Introduction
Myasthenia gravis (MG) is a common autoimmune
neurological disorder, with an incidence rate between 7.7 and 15
per 100,000 across all age groups, but with a female bias (1,2). MG
is mainly induced by production of an acetylcholine receptor (AChR)
antibody, which may cause abnormal immune responses of AChR at
neuromuscular junctions, thus depriving its normal neural
transmission function (3).
Clinical manifestation of MG is general skeletal muscle fatigue and
blepharoptosis, both of which are aggravated following periods of
activity and alleviated with rest, thus exhibiting relief in the
morning and progression of symptoms in night (4). MG progression may affect neural
tissues other than neuromuscular junction or may affect non-neural
tissues, which may lead to epilepsy, pyramidal tract sign and
memory deficit (5,6). The pathogenic mechanism of MG is
complex and involves genetic, environmental, physical and chemical
factors (7). As an autoimmune
disease, MG also involves abnormality in humoral immunity and
cellular immunity response (8,9).
T helper (Th) cells are subdivided into two
sub-populations, Th1 and Th2, based on the differential secretion
of cytokines (10). A recent study
demonstrated that Th1 and Th2 cells maintain the Th1/Th2 balance by
regulating cytokine secretion, thus serving a crucial role in
maintaining normal immune functions (11). Th1 cells mainly secrete interleukin
(IL)-2 and interferon (IFN)-γ, whereas Th2 cells mainly produce
IL-4 and IL-6 cytokines (12).
Through self-regulation and cross-regulation of secreted cytokines,
Th1 and Th2 maintain a homeostasis and server crucial roles in
regulating both cellular and humoral immunity (13). Another recent study demonstrated
the role of Th1/Th2 secreted factors in the occurrence and
progression of MG (14). FTY720,
also known as fingolimod, is a new generation immune suppressant
that is extracted from the Traditional Chinese Medicine
Cordyceps sinensis, with the major component ISP-I (also
known as myriocin) having immune suppressing roles that induce
lymphocyte apoptosis, accelerate matured lymphocyte nesting and
inhibit translocation of T cells from the thymus to the peripheral
blood circulation, and thus serving a role in transplantation
immunity with strong effects and minor side effects (15). A recent study confirmed the role of
FTY720 in regulating autoimmune diseases such as encephalomyelitis
(16); however, the role of FTY720
in MG remained unknown. The aim of the present study was to
investigate the role of FTY720 in MG and its underlying therapeutic
mechanism, as well as providing a basis for the discovery of novel
treatment approaches for MG.
Materials and methods
Experimental animal selection
A total of 60 healthy female specific-pathogen-free
(SPF)-grade Lewis rats (age, 2–3 months; weight, 130±20 g) were
purchased from the Laboratory Animal Center of Shanghai Jiaotong
University (Shanghai, China) and were maintained in an SPF-grade
facility at a fixed temperature of 21±1°C, relative humidity
between 50 and 70% and a 12 h light/dark cycle with free access to
food and water. This study was approved by the Laboratory Animal
Management and Ethics Committee and followed animal welfare codes
in Shanghai Jiaotong University Affiliated Sixth People's
Hospital.
Materials and equipment
Pentobarbital sodium and lidocaine were purchased
from Shanghai Zhaohui Pharmaceutical Co., Ltd. (Shanghai, China).
FTY720 was purchased from Jiangxi Haoran Bio-Pharma Co., Ltd.
(Jiangxi, China). R-AChR subunit α region 97–116 was purchased from
Xi'an Lianmei Biotechnology Co., Ltd. (Xi'an, China). Complete
Freund's adjuvant (CFA) was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). ELISA kits for IL-2 (cat. no. R2000; Rat
IL-2 Quantikine ELISA Kit), IFN-γ (cat. no. RIF00; Rat IFN-gamma
Quantikine ELISA Kit), IL-4 (cat. no. R4000; Rat IL-4 Quantikine
ELISA Kit) and IL-6 (cat. no. R6000B; Rat IL-6 Quantikine ELISA
Kit) were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). TRIzol reagent, Total RNA Extraction kit, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
primers, and High-Capacity cDNA Reverse Transcription kit were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). A LabSystem 1.3.1 microplate reader was purchased from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA). The ABI 7700 Fast
Real-Time PCR cycler was purchased from Applied Biosystems (Thermo
Fisher Scientific, Inc.). An ultrapure workstation was purchased
from Sutai High-Tech Materials Co., Ltd. (Shanghai, China).
Animal grouping and treatment
Healthy female SPF-grade Lewis rats were randomly
assigned into 4 groups (n=15/group): i) Control group, which
received no treatment; ii) experimental autoimmune myasthenia
gravis (EAMG) model group, which received triplicate subcutaneous
injections of the immunogen R-AchR-α97–116 (200 µl of CFA per
injection at each body site) at 3 different body sites (tail, back
and foot) on days 0, 30 and 60; iii) 0.5 mg/kg FTY720 group, which
received FTY720 (0.5 mg/kg) by gavage 15 days post-EAMG
immunization; and iv) 1.0 mg/kg FTY720 group, which received FTY720
(1.0 mg/kg) 15 days post-EAMG immunization. The EAMG model was
prepared via injection in the rats with immunogen R-AchR-α 97–116
on days 0, 30 and 60 using the same method.
