 10 µl of a 10 mM NADPH solution was added and
A550 was recorded using the spectrophotometer as a
function of time (about 3 min). The rate of reduction of cytochrome
c was calculated as ΔA550 min−1/0.021=nmol of
cytochrome c reduced per min.
Detection of mitochondrial
functions
The mitochondrial functions of U87 cells at a
density of 5.0×104 viable cells/cm2 cultured
with 0, 1, 10 and 20 µM Aβ1–42 in a cell culture
incubator at 37°C for 48 h was detected. ATP generation was
detected using an ATP assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Briefly,
200 µl lysis buffer was added to U87 cells cultured in 6-well
plates, which were subsequently centrifuged at 12,000 × g at 4°C
for 5 min. The supernatant was collected and detected together with
ATP standard solution on the luminometer. Mitochondrial membrane
potential (MMP) was detected using a JC-1 staining kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. In addition, the activity of the four complexes of the
electron transport chain (ETC) following treatment of U87 cells
with 10 µM Aβ1–42 for 48 h were also detected using
MitoCheck Complex Activity Assay Kit (I: 700930, II: 700940, III:
700950 and IV: 700990; Cayman Chemical Company, Ann Arbor, MI, USA,
Catalog No.). The rate of NADH oxidation was measured at 340 nm for
the activity of complex I. A decrease in absorbance at 600 nm for
complex II activity, the reduction of excess cytochrome c (550 nm
absorbance) for complex III and the direct oxidation of cytochrome
c for complex IV. Results were presented relative to values in the
control (0 µM) group.
Statistical analysis
Data are presented as the mean ± standard deviation
and analysis was performed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). Results were evaluated using Student's t-test
for two-group comparison or one-way analysis of variance followed
by a post hoc Dunnett's test for multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Aβ1–42 on the
proliferation of U87 cells
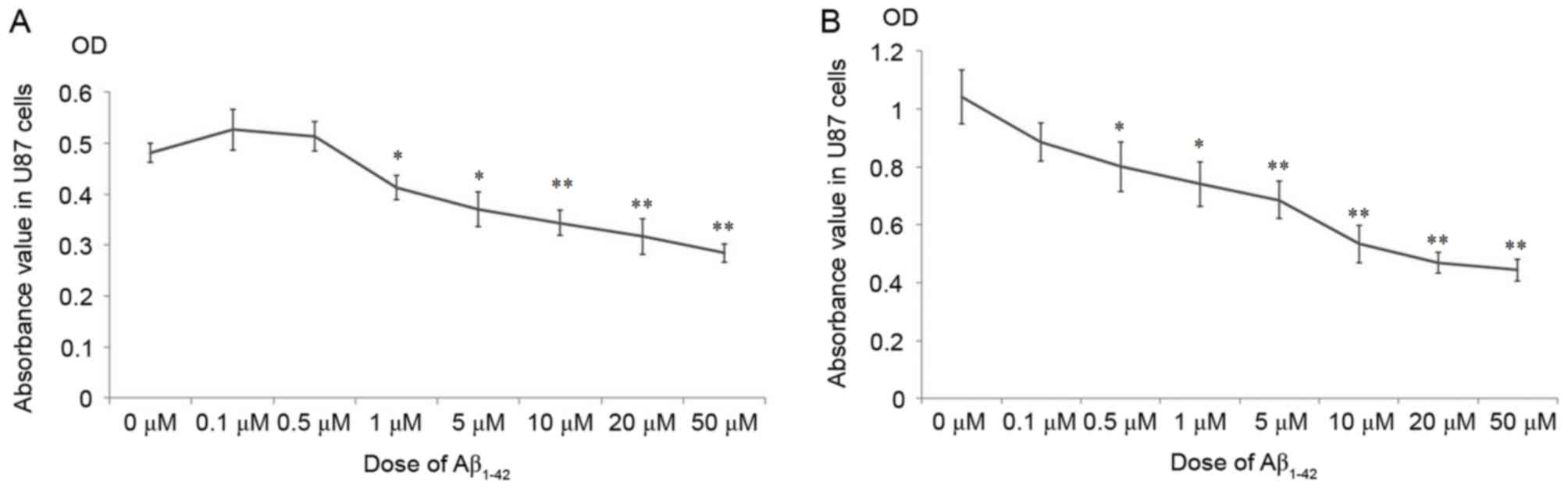
As demonstrated in Fig.
1, the cellular growth of U87 was significantly inhibited
following treatment with >1 µM Aβ1–42 at 24 h
(Fig. 1A) and 48 h (Fig. 1B), compared with the 0 µM group.
