Introduction
Pancreatic cancer (PC) is an aggressive tumor with a
7% 5-year survival rate worldwide (1,2).
Surgical resection may be the only way to cure PC. However, due to
the unobservable symptoms (3),
80–95% patients present with locally advanced or metastatic disease
when diagnosed (4). Even following
surgical resection of the tumor, there are still a number of
patients that present relapsed or with metastatic disease (1,5).
Gemcitabine (GEM), a type of first-line chemotherapy for advanced
PC (6,7), is able to induce cell apoptosis at
the S stage of the cell cycle thereby inhibiting PC cell
proliferation (8). GEM may
effectively relieve symptoms of patients suffering PC. However, the
therapeutic efficacy was frequently unsatisfactory for the
development of drug resistance in PC cells, commonly leading to
increased cell metastasis and increased therapeutic difficulty.
Therefore, investigating the mechanism underlying GEM resistance
and researching an effective therapy strategy to enhance the
sensitivity of tumor cells to the drug is of significance for the
treatment of PC.
A variety of molecular mechanisms were involved in
the acquisition of drug resistance, including
epithelial-mesenchymal transition (EMT) (9). EMT is a cellular process
characterized by morphological alterations from epithelial
phenotype to mesenchymal phenotype, by which cells lose epithelial
cell-cell adhesion and are able to move through the extracellular
matrix. EMT is frequently demonstrated to be associated with
enhanced proliferation, invasion and metastasis in cancer cells
(10). During EMT, cells
demonstrated the acquisition of mesenchymal markers including
Vimentin, type I collagen, fibronectin and Snail, and the
inhibition of cell adhesion molecules including epithelial
(E)-cadherin and catenin expression (11). Additionally, it has been
demonstrated that EMT is correlated with chemotherapeutic
sensitivity in human cancers and sensitivity to GEM in PC cells
(12–14).
It has been reported that heating tumors up to a
temperature of 42°C synergistically enhances the antitumor effect
of GEM (15,16), but the mechanism is not clearly
known. Furthermore, hyperthermia has been demonstrated to exhibit
an inhibitory effect against tumor growth factor-β1 induced EMT
(17). Therefore it was
hypothesized in the present study that EMT in GEM-resistant PC
cells may be suppressed by hyperthermia.
In the present study, the underlying mechanism of
GEM resistance in the PC cell line PANC-1 was investigated and the
effect of hyperthermia on the sensitivity and motility of
GEM-resistant PANC-1 cells were analyzed. GEM-resistant PANC-1
cells were demonstrated to exhibit elevated migratory and invasive
abilities. Additionally, hyperthermia combined with GEM had a
synergistic inhibitory effect on cellular motility by inhibiting
EMT in GEM-resistant PANC-1 cells and attenuated PC cell invasion
by downregulating matrix metalloproteinase (MMP)2 and MMP9.
Materials and methods
Cell culture
The human PC cell line PANC-1 was purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in Dulbecco's modified Eagle
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Lonza Group,
Ltd., Basel, Switzerland), 5 mM L-glutamine, 5 mM non-essential
amino acids, 100 U/ml penicillin and streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.), in a humidified 5% CO2
incubator at 37°C. Cells were treated with hyperthermia for 1 h in
a 42°C 5% CO2 incubator.
Development of GEM-resistant PANC-1
cell line
GEM-resistant PANC-1 cells was developed by exposing
PANC-1 cells to escalating concentrations of GEM between 0.5 and 5
µM in complete medium at 37°C (18). Following exposure to increasing
concentrations of GEM for at least 3 months until clones developed
resistance to 5 µM GEM, living cells were collected and called drug
resistant cells (PAN/GEM), which were used for subsequent
experiments.
Morphological observation of
GEM-resistant cells
A total of 2×105 cells/ml were cultured
in 60 mm culture dishes for 24 h and then cells were treated with
or without 5 µM GEM for 48 h. Medium was removed and cells were
washed once with DMEM. Cell morphology was observed and images were
captured using a vertical light microscope at magnification,
×100.
Migration and invasion assay
Cell migration was assessed using a Transwell assay
using 6.5 mm chambers (Corning Incorporated, Corning, New York,
USA) with 8-µm pore membranes (19). A total of 600 µl DMEM with GEM was
added to the lower chamber. The suspension of 5×104
cells in 100 µl DMEM with 1% FBS was plated into the upper chamber
following by adding GEM. After 20 h, cells on the undersurface of
the polycarbonate membranes were stained with crystal violet
(Amresco, Inc., Framingham, MA, USA) for 10 min at room temperature
and were observed using a light microscope at magnification, ×100,
and six fields were chosen at random to measure the average cell
coverage using Image J software (version 1.47; National Institutes
of Health, Bethesda, MD, USA). Relative migratory cell number was
expressed as a percentage of the control. Invasion was assayed
using the same procedure as the migration assay, except that 70 µl
1 mg/ml Matrigel (BD Biosciences, San Jose, CA, USA) was added into
the upper surface of the membrane.