Observation of symptoms and Lennon
score
Lennon scoring was used to evaluate EAMG model rats
in a double-blinded manner (17).
Major symptoms of EAMG include muscle weakness, shakiness, body
curvature, fatigue and hoarse vocalization. The clinical evaluation
was performed based on the EAMG clinical grading scales in animal
models (rat), which were developed by Lennon et al (18). A score of 0 was defined as normal,
in which rats exhibited muscle strength without abnormality. A
score of 1 was assigned to rats exhibiting mild abnormality of
muscle strength, minor decrease of activity, weakness of grasping
and fatigue. A score of 2 was assigned to rats that exhibited
significant abnormality in motility, decreased activity or body
weight, head/tail falling, unstable walking, body curvature under
resting conditions, curved forelimbs and shaking. A score of 3 was
assigned to rats that exhibited whole body weakness, no activity,
body shakiness and dying status, which were consistent with
previous studies (18,19); a score of 4 was defined as death.
Rats exhibiting boundary phenotypes were assigned an additional 0.5
point. All rats were weighted prior to tissue collection. The
humane cut-off points were established according to a previous
study (19).
Sample collection
A total of 20 days post-EAMG immunization, a blood
sample (5 ml) was collected from the tail vein from rats in each of
the 4 groups. Blood samples were centrifuged at 3,000 × g for 15
min, and serum was collected and stored at −80°C for further
assays. Subsequently, rats were sacrificed and thymus tissues were
collected and stored at −80°C.
ELISA for serum levels of IL-2, IFN-γ,
IL-4 and IL-6
Serum samples were examined for the expression
levels of IL-2, IFN-γ, IL-4 and IL-6 using ELISA, following the
manufacturer's protocol. Using a blank control well as the
reference, absorbance values at 450 nm were measured by a
microplate reader. A linear regression model was plotted based on
the concentration of standard samples and respective optical
