Introduction
The cytoskeleton is a reticular structure involved
in maintaining cellular morphology and physiological function, and
it is susceptible to the calpain system, which may be activated by
high concentrations of calcium during myocardial
ischemia/reperfusion (I/R) injury (MIRI). The endoplasmic reticulum
(ER) is a dynamic membranous network which is involved in various
physiological processes, including protein synthesis, steroid
hormone synthesis, intracellular calcium homeostasis regulation and
molecular signal transmission (1).
ER stress that disrupts ER function may occur in response to
radiation, ischemic stress, glucose and lipid metabolic disorders,
and other cellular stressors, which lead to the accumulation of
unfolded and misfolded proteins in the ER and calcium
dyshomeostasis. In the early stages of ER stress, the ER may
decrease the damage to cells caused by unfolded and misfolded
proteins, via activation of transcriptional and translational
pathways. However, sustained or serious ER stress may cause
alterations in the lipid composition and calcium reserves of the
ER, and cytoskeletal degradation and inflammation may be induced by
the imbalance of homeostasis in the cells (2). Studies have confirmed that ER stress
is implicated in the pathogenesis of diseases, including I/R
injury, shock and cancer (3,4).
Previously, reperfusion injury was significantly alleviated by ER
stress inhibitors in an in vivo model of liver, myocardial
and cerebral acute I/R (5–7).
Peroxisome proliferator-activated receptor α (PPARα)
is a subtype of transcription factors of the nuclear receptor
superfamily. It is highly expressed in various metabolically active
organs, including the liver, heart and kidney. PPARα serves a
critical biological role in regulating the expression of target
genes by forming heterodimers with the retinoid X receptor,
including genes involved in mitochondrial β-oxidation, ketogenesis,
lipoprotein transport and glycolysis (8). A previous study revealed that,
besides regulating energy metabolism and inflammatory responses,
PPARα serves a key role in intervening in the occurrence and
development of disease by regulating stress reactions (9). Research in the liver has shown that
ER stress may be suppressed by activating PPARα; in fatty liver
tissue, PPARα activation reduced the accumulation of lipid droplets
and apolipoprotein B-100 in the hepatic ER, corrected the
disturbance in the lipid composition of the ER and upregulated the
expression of sarcoplasmic reticulum Ca2+ ATPase,
resulting in the prevention of the induction of ER stress and an
improvement in hepatic steatosis associated with fructose
consumption (10). In addition,
PPARα was associated with the prevention of ER stress induced by
the disturbance of lipid metabolism in cardiac cells by enhancing
AMP-activated protein kinase activity (11). A previous report demonstrated that
PPARα activation may alleviate acute I/R-induced injury to the
mitochondrial ultrastructure, and the underlying protective
mechanism involves the antioxidant effect of PPARα (12). However, there are few studies that
demonstrate the protective effect of the PPARα on the cytoskeleton
and ER stress during MIRI. Therefore, the present study aimed to
determine the protective effect of PPARα activation on myocardial
I/R injury in rats and to investigate its possible underlying
mechanisms in ER stress. The present study demonstrated the
protective effects of fenofibrate on I/R heart tissue through ER
stress inhibition in the myocardium. These results provide a
theoretical basis for future clinical trials for fenofibrate in
patients with reperfusion injury.
Materials and methods
Animals
A total of 48 male Wistar rats (age, 6–8 weeks;
weight, 160–220 g) were bred in-house (Laboratory Animal Center,
The Second Affiliated Hospital of Harbin Medical University,
Harbin, China) and had ad libitum access to food and water. Rats
were maintained at a controlled temperature (22±2°C) and humidity
(50–70%). All animals were housed in a controlled pathogen free
environment with 12-h light and dark cycles. All protocols for
animals were performed strictly in accordance with the guidelines
for the Care and Use of Laboratory Animals, and the present study
was approved by the Ethical Committee of The Second Affiliated
Hospital of Harbin Medical University.
Myocardial I/R injury model
Wistar rats were randomly divided into four groups
(n=8 rats/group): Normal group (normal); sham group (sham); I/R
group (I/R); and fenofibrate pretreatment + I/R group (FF+I/R).
Fenofibrate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
suspended in 3% gum acacia (Sigma-Aldrich; Merck KGaA) and
administered for 7 days at a dose of 80
mg·kg−1·day−1 by oral gavage in the FF+I/R
group. The other three groups of rats were given a similar amount
of the solvent (3% gum acacia) for 7 days. The dose of fenofibrate
and the concentration of gum acacia were based on a previous study
(12,13).
A total of 1 h subsequent to the final intragastric
administration, the myocardial I/R model was generated. Briefly,
rats were intraperitoneally injected with 45 mg/kg pentobarbital
sodium. Following oral endotracheal intubation, the rats were
mechanically ventilated with air using a rodent ventilator (room
air; rate, 75 cycles/min; 3 ml/100 g tidal volume). The
electrocardiogram (ECG) was recorded. The left anterior descending
(LAD) coronary artery was ligated (1–2 mm region under the boundary
pulmonary artery pyramid and left auricle of heart) with a 5-0
polyester suture. A small polyethylene tube was placed between the
ligature and the myocardial tissues. The rats in the sham group
underwent the same surgical procedures, although the suture was not
fastened. Following ischemia for 45 min, the ligature was released
to permit reperfusion for 120 min. At the end of reperfusion, the
rats were sacrificed by overdose of pentobarbital sodium (100
mg/kg), and the left ventricle tissues were harvested and frozen in
liquid nitrogen (−196°C) immediately for further measurement.
Heart functional examination
Standard echocardiography was performed at room
temperature for all groups of rats following 45 min acute
myocardial ischemia and 120 min reperfusion. The left ventricular
ejection fraction (LVEF) and left ventricular end-diastolic
diameter (LVDd) were measured from the parasternal long-axis view
at the mid-papillary muscle level with an ultrasound imaging system
(Vevo770; FUJIFILM Visual Sonics, Inc., Toronto, ON, Canada).
Electron microscopy
Fresh myocardial tissues (1 mm3) were
excised from the tissue of the cardiac apex following reperfusion,
and fixed overnight in 3% glutaric dialdehyde at 4°C. Trimmed
tissues were post-fixed with 1% osmium tetraoxide for 2 h. Samples
were subsequently dehydrated in ethanol followed by acetone and
embedded in Epoxy resin (Ladd Research Industries, Williston, VT,
USA). The specimens were processed into ultrathin sections (50 nm).
The sections were stained with 1% uranium acetate and 1% lead
citrate for 10 min respectively at room temperature. A total of 64
tissue sections (eight fields of view per section), corresponding
to four sections from each rat (n=4), were observed via a
transmission electron microscope (H-7650; Hitachi, Ltd., Tokyo,
Japan) at an accelerating voltage of 80 kV.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Life
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
and the RNA concentration was determined using a UV
spectrophotometer. RT of total RNA to cDNA was performed using an
AccuPower RocketScript™ RT PreMix kit (Bioneer
Corporation, Daejeon, Korea), following the manufacturer's
protocol. The thermal cycle profile for RT was set for primer
annealing at 37°C for 10 min, cDNA synthesis at 50°C for 60 min and
heat inactivation at 95°C for 5 min. qPCR was performed using an
AccuPower 2× GreenStar™ qPCR Master Mix kit (Bioneer
Corporation) in a Bio-Rad iQ5 optical module (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). qPCR was performed under the following
conditions: 95°C for 10 min, and 40 cycles of denaturation at 95°C
for 10 sec and annealing at 58°C for 20 sec. Primer sequences were:
GRP78 forward, 5′-TGACTATGAAGAATCCCAAGA-3′ and reverse,
5′-TATCAACATCCAGTTCCACC-3′); GAPDH forward,
5′-GTTCAACGGCACAGTCAAGG-3′ and reverse, 5′-CACCAGTGGATGCAGGGAT-3′.