Western blot analysis
Cells were washed twice with PBS and extracted using
Mammalian Protein Extraction reagent (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
proteins (50 µg) were separated by 10% SDS-PAGE (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) and transferred
to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat dried milk in TBST
for 1 h at room temperature and incubated with specific primary
antibodies overnight at 4°C: Mouse monoclonal anti-E-cadherin (cat.
no. sc-21791; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), mouse monoclonal anti-Vimentin (cat. no. sc-6260; 1:2,000;
Santa Cruz Biotechnology, Inc.), mouse monoclonal anti-β-actin
(cat. no. sc47778; 1:1,000; Santa Cruz Biotechnology, Inc.), rabbit
polyclonal anti-MMP2 antibody (cat. no. 4022; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit polyclonal
anti-MMP9 antibody (cat. no. 3852; 1:1,000; Cell Signaling
Technology, Inc.) were used, followed by horseradish
peroxidase-conjugated secondary antibodies goat anti-mouse (cat.
no. sc-2005; 1:2,000; Santa Cruz Biotechnology, Inc.) and goat
anti-rabbit (cat. no. sc-2004; 1:2,000; Santa Cruz Biotechnology,
Inc.) IgG secondary antibodies for 2 h at room temperature.
Development was performed using enhanced
chemiluminescence-detecting reagent (GE Healthcare, Chicago, IL,
USA).
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in cells was extracted using a total RNA
mini plus kit (A&A Biotechnology, Gdynia, Poland). cDNA was
obtained by RT-PCR using a RevertAid™ First Strand cDNA
synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) in a 50 µl reaction mixture. Reverse
transcription was carried out at 45°C for 45 min. cDNA was
subsequently amplified using TaqMan® Gene Expression
assay (Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR
thermocycling conditions were as follows: Initial denaturation at
94°C for 3 min, 40 amplification cycles of 94°C for 10 sec, 53°C
for 30 sec and 72°C for 40 sec, followed by a final extension at
72°C for 10 min. The primers were as follows: E-cadherin forward
(F), 5′-GTCAGTTCAGACTCCAGCCC-3′ and reverse (R),
5′-AAATTCACTCTGCCCAGGACG-3′; Vimentin F, 5′-TCTACGAGGAGGAGATGCGG-3′
and R, 5′-GGTCAAGACGTGCCAGAGAC-3′; MMP2 F,
5′-TATGGCTTCTGCCCTGAGAC-3′ and R, 5′-CACACCACATCTTTCCGTCA-3′; MMP9
F, 5′-AGTCCACCCTTGTGCTCTTC-3′ and R, 5′-ACTCTCCACGCATCTCTGC-3′;
GAPDH F, 5′-GATCCCTCCAAAATCAAGTG-3′ and R,
5′-GAGTCCTTCCACGATACCAA-3′. RT-qPCR was performed using ABI PRISM
7,700 Sequence detector (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with SDS software version 1.7. mRNA expression of
target genes was calculated using the formula 2−ΔΔCq
(20) and was normalized to the
level of GAPDH. The value of mRNA in cells was demonstrated as the
relative value of mRNA in control cells.
Cytotoxicity assay
The cytotoxicity of GEM on cells was evaluated using
an MTT assay (Sigma Aldrich; Merck KGaA, Darmstadt, Germany). A
total of 1×104 cells were seeded into each well of a
96-well plate in 100 µl medium and incubated with various
concentration of GEM for 48 h at 37°C in a 5% CO2
incubator. Then cells were incubated with 20 µl 5 mg/ml MTT for 4 h
at 37°C and then cells were lysed for 10 min at room temperature by
addition of 200 µl dimethyl sulfoxide (OriGen Biomedical, Inc.,
Austin, TX, USA). Absorbance was measured at 490 nm using a
microplate reader. Cell survival was expressed as a percentage of
the untreated control.
Metalloproteinase inhibitor (TIMP)1
and TIMP2 adenoviral infection of PANC-1 cell line
Human TIMP1 and TIMP2 cDNA plasmids were obtained
from Wuhan Huamei Bioengineering Co., Ltd. (Wuhan, China). The
adenovirus serotype 2 vector system with Adβgal was obtained from
Genzyme (Framingham, MA, USA). The TIMP adenovirus vectors were
constructed as previously described (21). Briefly, the titer of the
recombinant adenoviral vectors was established by the logarithmic
limiting dilution in 293 cells. The lowest dilution at which CPE
occurred was taken to be the titer of the virus. Adenoviral stocks
were screened for replication-competent adenovirus by titration in
non-permissive PANC-1 cells (21–23).
Cells (1×105) were infected with Adβgal virus at
multiplicities of infection of 10, 50, 100, 500 and 1,000 in the
presence of 2% FCS (Lanzhou Minhai Bioengineering Co., Ltd.,
Lanzhou, China) for 18 h at 37°C using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The medium was subsequently removed
and replaced with fresh medium plus 10% FCS. Cells were washed with
PBS and used for subsequent experimentation after 24 h.
Statistical analysis
Data were obtained from at least three experiments.
Statistical analysis was preformed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). Values are presented as the mean ± standard
error of the mean. One-way analysis of variance was used to assess
differences between groups. The Duncan's new multiple range test
was subsequently employed for pairwise comparisons followed by the
Bonferroni correction. P<0.05 was considered to indicate a
statistically significant difference.
Results
EMT is involved in the development of
GEM-resistant PC PANC-1 cells
To investigate the mechanism underlying GEM
resistance in PC, a GEM-resistant PC PANC-1 cell model (PAN/GEM)
was first constructed. PAN/GEM cells were developed by exposing
PANC-1 cells to escalating concentrations of GEM in complete medium
and evaluating which cells demonstrated resistance to GEM. It was
observed that PAN/GEM cells grew faster compared with PANC1 cells
in medium without GEM (data not shown). The cytotoxicity of GEM on
PAN/GEM and PANC-1 cells was evaluated by analyzing cell growth
using an MTT assay. The IC50 of GEM was determined by
exposing PAN/GEM and PANC-1 cells to different concentrations of
GEM for 48 h. IC50 values of the two cell lines were
respectively calculated to be 29.3 and 5.1 mmol/l (Fig. 1A). Furthermore, it was demonstrated
that GEM-resistant PAN/GEM cells exhibited EMT phenotypes
characterized by spindle-like morphology (Fig. 1B). The two cell types were treated
with 5 µM GEM for 48 h and prior to visualizing their morphology.