Surprisingly, doses of Aβ1–42 <1 µM marginally
promoted the cellular growth at 24 h (Fig. 1A), however, after 48 h treatment
these doses inhibited growth compared with the 0 µM group (Fig. 1B). These results indicate that the
effects of Aβ1–42 on the proliferation of U87 cells were
complex. Small doses of Aβ1–42 promoted the
proliferation of U87 cells for the initial 24 h, while doses >1
µM inhibited the growth of U87 cells after 24 h. After a longer
treatment duration of 48 h, the proliferation of U87 cells was
arrested compared with the 0 µM group at all Aβ1–42
doses.
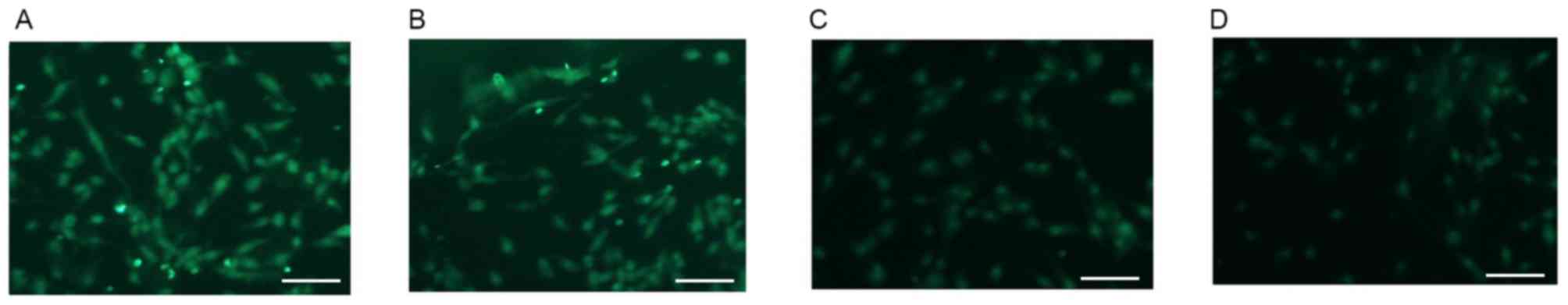
Alterations in the expression of GFAP
in U87 cells
Various reports have indicated that U87 human
glioblastoma cells may be used as astrocytes for AD neuropathology
and cytotoxicity studies (19–21).
As a cellular marker of astrocytes, GFAP (green staining) was
highly expressed on U87 cells that were not treated with
Aβ1–42 (0 µM group; Fig.
2A). The expression of GFAP was markedly decreased in a
dose-dependent manner in Aβ1–42 treatment groups after
48 h treatment. Representative images of GFAP staining in cells
treated with 1, 10 and 20 µM Aβ1–42 are presented in
Fig. 2B-D.
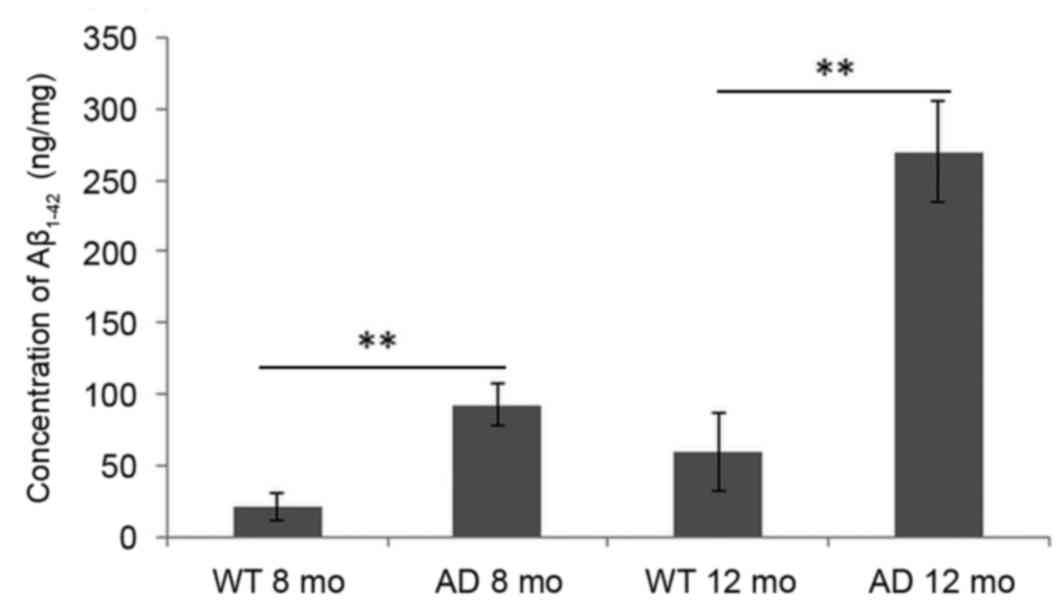
Alterations in the concentration of
Aβ1–42 in the mouse brain cortex
As demonstrated in Fig.