2−ΔΔCq was calculated for every sample, and the mRNA
expression levels were determined using the 2−ΔΔCq
method (14) and normalized to
GAPDH.
Western blotting
Heart tissues were homogenized in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The homogenates were centrifuged at
1,600 × g for 10 min at 4°C. A bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology) was used for protein
quantification. A total of 20 µg protein was electrophoresed on
10–15% SDS-PAGE gels and transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
subsequently blocked with 5% (w/v) non-fat milk in TBS containing
0.1% (v/v) Tween-20 and incubated overnight at 4°C with anti-PPARα
(cat no. WL00978; 1:1,000; Wanleibio, Co., Ltd., Shanghai, China),
anti-Desmin (cat no. ab32362; 1:8,000; Abcam, Cambridge, UK),
anti-GRP78 (cat no. WL00621; 1:1,000; Wanleibio, Co., Ltd.), and
anti-GADPH (cat no. ab181602; 1:8,000; Abcam). Following washing,
bound antibodies were detected following incubation for 1 h at room
temperature with peroxidase-conjugated goat anti-rabbit IgG (cat
no. ZB-2301; 1:10,000; OriGene Technologies, Inc., Beijing, China).
Blots were developed using Western Lightning BeyoECL Plus reagent
(Beyotime Institute of Biotechnology) and were quantified using
ImageJ software (version 2.1.4.7; National Institutes of Health,
Bethesda, MD, USA).
Measurement of calpain activity
Calpain activity was measured using the GENMED
Tissue Calpain Activity Assay kit (GenMed Scientifics, Inc.,
Wilmington, DE, USA). A total of ~100 mg tissue was homogenized in
1,000 µl nondenaturing lysis buffer provided with the kit, the
lysates were centrifuged for 15 min at 12,000 × g and the
supernatant was collected. Protein concentrations were quantified
using a micro bicinchoninic acid protein assay.
Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC) was
used as the calpain substrate. A total of 50 µl supernatant (100 µg
protein/50 µl) was added to 150 µl GENMED substrate (dissolved in
GENMED buffer), and AMC release was measured using a microplate
reader (Thermo Fisher Scientific, Inc.) using 380 nm excitation and
430 nm emission filters. Calpain activity was expressed as µmol AMC
released/mg tissue protein at 37°C and in pH 7.5 per hour. The
calcium activity levels in the different experimental groups were
comparable.
Statistical analysis
All data are expressed as the mean ± standard error.
All the statistical analyses were performed using SPSS version 20.0
software (IBM Corp., Armonk, NY, USA). All P-values were two sided,
and P<0.05 was considered to indicate a statistically
significant difference. Multiple group comparisons were analyzed
using one-way analysis of variance, and the post hoc test employed
was Fisher's least significant difference test. The Pearson
correlation method was performed to analyze the association between
the expression of GRP78 protein and desmin protein.
Results
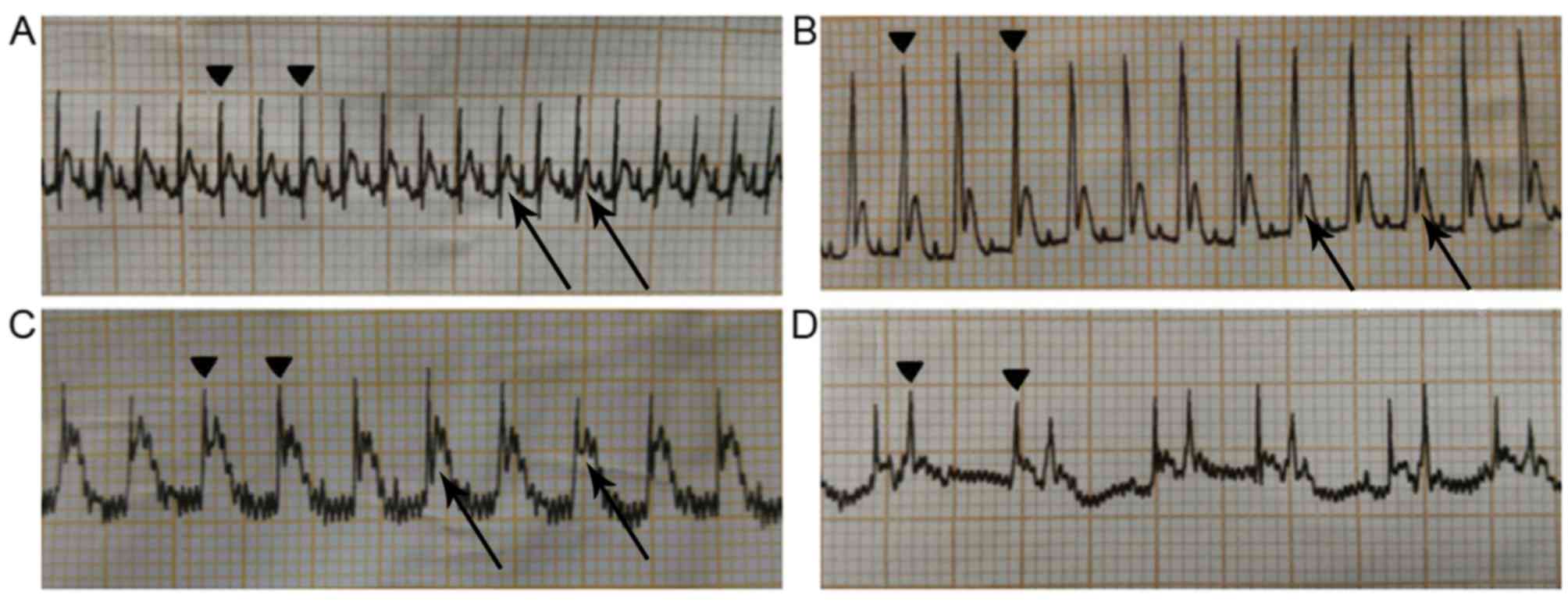
ECG of rats with acute I/R
Prior to LAD ligation, the ECG of each rat in the
I/R and FF+I/R groups was normal, as those in normal and sham group
(Fig. 1A). Following LAD ligation,
the distal myocardium may turn white or cyanosed. The ventricular
wall motion decreased and the ECG demonstrated that the amplitude
of the QRS wave increased significantly (Fig. 1B) and the ST segment was elevated
(Fig. 1C). Following reperfusion,
the myocardium was hyperemic and the cyanotic color disappeared.
The ST segment resolution and amplitude of QRS decreased, which
were additionally observed in the ECG. There are certain studies
that suggest that the ECG (such as ‘Wagner QRS score’) may be used
to estimate myocardial infarct size. However, arrhythmias,
including left/right bundle branch block and ventricular paced ECG,
are the confounders of QRS score calculation (15–17).
In the present study, certain rats of I/R group and FF+I/R group
exhibited arrhythmia waves in the ECG following reperfusion during
the experiments (Fig. 1D); the
most common reperfusion arrhythmias were ventricular tachycardia,
atrioventricular block and sinoatrial block. The present study
concluded that reperfusion arrhythmia may disturb the QRS score
calculation, and therefore did not consider using the ECG to
evaluate the degree of myocardial infarction, and it was only one
of the indicators to determine whether or not the model was
established successfully.