As expected, PANC-1 cells were rounded and exhibited membrane
blebbing, which is an apoptotic feature. However, no significant
alterations in PAN/GEM morphology were observed (Fig. 1B). Additionally, it was
demonstrated that migration and invasion rates in GEM-resistant
PAN/GEM cells were significantly increased (P<0.01; Fig. 1C and D). This was accompanied by
significantly decreased E-cadherin and increased Vimentin
expression (P<0.01; Fig. 1E),
compared with PANC-1 cells, suggesting that EMT was involved in the
development of GEM resistance in PANC-1 cells, and the enhancement
of cellular motility.
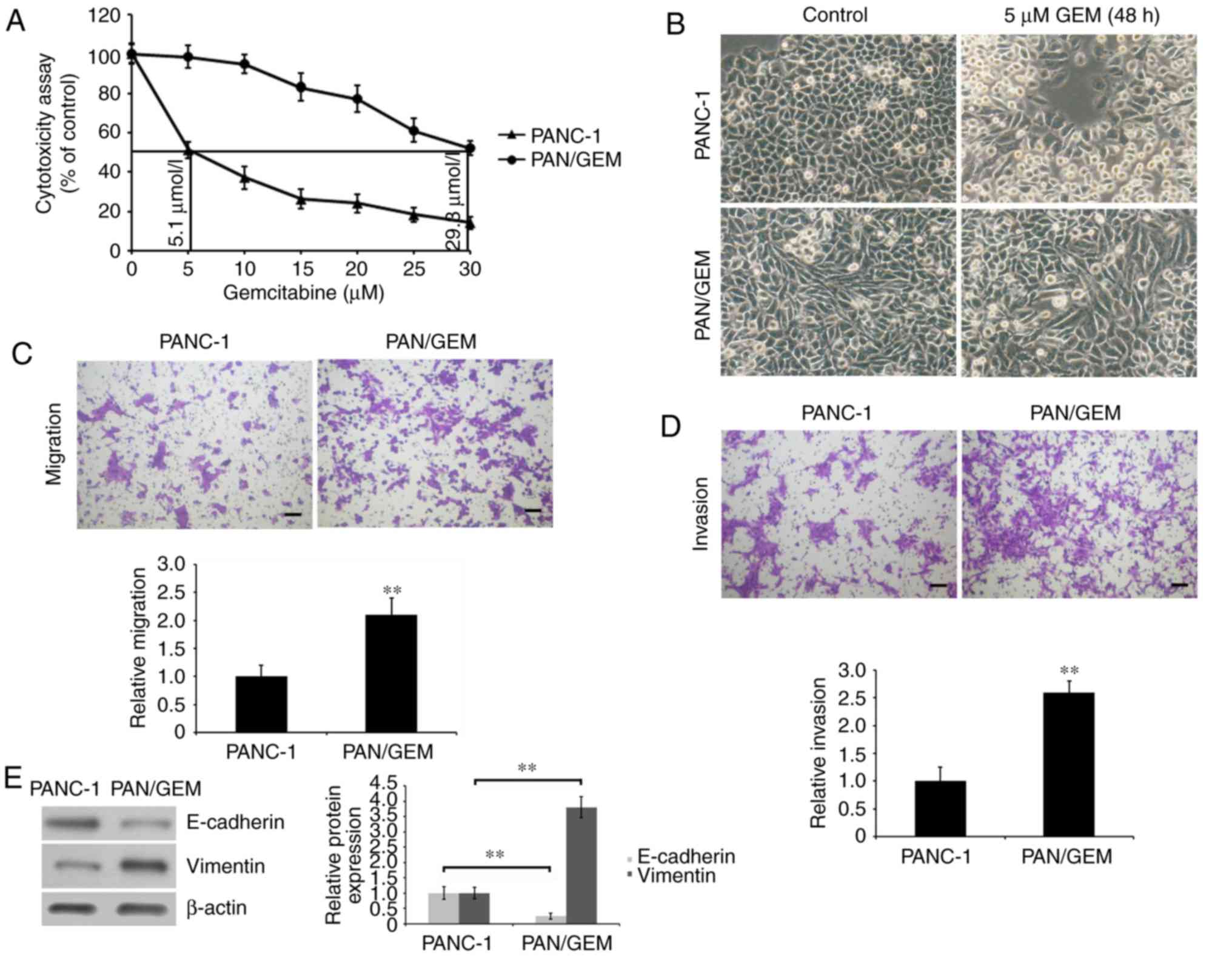 | Figure 1.Characterization of PAN/GEM and PANC-1
cells. (A) The cytotoxicity of GEM on cells was evaluated using a
MTT assay. Cells were incubated in 100 µl medium for 24 h, and then
were treated with 0, 5, 10, 15, 20, 25 and 30 µM GEM for 48 h. The
IC50 values of the two cells were calculated. The
cytotoxicity was expressed as a percentage of GEM-untreated
control. (B) PAN/GEM and PANC-1 cells were exposed to 5 µM GEM for
48 h and then the morphology was visualized using a vertical
microscope (magnification, ×100). (C) Cell migration and (D)
invasion were assessed using a Transwell assay. PAN/GEM cells
demonstrated an increased migratory and invasive ability compared
to PANC-1 cells. Migratory and invasive cells on the undersurface