3, compared with WT mice, the concentration of
Aβ1–42 in the cortex of mice brains was significantly
increased in AD mice at early AD stage (8 months) and AD progressed
stage (12 months). Levels were barely detected in WT mice at 8
months and marginally increased at 12 months. Even in AD mice, the
concentration of Aβ1–42 at the early stage (8 months)
was low compared with levels at 12 months.
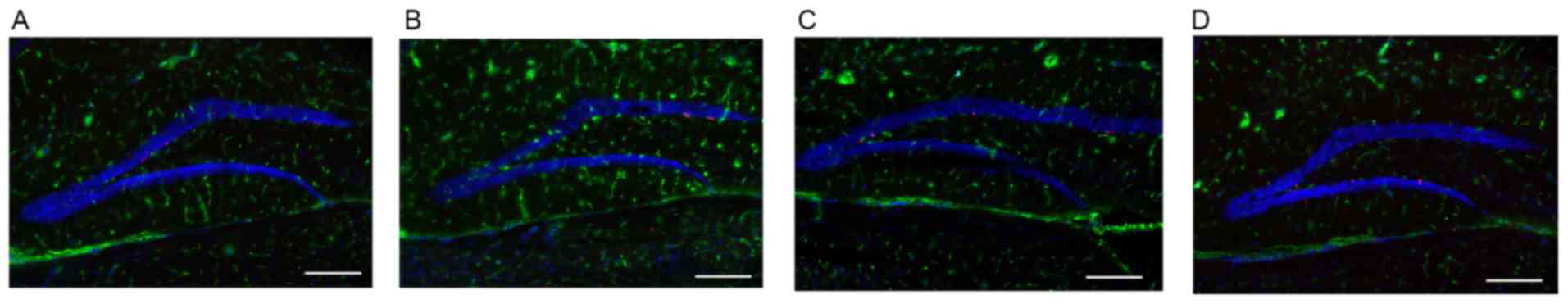
Abnormal astrocytes in hippocampus
region of AD mice brain
Compared with WT mice, the number of astrocytes in
the hippocampus region of the AD mouse brain was visibly increased
at the early stage of AD (8 months; Fig. 4A and B). This process is termed
astrocytosis. During this period (8 months), the concentration of
Aβ1–42 was at a low level (Fig. 3). However, at 12 months, the level
of Aβ1–42 was increased significantly in AD mice
(Fig. 3). The number of astrocytes
in the hippocampus region of the AD mouse brain was markedly
decreased in progressed AD (12 months) mice compared with the WT
group (Fig. 4C and D).
The effects of Aβ1–42 on
apoptosis in U87 cells
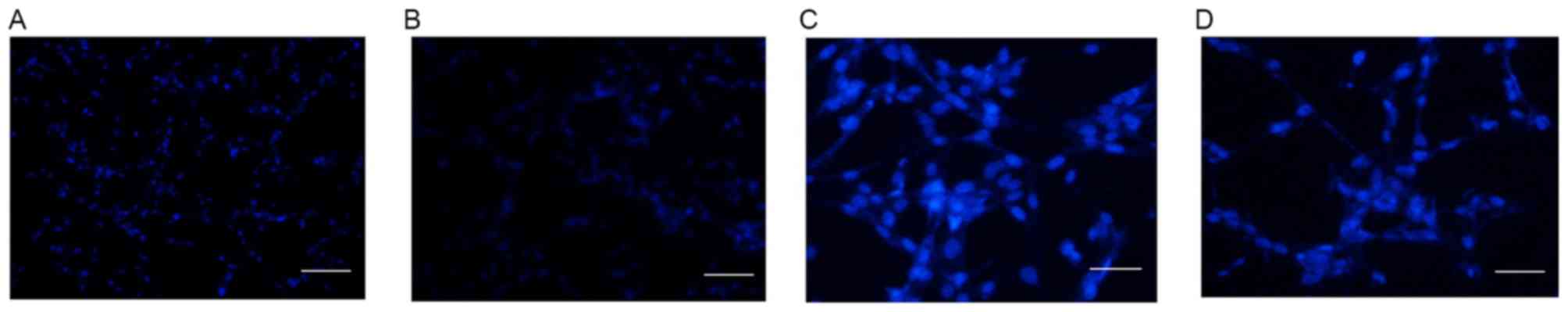
The cellular apoptosis of U87 cells was detected by
DAPI staining in control cells and cells treated with 10 µM
Aβ1–42 for 48 h at magnifications of ×100 (Fig. 5A and B, respectively) and ×200
(Fig. 5C and D, respectively). As
demonstrated in Fig. 5, no obvious
apoptosis was detected in the control group (Fig. 5A and C). However, following
treatment with 10 µM Aβ1–42 for 48 h, obvious apoptosis
was detected by abnormal cell nucleus staining (Fig. 5B and D). Furthermore, the protein
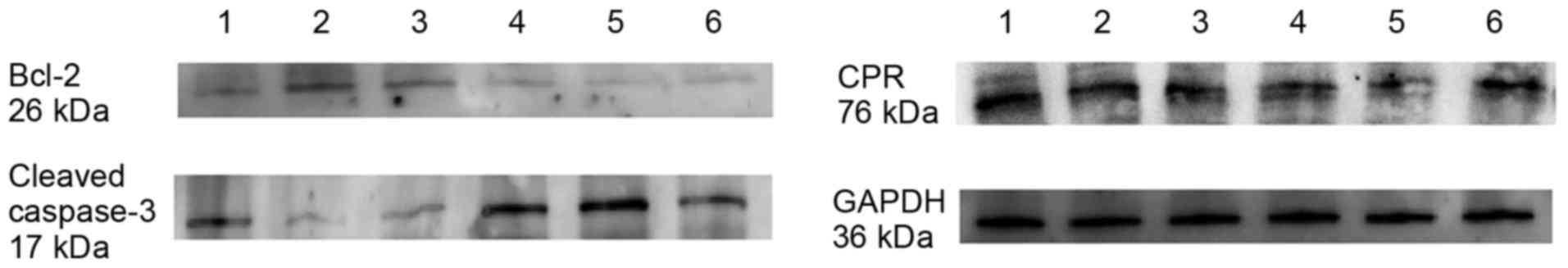
expression of the apoptosis factors Bcl-2 and cleaved caspase-3
were detected by western blot analysis following treatment of U87
cells with 10 µM Aβ1–42 for 48 h. Lanes 1–3 represent
protein expression in control U87 cells and lanes 4–6 represent
protein expression in U87 cells treated with 10 µM
Aβ1–42 for 48 h (Fig.
6). As demonstrated in Fig. 6,
the expression of Bcl-2 was markedly decreased following treatment
with 10 µM Aβ1–42 for 48 h compared with the control