density (OD) values. Sample concentration was further deduced based
on OD values and regression function.
RT-qPCR for expression of Th1 and Th2
cytokines
Total RNA was extracted from thymus tissue samples
(100 mg) of rats using TRIzol reagent. Total RNA purity and
quantification was examined by UV spectrometry. cDNA was
synthesized by reverse transcription using High-Capacity cDNA
Reverse Transcription Kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions via incubation for 5
min at 95°C followed by 5 min at 4°C. Primer Premier 6.0 (Premier
Biosoft International, Palo Alto, CA, USA) was used to design
gene-specific PCR primers (Table
I). qPCR was used to determine target gene mRNA expression
levels using the following thermocycling conditions: 1 cycle at
92°C for 30 sec, followed by 35 cycles of 92°C for 30 sec, 58°C for
45 sec and 72°C for 35 sec, and final extension step at 72°C for 1
min. Cq values were calculated based on internal reference gene
GAPDH for plotting standard curve, and gene expression analysis was
performed by the 2−ΔΔCq method (20).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
AGTGCCAGCCTCGTCTCATAG |
|
| R:
CGTTGAACTTGCCGTGGGTAG |
| IL-2 | F:
CCAGGATAGCGATACGAACTT |
|
| R:
GGCATCTCTGCTTCAACCG |
| IFN-γ | F:
CGGCTAAGAAATAGCGATCTC |
|
| R:
GGCTCAATCGCTCTCGACT |
| IL-4 | F:
GGATCTAGCCGGAACAATACT |
|
| R:
CGTCTGACATCTGGCAGGAT |
| IL-6 | F:
GAAGATCTCAATAGCACGT |
|
| R:
AATCTCTCACGTCATCCTTC |
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for statistical analysis. Data are presented as
the mean ± standard deviation. Comparison of means among multiple
groups was performed by one-way analysis of variance with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical symptoms, body weight and
Lennon score
Healthy female SPF-grade Lewis rats were randomly
assigned into four groups, including a Control group, an EAMG model
group, which received injection of the R-AchR-α97–116 immune
antigen, and two groups of EAMG rats that received either a low