of the polycarbonate membranes were observed using a light
microscope (magnification, ×100). Scale bar=100 µm. (E) E-cadherin
and Vimentin expression in PAN/GEM and PANC-1 cells were assessed
by western blot analysis using an anti-E-cadherin or anti-Vimentin
antibody. β-actin was detected as an internal standard. The protein
blots were quantified by densitometry and the amounts were
expressed relative to the internal reference β-actin. **P<0.01
vs. PANC-1 cells. PAN/GEM, gemcitabine-resistant pancreatic cancer
cell line PANC-1; E, epithelial. |
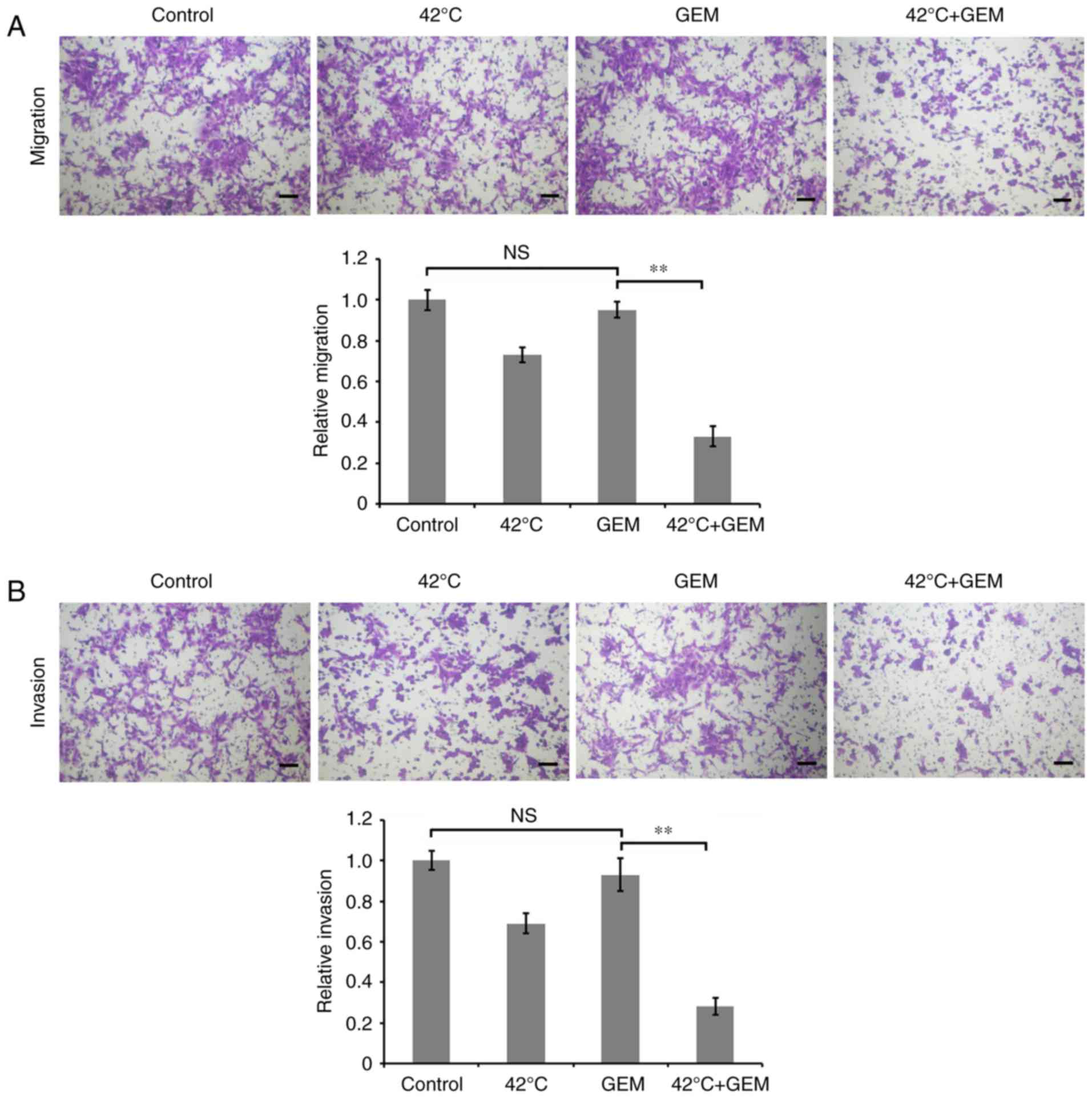
Hyperthermia combined with GEM
attenuates migration and invasion in GEM-resistant PAN/GEM
cells
To investigate the effect of hyperthermia on
migration and invasion of GEM-resistant PAN/GEM cells, PC cells
were treated with hyperthermia at 42°C for 1 h and then treated
with 5 µM GEM for 24 h. Transwell assays demonstrated that
hyperthermia combined with GEM significantly decreased the
migration and invasion in PAN/GEM cells compared with the GEM
treatment alone (P<0.01), while GEM alone treatment did not
significantly affect the migration and invasion compared with
untreated control group (Fig. 2A and
B). These data implicated that hyperthermia combined with GEM
was able to effectively eliminate cellular resistance to GEM by
inhibiting migration and invasion.