group, and the opposite was observed for the expression of cleaved
caspase-3, which was markedly increased following treatment with 10
µM Aβ1–42 for 48 h compared with control cells.
Furthermore, the expression of CPR was determined by western
blotting. Generally, the expression of CPR was downregulated
following treatment with 10 µM Aβ1–42 for 48 h. However,
the individual differences among samples were obvious (Fig. 6).
The effects of Aβ1–42 on
mitochondrial functions
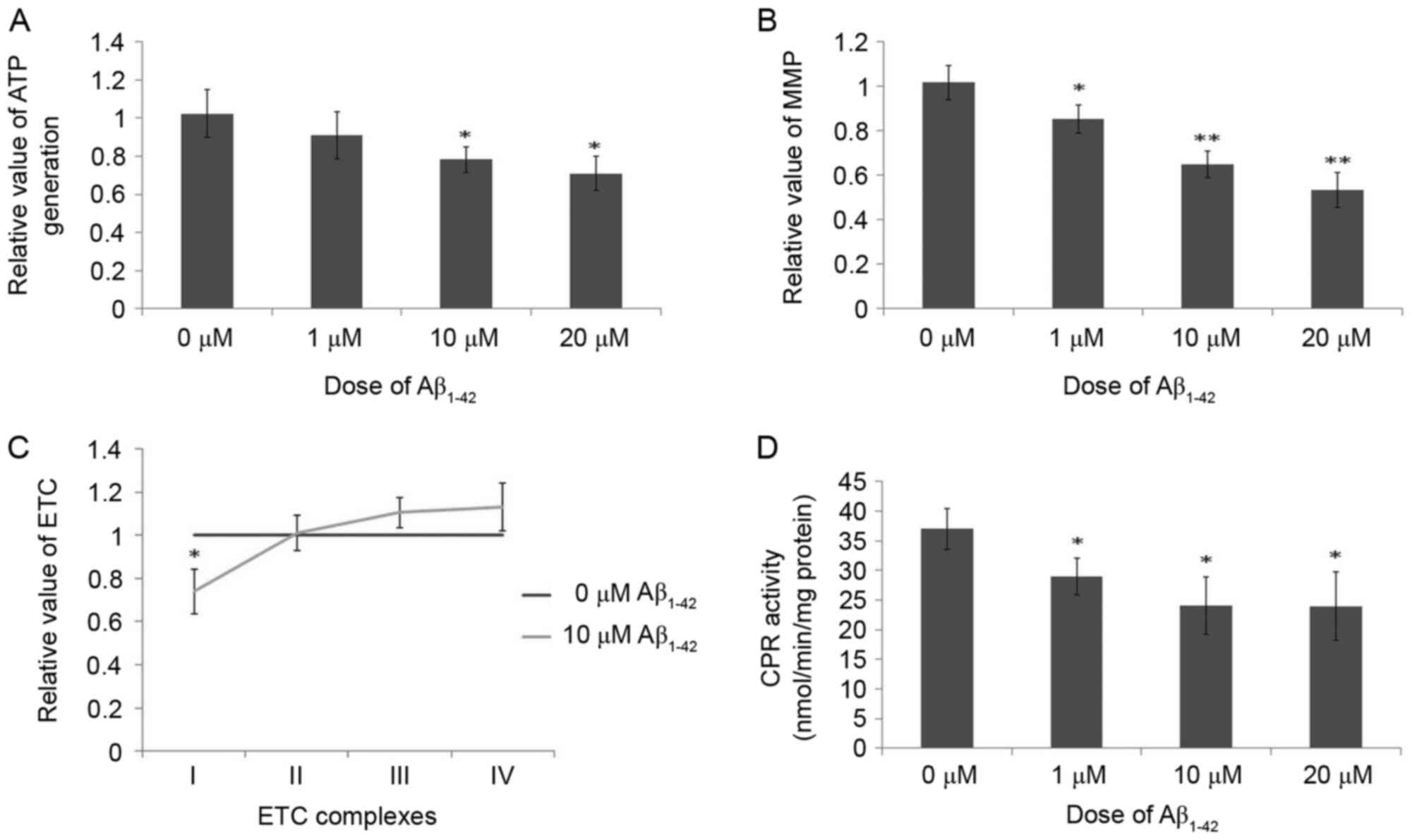
ATP generation was downregulated following treatment
with Aβ1–42 for 48 h in a dose-dependent manner compared
with the control group, and these differences were significant at
10 and 20 µM Aβ1–42 (Fig.
7A). As demonstrated in Fig.
7B, due to the toxicity of Aβ1–42 on mitochondria,
the MMP was significantly decreased in cells treated with
Aβ1–42 for 48 h in a dose-dependent manner compared with
control cells. Additionally, the activity of complex I of the ETC
was markedly decreased, while the activities of complexes III and
IV were marginally increased, following treatment with 10 µM
Aβ1–42 for 48 h compared with control cells (Fig. 7C). No obvious difference was
observed for complex II activity in control cells and cells treated
with 10 µM Aβ1–42 for 48 h (Fig. 7C). These results indicate the
obvious toxicity of Aβ1–42 on mitochondria as certain
functions of mitochondria were inhibited following treatment with
Aβ1–42.
The effects of Aβ1–42 on
the activity of CPR
CPR has a key role in the metabolism of chemicals
(22). Enzymes are regulated in
two ways, one is expression regulation (slow adjustment) and the
other is activity regulation (rapid adjustment). Generally, effects
due to the regulation of expression are slow while effects of
activity regulation occur more rapidly. However, expression and
activity regulation are both important for the catalyst function of
an enzyme. Therefore, the present study detected the expression and
activity of CPR. The results for CPR expression were described
above for western blotting results in Fig. 6. Concerning CPR activity, the CPR
activity, in the reduction of cytochrome c, was markedly inhibited