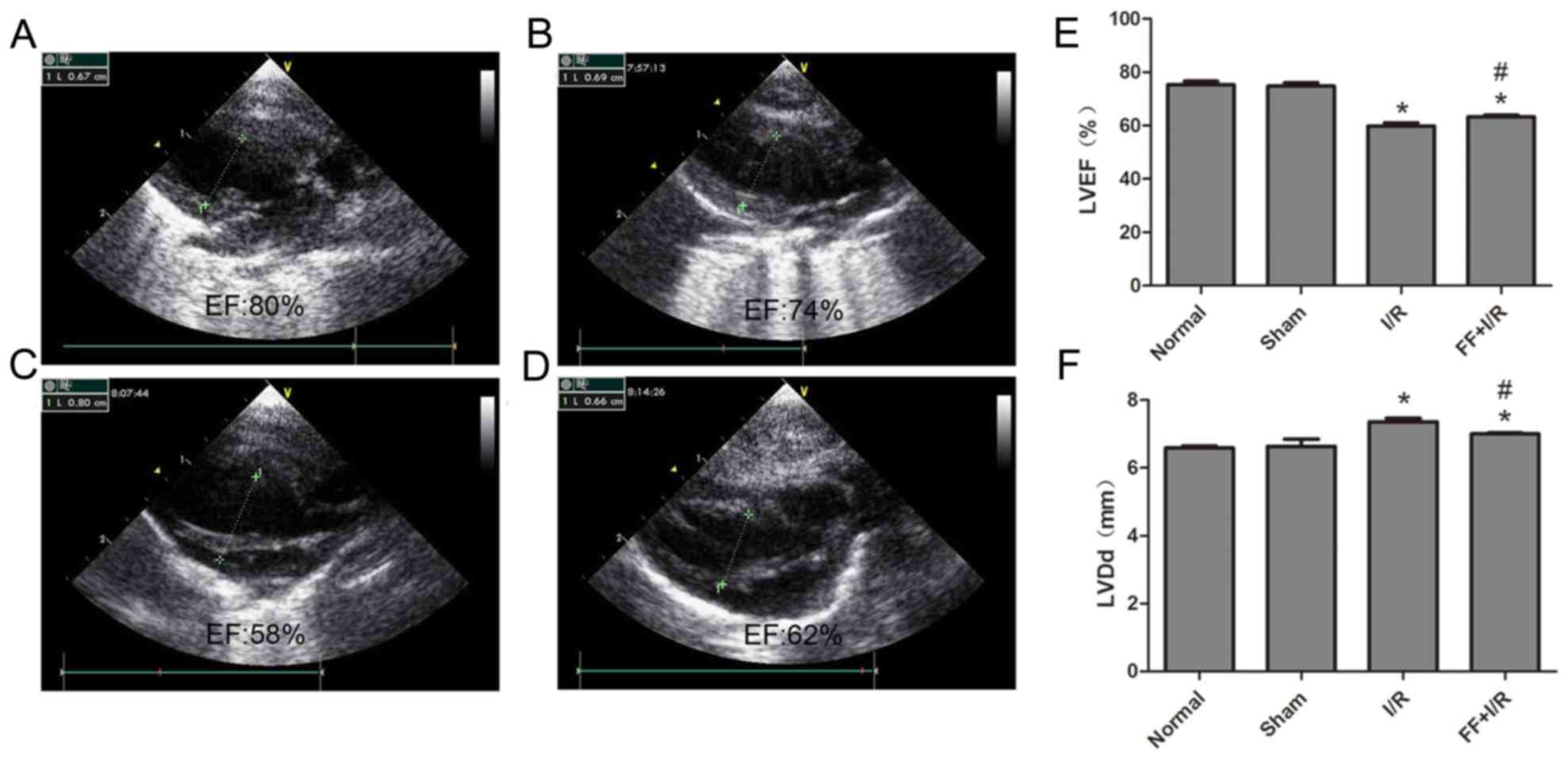
Cardiac function of each group
The cardiac function of each rat (n=8) was examined
by echocardiography. Compared with the normal group, the values of
LVEF and LVDd had no significant differences in the sham group
(normal, 75.25±3.81 vs. sham, 74.75±3.61, P>0.05; normal,
6.59±0.16 vs. sham, 6.64±0.60, P>0.05). Compared with the sham
group, the values of LVEF in the I/R group and FF+I/R group were
lower, while the LVDd values were higher (all P<0.05).
Furthermore, compared with the I/R group, the values of LVEF in the
FF+I/R group were higher (I/R, 59.75±3.62 vs. FF+I/R, 63.25±2.05,
P<0.05), and the LVDd values were lower (I/R, 7.33±0.16 vs.
FF+I/R, 7.00±0.19, P<0.05) (Fig.
2).
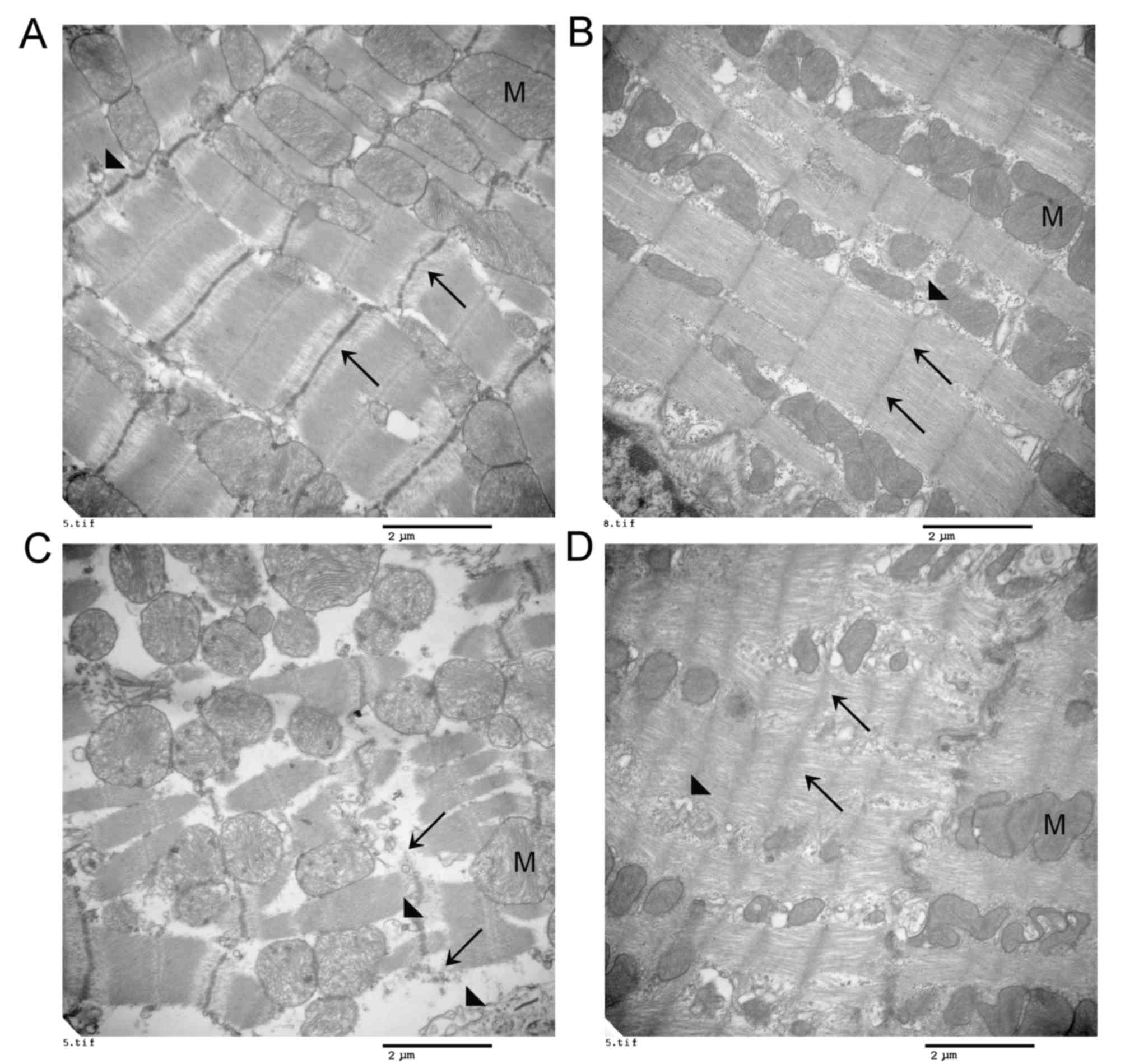
PPARα activation decreases
cytoskeletal structure damage caused by acute myocardial I/R
injury
Fresh myocardial tissues (1 mm3) were
excised from the cardiac apex following reperfusion, and the
mitochondrial ultrastructure was observed by transmission electron
microscopy (n=4). Regularly arranged endoplasmic reticulum,
mitochondria and complete Z lines were observed in the normal and
sham groups (Fig. 3A and B). In
the I/R group, the transmission electron microscopy observation
revealed destruction of the cytoskeleton and organelles, including
partial and even total rupture, in addition to disintegration of
the Z lines, dilation of the endoplasmic reticulum and turgidity of
the mitochondria, accompanied by distinct dislocation of the
endoplasmic reticulum and mitochondria (Fig. 3C). Notably, pretreatment with
fenofibrate alleviated these deleterious effects on the cardiac
subcellular structure induced by I/R injury (Fig. 3D).