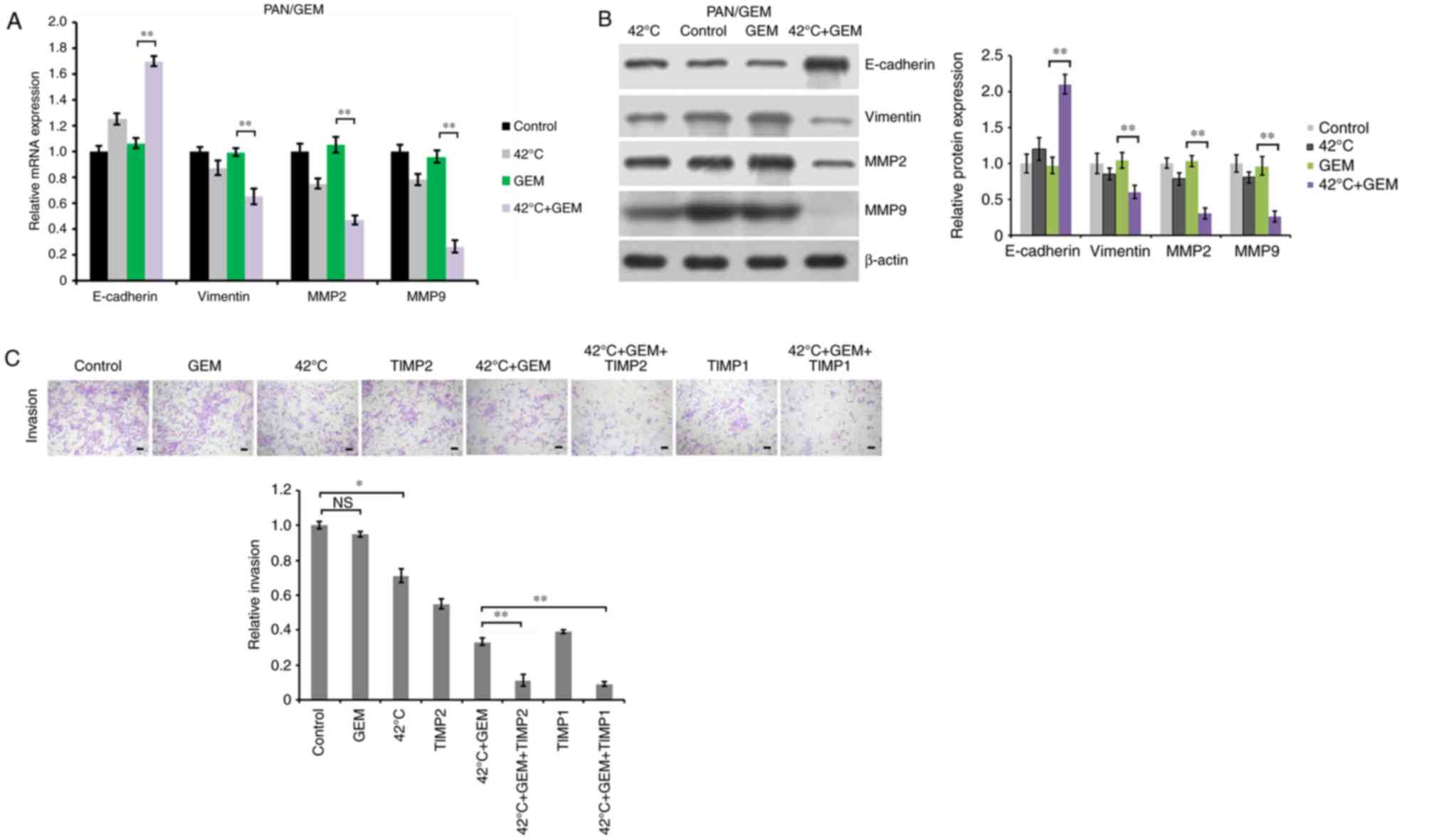
Hyperthermia reverses EMT in
GEM-resistant PAN/GEM cells
To investigate the underlying mechanism of
hyperthermia inhibiting migration and invasion of GEM-resistant
PAN/GEM cells, cells were treated with hyperthermia at 42°C for 1
h, and then treated with 5 µM GEM for 48 h. EMT molecular markers
were detected by RT-qPCR and western blot analysis. The results
demonstrated that GEM treatment alone did not significantly alter
the expression of E-cadherin, Vimentin, MMP2/9 compared with
untreated PAN/GEM cells, while hyperthermia treatment alone at 42°C
slightly increased E-cadherin expression, and decreased Vimentin
and MMP2/9 expression (Fig. 3A and
B). It was demonstrated that hyperthermia combined with GEM
significantly upregulated E-cadherin, and downregulated Vimentin,
MMP2 and MMP9 at the mRNA and protein levels to reverse EMT
compared with the GEM treatment alone (P<0.01; Fig. 3A and B). These findings identified
that hyperthermia at 42°C was able to reverse EMT to
mesenchymal-epithelial transition in PAN/GEM cells, which may imply
a mechanism for the effect of hyperthermia combined with GEM on PC
cell migration and invasion. MMP2 and MMP9 have been demonstrated
to be overexpressed in a number of aggressive tumors, and to be
associated with tumor invasion (24). To confirm the mechanism underlying
hyperthermia combined with GEM inhibiting PC cell invasion, TIMP1
and TIMP2 were introduced to inhibit their catalytic activity by
adenovirus Adβgal expressing TIMP1 or TIMP2 infection (21). Following infection for 24 h, cell
invasion was evaluated. The data demonstrated that TIMP2 and TIMP1
significantly augmented the inhibition of hyperthermia plus GEM on
cell invasion (P<0.01; Fig.