following treatment with 10 µM Aβ1–42 for 48 h compared
with control cells (Fig. 7D).
Discussion
AD was first observed, described and named by Alois
Alzheimer, a German psychiatrist and pathologist in 1906. It is a
serious chronic neurodegeneration without effective drugs
currently. The morbidity of AD has been increasing rapidly in
recent years. Aβ is formed following sequential cleavage of a
transmembrane glycoprotein termed amyloid precursor protein (APP)
(23). Various isoforms of 30–51
amino acid residues are generated by cleavage by α-, β- and γ-
secretases, of which the most common isoforms are Aβ1-40
and Aβ1–42. Aβ1–42 is the most fibrillogenic
and is highly associated with AD (24). An increased understanding of the
effects of Aβ1–42 on astrocytes, not only on neurons as
numerous previous studies have focused on, is essential. As a
common and routine method, the present study used U87 human
glioblastoma cells as astrocytes. Initially, MTT assays were
performed to investigate the effect of Aβ1–42 on the
growth of U87 cells. High doses of Aβ1–42 (>1 µM) led
to growth inhibition. However, low doses of Aβ1–42
(<1 µM) marginally promoted the cellular growth after 24 h, but
inhibited growth after 48 h. These effects were dose-dependent,
which means that different doses of chemicals lead to different
levels of effects. However, it is still surprising that low and
higher doses of Aβ1–42 led to opposing effects after 24
h treatment. Furthermore, U87 cells treated with a low dose of
Aβ1–42 (1 µM) exhibited abnormal growth termed
astrocytosis. These results were also confirmed by in vivo
experiments using double transgenic mice that express APP and the
mutant presenilin 1, and are widely used as AD mice. Compared with
WT mice, the number of astrocytes in the hippocampus region of the
AD mouse brain was increased at the early stage of AD with a low
Aβ1–42 level, while astrocytes were obviously decreased
in the brains of AD at 12 months with an increased
Aβ1–42 level. Astrocytosis, also termed reactive
astrogliosis, consists of abnormal reactive astrocytes and is often
observed as an early phenomenon in AD development (25,26).