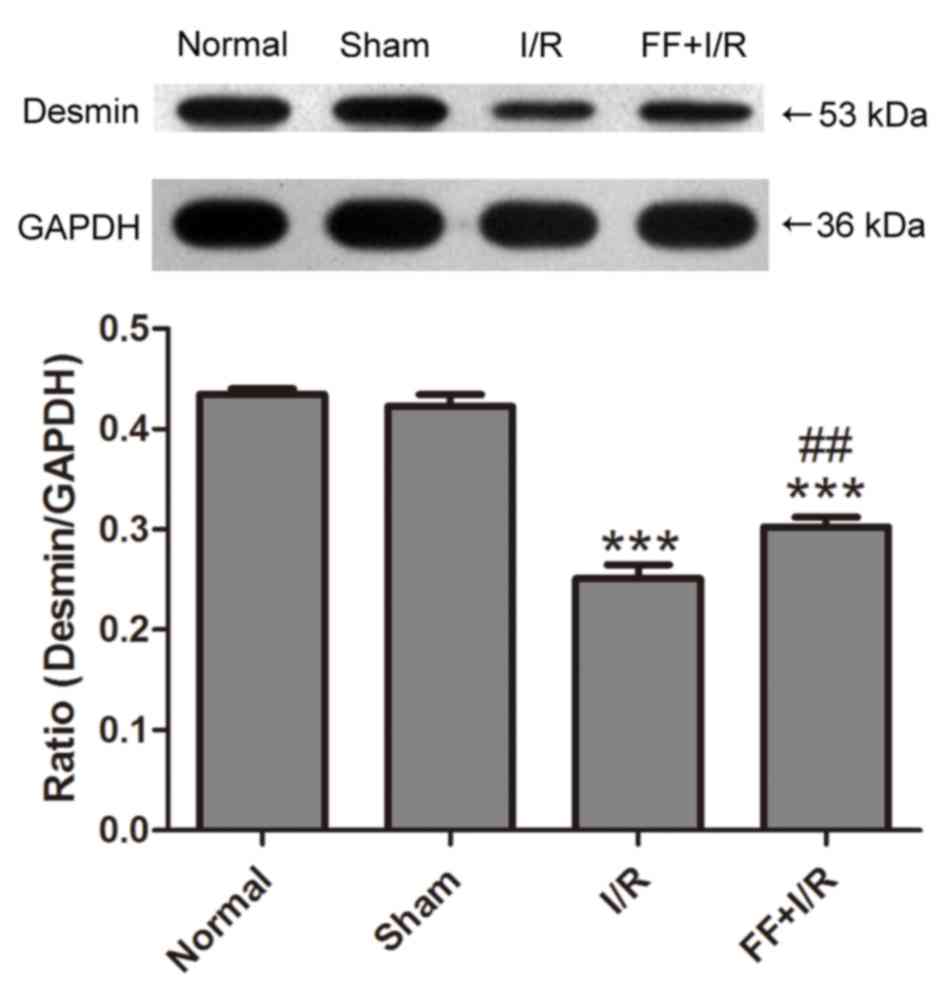
PPARα activation decreases the
degradation of desmin caused by acute myocardial I/R injury
The expression of desmin protein was detected by
western blotting (n=8). Compared with the normal group, the
expression of desmin protein in the myocardium was not
significantly different in the sham group (normal, 0.43±0.02 vs.
sham, 0.42±0.03; P>0.05). Compared with the sham group, I/R
induced a decrease in the expression levels of desmin, a sensitive
substrate of calpain (18), while
such alterations were suppressed by pretreatment with fenofibrate
(I/R, 0.25±0.04 vs. FF+I/R, 0.30±0.03; P<0.01; Fig. 4).