dose (0.5 mg/kg) or a high dose (1.0 mg/kg) of FTY720 15 days
post-immunization. Following the second immunization, rats
exhibited signs of muscle weakness, whereas typical MG symptoms
occurred following the third round of immunization injections. EAMG
model rats exhibited severe mental retardation, loss of glossy fur
or even detachment, hypo-activity, limb weakness, weakened
grasping, decreased appetite, body shaking and aggravated lower
muscle strength following activity. FTY720 treatment slowed the
onset of disease and alleviated symptoms, with more potent effects
observed in the high-dosage group. EAMG model rats exhibited
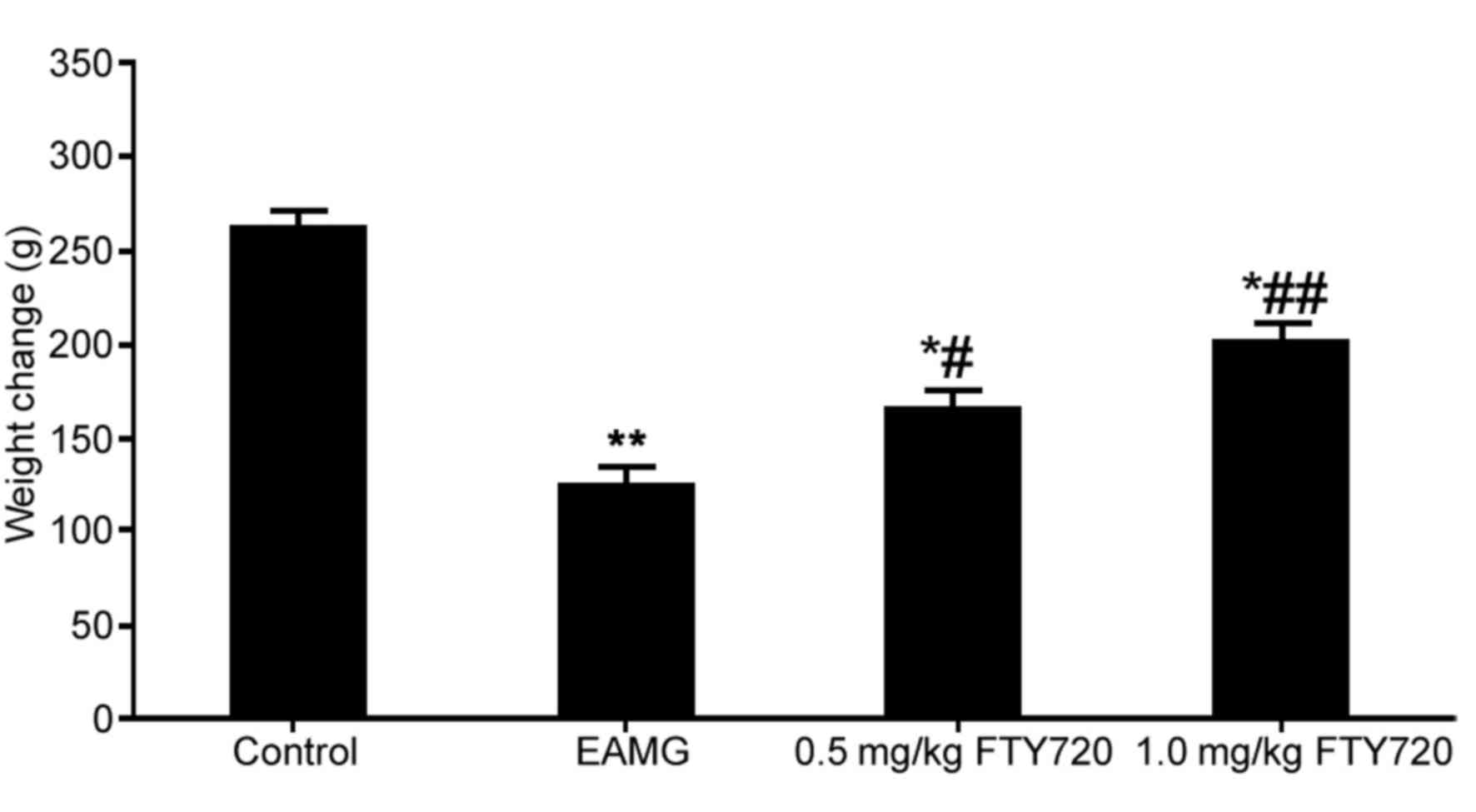
significantly lower body weight compared with the control group
(P<0.01; Fig. 1). EAMG rats
treated with either a low or high dose of FTY720 had significantly
higher body weights compared with untreated EAMG rats (P<0.05
and P<0.01, respectively; Fig.
1). The maximum body weight loss was 60 g (23% total body
weight), which was contemplated in the documents submitted to the
Ethics Committee of the Shanghai Jiaotong University Affiliated
Sixth People's Hospital for approval.
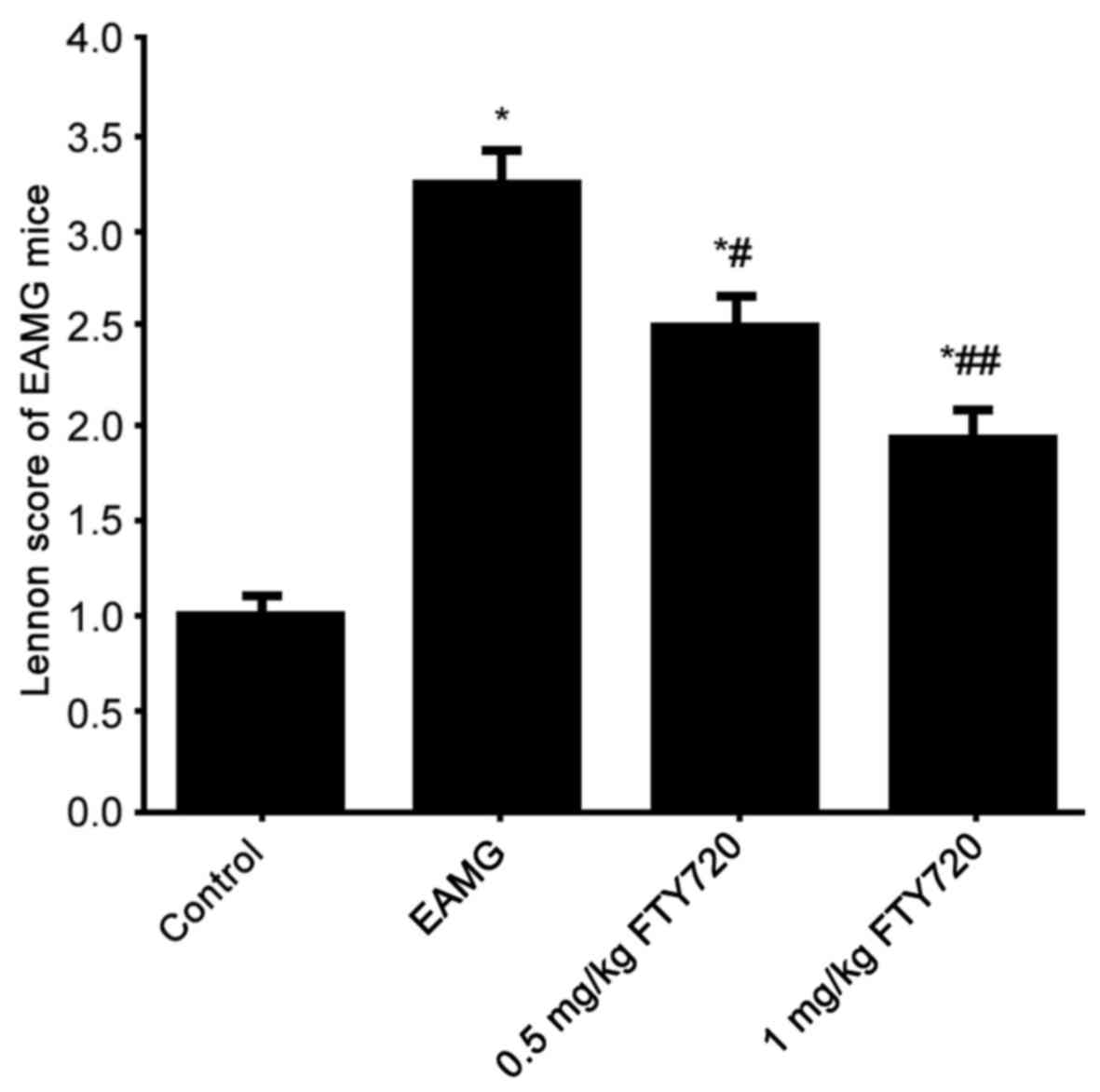
Lennon score of all rats (score was defined as 1 in
all control rats, data not shown) was evaluated. Rats in the EAMG
group demonstrated significantly elevated Lennon scores compared
with control rats (Fig. 2).
FTY7210 treatment significantly suppressed Lennon scores in a
dose-dependent manner compared with both the control group and the
EAMG group (Fig. 2). These results
indicated that FTY720 treatment may improve the clinical symptoms
EAMG.
Effects of FTY720 treatment on mRNA
expression levels of Th1 cytokines IL-2 and IFN-γ in thymus
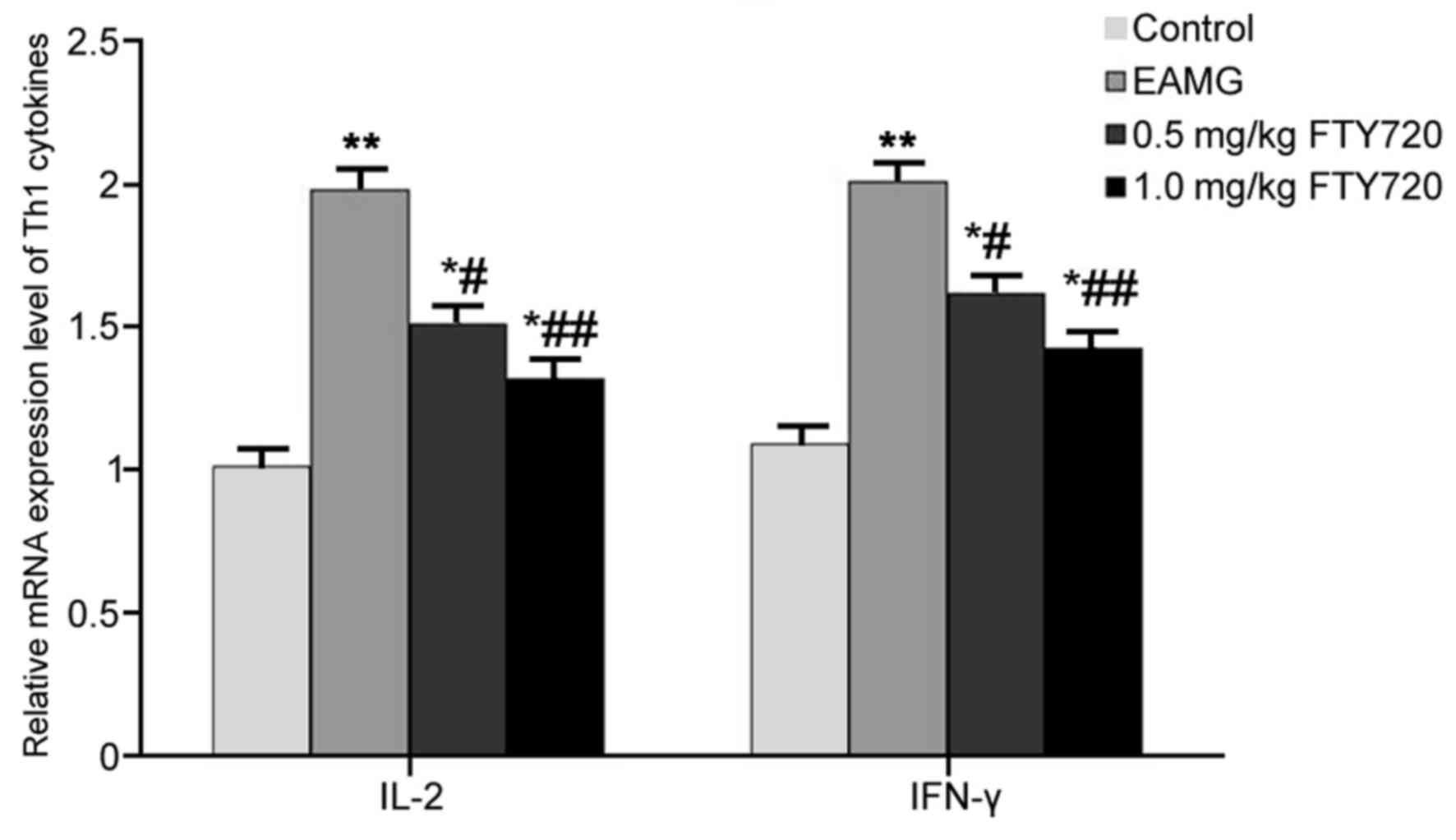
RT-qPCR was used to examine mRNA expression levels
of Th1 cytokines IL-2 and IFN-γ in rat thymus tissues. In EAMG
model rats, the mRNA expression levels of IL-2 and IFN-γ were
significantly higher compared with the respective expression levels
in Control rats (P<0.01; Fig.