Reactive astrocytes colocalize with Aβ plaques in AD brains.
Astrocytosis harms surrounding neural cells, resulting in damage to
neural functions, glial scarring and inflammation in the brain
(27). Reactive astrocytes may
promote neural toxicity via the generation of cytokines, which may
subsequently damage nearby neurons. In addition, they may also
promote secondary damage or degeneration following CNS injury
(28).
As a form of abnormal proliferation, astrocytosis is
temporary, occurring only at the beginning of cellular growth or in
the early stage of AD. Two different abnormal pathological
astrocytes have been reported (29–31).
To detect the effect of Aβ1–42 on apoptosis, DAPI
staining was performed. As a fluorescent stain, DAPI binds strongly
to DNA and is used extensively in apoptosis detection. Apoptosis, a
process of programmed cell death that is regulated by various
factors, inevitably leads to cellular death. Caspase-3 and Bcl-2
are key factors involved in apoptosis. Caspase-3 is activated,
following cleavage by an initiator caspase, in apoptotic tissues
and cells partially by mitochondrial regulation and has a dominant
role in apoptosis (32). In the
present study, increased activation of caspase-3 was observed
following treatment with Aβ1–42. As an important
anti-apoptotic oncogene, Bcl-2 is localized to the outer membrane
of mitochondria, and has a key role in the promotion of cellular
survival and inhibition of apoptosis (33). Reduced expression of Bcl-2 was
observed following treatment of cells with Aβ1–42 in the
present study. As caspase-3 and Bcl-2 are located on and regulated
via mitochondria, the present study subsequently investigated the
function of mitochondria.
As a source of chemical energy in the form of ATP,
the mitochondrion is a double membrane-bound organelle that is
widely distributed in the majority of eukaryotic cells. In the
current study, the results demonstrated that ATP generation was
significantly decreased in a dose-dependent manner following 48 h
treatment with Aβ1–42, which indicates that the primary
function of mitochondria was inhibited by Aβ1–42. In
addition to supplying cellular energy, mitochondria are also
involved in cell growth and apoptosis in AD (34,35).
Mitochondrial dysfunction has been implicated in several human
diseases, including Alzheimer's disease, Parkinson's disease and
schizophrenia (13–15). All of these reports are in
accordance with the results of the present study. ETC, a chain that
consists of a series of compounds containing certain complexes,
transfers electrons from donors to acceptors via redox reactions
and couples electron transfer with the transfer of protons. This
process creates an electrochemical proton gradient that drives the
generation of Mitochondrial ATP (36). The present study demonstrated that,
compared with other complexes and the activity in control cells,
the activity of complex I of the ETC was decreased following
treatment with Aβ1–42. MMP is critical for maintaining
the normal functions of the ETC in mitochondria to generate ATP.
Loss of MMP leads to the depletion of ATP and therefore energy in
cells, eventually resulting in apoptosis or necrosis. The current
study demonstrated that the MMP was markedly decreased following
treatment with Aβ1–42 for 48 h in a dose-dependent
manner, which further impairs the generation of ATP. These
observations may partially explain the reduced ATP generation
following treatment with Aβ1–42, indicating that
Aβ1–42 may enter the mitochondria and interfere with the
ETC and MMP, subsequently leading to inhibition of ATP
production.