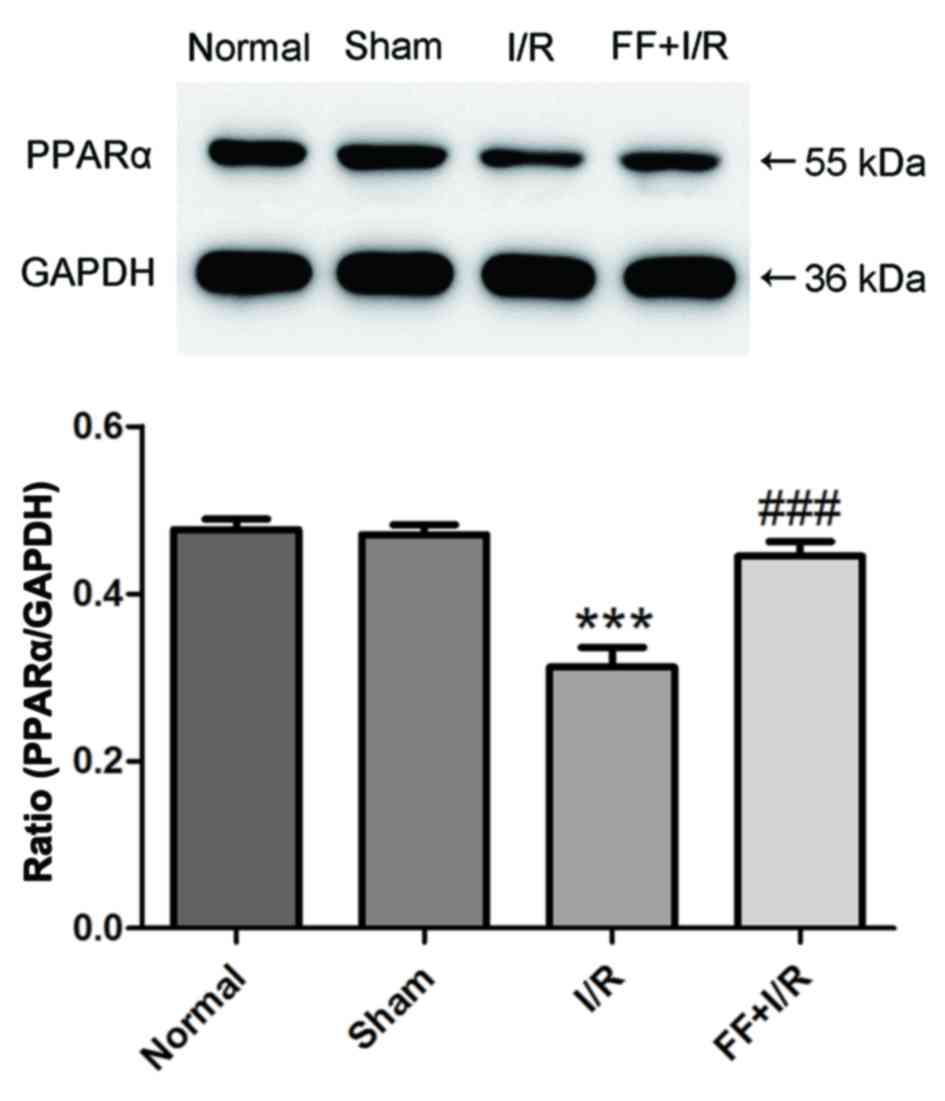
PPARα levels in each group
The expression of PPARα was measured by western
blotting (n=8). Compared with the normal group, the expression of
PPARα protein in the myocardium was not significantly different
compared with the sham group (normal, 0.48±0.04 vs. sham,
0.47±0.03; P>0.05). Compared with the sham group, I/R caused
marked decreases in PPARα protein expression levels (P<0.001),
but it was significantly upregulated by fenofibrate treatment (I/R,
0.31±0.07 vs. I/R+FF, 0.44±0.05; P<0.001; Fig. 5).
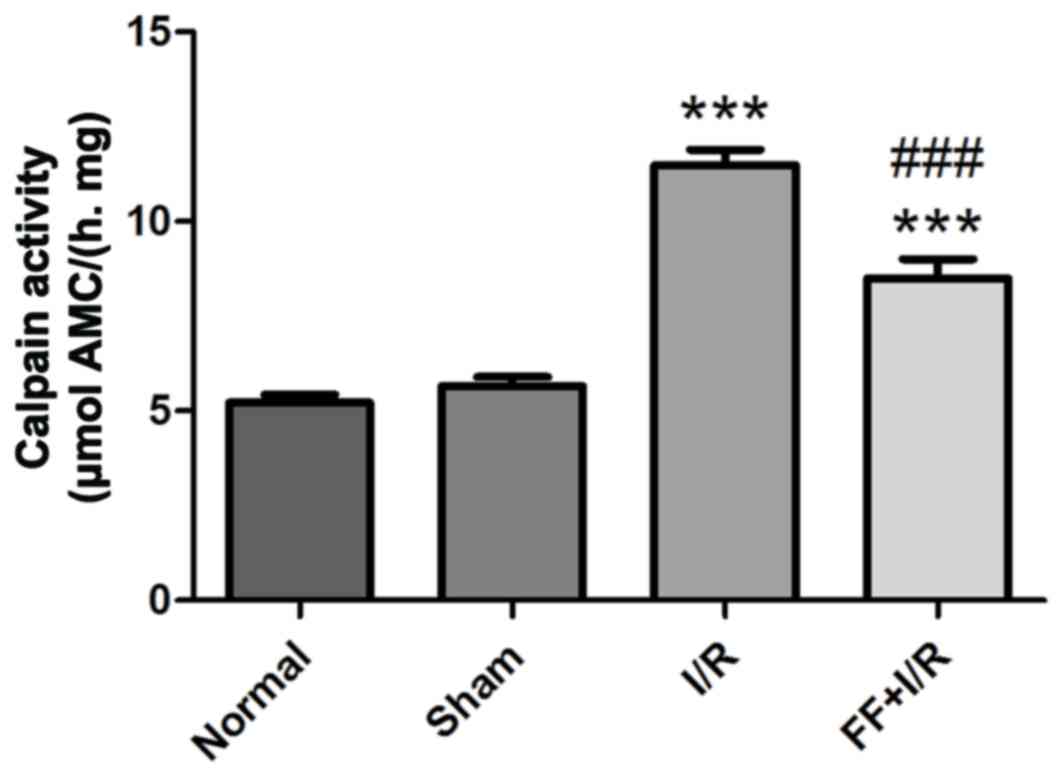
Calpain activity in myocardium
Calpain activity was measured in each group (n=8).
Compared with the normal group, there was no significant difference
in calpain activity in the sham group (normal, 5.21±0.61 vs. sham,
5.65±0.67; P>0.05). I/R injury had resulted in a marked increase
in calpain activity compared with that in non-I/R hearts
(P<0.001), whereas the increased calpain activity was
significantly attenuated by pretreatment with fenofibrate (I/R,
11.47±1.19 vs. FF+I/R, 8.49±1.44; P<0.001; Fig. 6).
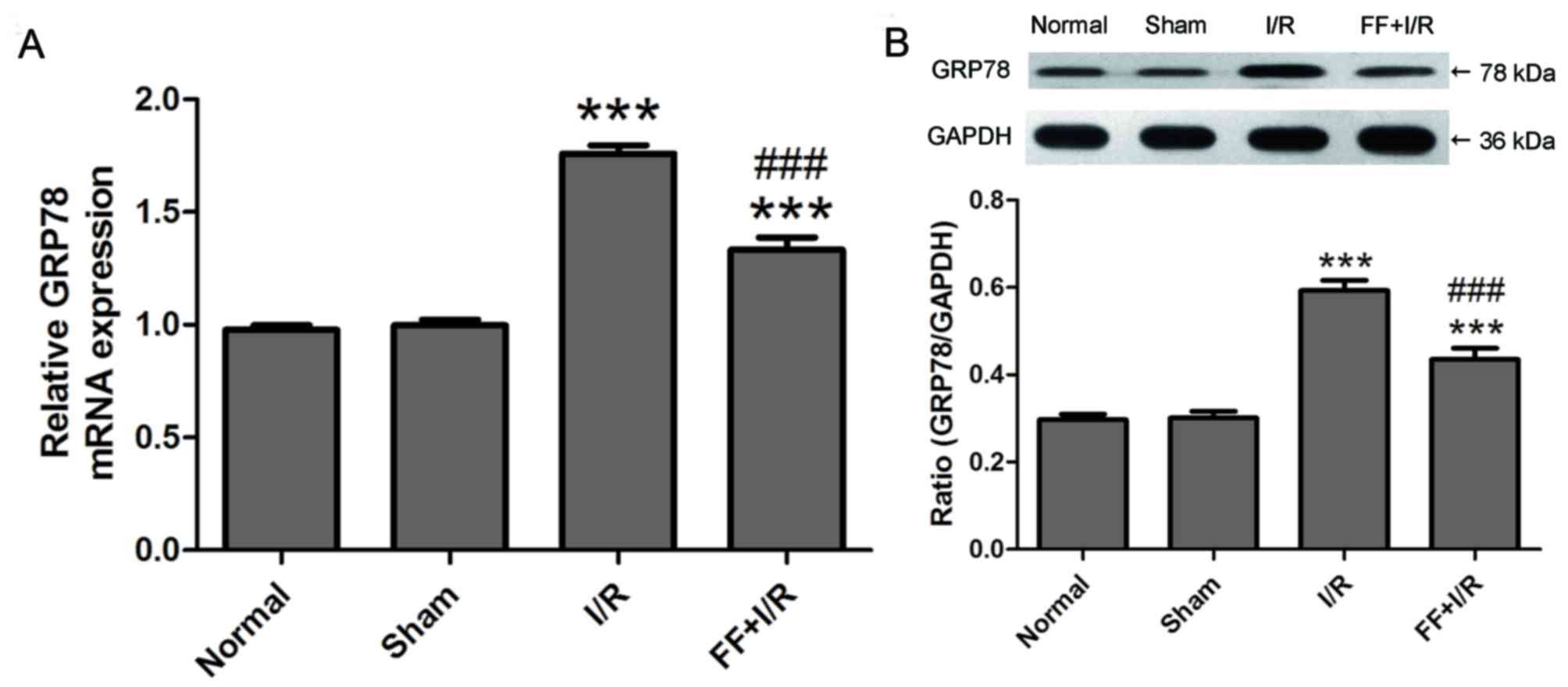
PPARα activation inhibits GRP78
overexpression caused by acute myocardial I/R injury
I/R injury in the tissues has been demonstrated to
be associated with ER stress induction. Thus, possible alterations
in the mRNA and protein expression of ER stress parameters were
evaluated (n=8). As presented in Fig.