3C). The inhibitory effect of TIMP1 and TIMP2 on MMP2/9 was
confirmed by MMP2/9 activity detection using MMP2/9 activity assay
kit (gelatin zymography) (data not shown). These results suggest
that hyperthermia plus GEM may attenuate PC cell invasion by
downregulating MMP2 and MMP9.
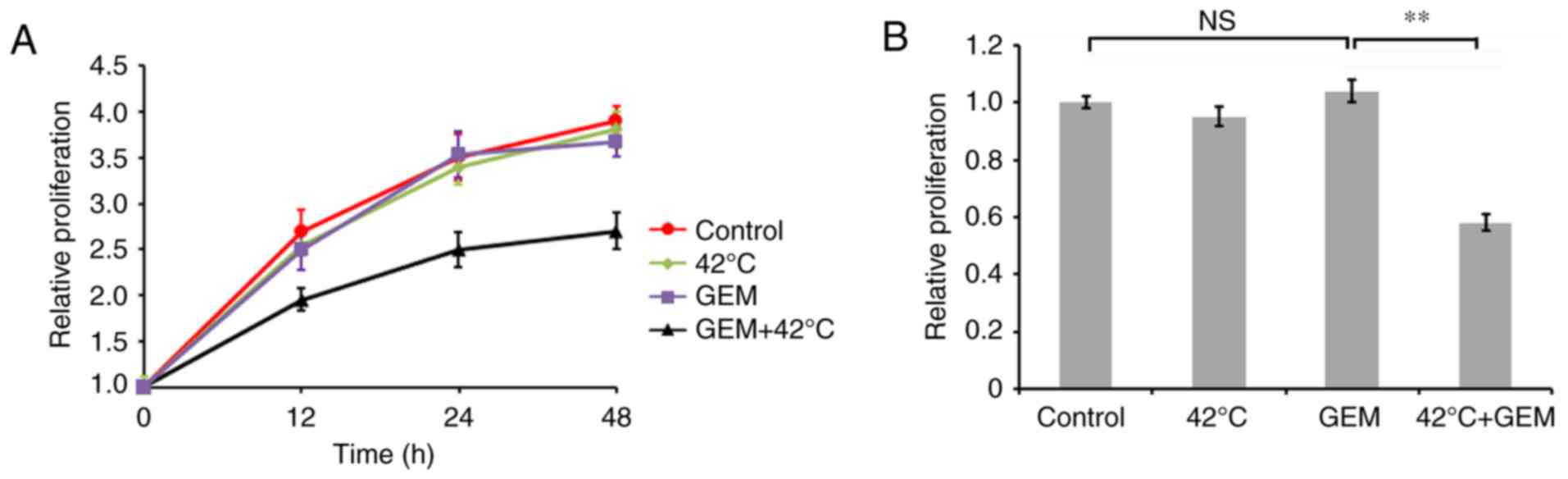
Hyperthermia enhances sensitivity of
GEM-resistant PAN/GEM cells to GEM
To determine whether hyperthermia increases the
sensitivity of PAN/GEM cells to GEM, an MTT assay on PAN/GEM cells
treated with or without hyperthermia was performed. It was
demonstrated that hyperthermia significantly enhanced cell growth
inhibition induced by 5 µM GEM in PAN/GEM cells, while hyperthermia
or GEM alone treatment did not significantly affect cell
proliferation compared with the control (P<0.01; Fig. 4). These data indicated that PAN/GEM
cells with hyperthermia treatment exhibited markedly increased
sensitivity to GEM compared with without hyperthermia
treatment.
Discussion
PC is an aggressive disease and a major cause of
cancer-associated mortality with the 5-year survival rate being
<10% worldwide (1,25). It has been demonstrated that
~80–95% of patients are at the locally advanced or metastatic
disease stage when diagnosed (4)
due to the unobservable symptoms of PC (3). Thus, there is a need to identify
strategies to treat and manage PC. GEM is one of the
chemotherapeutic agents used to treat advanced PC. However, nearly
all patients eventually succumb to relapse due to drug resistance,
thus making the therapeutic efficacy of GEM significantly limited.
Therefore, investigating effective therapeutic strategies are
necessary to overcome the acquisition of drug resistance. In the
present study it was demonstrated that hyperthermia was able to
attenuate the drug resistance of PC PANC-1 cells to GEM, and
hyperthermia in combination with GEM was able to inhibit cell
migration and invasion through EMT inhibition.
In the present study, a GEM-resistant PC cell line,
PAN/GEM, was developed by simulating the condition of drug
resistance development in vivo using increasing
concentrations of GEM. The cell proliferation assay demonstrated
that the IC50 of GEM in drug-resistant PAN/GEM cells at
48 h was significantly increased compared with the parental PANC-1
cells. GEM at 5 µM did not significantly affect the proliferation
of PAN/GEM cells, while under identical conditions, the
proliferation of parental PANC-1 cells was reduced by ~50%,
suggesting that PAN/GEM cells demonstrated the resistance to GEM
and could be used in subsequent experiments. In addition, it was
demonstrated that generation of GEM resistance caused the
alteration of PANC-1 cell morphology transforming from
cobblestone-like epithelial cells into slender spindle-shaped
cells, suggesting the occurrence of the EMT phenotype in resistant
cells. In addition, EMT is characterized by the loss of cell
adhesion molecules, including E-cadherin and elevation of
mesenchymal markers such as Vimentin (11). It was demonstrated that that
GEM-resistant PAN/GEM cells exhibited significant downregulation of
E-cadherin expression and upregulation of Vimentin expression
compared with parental PANC-1 cells. These data supported that the
development of PANC-1 cells resistant to GEM induced EMT. A
previous study indicated that EMT was involved in the acquisition
of drug resistance (9). Therefore,
in the present study it was hypothesized that EMT may be the
mechanism underlying the acquisition of GEM resistance in PANC-1
cells.