3). Low-dose FTY720 treatment significantly decreased IL-2 and
IFN-γ mRNA expression in the thymus tissues compared with
expression in the EAMG model group (P<0.05; Fig. 3). Rats in the high-dosage FTY720
treatment group exhibited more potent inhibitory effects on IL-2
and IFN-γ mRNA expression levels (P<0.01 vs. EAMG; Fig. 3).
Effects of FTY720 treatment on mRNA
expression levels of Th2 cytokines IL-4 and IL-6 in thymus
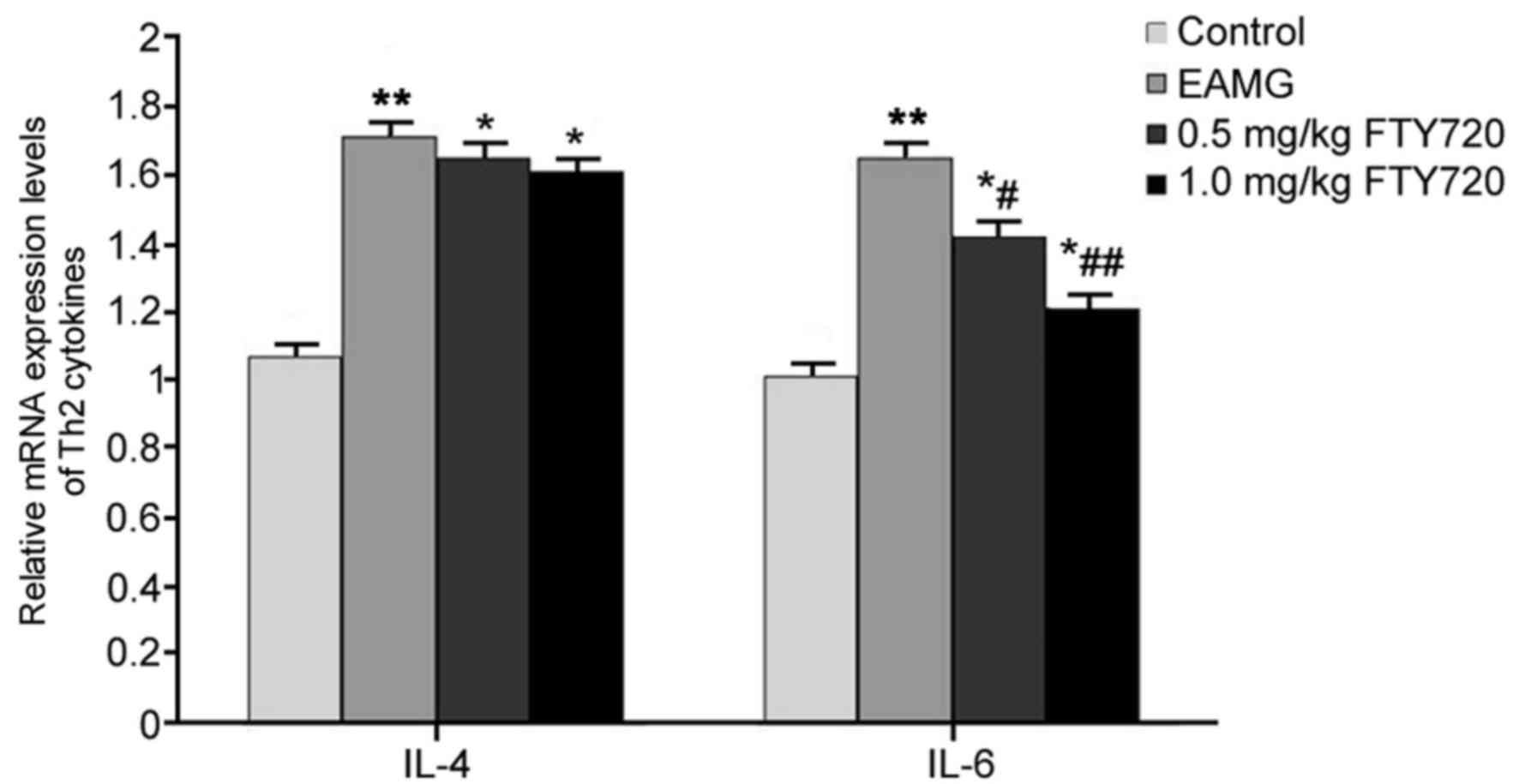
RT-qPCR was used to examine the mRNA expression
levels of Th2 cytokines IL-4 and IL-6 in rat thymus tissues. In
EAMG model rats, the mRNA expression levels of IL-4 and IL-6 were
significantly elevated compared with the respective expression
levels in Control rats (P<0.01; Fig. 4). Rats in the low-dose FTY720
treatment group exhibited a significantly decreased expression of
IL-6 mRNA compared with untreated EAMG model rats (P<0.05;
Fig. 4), and high-dose FTY720
treatment had a more potent inhibitory effect on IL-6 mRNA
(P<0.01 vs. EAMG). However, neither of the FTY720 treatments
significantly affected IL-4 mRNA expression (P>0.05 vs. EAMG;
Fig. 4).