7, compared with the normal group, there was no difference in
the expression of GRP78 mRNA and protein in the sham group (normal,
0.98±0.06 vs. sham, 1.00±0.07, P>0.05; normal, 0.30±0.04 vs.
sham, 0.30±0.04, P>0.05). Compared with the sham group, the
expression of GRP78 mRNA and protein was elevated by I/R
(P<0.001), and expression was reduced by pretreatment with the
PPARα agonist fenofibrate (I/R, 1.76±0.11 vs. FF+I/R, 1.33±0.16,
P<0.001; I/R, 0.59±0.07 vs. FF+I/R, 0.43±0.07, P<0.001;
Fig. 7).
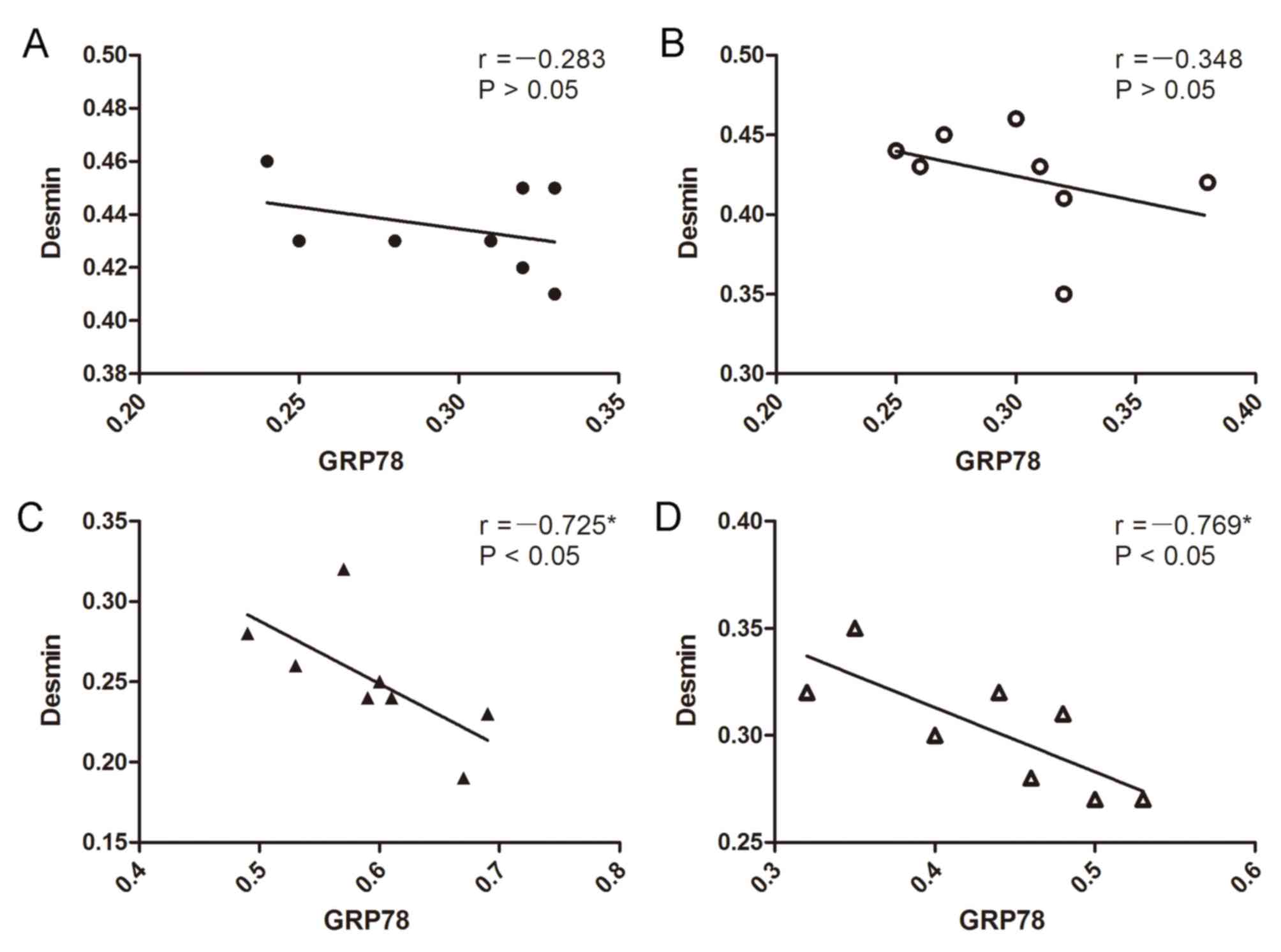
Correlation analysis between the
expression of GRP78 protein and desmin protein
Negative correlations were observed between the
expression of the GRP78 protein and desmin protein in the I/R group
and FF+I/R group (I/R, r=−0.725, P<0.05; FF+I/R, r=−0.769,
P<0.05; Fig. 8).
Discussion
In order to evaluate the severity of cardiac damage,
the present study observed the myocardial cytoskeleton structure of
a myocardial I/R injury rat model which was generated by a LAD
coronary artery ligation for 45 min and reperfusion for 120 min.
Following LAD ligation, the distal myocardium turned white or
cyanosed, ventricular wall motion decreased, and the ECG
illustrated that the amplitude of the QRS wave increased
significantly and the ST segment was elevated. Following
reperfusion, the myocardium was hyperemic and the cyanotic color
disappeared. ST segment resolution, a decrease in the amplitude of
the QRS wave and reperfusion arrhythmia were additionally observed
on the ECG, which confirmed that the acute MIRI models were
successfully established. Myocardial injury in the I/R group was
more serious than that in the normal and sham groups; swelling and
absence of the Z line were observed ultrastructurally, and the
cardiac function was decreased markedly. In addition, it was
demonstrated that myocardial PPARα mRNA and protein expression
levels decreased significantly in the I/R group, whereas
pretreatment with fenofibrate upregulated the expression of the
PPARα protein, and the subcellular structural injury and cardiac
dysfunction were relieved. These experiments suggested that PPARα
activation protected against acute myocardial I/R injury.