It has been reported that heating tumors may
synergistically enhance antitumor effect of GEM (15,26)
and that hyperthermia exhibits an inhibitory effect on EMT
(17). Thus, PC cells were treated
with different temperatures for different durations and the
optimized temperature at 42°C for 1 h was chosen (data not shown).
Under these conditions, hyperthermia treatment alone did not
significantly affect cell proliferation and minimized cytotoxicity.
Additionally, it was demonstrated that the temperature of 42°C was
able to effectively enhance the effect of GEM on inhibiting PC cell
migration and invasion.
E-cadherin has been identified as a tumor suppressor
in multiple cancer types (27),
and has been demonstrated to serve a role in limiting cell motility
and migration (28). In the
present study, the expression level of E-cadherin was analyzed at
the mRNA and protein levels. When treated with hyperthermia plus
GEM, PC PANC-1 cells demonstrated significantly increased
E-cadherin expression, implying that hyperthermia plus GEM may
affect PC cell migration by upregulating E-cadherin. MMPs are a
family of zinc-dependent endopeptidases (29). MMP2 and MMP9 are family members of
MMPs, and facilitate the invasion of cancer cells (30,31).
MMP2 and MMP9 expression are associated with various morphological
features in pancreatic ductal adenocarcinoma (32). A previous study reported that MMP2
was involved in promoting invasion of hepatocellular carcinoma
cells (31). Furthermore,
hyperthermia has been reported to be able to inhibit cell
metastases and downregulate the expression of cancer metastasis
associated genes, including membrane type 1-matrix
metalloproteinase, which is a known activator of latent MMP2
(33). In the present study, it
was demonstrated that hyperthermia at 42°C significantly decreased
cell migration and invasion in resistant PAN/GEM cells, and then
the expression levels of MMP2 and MMP9 in PANC-1/GEM cells
following treated with hyperthermia plus GEM were analyzed. The
results demonstrated a significant decrease in MMP2 and MMP9
expression at the mRNA and protein levels in PANC-1/GEM cells
treated with hyperthermia plus GEM compared with GEM treatment
alone. It suggested that hyperthermia combined with GEM affected PC
cell invasion through downregulating MMP2 and MMP9. TIMP is an
inhibitor of MMPs, which binds Zn2+ at the active site
of MMPs to block catalytic activity (34). MMP2 and MMP9 inhibitors, TIMP2 and
TIMP1 were used in the present study to inhibit MMP activity. The
inhibitory effect of TIMP1 and TIMP2 on MMP2/9 was confirmed by
MMP2/9 activity detection using MMP2/9 activity assay kit (gelatin
zymography; data not shown). TIMP2 and TIMP1 enhanced the
suppression of hyperthermia plus GEM on the invasion of PC cells
even if hyperthermia combined with GEM itself could suppress the
invasion of PC cells. Therefore, the present study demonstrated
that hyperthermia combined with GEM probably affected GEM-resistant
PC PANC-1 cell invasion by downregulating MMP2 and MMP9.
In conclusion, the results of the present study
demonstrated that hyperthermia at 42°C combined with GEM may be a
more effective way for improving the sensitivity of PC PANC-1 cells
to GEM and inhibiting cell invasion by reversing EMT. The present
study may provide a promising clinical therapeutic strategy for PC.
To verify the universality of the therapeutic strategy in PC,
additional PC cell lines are required to complete the present
study.
References
|
1
|
Worni M, Guller U, White RR, Castleberry
AW, Pietrobon R, Cerny T, Gloor B and Koeberle D: Modest
improvement in overall survival for patients with metastatic
pancreatic cancer: A trend analysis using the surveillance,
epidemiology, and end results registry from 1988 to 2008. Pancreas.
42:1157–1163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bednar F and Simeone DM: Recent advances
in pancreatic surgery. Curr Opin Gastroenterol. 30:518–523. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ottaiano A, Capozzi M, De Divitiis C, De
Stefano A, Botti G, Avallone A and Tafuto S: Gemcitabine
mono-therapy versus gemcitabine plus targeted therapy in advanced
pancreatic cancer: A meta-analysis of randomized phase III trials.
Acta Oncol. 56:377–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghaneh P, Smith R, Tudor-Smith C, Raraty M
and Neoptolemos JP: Neoadjuvant and adjuvant strategies for
pancreatic cancer. Eur J Surg Oncol. 34:297–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massari F, Santoni M, Ciccarese C,
Brunelli M, Conti A, Santini D, Montironi R, Cascinu S and Tortora
G: Emerging concepts on drug resistance in bladder cancer:
Implications for future strategies. Crit Rev Oncol Hematol.
96:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Sousa Cavalcante L and Monteiro G:
Gemcitabine: Metabolism and molecular mechanisms of action,
sensitivity and chemoresistance in pancreatic cancer. Eur J
Pharmacol. 741:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao X, Koch G, Ait-Oudhia S, Straubinger
RM and Jusko WJ: Pharmacodynamic modeling of cell cycle effects for
gemcitabine and trabectedin combinations in pancreatic cancer
cells. Front Pharmacol. 7:4212016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu P, Zhu Y, Yang C, Wang Y and Wang G:
Department of oncology, huangshan people's hospital, affiliated to
wangnan medical college: The mechanism and countermeasures on the
secondary resistance of epidermal growth factor receptor tyrosine
kinase inhibitor (EGFR-TKI). Anti-Tumor Pharm. 5:42015.
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert G, Gaggioli C, Bailet O, Chavey C,
Abbe P, Aberdam E, Sabatié E, Cano A, Garcia de Herreros A,
Ballotti R and Tartare-Deckert S: SPARC represses E-cadherin and
induces mesenchymal transition during melanoma development. Cancer
Res. 66:7516–7523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
13
|
Neel DS and Bivona TG: Secrets of drug
resistance in NSCLC exposed by new molecular definition of EMT.
Clin Cancer Res. 19:3–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng
L, Shi Y, Wang H, Yin B, Xia J and Wang Z: Down-regulation of
miR-223 reverses epithelial-mesenchymal transition in
gemcitabine-resistant pancreatic cancer cells. Oncotarget.
6:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maeda H, Wu J, Sawa T, Matsumura Y and
Hori K: Tumor vascular permeability and the EPR effect in
macromolecular therapeutics: A review. J Control Release.
65:271–284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirui DK, Celia C, Molinaro R, Bansal SS,
Cosco D, Fresta M, Shen H and Ferrari M: Mild hyperthermia enhances
transport of liposomal gemcitabine and improves in vivo therapeutic
response. Adv Healthc Mater. 4:1092–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura-Tsuchiya R, Ishikawa T, Kokura S,
Mizushima K, Adachi S, Okajima M, Matsuyama T, Okayama T, Sakamoto
N, Katada K, et al: The inhibitory effect of heat treatment against
epithelial-mesenchymal transition (EMT) in human pancreatic
adenocarcinoma cell lines. J Clin Biochem Nutr. 55:56–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meena AS, Sharma A, Kumari R, Mohammad N,
Singh SV and Bhat MK: Inherent and acquired resistance to
paclitaxel in hepatocellular carcinoma: Molecular events involved.
PLoS One. 8:e615242013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y, Zhang J, Mi W, Yang J, Han F, Lu X
and Yu W: Silencing of GM3 synthase suppresses lung metastasis of
murine breast cancer cells. Breast Cancer Res. 10:R12008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rigg AS and Lemoine NR: Adenoviral
delivery of TIMP1 or TIMP2 can modify the invasive behavior of
pancreatic cancer and can have a significant antitumor effect in
vivo. Cancer Gene Ther. 8:869–878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerald R and Meidel RS: Adenoviral
vectorsGlover DM and Hames BD: DNA Cloning 4. A Prac Appr. Oxford
Univ Press; Oxford: pp. 285–305. 1996
|
|
23
|
Mittereder N, March KL and Trapnell BC:
Evaluation of the concentration and bioactivity of adenovirus
vectors for gene therapy. J Virol. 70:7498–7509. 1996.PubMed/NCBI
|
|
24
|
Sun XF, Shao YB, Liu MG, Chen Q, Liu ZJ,
Xu B, Luo SX and Liu H: High-concentration glucose enhances
invasion in invasive ductal breast carcinoma by promoting
Glut1/MMP2/MMP9 axis expression. Oncol Lett. 13:2989–2995. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim CE, Lim SK and Kim JS: In vivo
antitumor effect of cromolyn in PEGylated liposomes for pancreatic
cancer. J Control Release. 157:190–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shamir ER and Ewald AJ: Adhesion in
mammary development: Novel roles for E-cadherin in individual and
collective cell migration. Curr Top Dev Biol. 112:353–382. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kowalski PJ, Rubin MA and Kleer CG:
E-cadherin expression in primary carcinomas of the breast and its
distant metastases. Breast Cancer Res. 5:R217–R222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baruch RR, Melinscak H, Lo J, Liu Y, Yeung
O and Hurta RA: Altered matrix metalloproteinase expression
associated with oncogene-mediated cellular transformation and
metastasis formation. Cell Biol Int. 25:411–420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacob A and Prekeris R: The regulation of
MMP targeting to invadopodia during cancer metastasis. Fron Cell
Dev Biol. 3:42015.
|
|
31
|
Chen Y, Yu Y, Sun S, Wang Z, Liu P, Liu S
and Jiang J: Bradykinin promotes migration and invasion of
hepatocellular carcinoma cells through TRPM7 and MMP2. Exp Cell
Res. 349:68–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jakubowska K, Pryczynicz A, Januszewska J,
Sidorkiewicz I, Kemona A, Niewiński A, Lewczuk Ł, Kędra B and
Guzińska-Ustymowicz K: Expressions of matrix metalloproteinases 2,
7 and 9 in carcinogenesis of pancreatic ductal adenocarcinoma. Dis
Markers. 2016:98957212016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sawaji Y, Sato T, Seiki M and Ito A: Heat
shock-mediated transient increase in intracellular 3′,5′-cyclic AMP
results in tumor specific suppression of membrane type 1-matrix
metalloproteinase production and progelatinase A activation. Clin
Exp Metastasis. 18:131–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|