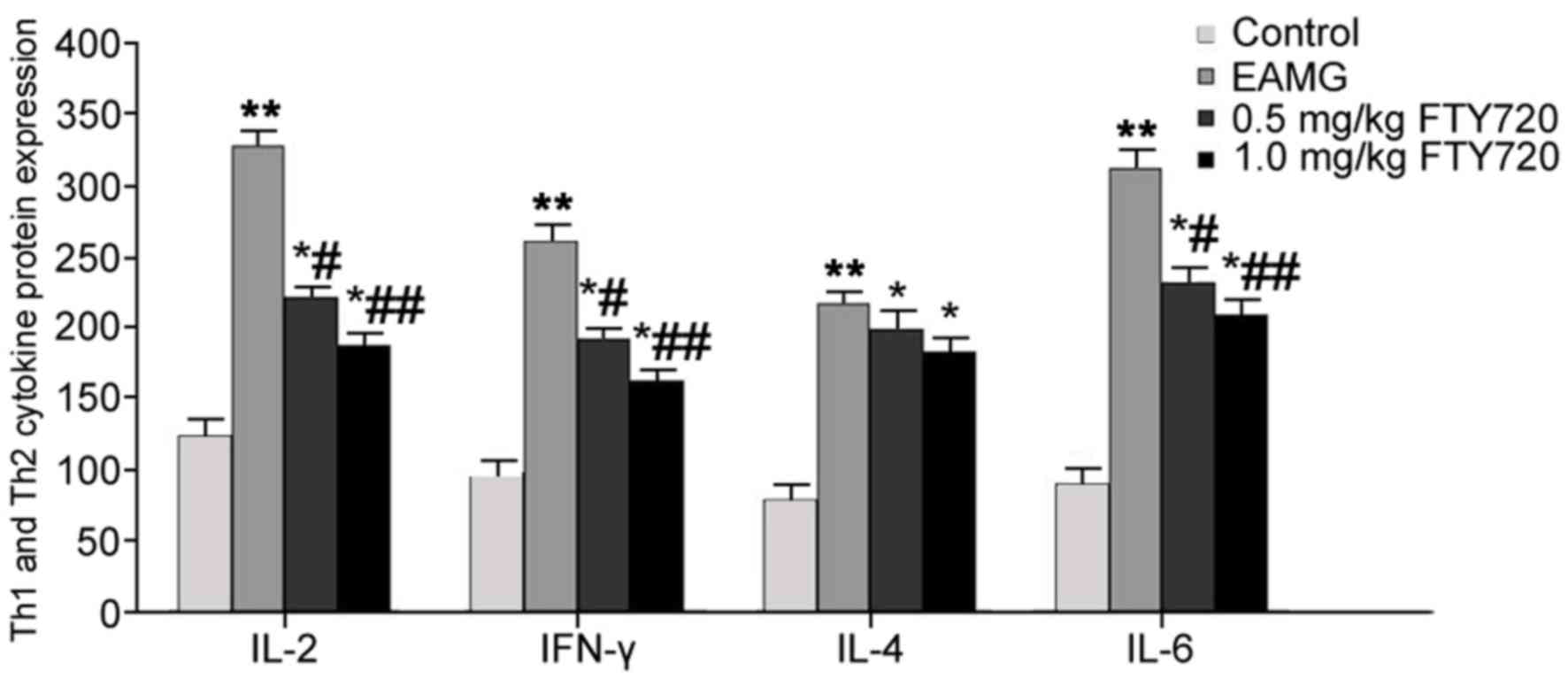
Effects of FTY720 treatment on protein
expression levels of Th1 and Th2 cytokines in serum
ELISA analysis was used to determine the effects of
FTY720 treatments on Th1 and Th2 cytokines in EAMG rat serum. In
EAMG model rats, serum levels of IL-2, IFN-γ, IL-4 and IL-6 were
significantly elevated compared with protein expression levels in
Control group rats (P<0.01). IL-2, IFN-γ and IL-6 expression
levels were significantly reduced following low-dose FTY720
treatment (P<0.05 vs. EAMG), with more potent inhibitory effects
observed in the high-dosage FTY720 treatment group (P<0.01 vs.