Acute myocardial I/R injury is one of the foci of
basic research and clinical studies, and its pathogenesis remains
to be clarified. Intracellular overproduction of oxygen free
radicals and calcium overload have been demonstrated to be
important pathological factors of myocardial I/R injury (19). In addition, calcium overload may
activate a number of calcium-dependent enzyme systems, including
phospholipase and calpain, promote the hydrolysis of membrane
phospholipids, which leads to damage to and degradation of the
cytoskeleton, and induce cellular morphological damage, dysfunction
and remodeling (20,21). In this process, if the degradation
of the cytoskeleton may be reduced, the damage to and dysfunction
of cells due to reperfusion may be relieved effectively.
Calpain is a neutral cysteine amino acid proteolytic
enzyme whose activation depends on the calcium concentration.
Calpain exists in various cells with inactive zymogen in normal
circumstances, and in pathological conditions, including I/R, the
sustained increase in intracellular Ca2+ concentration
may lead to the hydrolysis and activation of calpain, resulting in
the excessive degradation of its substrates, including desmin and
fibronectin (22–24). The present study revealed that
myocardial calpain activity was enhanced in the I/R group, and the
expression of desmin, a cytoskeletal protein, was significantly
decreased compared with the sham group; this suggested severe
biodegradation of the cytoskeleton. Desmin is one of the important
cytoskeletal structures of the myocardium, and structural damage to
desmin, which primarily exists in the Z line and the intercalated
disk of myocardial fibers, severely affects the systolic and
diastolic function of muscle fibers (25). In addition, the degradation
products of desmin may induce alterations in gene regulation in the
nucleus, and therefore cause myocardial remodeling (21,26).
In conclusion, the damage to desmin induced by the overactivation
of calpain has been identified to be one of the most important
factors of myocardial I/R injury. In the present study, it was
observed that compared with the I/R group, PPARα activation
effectively reduced the activity of calpain in I/R rat hearts, and
the expression levels of desmin were similar to those in the normal
and sham groups; this suggested that PPARα activation may
significantly alleviate calcium overload induced by I/R, and
protect the myocardial ultrastructure from the ‘secondary attack’
of alterations in the intracellular environment of the
myocardium.
In the process of MIRI, ER stress induced by
hypoxia-ischemia of tissues, oxidative stress, acidosis and energy
deficiency may continue by disrupting intracellular calcium
homeostasis, inducing injury to the I/R myocardium (27). Previous studies have suggested that
ER stress and calcium overload are essential prerequisites, and
form a vicious circle (28,29).
The ER becomes weak due to the swelling of myocardial cells and
endothelial cells at the early stage of reperfusion, and ER stress
may be aggravated. The calcium reserves of the ER tend to collapse
and calcium may be released sustainably. Finally, damage to cells
following reperfusion is aggravated by calpain system activation
(30). Thus, it may be seen that
the overload of calcium is associated with the function of the ER
in the myocardium in MIRI rats. The mitigation of ER stress
maintains the intracellular calcium homeostasis and reduces
cytoskeletal damage. GRP78, as a co-chaperone of ER stress, may
bind unfolded proteins which accumulate in the ER and protect cells
against ER dysfunction during the early stages of ER stress.
Elevated protein expression of GRP78 was positively associated with
the intensity of ER stress (31).
However, overexpression of GRP78 may not be enough to completely
prevent ER stress induced by I/R and, subsequently, structural
damage and functional disorders may emerge in the myocardium
(32). The present study revealed
that GRP78 mRNA and protein expression was increased significantly
in the I/R group compared with the sham group, which suggested that
I/R induced ER stress in cardiomyocytes, and GRP78 was not
sufficient to reduce the myocardial damage caused by ER stress;
this phenomenon was consistent with that reported in the literature
(32). Studies have demonstrated
that PPARα activation may relieve ER stress induced by I/R in the
liver and hypertrophied neonatal hearts (33,34).
In the present study, the expression of GRP78 mRNA and protein was
significantly decreased in the FF+I/R group, which suggested that
ER stress was reduced in FF+I/R cardiomyocytes. The trend between
the alterations in myocardial calpain activation and the intensity
of ER stress was similar in all groups, and a correlation was
identified between GRP78 and desmin. Thus, calpain activity
alleviation caused by PPARα activation may be associated with
alleviating the intensity of ER stress. The specific underlying
mechanism underlying the effect of PPARα activation on alleviating
ER stress requires further research.
Morphological alterations of mitochondria were
additionally observed under transmission electron microscopy in the
I/R group, including cytoskeletal damage, and PPARα activation
promoted the recovery of the morphology of the mitochondria. These
findings were consistent with a previous study (12). A previous study demonstrated that
ER stress may induce mitochondrial dysfunction (35). The present study predicts that the
effect of PPARα activation by fenofibrate protects against acute
myocardial I/R injury, and may be associated with the inhibition of
ER stress and ER stress-induced mitochondrial dysfunction in MIRI,
although this requires further clarification.
In conclusion, the present study may aid the
identification of the underlying mechanisms involved in the
protective effects of PPARα activation on acute myocardial I/R
injury. PPARα activation may suppress I/R-induced ER stress (with a
reduction in GRP78 expression), and thus the overactivation of
calpain may be prevented, as demonstrated by a reduction in the
damage to the cytoskeleton of cardiomyocytes and cardiac
dysfunction. The signal transduction involved requires further
investigation. Suppression of ER stress may be a new useful target
for protecting the I/R myocardium.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Open
Foundation of Key Laboratory of Myocardial Ischemia, Harbin Medical
University, Ministry of Education (China) (grant nos. KF201504 and
KF201706), the Postdoctoral Science-research Developmental
Foundation of Heilongjiang Province (grant no. LBH-Q12030) and the
Natural Science Foundation of Heilongjiang Province of China (grant
no. D201266).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM and JY designed the research; HM, JL, SZ, SL, MZ
and YJ performed the experiments; HM analyzed the data; HM and JL
wrote the paper; and all authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of The Second Affiliated Hospital of Harbin Medical
University (Harbin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare no that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMPK
|
AMP activated protein kinase
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein-78
|
|
I/R
|
ischemia/reperfusion
|
|
LAD
|
left thoracotomy and the left anterior
descending
|
|
LVDd
|
left ventricular end-diastolic
diameter
|
|
LVEF
|
left ventricular ejection fraction
|
|
PPARα
|
peroxisome proliferator-activated
receptor α
|
|
ROS
|
reactive oxygen species
|
|
RXR
|
retinoid X receptor
|
|
SERCA
|
sarcoplasmic reticulum Ca2+
ATPase
|
|
Suc-LLVY-AMC
|
Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin
|
|
MIRI
|
myocardial ischemia/reperfusion
injury
|
|
ECG
|
electrocardiogram
|
References
|
1
|
Westrate LM, Lee JE, Prinz WA and Voeltz
GK: Form follows function: The importance of endoplasmic reticulum
shape. Annu Rev Biochem. 84:791–811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Zhao D, Ren J and Yang J:
Endoplasmic reticulum stress and protein quality control in
diabetic cardiomyopathy. Biochim Biophys Acta. 1852:209–218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou H, Zhu J, Yue S, Lu L, Busuttil RW,
Kupiec-Weglinski JW, Wang X and Zhai Y: The dichotomy of
endoplasmic reticulum stress response in liver ischemia-reperfusion
injury. Transplantation. 100:365–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iurlaro R and Muñoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Ren F, Cheng Q, Bai L, Shen X, Gao
F, Busuttil RW, Kupiec-Weglinski JW and Zhai Y: Endoplasmic
reticulum stress modulates liver inflammatory immune response in
the pathogenesis of liver ischemia and reperfusion injury.