Cytochrome P450s, which have maximum absorption at
~450 nm, are a superfamily of hemoproteins that function as the
terminal oxidase enzymes in the ETC. As a microsomal flavoprotein,
CPR is the obligate redox partner for cytochrome P450s,
transferring electrons from NADPH to cytochrome P450s. CRP is
required for all microsomal P450-catalyzed monooxygenase reactions
(37). Although various P450
subfamilies have important roles in the normal and pathological
functions of astrocytes, CPR is the key enzyme. Inhibition of CPR
would widely and largely reduce the function of all P450-mediated
metabolism. Our previous research indicated that CPR was highly
associated with astrocytosis (38). Furthermore, another study reported
widespread distribution of CPR in neurons and glial cells in the
brain, including in the cerebral cortex and hippocampus (39). The results of the present study
indicated that Aβ1–42 marginally inhibited the
expression of CPR in certain samples. However, the individual
differences between individual samples was obvious, which indicates
that the expression of CPR may be complex and regulated by various
factors.
In conclusion, the current study indicated that low
doses of Aβ1–42 (<1 µM) promoted astrocytosis
temporarily after 24 h, but led to reduced astrocyte numbers with
higher doses of Aβ1–42, which may occur by induction of
apoptosis in U87 cells via regulation of Bcl-2 and caspase-3
expression. Furthermore, the functioning of mitochondria was
inhibited by treatment with Aβ1–42. During this process,
the expression, in certain samples, and activity of CPR were
downregulated compared with control cells. The present study
provides novel insights into the effects of Aβ1–42 on
astrocytes and highlights astrocytes as a potential target for
protection against AD.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20130219), the
National Nature Science Foundation of China (grant no. 81401045)
and the Qing Lan Project of Jiangsu Province.
References
|
1
|
Iqbal K and Grundke-Iqbal I: Developing
pharmacological therapies for Alzheimer disease. Cell Mol Life Sci.
64:2234–2244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Awasthi M, Singh S, Pandey VP and Dwivedi
UN: Alzheimer's disease: An overview of amyloid beta dependent
pathogenesis and its therapeutic implications along with in silico
approaches emphasizing the role of natural products. J Neurol Sci.
361:256–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark IA and Vissel B: Amyloid β: One of
three danger-associated molecules that are secondary inducers of
the proinflammatory cytokines that mediate Alzheimer's disease. Br
J Pharmacol. 172:3714–3727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blennow K, Mattsson N, Schöll M, Hansson O
and Zetterberg H: Amyloid biomarkers in Alzheimer's disease. Trends
Pharmacol Sci. 36:297–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ZX, Tan L, Liu J and Yu JT: The
essential role of soluble Aβ oligomers in Alzheimer's disease. Mol
Neurobiol. 53:1905–1924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thal DR, Walter J, Saido TC and Fändrich
M: Neuropathology and biochemistry of Aβ and its aggregates in
Alzheimer's disease. Acta Neuropathol. 129:167–182. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebenezer PJ, Weidner AM, LeVine H III,
Markesbery WR, Murphy MP, Zhang L, Dasuri K, Fernandez-Kim SO,
Bruce-Keller AJ, Gavilán E and Keller JN: Neuron specific toxicity
of oligomeric amyloid-β: Role for JUN-kinase and oxidative stress.
J Alzheimers Dis. 22:839–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bloom GS: Amyloid-β and tau: The trigger
and bullet in Alzheimer disease pathogenesis. JAMA Neurol.
71:505–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drolle E, Hane F, Lee B and Leonenko Z:
Atomic force microscopy to study molecular mechanisms of amyloid
fibril formation and toxicity in Alzheimer's disease. Drug Metab
Rev. 46:207–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rottkamp CA, Raina AK, Zhu X, Gaier E,
Bush AI, Atwood CS, Chevion M, Perry G and Smith MA: Redox-active
iron mediates amyloid-beta toxicity. Free Radic Biol Med.
30:447–450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liem RK and Messing A: Dysfunctions of
neuronal and glial intermediate filaments in disease. J Clin
Invest. 119:1814–1824. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao YQ and Liang GB: Pathologic changes
and dysfunctions of astrocytes in the complex rat AD model of
ovariectomy combined with D-galactose injection. Bratisl Lek Listy.
115:692–698. 2014.PubMed/NCBI
|
|
13
|
Onyango IG, Dennis J and Khan SM:
Mitochondrial dysfunction in Alzheimer's disease and the rationale
for bioenergetics based therapies. Aging Dis. 7:201–214. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haddad D and Nakamura K: Understanding the
susceptibility of dopamine neurons to mitochondrial stressors in
Parkinson's disease. FEBS Lett. 589:3702–3713. 2005. View Article : Google Scholar
|
|
15
|
Rajasekaran A, Venkatasubramanian G, Berk
M and Debnath M: Mitochondrial dysfunction in schizophrenia:
pathways, mechanisms and implications. Neurosci Biobehav Rev.
48:10–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zanger UM and Schwab M: Cytochrome P450
enzymes in drug metabolism: Regulation of gene expression, enzyme
activities, and impact of genetic variation. Pharmacol Ther.