EAMG). No significant differences were identified for either high-
or low-dose FTY720 treatment on IL-4 expression levels, compared
with EAMG model rats (Fig. 5).
Discussion
A previous study has demonstrated the close
correlation between MG pathogenesis and humoral and cellular immune
functions, particularly for cellular immunity (21). The Th1-induced immune response
mainly serves a role in pathogenic immune responses by increasing
IL-2 or IFN-γ secretion, thus activating inflammatory response,
whereas the Th2 subpopulation of T cells exerts protective
functions through the secretion of cytokines that induce an immune
response (22). During MG
pathogenesis, both Th1 and Th2 cells are able to regulate MG
progression through the synergistic effects of the secreted
cytokines, with more potent effects being attributed to Th1
cytokines (23).
The present study used a R-AChR-α97–116 immunogen to
generate EAMG model rats, in which the effects of treatment with
the novel immune suppressant drug FTY720 were examine. The present
results indicated that FTY720 treatment was able to reduce muscle
weakness symptoms of EAMG rats, increase body weight and decrease
Lennon score, which indicated that FTYP720 treatment may be used to
examine the mechanisms of MG development, as well as a potential
immune therapy. Previous results confirmed that Th1 cells secreted
IFN-γ to facilitate antigen-specific Th1 cell to generate AchR-α
antibodies, the levels of which are increased in muscle and thymus
epithelium cells and thus indicate a correlation between IFN-γ and
MG production (24). Th1 secreted
IL-2 cytokines, which may further facilitate the activation of Th
cells and natural killer cells, lead to cytokine production, as
indicated by decreased IL-2 levels in MG patients (25). Th2-cell secreted self-activating
factor IL-4 is an important factor for inducing the humoral immune
response and is important for AchR-α antibody production, thus
serving a crucial role in MG pathogenesis (26); however its functional mechanism
remains unknown. Expression levels of the Th2-cell secreted IL-6
cytokine and its receptors have been reported to be upregulated in
patients with MG, which suggested that IL-6 receptor upregulation
may activate T cells and facilitate MG pathogenesis. In clinical
assessments, increase in IL-6 activity or content may be used as a
clinical marker for MG (27,28).
The present study analyzed the effects of FTY720 treatment on Th1
and Th2 cytokine production, and confirmed that FTY720 treatment
was able to suppress IL-2, IFN-γ and IL-6 expression levels in
thymus tissues and sera, but did not affect IL-4 levels.
Although the present study obtained significant
results, there were also a few limitations. First, the specific
pharmaceutical mechanisms for the function of FTY720 have not been
fully investigated, which maybe clarified in future studies.
Second, the sample size of the experimental animals used was
relatively small. Third, the protective function of FTY720 has not
been investigated in other animal models, which may also be
examined in the future studies.
In conclusion, FTY720 treatment suppressed the
inflammatory response by inhibiting the secretion of inflammatory
factors, thus improving the symptoms of EAMG. Results from the
present study may provide additional evidence for EAMG pathogenesis
mechanism and clinical treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by The Medical Professional
Cross Research Fund, Project of Shanghai Jiao Tong University
(grant no. YG2015MS14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH substantially contributed to the design of the
study and the performance of the experiments. YZ, TZ and HW
contributed to acquisition, analysis and interpretation of the
data. YZ provided final approval of the version of the manuscript
to be published and agreed to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
This study has been reviewed and approved by the
Ethical Committee of Shanghai Jiaotong University Affiliated Sixth
People's Hospital (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lizarraga AA, Lizarraga KJ and Benatar M:
Getting rid of weakness in the ICU: An updated approach to the
acute management of myasthenia gravis and guillain-barré syndrome.
Semin Neurol. 36:615–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sonkar KK, Bhoi SK, Dubey D, Kalita J and
Misra UK: Direct and indirect cost of myasthenia gravis: A
prospective study from a tertiary care teaching hospital in India.
J Clin Neurosci. 38:114–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beck G, Yabumoto T, Baba K, Sasaki T,
Higuchi O, Matsuo H and Mochizuki H: Double seronegative myasthenia
gravis with anti-LRP4 antibodies presenting with dropped head and
acute respiratory insufficiency. Intern Med. 55:3361–3363. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xin Y, Cai H, Lu T, Zhang Y, Yang Y and
Cui Y: miR-20b Inhibits T Cell Proliferation and Activation via
NFAT signaling pathway in thymoma-associated myasthenia gravis.
Biomed Res Int. 2016:95957182016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee HE, Kim YH, Kim SM and Shin HY:
Clinical significance of repetitive compound muscle action
potentials in patients with myasthenia gravis: A predictor for
cholinergic side effects of acetylcholinesterase inhibitors. J Clin
Neurol. 12:482–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gung Y, Zhang H, Li S and Wang Y:
Sternotomy versus video-assisted thoracoscopic surgery for
thymectomy of myasthenia gravis patients: A meta-analysis. Asian J
Endosc Surg. 9:285–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng QF, Zhang Z, Wang YJ, Chen W, Li FF,
Yue LT, Zhang CJ, Li H, Zhang M, Wang CC, et al: Astilbin
ameliorates experimental autoimmune myasthenia gravis by decreased
Th17 cytokines and up-regulated T regulatory cells. J Neuroimmunol.
298:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Geng Y, Zheng Y and Wang Y: A
case of anterior mediastinitis and bilateral multiple lung
abscesses occurring after trans-subxiphoid video-assisted
thoracoscopic extended thymectomy for thymoma with myasthenia
gravis. J Thorac Dis. 8:E970–E973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nikolic AV, Bojic SD, RakocevicStojanovic
VM, Basta IZ and Lavrnic DV: Electrophysiological findings in
patients with low density lipoprotein receptor related protein 4
positive myasthenia gravis. Eur J Neurol. 23:1635–1641. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vargas-Rojas MI, Solleiro-Villavicencio H
and Soto-Vega E: Th1, Th2, Th17 and Treg levels in umbilical cord
blood in preeclampsia. J Matern Fetal Neonatal Med. 29:1642–1645.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian T, Yu S, Liu L, Xue F, Yuan C, Wang
M, Ji C and Ma D: The profile of T helper subsets in bone marrow
microenvironment is distinct for different stages of acute myeloid
leukemia patients and chemotherapy partly ameliorates these
variations. PLoS One. 10:e01317612015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kornete M, Mason ES, Girouard J, Lafferty
EI, Qureshi S and Piccirillo CA: Th1-Like ICOS+
Foxp3+Treg cells preferentially express CXCR3
and home to β-islets during pre-diabetes in BDC2.5 NOD Mice. PLoS
One. 10:e01263112015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Cao H, Wang H, Yin G, Du J, Xia F,
Lu J and Xiang M: Multiple mechanisms involved in diabetes
protection by lipopolysaccharide in non-obese diabetic mice.
Toxicol Appl Pharmacol. 285:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi H, Noto YI, Makita N,
Kushimura-Okada Y, Ishii R, Tanaka A, Ohara T, Nakane S, Higuchi O,
Nakagawa M and Mizuno T: Myasthenic symptoms in anti-low-density
lipoprotein receptor-related protein 4 antibody-seropositive
amyotrophic lateral sclerosis: Two case reports. BMC Neurol.
16:2292016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahman MM, Prunte L, Lebender LF, Patel
BS, Gelissen I, Hansbro PM, Morris JC, Clark AR, Verrills NM and
Ammit AJ: The phosphorylated form of FTY720 activates PP2A,
represses inflammation and is devoid of S1P agonism in A549 lung
epithelial cells. Sci Rep. 6:372972016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Breart B and Bousso P: S1P1
downregulation tailors CD8+ T-cell residence time in
lymph nodes to the strength of the antigenic stimulation. Eur J
Immunol. 46:2730–2736. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaminski HJ, Himuro K, Alshaikh J, Gong B,
Cheng G and Kusner LL: Differential RNA expression profile of
skeletal muscle induced by experimental autoimmune myasthenia
gravis in rats. Front Physiol. 7:5242016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lennon VA, Lindstrom JM and Seybold ME:
Experimental autoimmune myasthenia: A model of myasthenia gravis in
rats and guinea pigs. J Exp Med. 141:1365–1375. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng X, Liu Y, Wang H, Liu L and Gong H:
Effcacy of tripterygium glycosides on experimentalautoimmune
myasthenia gravis in rats. Int J Clin Exp Med. 10:12235–12239.
2017.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anand G, Vasanthakumar R, Mohan V, Babu S
and Aravindhan V: Increased IL-12 and decreased IL-33 serum levels
are associated with increased Th1 and suppressed Th2 cytokine
profile in patients with diabetic nephropathy (CURES-134). Int J
Clin Exp Pathol. 7:8008–8015. 2014.PubMed/NCBI
|
|
22
|
Dalakas MC: Future perspectives in
target-specific immunotherapies of myasthenia gravis. Ther Adv
Neurol Disord. 8:316–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo J and Lindstrom J: AChR-specific
immunosuppressive therapy of myasthenia gravis. Biochem Pharmacol.
97:609–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Cao F, Ran Q and Sun X: Regulatory T
cells exhibit neuroprotective effect in a mouse model of traumatic
brain injury. Mol Med Rep. 14:5556–5566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uzawa A, Kanai T, Kawaguchi N, Oda F,
Himuro K and Kuwabara S: Changes in inflammatory cytokine networks
in myasthenia gravis. Sci Rep. 6:258862016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Wang CC, Zhang M, Li XL, Zhang P,
Yue LT, Miao S, Wang S, Liu Y, Li YB and Duan RS: Statin-modified
dendritic cells regulate humoral immunity in experimental
autoimmune myasthenia gravis. Mol Cell Neurosci. 68:284–292. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan X, Lin C, Han J, Jiang X, Zhu J and
Jin T: Follicular helper CD4+ T cells in human
neuroautoimmune diseases and their animal models. Mediators
Inflamm. 2015:6389682015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yilmaz V, Oflazer P, Aysal F, Durmus H,
Poulas K, Yentur SP, Gulsen-Parman Y, Tzartos S, Marx A, Tuzun E,
et al: Differential cytokine changes in patients with myasthenia
gravis with antibodies against AChR and MuSK. PLoS One.
10:e01235462015. View Article : Google Scholar : PubMed/NCBI
|