Transplantation. 94:211–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou JY, Liu Y, Liu L and Li XM: Protective
effect of hyperoside on cardiac ischemia reperfusion injury through
inhibition of ER stress and activation of Nrf2 signaling. Asian Pac
J Trop Med. 9:76–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kersten S: Integrated physiology and
systems biology of PPARα. Mol Metab. 3:354–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nan YM, Wang RQ and Fu N: Peroxisome
proliferator-activated receptor α, a potential therapeutic target
for alcoholic liver disease. World J Gastroenterol. 20:8055–8060.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Q, Baker C, Christian P, Naples M, Tong
X, Zhang K, Santha M and Adeli K: Hepatic mitochondrial and ER
stress induced by defective PPARα signaling in the pathogenesis of
hepatic steatosis. Am J Physiol Endocrinol Metabol.
306:E1264–E1273. 2014. View Article : Google Scholar
|
|
11
|
Palomer X, Capdevila-Busquets E, Garreta
G, Davidson MM and Vázquez-Carrera M: PPARα attenuates
palmitate-induced endoplasmic reticulum stress in human cardiac
cells by enhancing AMPK activity. Clin Investig Arterioscler.
26:255–267. 2014.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mo H, Zhao S, Luo J and Yuan J: PPARα
activation by fenofibrate protects against acute myocardial
ischemia/reperfusion injury by inhibiting mitochondrial apoptosis.
Int J Clin Exp Pathol. 9:10955–10964. 2016.
|
|
13
|
Yuan J, Wu J and Han ZG: Fenofibrate
improves energy metabolism and attenuates isoproterenol induced
acute myocardial ischemic injury in rats via PPAR alpha activation.
Zhonghua Xin Xue Guan Bing Za Zhi. 36:847–850. 2008.(In Chinese).
PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones MG, Anderson KM, Wilson PW, Kannel
WB, Wagner NB and Wagner GS: Prognostic use of a QRS scoring system
after hospital discharge for initial acute myocardial infarction in
the Framingham cohort. Am J Cardiol. 66:546–550. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tjandrawidjaja MC, Fu Y, Westerhout CM,
Wagner GS, Granger CB and Armstrong PW: APEX-AMI Investigators:
Usefulness of the QRS score as a strong prognostic marker in
patients discharged after undergoing primary percutaneous coronary
intervention for ST-segment elevation myocardial infarction. Am J
Cardiol. 106:630–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shiomi H, Kosuge M, Morimoto T, Watanabe
H, Taniguchi T, Nakatsuma K, Toyota T, Yamamoto E, Shizuta S, Tada
T, et al: QRS score at presentation electrocardiogram is correlated
with infarct size and mortality in ST-segment elevation myocardial
infarction patients undergoing primary percutaneous coronary
intervention. Circ J. 81:1129–1136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blunt BC, Creek AT, Henderson DC and
Hofmann PA: H2O2 activation of HSP25/27 protects desmin from
calpain proteolysis in rat ventricular myocytes. Am J Physiol.
293:1518–1525. 2007.
|
|
19
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh RB, Chohan PK, Dhalla NS and
Netticadan T: The sarcoplasmic reticulum proteins are targets for
calpain action in the ischemic-reperfused heart. J Mol Cell
Cardiol. 37:101–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumarapeli AR and Wang X: Genetic
modification of the heart: Chaperones and the cytoskeleton. J Mol
Cell Cardiol. 37:1097–1109. 2004.PubMed/NCBI
|
|
22
|
Chohan PK, Singh RB, Dhalla NS and
Netticadan T: L-arginine administration recovers sarcoplasmic
reticulum function in ischemic reperfused hearts by preventing
calpain activation. Cardiovasc Res. 69:152–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang CM, Gao L, Zheng YJ and Yang HT:
Berbamine protects the heart from ischemia/reperfusion injury by
maintaining cytosolic Ca(2+) homeostasis and preventing calpain
activation. Circ J. 76:1993–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
French JP, Quindry JC, Falk DJ, Staib JL,
Lee Y, Wang KKW and Powers SK: Ischemia-reperfusion-induced calpain
activation and SERCA2a degradation are attenuated by exercise
training and calpain inhibition. Am J Physiol Heart Circulat
Physiol. 290:128–136. 2006. View Article : Google Scholar
|
|
25
|
Wilding JR, Joubert F, de Araujo C, Fortin
D, Novotova M, Veksler V and Ventura-Clapier R: Altered energy
transfer from mitochondria to sarcoplasmic reticulum after
cytoarchitectural perturbations in mice hearts. J Physiol.
575:191–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calaghan SC, Guennec JYL and White E:
Cytoskeletal modulation of electrical and mechanical activity in
cardiac myocytes. Prog Biophys Mol Biol. 84:29–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krebs J, Agellon LB and Michalak M:
Ca2+ homeostasis and endoplasmic reticulum (ER) stress:
An integrated view of calcium signaling. Biochem Biophys Res
Communicat. 460:114–121. 2015. View Article : Google Scholar
|
|
28
|
Kim J, Choi TG, Ding Y, Kim Y, Ha KS, Lee
KH, Kang I, Ha J, Kaufman RJ, Lee J, et al: Overexpressed
cyclophilin B suppresses apoptosis associated with ROS and Ca2+
homeostasis after ER stress. J Cell Sci. 121:3636–3648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Zhu W, Tao J, Xin P, Liu M, Li J and
Wei M: Fasudil protects the heart against ischemia-reperfusion
injury by attenuating endoplasmic reticulum stress and modulating
SERCA activity: The differential role for PI3K/Akt and JAK2/STAT3
signaling pathways. PLoS One. 7:e481152012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Armstrong SC, Shivell CL and Ganote CE:
Sarcolemmal blebs and osmotic fragility as correlates of
irreversible ischemic injury in preconditioned isolated rabbit
cardiomyocytes ☆. J Mol Cell Cardiol. 33:149–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pavli M, Farmaki E, Merkourea S, Vastardis
H, Sklavounou A, Tzerbos F and Chatzistamou I: Endoplasmic
reticulum stress-associated chaperones, Bip/GRP78 and calnexin are
overexpressed in keratocystic odontogenic tumours. J Oral
Maxillofac Res. 5:e32014.PubMed/NCBI
|
|
32
|
Minamino T, Komuro I and Kitakaze M:
Endoplasmic reticulum stress as a therapeutic target in
cardiovascular disease. Circ Res. 107:1071–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lam VH, Zhang L, Huqi A, Fukushima A,
Tanner BA, Onay-Besikci A, Keung W, Kantor PF, Jaswal JS, Rebeyka
IM and Lopaschuk GD: Activating PPARα prevents post-ischemic
contractile dysfunction in hypertrophied neonatal hearts. Circ Res.
117:41–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pantazi E, Folch-Puy E, Bejaoui M,
Panisello A, Varela AT, Rolo AP, Palmeira CM and Roselló-Catafau J:
PPARα agonist WY-14643 induces SIRT1 activity in rat fatty liver
ischemia-reperfusion injury. Biomed Res Int. 2015:8946792015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luciani DS, Gwiazda KS, Yang TL, Kalynyak
TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM and
Johnson JD: Roles of IP3R and RyR Ca2+ channels in endoplasmic
reticulum stress and beta-cell death. Diabetes. 58:422–432. 2009.
View Article : Google Scholar : PubMed/NCBI
|