138:103–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laursen T, Jensen K and Møller BL:
Conformational changes of the NADPH-dependent cytochrome P450
reductase in the course of electron transfer to cytochromes P450.
Biochim Biophys Acta. 1814:132–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coon MJ: Cytochrome P450: Nature's most
versatile biological catalyst. Annu Rev Pharmacol Toxicol. 45:1–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Cheng D, Cheng R, Zhu X, Wan T, Liu
J and Zhang R: Mechanisms of U87 astrocytoma cell uptake and
trafficking of monomeric versus protofibril Alzheimer's disease
amyloid-β proteins. PLoS One. 9:e999392014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deb S, Zhang JW and Gottschall PE:
Activated isoforms of MMP-2 are induced in U87 human glioma cells
in response to beta-amyloid peptide. J Neurosci Res. 55:44–53.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai JC, Ananthakrishnan G, Jandhyam S,
Dukhande VV, Bhushan A, Gokhale M, Daniels CK and Leung SW:
Treatment of human astrocytoma U87 cells with silicon dioxide
nanoparticles lowers their survival and alters their expression of
mitochondrial and cell signaling proteins. Int J Nanomedicine.
5:715–723. 2010.PubMed/NCBI
|
|
22
|
Iyanagi T: Structure and function of
NADPH-cytochrome P450 reductase and nitric oxide synthase reductase
domain. Biochem Biophys Res Commun. 338:520–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Brien RJ and Wong PC: Amyloid precursor
protein processing and Alzheimer's disease. Annu Rev Neurosci.
34:185–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao J, Zhang W, Zhang P, Liu R, Liu L, Lei
G, Su C, Miao J and Li Z: Abeta20-29 peptide blocking apoE/Abeta
interaction reduces full-length Abeta42/40 fibril formation and
cytotoxicity in vitro. Neuropeptides. 44:305–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jain P, Wadhwa PK and Jadhav HR: Reactive
astrogliosis: Role in Alzheimer's disease. CNS Neurol Disord Drug
Targets. 14:872–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodríguez JJ, Olabarria M, Chvatal A and
Verkhratsky A: Astroglia in dementia and Alzheimer's disease. Cell
Death Differ. 16:378–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Członkowska A and Kurkowska-Jastrzębska I:
Inflammation and gliosis in neurological diseases-clinical
implications. J Neuroimmunol. 231:78–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agostinho P, Cunha RA and Oliveira C:
Neuroinflammation, oxidative stress and the pathogenesis of
Alzheimer's disease. Curr Pharm Des. 16:2766–2778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh S and Joshi N: Astrocytes:
Inexplicable cells in neurodegeneration. Int J Neurosci.
127:204–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robel S and Sontheimer H: Glia as drivers
of abnormal neuronal activity. Nat Neurosci. 19:28–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pekny M, Pekna M, Messing A, Steinhäuser
C, Lee JM, Parpura V, Hol EM, Sofroniew MV and Verkhratsky A:
Astrocytes: A central element in neurological diseases. Acta
Neuropathol. 131:323–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu D, Corbett B, Yan Y, Zhang GX, Reinhart
P, Cho SJ and Chin J: Early cerebrovascular inflammation in a
transgenic mouse model of Alzheimer's disease. Neurobiol Aging.
33:2942–2947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Obulesu M and Lakshmi MJ: Apoptosis in
Alzheimer's disease: An understanding of the physiology, pathology
and therapeutic avenues. Neurochem Res. 39:2301–2312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ajith TA and Padmajanair G: Mitochondrial
Pharmaceutics: A new therapeutic strategy to ameliorate oxidative
stress in Alzheimer's disease. Curr Aging Sci. 8:235–240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jonckheere AI, Smeitink JA and Rodenburg
RJ: Mitochondrial ATP synthase: Architecture, function and
pathology. J Inherit Metab Dis. 35:211–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Estabrook RW: Steroid hydroxylations: A
paradigm for cytochrome P450 catalyzed mammalian monooxygenation
reactions. Biochem Biophys Res Commun. 338:290–298. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao Y, Liu S, Wang Y, Yuan W, Ding X,
Cheng T, Shen Q and Gu J: Suppression of cytochrome P450 reductase
expression promotes astrocytosis in subventricular zone of adult
mice. Neurosci Lett. 548:84–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Norris PJ, Hardwick JP and Emson PC:
Localization of NADPH cytochrome P450 oxidoreductase in rat brain
by immunohistochemistry and in situ hybridization and a comparison
with the distribution of neuronal NADPH-diaphorase staining.
Neuroscience. 61:331–350. 1994. View Article : Google Scholar : PubMed/NCBI
|